Abstract
The aim of this study was a comparison of the micro-tensile bond strength (μTBS) to dentin and microleakage of in vitro class V restorations of three different ion-releasing restorative materials under various pH conditions: giomer, a resin-modified glass ionomer (RMGI), and a new alkasite material. A μTBS test was performed using a universal testing machine, immediately and after storage at different pH (4, 7, and 10) buffer solutions (n = 15) over 24 h, and the failure mode was analyzed. For microleakage analysis, class V restorations were performed on extracted premolars, which were sectioned and stored in pH 4-, 7-, and 10-buffered fluorescent 0.02% rhodamine B dye. The specimens were observed under a confocal laser scanning microscope (CLSM) and scored using the acquired images. There were no significant differences in the μTBS according to the type of material (p = 0.518). The giomer showed a decreased bond strength under the pH 4 condition compared with the immediately tested or pH 7-stored specimens (p ≤ 0.043). In the microleakage analysis, the class V restoration with giomer showed a higher microleakage than RMGI or alkasite (p = 0.001). For RMGI and alkasite, the specimens stored at pH 4 showed a significantly lower microleakage than those stored at pH 7 (p = 0.028). RMGI and alkasite can be adopted as restorative materials in generalized or localized low-pH conditions.
1. Introduction
Ion-releasing dental restoratives, especially fluoride, have garnered increased attention, owing to their anti-cariogenic effects and enhanced remineralization of dental hard tissues, especially in pathologic conditions [1,2,3]. There are several fluoride-containing dental restoratives available on the market, including glass ionomers, resin-modified glass ionomers (RMGI), polyacid-modified composites (compomers), giomers, and composites [1]. Glass ionomer cements chemically bond to tooth structure, and their fluoride release ability provides a caries-preventive effect [4]. RMGI is a hybrid material of traditional glass ionomer cement with a small supplement of light-curing resin [5]. Giomer is based on the incorporation of surface pre-reacted glass-ionomer fillers into a resin matrix. It has fluoride-releasing property which might enhance the antibacterial effects in restoration [6]. Recently, a new ‘alkasite’ restorative material was introduced, comprising an alkaline filler, which releases acid neutralizing ions along with fluorides, calcium and hydroxide ions along changes of the oral environment [7]. Alkaline glass fillers were shown to be able to raise the pH and form fluorapatite in media containing phosphate, which could explain the low incidence of secondary caries in alkasite restorations [8]. Ion release from fillers has been reported to induce the remineralization of dental hard tissues in previous studies [9]. Fluoride-containing restorative materials may feature greater longevity, a reduced incidence of marginal failure, an elevated concentration of fluoride in contingent plaques, and antibacterial action when compared with non-fluoride-releasing materials [10].
Intraoral pH can be largely influenced by dietary intake, but also by intrinsic factors, such as regurgitation [11], and by the flow rate and buffering capacity of saliva [12]. In a healthy oral cavity, the pH is maintained near neutrality (6.7–7.3) by saliva [13]. In pathological conditions, such as periodontitis and dental caries, changes in the microbial and environmental dynamics may yield a shift of the pH level in lesions. The pH, after a sucrose rinse on localized plaque, can decrease to a cariogenic pH of 4–4.5 [14]. Bacterial acid production results in a decrease in pH, which may be the driving force behind the carious process [15]. Natural tooth surfaces and filling materials are not only affected by bacterial acids, but also by strong acids that induce erosion. This may be induced by extrinsic acids (soft drinks) and intrinsic acids (refluxed gastric acids). During reflux episodes, gastric acid attacks the tooth structure with a pH of 1.5–3.1 [16,17].
The aim of this study was to evaluate the bond strength to the dentin of three different ion-releasing restorative materials: a giomer, an RMGI, and an alkasite restorative material and the marginal integrity of class V restorations. The null hypotheses were that (1) the type of materials, (2) various pH conditions, and (3) the locations in the margin (enamel or dentin) would have no significant effect on the bond strength to the dentin and the marginal microleakage of the class V restorations.
2. Materials and Methods
2.1. Materials
For the in vitro study, three ion-releasing materials were used and are given as follows: giomer (Beautifil Flow Plus; Shofu, Kyoto, Japan); RMGI (Fuji II LC; GC, Tokyo, Japan); and alkasite (Cention N; Ivoclar Vivadent, Schaan, Liechtenstein). In addition, one dentin adhesive (All-Bond Universal; Bisco, Schaumburg, IL, USA) was used with Beautifil Flow Plus and Cention N. The compositions of the materials used in this study are summarized in Table 1.

Table 1.
The composition of materials used in this study. % indicates vol% in the table.
For micro-tensile bond strength (μTBS) test, 48 extracted human third molars were prepared and disinfected with 0.2% thymol solution. For microleakage analysis, 27 human caries-free mandibular premolars were obtained from patients for orthodontic reasons and were disinfected with a 0.2% thymol solution. The included teeth were obtained from patients, as approved by the Kyung Hee University Dental Hospital Institutional Review Board (KH-DT21026), and all methods were performed in accordance with the relevant guidelines and regulations.
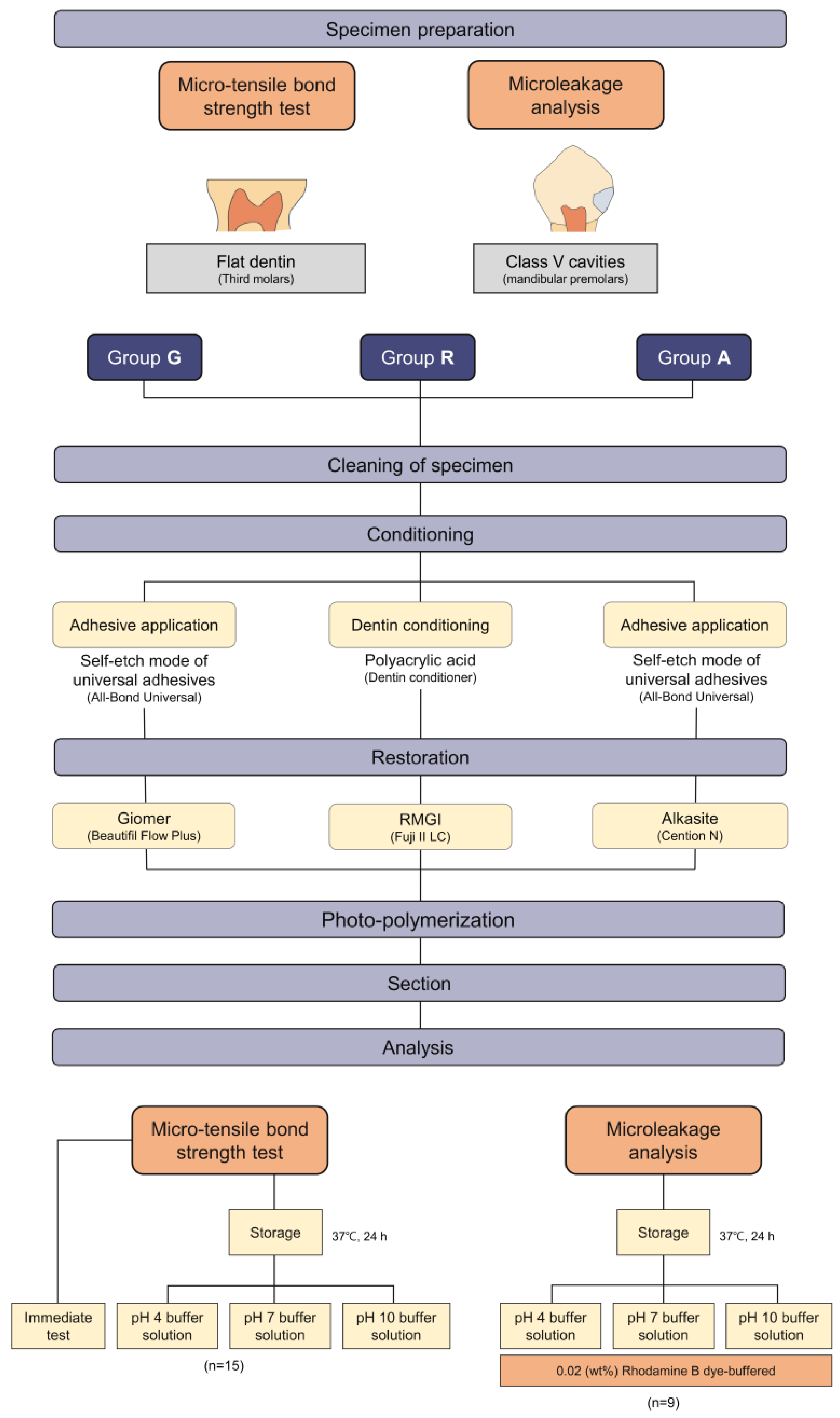
The scheme of this study and the experimental groups and each restoration procedures can be seen in Figure 1.
