The Effect of a Topcoat with Amorphous Polymer Layers on the Mesogen Orientation and Photoalignment Behavior of Side Chain Liquid Crystalline Polymer Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Measurements
2.4. Photoirradiation
3. Results and Discussion
3.1. Mesogen Orientation of PAz Films
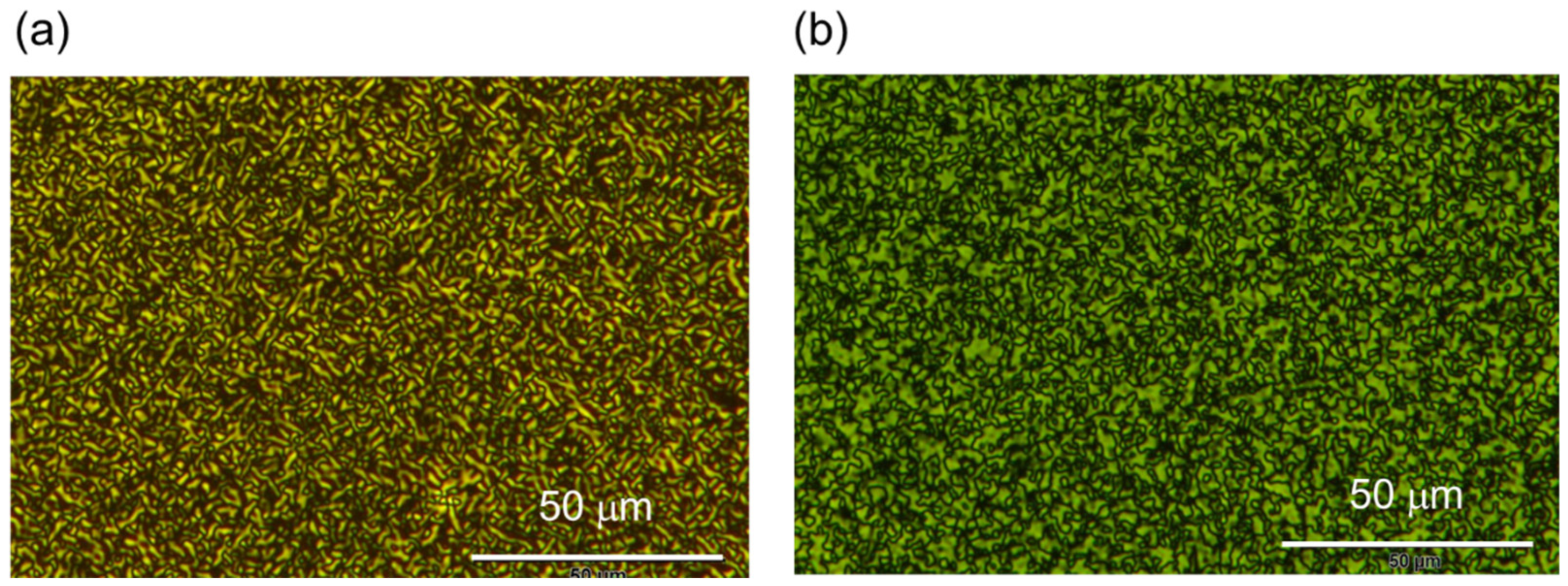
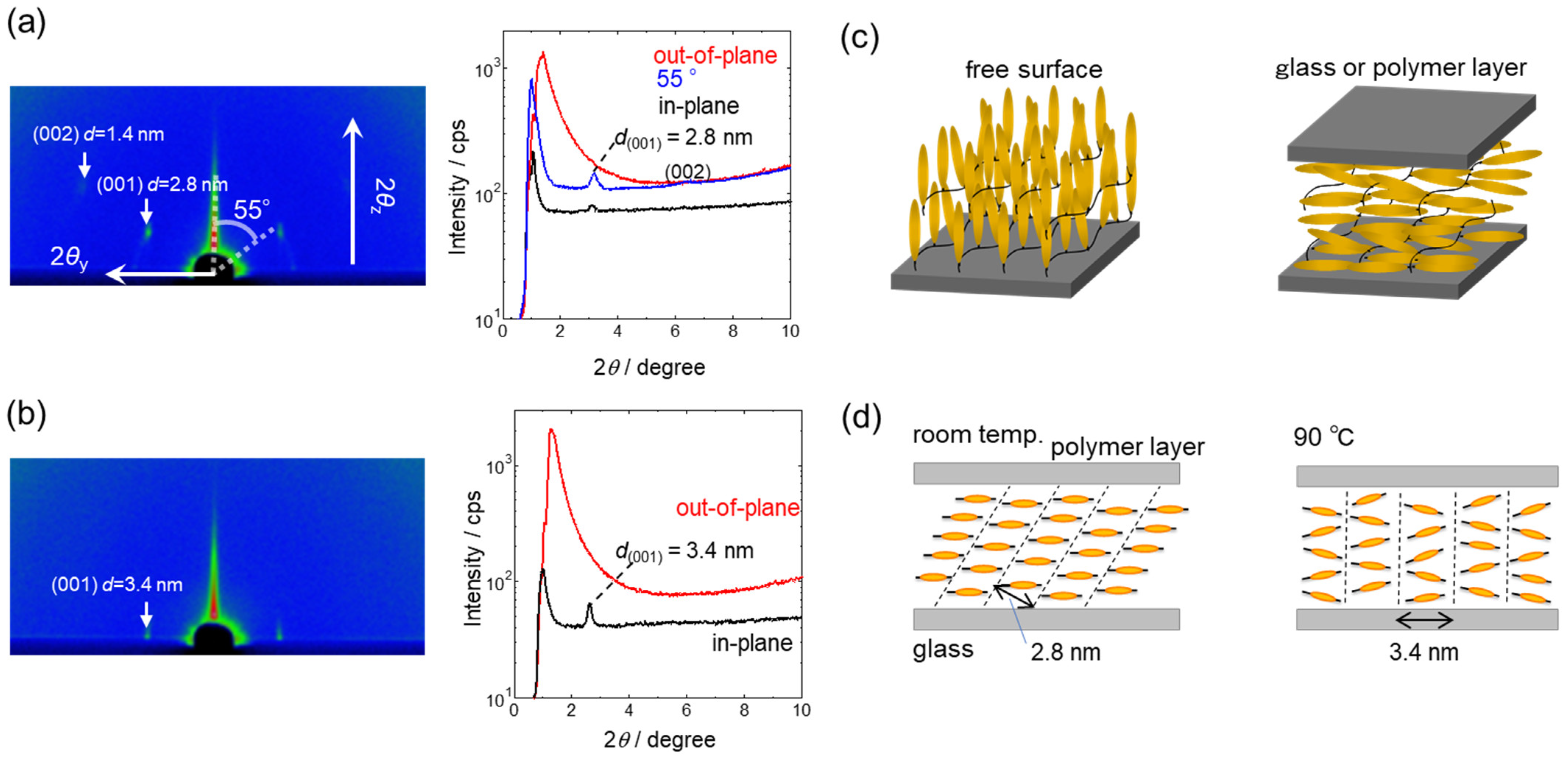
3.1.1. PAz Film Contacting with Air
3.1.2. PAz Film Sandwiched between Two Glass Substrates
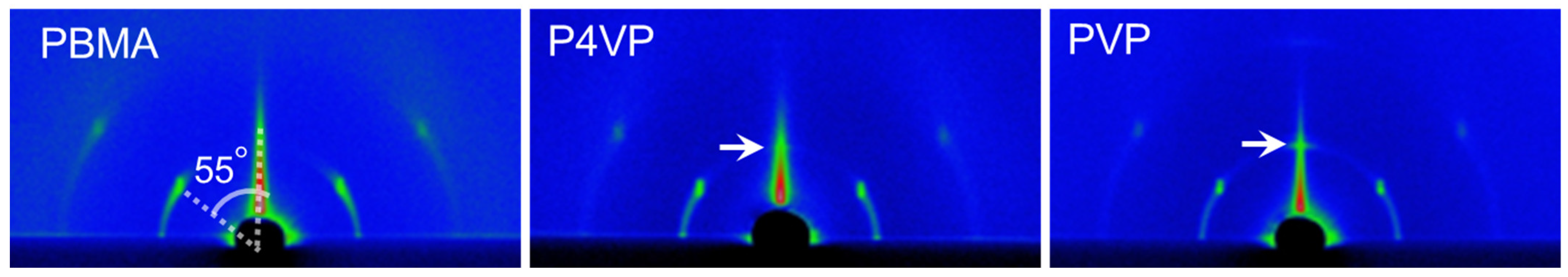
3.1.3. PAz Film with Top-Coated Amorphous Polymers
3.1.4. Reversible Orientation Switching by Coating and Removal of the Top Layer
3.2. Photoalignment of PAz and P(Az-CB) Films
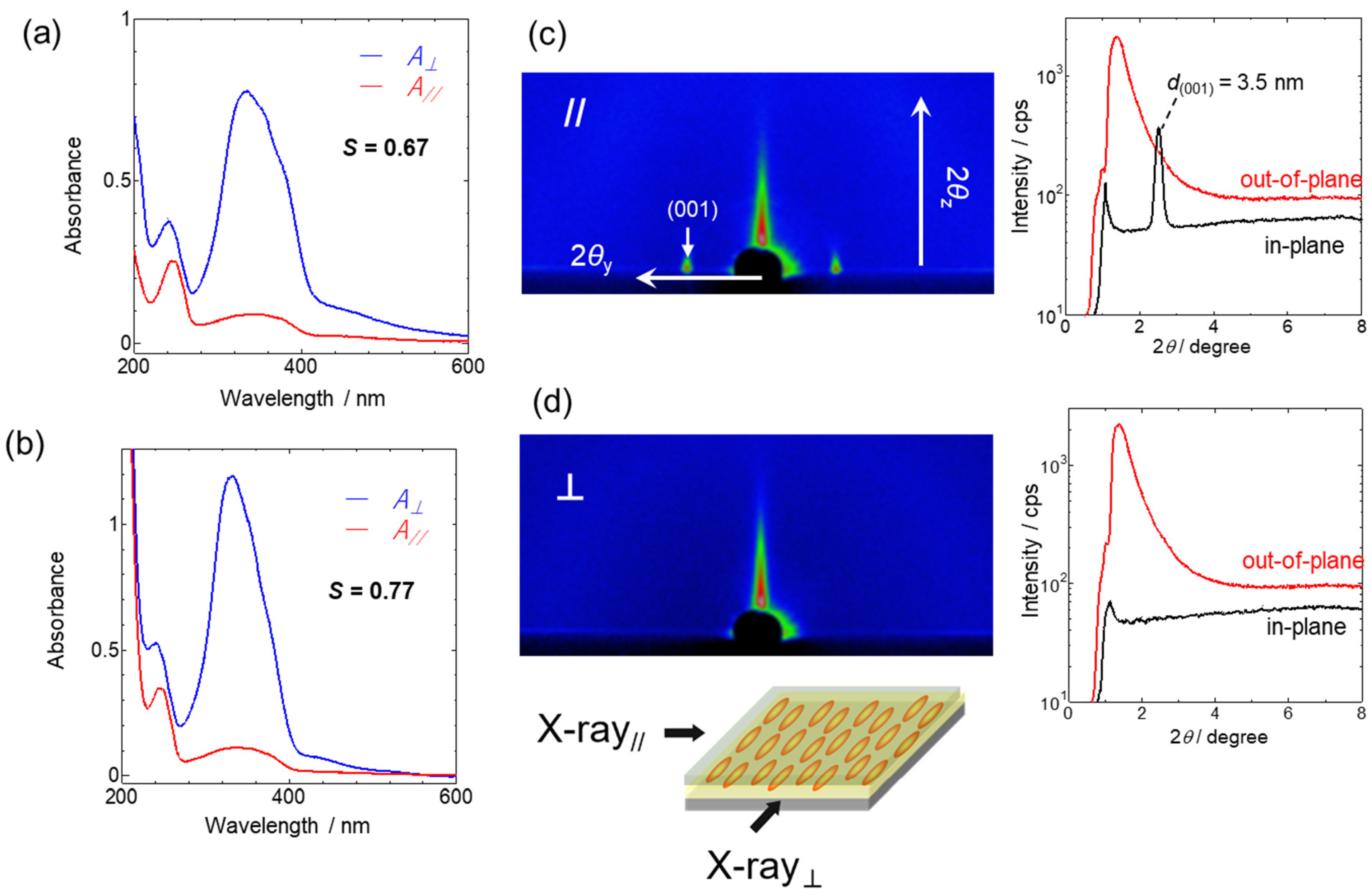
3.2.1. In-Plane Photoalignment of PAz Coated with Polymer Layers
3.2.2. In-Plane Photoalignment of P(Az-CB)