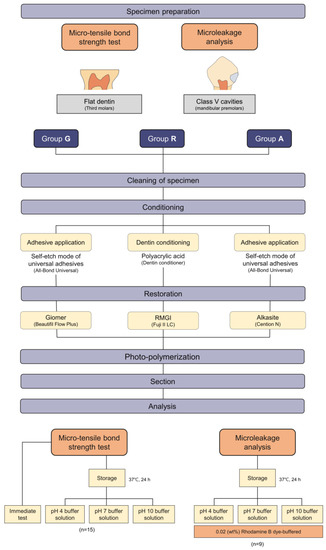
Figure 1.
The scheme of the study and classification of experimental groups.
2.2. Micro-Tensile Bond Strength (μTBS) Test
2.2.1. Dentin Specimen Preparation
The occlusal surfaces of the extracted third molars (n = 48) were flattened until superficial, fresh dentin was exposed. The top surfaces of the dentin specimens were serially polished with 320-, 400-, and 600-grit silicon carbide paper.
2.2.2. Group Assignment, Surface Treatment and Restoration Procedure
The specimens were randomly divided and allocated into three groups (n = 16 per each group): group G (giomer), group R (RMGI), and group A (alkasite).
For group G, the dentin surfaces were thoroughly cleaned with a 3-way syringe for 20 s and dried with air. All-Bond Universal was applied to the cleaned surface, and air-dried for 10 s. An adhesive layer was photopolymerized (Bluephase G2; Ivoclar Vivadent) for 20 s at the intensity of 1200 mw/cm2. Beautifil Flow Plus was applied to a cylindrical Teflon-coated mold (diameter = 10 mm, depth = 10 mm). The thickness of each increment was approximately 2 mm, and a restoration with a total thickness of 10 mm was formed. Each increment was light-cured for 10 s.
For group R, the dentin surfaces were conditioned with dentin conditioner (GC) for 20 s, rinsed for 30 s, and left moist. Fuji II LC was mixed and filled into the mold (diameter = 10 mm, depth = 10 mm) according to the manufacturer’s instructions. The restoration was light-cured for 20 s.
For group A, the dentin surfaces were thoroughly cleaned with a 3-way syringe for 20 s. All-Bond Universal was applied to the surface, and photopolymerized like as in Group G. Cention N was mixed and filled into the mold (diameter = 10 mm, depth = 10 mm) according to the manufacturer’s instructions. The restoration was light-cured for 20 s.
2.2.3. Micro-Tensile Bond Strength (μTBS) Test and Failure Mode Analysis
Each specimen was sectioned perpendicular to the bonded surface to obtain dentin restoration beams with a surface area of 1 × 1 mm2 using a high-speed diamond saw (Isomet 5000; Buehler, Lake Bluff, IL, USA). The sectioned beams were divided into four subgroups; immediate, pH 4 storage, pH 7 storage, and pH 10 storage (n = 15). For the immediate group, after the section, the specimens were fixed to the μTBS jig with a cyanoacrylate adhesive (Zapit; Dental Ventures of America, Corona, CA, USA). The μTBS was measured using a universal testing machine (AGS-X; Shimadzu, Kyoto, Japan) at a crosshead speed of 1 mm/min until bond failure occurred. For the pH 4-, pH 7- and pH 10-storage subgroups, the sectioned beams of each group were stored in pH 4, 7, and 10 buffer solutions: pH 4 (0.05 M potassium biphthalate aqueous solution); pH 7 (0.003 M potassium dihydrogen phosphate and 0.004 M disodium hydrogen phosphate); and pH 10 (0.006 M sodium borate decahydrate and 0.038 M sodium carbonate anhydrate aqueous solution) (Samchun Chemical, Seoul, South Korea) at 37 °C for 24 h. pH measurements were carried out before and after completed storage using pH meter (ORION Star A211, Thermo Scientific, Waltham, MA, USA). μTBS was measured using a universal testing machine (AGS-X) until bond failure occurred. The obtained data were converted to MPa using the Trapezium-X software (Shimadzu).
The specimens were then examined under a stereo-microscope (SZN745; Sunny, Shanghai, China) at ×40 magnification to identify the location of bond failure. Failure patterns were classified into one of four modes: adhesive failure, mixed failure, cohesive failure in dentin and cohesive failure in restorative.
2.3. Microleakage Evaluation
2.3.1. Specimen Preparation for Microleakage Evaluation
After surface debridement with a hand-scaling instrument and cleaning with a rubber cup and pumice slurry of extracted premolars (n = 27), a trapezoidal Class V cavity preparation (which has a long base on the buccal surface and a short one opposite on the pulpal side) was placed on the buccal surface at the cementoenamel junction. Preparations were made using a no. 170 carbide bur (Kerr, Orange, CA, USA). The prepared sections were measured to be 5 mm long in the occlusal margin, 4 mm in the gingival margin, 3 mm in width, and 2 mm deep with the occlusal margin in enamel and the gingival margin in dentin or cementum.
2.3.2. Group Assignment and Confocal Laser Scanning Microscopic Analysis
The teeth were randomly divided into three groups (n = 9) as described in Section 2.2.2. The same restoration procedures were performed into the class V cavities. After the restoration procedures, finishing and polishing were performed with an aluminum oxide disc system (Soflex discs; 3M ESPE, St. Paul, MN, USA) with serial application according to the manufacturer’s instructions. The specimens were coated with two layers of nail polish, leaving a 1 mm space around the cavity margins to avoid ingress of dye through other microfissures and cracks. The specimens were immersed for 24 h in a buffered fluorescent 0.02% rhodamine B dye (pH 4, 7, and 10). After drying at ambient temperature for 2 h, the specimens were embedded in a self-curing acrylic resin (MTDI, Daejeon, Korea) for cold mounting and were sectioned parallel to the long axis of the tooth to observe the bucco-lingually sectioned surfaces with Isomet 5000. Three sectioned specimens were selected from the middle segments of each tooth. The specimens were examined using a confocal laser scanning microscope (CLSM; LSM700, Carl Zeiss, Oberkochen, Germany). The absorption and emission wavelengths of rhodamine B were 540 nm and 590 nm, respectively. Each image was acquired in the Z-stack mode using 30 sections with a 10–15 μm thickness along the z-axis and in tile scan mode to capture the entire surface of the specimens. Image analysis was performed using ZEN 3.1 (Blue-edition, Zen Software Ltd., Manchester, UK), and images were acquired in the superimposition mode and fluorescent mode.
2.3.3. Assessment of Microleakage
The microleakage score was recorded using the 0–6 scoring method [18]. The score was measured in both the enamel and gingival margins of class V cavities using both the superimposed and fluorescent modes of images. The scoring standards were as follows.
- No dye penetration.
- Dye penetration to one-half of the occlusal or gingival wall.
- Dye penetration extending to the end of the occlusal or gingival walls.
- Dye penetration through the gingival or occlusal wall to one-third of the axial wall.
- Dye penetration through the gingival or occlusal wall to two-thirds of the axial wall.
- Dye penetration throughout the axial wall.
According to different restorative materials and pH conditions, nine subgroups were categorized and scored by the author (H.-J. Kim), and the data were acquired and analyzed using the mean and standard deviation of each group (n = 9).
3. Statistical Analysis
The sample size was determined according to previous studies on the μTBS test [19,20] and microleakage [19,21] using G Power 3.1.9.7.
The μTBS data were analyzed by two-way analyses of the variance (ANOVA) and a Bonferroni test for post hoc analysis (α = 0.05) using the SPSS software (version 25.0; IBM Corp, Armonk, NY, USA) for determining the effects of the pH or the restorative materials. The microleakage scores were analyzed using three-way ANOVA. The data were analyzed and interpreted using the main effect model.
4. Results
4.1. Micro-Tensile Bond Strength (μTBS) Test and Failure Mode Analysis
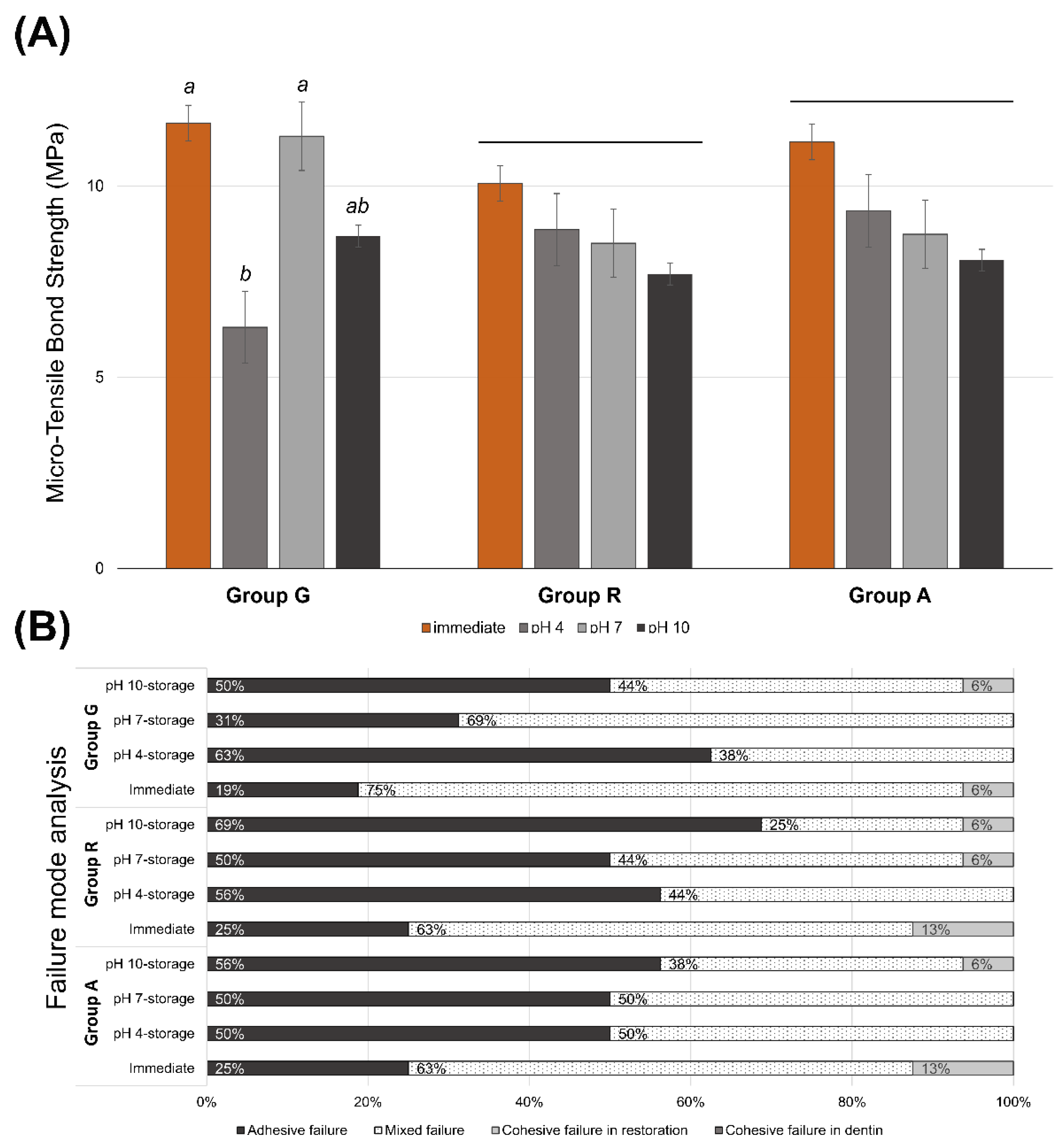
Figure 2A shows the μTBS values of all experimental groups. There were no significant differences in the bond strength among the restorative materials (p = 0.518). In groups R and A, there were no significant differences in bond strength between the immediate test and the pH 4-, pH 7-, and pH 10-buffered storage (p ≥ 0.487). However, in group G, the immediately tested (p = 0.035) and the pH 7-stored specimens (p = 0.043) exhibited a significantly higher bond strength than the pH 4-stored specimens. Table 2 presents the mean and standard deviation of the μTBS data used in this study. At pH 4, group A showed significantly higher bond strength than group G (p = 0.042) (Table 2). Figure 2B exhibits the distribution of failure analysis of the debonded surface. There was a tendency to increase the proportion of adhesive failure in the sub-groups, which showed relatively lower bond strength. Cohesive failure in dentin was not observed in all experimental groups.
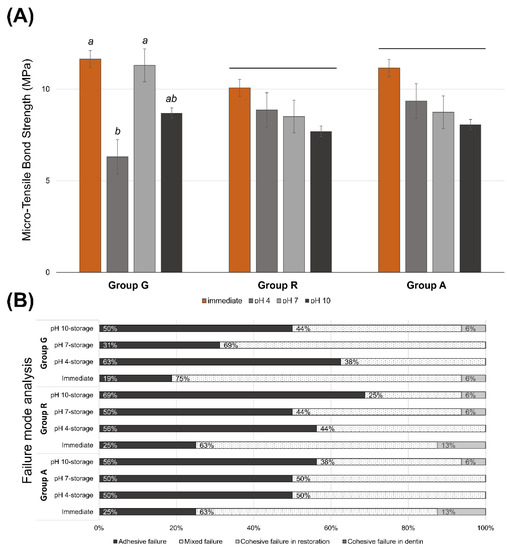
Figure 2.
(A) Micro-tensile bond strength of the experimental groups. Same lower letters on top of the bar denote no statistical differences between groups (p > 0.05). Long bar means no significant differences among groups. (B) Distribution of failure modes.

Table 2.
Micro-tensile bond strength (MPa).
4.2. Microleakage Evaluation with CLSM Images
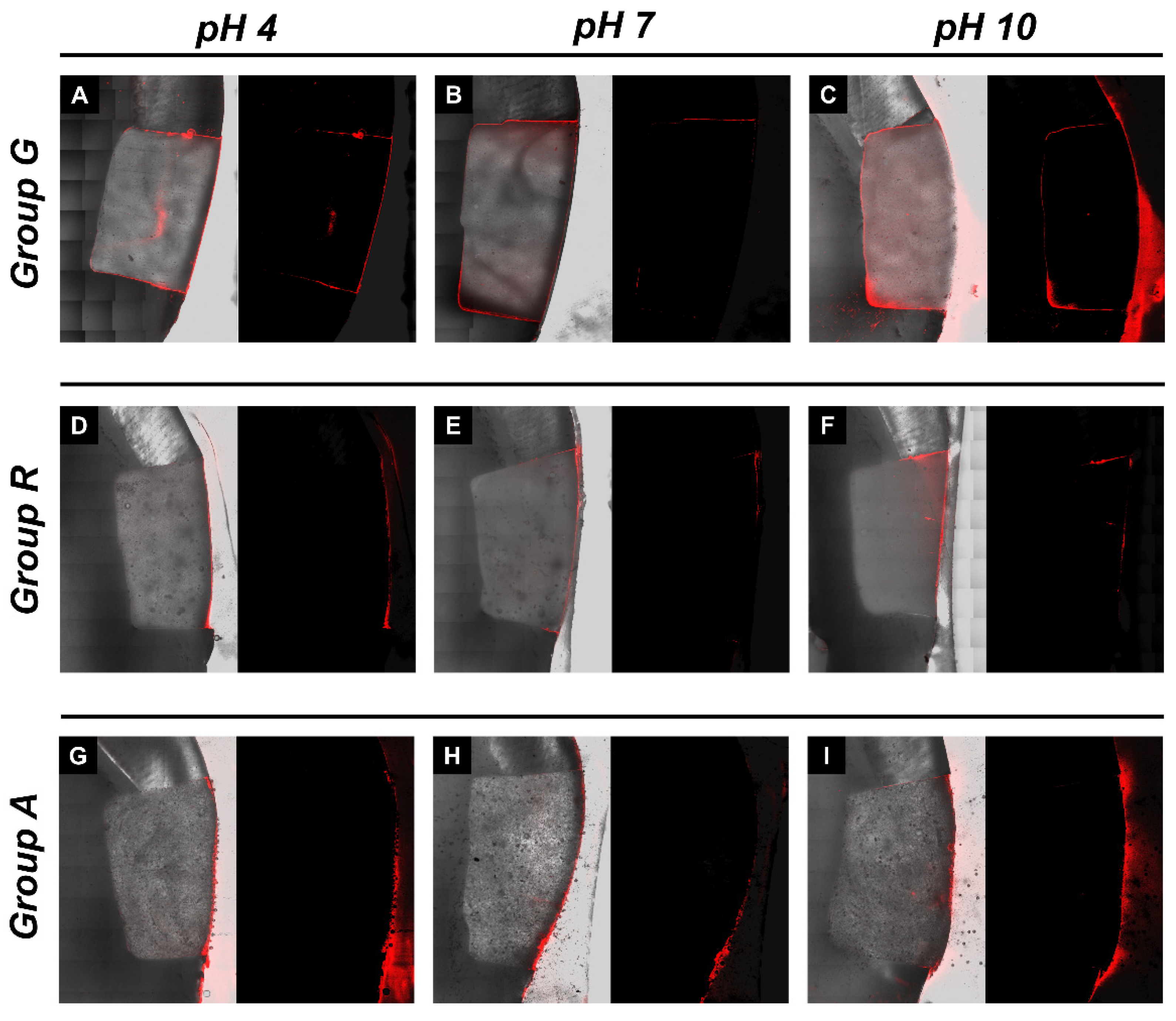
Figure 3 shows representative CLSM images for an assessment of the microleakage of class V restorations with different restorative materials and under different pH conditions. All images exhibited red fluorescence on the buccal surface of the class V restorations. In group G, more dye penetration into the axial wall was observed (Figure 3A–C). In groups R and A, less dye penetration was observed, and penetration of the axial wall was rarely observed. In Figure 3, the left image with a gray background is a superimposed image with a sectioned microscopic view and red fluorescence, and the right image with a black background is the fluorescent mode. Both images were assessed for microleakages.
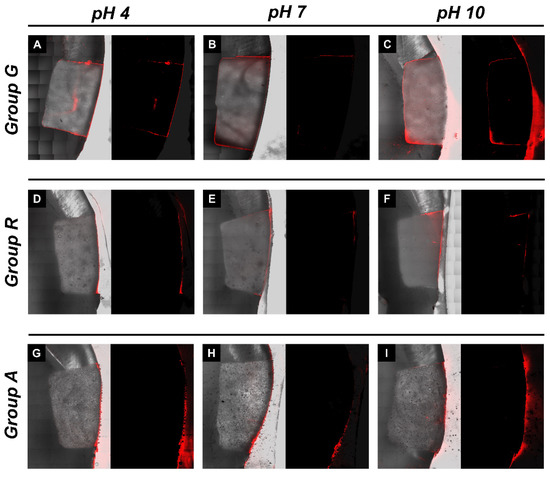
Figure 3.
Microleakage analysis of the experimental groups. Representative images of confocal laser scanning microscopic photograph showing the rhodamine B dye penetration. (A–C) Representative images of pH 4, pH 7 and pH 10-stored giomer restorations, respectively; (D–F) pH 4-, pH 7- and pH 10-stored RMGI restorations, respectively; (G–I) pH 4-, pH 7- and pH 10-stored alkasite restorations, respectively.
4.3. Microleakage Evaluation with Score
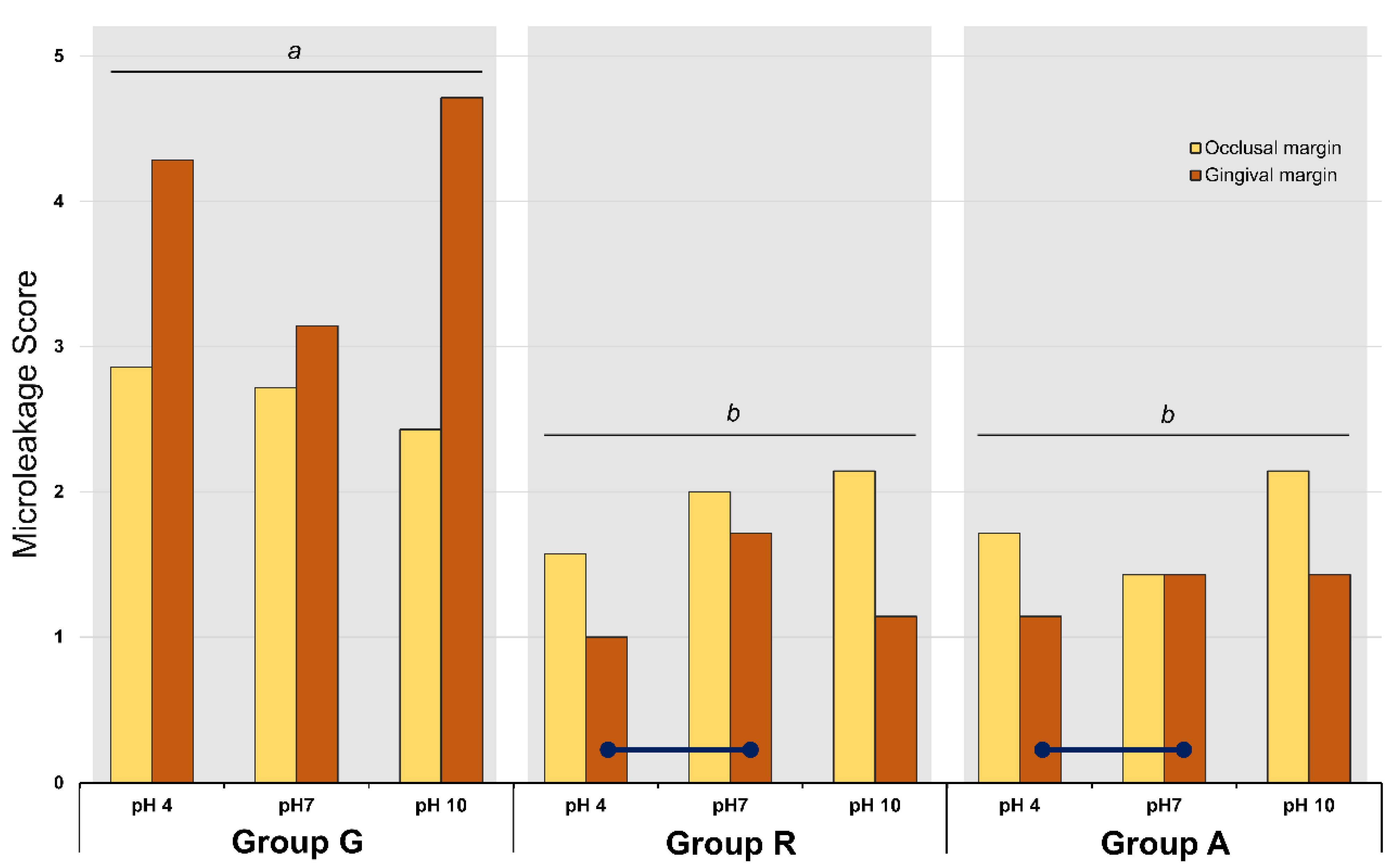
Figure 4 and Table 3 present the microleakage scores of all experimental groups. Three-way ANOVA revealed that the factors ‘pH’, ‘location of margin’, and ‘material’ significantly affected the microleakage score (p = 0.028). Although the interactions between these three factors were statistically significant, the data were interpreted using the main effect model. With regards to the materials, groups R and A exhibited a significantly lower microleakage score than group G (p = 0.001). In group G, statistically significant differences were noted between the occlusal and gingival margin scores (p = 0.001); however, no significant difference was detected between the different pH conditions (p > 0.05). The microleakage score in the gingival margin was higher than that in the occlusal margin (p = 0.001). In group R, there were significant differences between the different pH conditions and between the locations of the margin. In that group, the microleakage scores in the occlusal margin were significantly higher than those in the gingival margin (p = 0.001). In the gingival margin of group R, the microleakage score of the pH 4-stored specimen was significantly lower than that of the pH 7-stored specimens (p = 0.028). In the gingival margin of group A, the pH 4-stored specimens showed lower microleakage scores than the pH 7-stored specimens (p = 0.028). The enamel margins showed a higher microleakage than the gingival margins (p = 0.001).
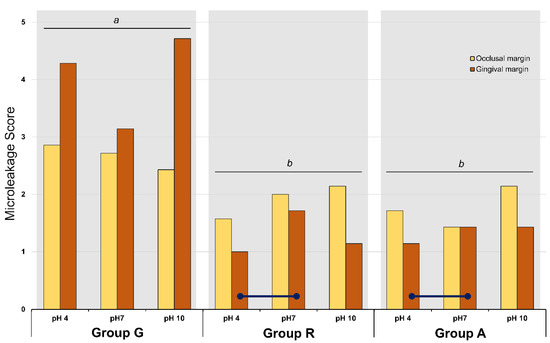
Figure 4.
Mean scores of microleakage analysis of the experimental groups. Different lower letters on top of the long bar denote statistically significant difference among groups (p = 0.001). Navy lines mean statistically difference between the marked sub-groups (p < 0.05).

Table 3.
Microleakage score assessment.
5. Discussion
Despite the remarkable developments of restorative dentistry, there have been demands for ion-releasing restorative materials, especially fluoride, owing to their inherent functions. In hypomineralized conditions, such as molar incisor hypomineralization, enamel hypoplasia, amelogenesis imperfecta, and white spot lesions, fluoride-releasing materials are considered anti-caries agents [22,23]. Several fluoride-containing restorative materials are available on the market, including RMGI, compomers, giomers, and bioactive composite resins. These hybrid materials have been introduced to overcome the problems associated with conventional glass ionomers and composite resins and maintain their clinical advantages [7]. Fluoride-releasing materials have some limitations, such as insufficient physical properties and brittleness in high-stress-bearing areas. The need to improve fluoride-releasing dental materials has led to increasing research efforts focused on reinforcement techniques.
In this study, the bond strengths of three different ion-releasing materials were evaluated under various pH conditions. There was no significant difference in bond strength among the materials. For RMGI and alkasite, there was no difference in bond strength among the specimens immediately tested and those tested after storage under different pH conditions. However, for giomer, the bond strength after storage in a pH 4 buffer was significantly lower than for those immediately tested or tested after pH 7-storage.
Some functional monomers in dentin adhesives, especially 10-MDP, can produce an acid-base resistant zone (ABRZ) on the adhesive interface [24]. This is characterized as a structure at the resin-dentin interface that is resistant to acid-base challenges and differs from the hybrid layer [25]. In universal dentin adhesives, a thin ABRZ was observed in a self-etch application with a thick hybrid layer, and no ABRZ was observed in the etch-and-rinse mode [26]. In situations of acid challenge, ABRZ might be a protective layer against attack. Although the giomer exhibited a comparable bonding performance under other pH conditions (pH 7 and 10) in this study, it showed a significantly lower bond strength under acidic conditions at pH 4. It is speculated that the thin ABRZ might not resist acid attacks on the bonding interface, which decreases the bonding durability of the giomer stored at pH 4. In this study, universal dentin adhesive with self-etch application was used, for the theoretical ability to etch and infiltrate simultaneously, thus preventing discrepancies between demineralization and infiltration [27] and the capability of ABRZ formation [26]. The ABRZ in self-etch mode of universal adhesives might not be enough to confront the acidic changes of environment in giomer restoration in this study. Universal dentin adhesive with self-etch application was also used in alkasite restoration. However, there was no significant difference among various pH conditions. It is assumed that alkasite has high alkalizing potential with the release of hydroxyl ions [28]. Kasraei S et al. reported that the RMGI and alkasite showed a significant increase in the pH following acidification of the environment [28].
Three factors (‘pH’, ‘location of margin’, and ‘materials’) significantly affected the microleakage scores of in vitro class V restorations. There were significant differences in the microleakage scores between the three materials (p = 0.001). Giomers showed a higher microleakage score than RMGI and alkasite. Beautifil Flow Plus, which was used in this study, has a relatively low (47%) volume of the filler and a higher volume of the resin matrix, which makes it susceptible to polymerization shrinkage. The resin matrix of giomer is similar to that contained in a composite, with a base around bis-GMA and other monomers added for modifying its rheological and polymerization properties [29]. The setting reaction of the giomer is performed only by photopolymerization, which is the same as that of a composite. This involves the creation of a resin network and covalent bonds with inorganic (especially S-PRG) fillers. Tsujimoto et al. reported that bulk-fill giomers exhibit significantly higher volumetric shrinkage than bulk-fill composite resins [30]. RMGI and alkasite have a compensatory mechanism against polymerization shrinkage of the matrix, which is a hygroscopic expansion with water sorption [7,31]. In addition, the presence of a special filler (isofiller) in the alkasite, which acts as a stress reliever, may minimize the shrinkage force [32]. In the case that the composite material has the lower-molecular weighed monomer (e.g., triethylene glycol dimethacrylate, TEGDMA; 2-hydroxyethyl methacrylate, HEMA) in the matrix, the tendency of polymerization shrinkage decreases [33]. However, in addition to the composition of composite matrix, the amount of matrix and the existence of the functional monomer or modulator to reduce the polymerization shrinkage can influence the final volume of polymerization shrinkage. Although polymerization shrinkage stress must be considered a multi-factorial phenomenon [34], in this study, it is speculated that higher volume of the matrix might have mainly contributed the higher contraction and consequential higher microleakage of the giomer. Deliperi et al. exhibited that giomer showed high microleakage, especially in enamel margin with self-etch adhesive [6]. Sunico et al. reported reduced marginal adaptation and increased marginal discoloration of class V restorations with giomer in a two-year clinical study [35].
In addition, for giomer, a higher microleakage was observed at the gingival margin than at the occlusal margin (p = 0.001). This may be related to a difference between the bonding ability of enamel (occlusal) and of dentin (gingival). Dentin provides a weaker bond than enamel, and polymerization shrinkage in one increment may cause debonding at the weaker interface [36]. Previous studies reported that several composite resin restorations on non-cervical caries lesions (class V cavities) exhibited more microleakage on the gingival margins than on the occlusal margins because the flexural stresses at the cervical margins are much higher than those at the occlusal margins [37,38]. Polymerization shrinkage causes material shrinkage in all directions, and most often, the dentin margins are unprotected against microleakage [39]. However, RMGI and alkasite exhibited the opposite result; higher microleakage scores were observed at the enamel margin than at the gingival margin in the current study. For RMGI, adhesion is mainly due to a physicochemical reaction with dentin and enamel due to the polar nature of the polyacrylates and minerals in dental hard tissues. Alkasite is deficient in adhesive potential and does not contain any acidic monomers or water. Thus, it was applied after universal dentin adhesive application to class V cavities. For both materials, compensation mechanisms, such as water sorption and the existence of a special stress reliever, might reduce polymerization shrinkage and stress. Selective enamel etching was not performed in the absence of shrinkage stress, which might have increased the tendency of microleakage in the enamel margin.
An intragroup analysis of microleakages in the gingival margin of the RMGI and alkasite restorations showed significant differences between the pH 4 and pH 7 storage specimens (Table 3). In the gingival margin, pH 4 resulted in a significantly lower microleakage than pH 7. This result suggests that the strong buffering capacity with ion-releasing property of these materials might counteract a low pH environment.
The current results indicated that the bond strength to dentin is not affected according to the type of restorative materials. Although the pH condition did not influence to the bond strength of RMGI and alkasite, that of giomer significantly decreased in acidic pH, which suggested a relatively lower ion-releasing capacity (esp, hydroxyl ions) of giomer than RMGI or alkasite. The microleakage scores were affected by three experimental factors (the type of materials, the location of margin and pH). Related to the types of materials and the location of margin, it is assumed that the experimental result is mainly due to polymerization shrinkage, which occurred more in giomer. RMGI and alkasite showed a lower microleakage in pH 4 than pH 7 (p = 0.028). It is assumed that the ion-releasing capacities of the materials can be activated under acidic conditions.
Several attempts have been made to develop hybrid restorative materials that combine the advantages of the resin composite and glass ionomer cement families. In this study, although the three materials showed no significant difference in bond strength to the dentin surface, the bond strength of the giomer decreased significantly at pH 4. RMGI and alkasite exhibited a lower microleakage than the giomer, especially under acidic pH conditions, and the microleakage under low-pH conditions was decreased compared to when under neutral pH conditions. It is suggested that the ion-releasing capacity of these materials is activated under acidic conditions. RMGI and alkasite might be adopted in generalized or localized low pH clinical conditions (e.g., oral pathologic conditions, and lowered pH induced by reflux episodes).
In this study, thermocycling was not considered in the microleakage test. Microleakage specimens were stored at 37 °C in a buffer solution at various pH values to control a variable, ‘linear coefficient of thermal expansion (LCTE)’, which influences the volumetric change of restorative materials in the oral cavity. The LCTE of resin-based materials is affected by the amount of filler [40]. Beautifil flow plus has a 47 vol% of filler [41], RMGI has 55 vol% filler [28], and alkasite has a 58 vol% (78 wt%) filler content in the final mass [28,42]. Thus, the harsh oral environment could not be simulated, which is a limitation of this study. The buffer solution was stored for 24 h. The short storage period was intended to limit the hygroscopic expansion of the resin-based restorative materials. Little is known about the hygroscopic expansion and water sorption of alkasite; however, a previous study reported that alkasite exhibited the same or a slightly lower water sorption than conventional glass ionomer cement [43]. The bulk marginal gap reduction in RMGI occurred within the first week of water storage [44]. Thus, Toledano et al. reported that 1 week of water storage can allow for a hygroscopic expansion of the material, which may fully compensate for the original polymerization shrinkage of the material, allowing for less microleakage [31].
This study focused the mechanical properties of ion-releasing restorative materials from a clinical aspect (e.g., bond strength and microleakage of class V cavities). In further studies, new restorative material will be analyzed about the inherent mechanical properties of polymerization shrinkage, shrinkage stress in various restorative configuration, and also about the pH buffering capacity in a simulated oral environment, as well as the remineralizing capacity to the underneath dentin. Alkalizing potential of the materials can be proved also through in vitro or in vivo anti-bacterial activity in the future.
Although comparison of the commercial materials is not simple, within the limitations of this study, RMGI and new alkasite might be clinically adopted under low-pH condition. In further studies, the effect of long-term storage on the mechanical properties of three ion-releasing materials should be investigated.
6. Conclusions
Based on the findings of this in vitro study, the following conclusions were drawn.
- Giomer, RMGI, and alkasite exhibited no significant difference in the bond strength with dentin in this study. The pH 4 condition yielded a decreased bond strength for the giomer compared with that of the subgroups, which were immediately tested or stored at a neutral pH.
- RMGI and alkasite showed a lower microleakage than the giomers. There was a significant difference in the microleakage scores according to the margin location. The difference in scores varied according to the materials; a higher microleakage score was observed in the enamel margin than in the gingival margin for RMGI and alkasite, and higher microleakage scores were observed in the gingival margin than in the enamel margin for giomer.
- For RMGI and alkasite, the specimens stored at pH 4 showed significantly lower microleakage than those stored at pH 7.
- RMGI and alkasite might be adopted in generalized or localized low-pH clinical conditions.
Funding
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. 2021R1F1A1045987).
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the institutional review board (KH-DT21026).
Informed Consent Statement
Patient consent was waived because comprehensive consent was obtained when the patient’s teeth were extracted at our institution. This indicates that the extracted teeth can be used in future research. Therefore, individual patient consent is not required in studies that use only previously extracted teeth.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Dionysopoulos, D.; Koliniotou-Koumpia, E.; Helvatzoglou-Antoniades, M.; Kotsanos, N. Fluoride release and recharge abilities of contemporary fluoride-containing restorative materials and dental adhesives. Dent. Mater. J. 2013, 32, 296–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, J.-Y.; Li, Y.-Q.; Smales, R.; Yip, K.-K. Restoration of teeth with more-viscous glass ionomer cements following radiation-induced caries. Int. Dent. J. 2002, 52, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Loudon, J.A. Next generational fuji IX-a proposed universal dental material—But not yet ‘set in cement’. Oral Biol. Dent. 2014, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Ngo, H.; Mount, G.; Peters, M. A study of glass-ionomer cement and its interface with enamel and dentin using a low-temperature, high-resolution scanning electron microscopic technique. J. Esthet. Resto. Dent. 1999, 11, 223. [Google Scholar]
- Sidhu, S.K.; Watson, T.F. Resin-modified glass ionomer materials. A status report for the American Journal of Dentistry. Am. J. Dent. 1995, 8, 59–67. [Google Scholar]
- Deliperi, S.; Bardwell, D.N.; Wegley, C.; Congiu, M.D. In vitro evaluation of giomers microleakage after exposure to 33% hydrogen peroxide: Self-etch vs total-etch adhesives. Oper. Dent. 2006, 31, 227–232. [Google Scholar] [CrossRef]
- Francois, P.; Fouquet, V.; Attal, J.-P.; Dursun, E. Commercially available fluoride-releasing restorative materials: A review and a proposal for classification. Materials 2020, 13, 2313. [Google Scholar] [CrossRef]
- Khalid, H.; Aleesa, N.; Grosjean, M.; Hill, R.; Wong, F. Characterisation of a bioactive SiO2-CaO-CaF2-Na2O glass used in composites. Dent. Mater. 2021, 37, 1–9. [Google Scholar] [CrossRef]
- Vallittu, P.K.; Boccaccini, A.R.; Hupa, L.; Watts, D.C. Bioactive dental materials-Do they exist and what does bioactivity mean? Dent. Mater. 2018, 34, 693–694. [Google Scholar] [CrossRef]
- Burke, F.; Ray, N.; McConnell, R. Fluoride-containing restorative materials. Int. Dent. J. 2006, 56, 33–43. [Google Scholar] [CrossRef]
- Ranjitkar, S.; Kaidonis, J.A.; Smales, R.J. Gastroesophageal reflux disease and tooth erosion. Int. J. Dent. 2012, 2012, 479850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sreebny, L.M. Saliva in health and disease: An appraisal and update. Int. Dent. J. 2000, 50, 140–161. [Google Scholar] [CrossRef] [PubMed]
- Baliga, S.; Muglikar, S.; Kale, R. Salivary pH: A diagnostic biomarker. J. Indian Soc. Periodontol. 2013, 17, 461. [Google Scholar] [CrossRef] [PubMed]
- Moreau, J.L.; Xu, H.H. Fluoride releasing restorative materials: Effects of pH on mechanical properties and ion release. Dent. Mater. 2010, 26, e227–e235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, N.; Nyvad, B. Caries ecology revisited: Microbial dynamics and the caries process. Caries Res. 2008, 42, 409–418. [Google Scholar] [CrossRef]
- Gudmundsson, K.; Kristleifsson, G.; Theodors, A.; Holbrook, W.P. Tooth erosion, gastroesophageal reflux, and salivary buffer capacity. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1995, 79, 185–189. [Google Scholar] [CrossRef]
- Holbrook, W.; Furuholm, J.; Gudmundsson, K.; Theodors, A.; Meurman, J.H. Gastric reflux is a significant causative factor of tooth erosion. J. Dent. Res. 2009, 88, 422–426. [Google Scholar] [CrossRef]
- Muppalla, J.K.; Harikumar, V.; Sarathchandra, P.; Reddy, S.J.; Rajani, P. Effect of modulated photoactivation of bulkfill composite on microleakage in fluorosed and nonfluorosed teeth: A confocal laser scanning microscopy study. J. Conserv. Dent. 2020, 23, 180. [Google Scholar] [CrossRef]
- Mohamed, N.I.; Safy, R.K.; Elezz, A.F.A. Microtensile bond strength, marginal leakage, and antibacterial effect of bulk fill resin composite with alkaline fillers versus incremental nanohybrid composite resin. Eur. J. Dent. 2021, 15, 425–432. [Google Scholar] [CrossRef]
- Saad, A.; Inoue, G.; Nikaido, T.; Ikeda, M.; Burrow, M.; Tagami, J. Microtensile Bond Strength of Resin-Modified Glass Ionomer Cement to Sound and Artificial Caries—Affected Root Dentin with Different Conditioning. Oper. Dent. 2017, 42, 626–635. [Google Scholar] [CrossRef]
- Bollu, I.P.; Hari, A.; Thumu, J.; Velagula, L.D.; Bolla, N.; Varri, S.; Kasaraneni, S.; Nalli, S.V.M. Comparative evaluation of microleakage between nano-ionomer, giomer and resin modified glass ionomer cement in class V cavities-CLSM study. J. Clin. Diagnostic Res. 2016, 10, ZC66. [Google Scholar] [CrossRef] [PubMed]
- Restrepo, M.; Jeremias, F.; Santos-Pinto, L.; Cordeiro, R.C.; Zuanon, A.C. Effect of fluoride varnish on enamel remineralization in anterior teeth with molar incisor hypomineralization. J. Clin. Pediatr. Dent. 2016, 40, 207–210. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.F.D.; Fragelli, C.M.B.; Paschoal, M.A.B.; Campos, E.A.; Cunha, L.F.; Losso, E.M.; Cordeiro, R.D.C.L. Noninvasive and multidisciplinary approach to the functional and esthetic rehabilitation of amelogenesis imperfecta: A pediatric case report. Case Rep. Dent. 2014, 2014, 127175. [Google Scholar] [CrossRef] [PubMed]
- Carrilho, E.; Cardoso, M.; Marques Ferreira, M.; Marto, C.M.; Paula, A.; Coelho, A.S. 10-MDP based dental adhesives: Adhesive interface characterization and adhesive stability—A systematic review. Materials 2019, 12, 790. [Google Scholar] [CrossRef] [Green Version]
- Tsuchiya, S.; Nikaido, T.; Sonoda, H.; Foxton, R.M.; Tagami, J. Ultrastructure of the dentin-adhesive interface after acid-base challenge. J. Adhes. Dent. 2004, 6, 183–190. [Google Scholar]
- Guan, R.; Takagaki, T.; Matsui, N.; Sato, T.; Burrow, M.F.; Palamara, J.; Nikaido, T.; Tagami, J. Dentin bonding performance using Weibull statistics and evaluation of acid-base resistant zone formation of recently introduced adhesives. Dent. Mater. J. 2016, 35, 684–693. [Google Scholar] [CrossRef] [Green Version]
- Van Meerbeek, B.; Yoshihara, K.; Yoshida, Y.; Mine, A.; De Munck, J.; Van Landuyt, K. State of the art of self-etch adhesives. Dent. Mater. 2011, 27, 17–28. [Google Scholar] [CrossRef]
- Kasraei, S.; Haghi, S.; Valizadeh, S.; Panahandeh, N.; Nejadkarimi, S. Phosphate Ion release and alkalizing potential of three bioactive dental materials in comparison with composite resin. Int. J. Dent. 2021, 2021, 5572569. [Google Scholar] [CrossRef]
- Gonulol, N.; Ozer, S.; Sen Tunc, E. Water sorption, solubility, and color stability of giomer restoratives. J. Esthet. Restor. Dent. 2015, 27, 300–306. [Google Scholar] [CrossRef]
- Tsujimoto, A.; Barkmeier, W.W.; Takamizawa, T.; Latta, M.A.; Miyazaki, M. Depth of cure, flexural properties and volumetric shrinkage of low and high viscosity bulk-fill giomers and resin composites. Dent. Mater. J. 2017, 36, 205–213. [Google Scholar] [CrossRef] [Green Version]
- Toledano, M.; Osorio, E.; Osorio, R.; García-Godoy, F. Microleakage of Class V resin-modified glass ionomer and compomer restorations. J. Prosthet. Dent. 1999, 81, 610–615. [Google Scholar] [CrossRef]
- Afraaz, A.; Borugadda, R.; Mandava, J.; Chalasani, U.; Ravi, R.; Pamidmukkala, S.; Boddeda, M.R.; Athkuri, S. Evaluation of Marginal Adaptation and Wear Resistance of Nanohybrid and Alkasite Restorative Resins. J. Clin. Diagn. Res. 2020, 14, 16–20. [Google Scholar] [CrossRef]
- Szczesio-Wlodarczyk, A.; Polikowski, A.; Krasowski, M.; Fronczek, M.; Sokolowski, J.; Bociong, K. The Influence of Low-Molecular-Weight Monomers (TEGDMA, HDDMA, HEMA) on the Properties of Selected Matrices and Composites Based on Bis-GMA and UDMA. Materials 2022, 15, 2649. [Google Scholar] [CrossRef] [PubMed]
- Braga, R.R.; Ballester, R.Y.; Ferracane, J.L. Factors involved in the development of polymerization shrinkage stress in resin-composites: A systematic review. Dent. Mater. 2005, 21, 962–970. [Google Scholar] [CrossRef] [PubMed]
- Sunico, M.C.; Shinkai, K.; Katoh, Y. Two-year clinical performance of occlusal and cervical giomer restorations. Oper. Dent. 2005, 30, 282. [Google Scholar]
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification review of dental adhesive systems: From the IV generation to the universal type. Ann. Stomatol. 2017, 8, 1. [Google Scholar]
- Gupta, S.K.; Gupta, J.; Saraswathi, V.; Ballal, V.; Acharya, S.R. Comparative evaluation of microleakage in Class V cavities using various glass ionomer cements: An in vitro study. J. Interdiscip. Dent. 2012, 2, 164. [Google Scholar] [CrossRef]
- Sooraparaju, S.G.; Kanumuru, P.K.; Nujella, S.K.; Konda, K.R.; Reddy, K.; Penigalapati, S. A comparative evaluation of microleakage in class v composite restorations. Int. J. Dent. 2014, 2014, 685643. [Google Scholar] [CrossRef]
- Davidson, C.L. Resisting the curing contraction with adhesive composites. J. Prosthet. Dent. 1986, 55, 446–447. [Google Scholar] [CrossRef]
- Pinto-Sinai, G.; Brewster, J.; Roberts, H. Linear coefficient of thermal expansion evaluation of glass ionomer and resin-modified glass ionomer restorative materials. Oper. Dent. 2018, 43, E266–E272. [Google Scholar] [CrossRef]
- Nie, J.; Yap, A.; Wang, X. Influence of shrinkage and viscosity of flowable composite liners on cervical microleakage of class II restorations: A micro-CT analysis. Oper. Dent. 2018, 43, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Ilie, N. Comparative effect of self-or dual-curing on polymerization kinetics and mechanical properties in a novel, dental-resin-based composite with alkaline filler. Materials 2018, 11, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nayak, M.; Shenoy, V. Sorption and Solubility of Alkasite Restorative Material-An In Vitro Study. IOSR J. Dent. Med. Sci. 2019, 18, 69–73. [Google Scholar]
- Huang, C.; Kei, L.H.; Wei, S.H.; Cheung, G.S.; Tay, F.R.; Pashley, D.H. The influence of hygroscopic expansion of resin-based restorative materials on artificial gap reduction. J. Adhes. Dent. 2002, 4, 61–71. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).