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ichimura, K. Photoalignment of Liquid-Crystal Systems. Chem. Rev. 2000, 100, 1847–1873. [Google Scholar] [CrossRef] [PubMed]
- Chigrinov, V.G.; Kozenkov, V.M.; Kwok, H.-S. Photoalignment of Liquid Crystalline Materials; John Wiley & Sons: West Sussex, UK, 2008. [Google Scholar]
- Kawatsuki, N. Photoalignment and Photoinduced Molecular Reorientation of Photosensitive Materials. Chem. Lett. 2011, 40, 548–554. [Google Scholar] [CrossRef]
- Yaroshchuk, O.; Reznikov, Y. Photoalignment of liquid crystals: Basics and current trends. J. Mater. Chem. 2012, 22, 286–300. [Google Scholar] [CrossRef]
- Seki, T.; Nagano, S.; Hara, M. Versatility of photoalignment techniques: From nematics to a wide range of functional materials. Polymer 2013, 54, 6053–6072. [Google Scholar] [CrossRef]
- Seki, T. New strategies and implications for the photoalignment of liquid crystalline polymers. Polym. J. 2014, 46, 751–768. [Google Scholar] [CrossRef]
- Nagano, S. Inducing Planar Orientation in Side-Chain Liquid-Crystalline Polymer Systems via Interfacial Control. Chem. Rec. 2016, 16, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Seki, T. A Wide Array of Photoinduced Motions in Molecular and Macromolecular Assemblies at Interfaces. Bull. Chem. Soc. Jpn. 2018, 41, 1026–1057. [Google Scholar] [CrossRef]
- Nagano, S. Random Planar Orientation in Liquid-Crystalline Block Copolymers with Azobenzene Side Chains by Surface Segregation. Langmuir 2019, 35, 5673–5683. [Google Scholar] [CrossRef]
- Shibaev, V.; Bobrovsky, A.; Boiko, N. Photoactive liquid crystalline polymer systems with light-controllable structure and optical properties. Prog. Polym. Sci. 2003, 28, 729–836. [Google Scholar] [CrossRef]
- Zhao, Y.; Ikeda, T. Smart Light-Responsive Materials; John Wiley & Sons: Hoboken, NJ, USA, 2009. [Google Scholar]
- Ikeda, T. Photomodulation of liquid crystal orientations for photonic applications. J. Mater. Chem. 2003, 13, 2037–2057. [Google Scholar] [CrossRef]
- Priimagi, A.; Shevchenko, A. Azopolymer-based micro- and nanopatterning for photonic applications. J. Polym. Sci. Part B Polym. Phys. 2014, 52, 163–182. [Google Scholar] [CrossRef]
- Yu, Y.; Nakano, M.; Ikeda, T. Directed bending of a polymer film by light. Nature 2003, 425, 145. [Google Scholar] [CrossRef] [PubMed]
- Ube, T.; Ikeda, T. Photomobile Polymer Materials with Crosslinked Liquid-Crystalline Structures: Molecular Design, Fabrication, and Functions. Angew. Chem. Int. Ed. 2014, 53, 10290–10299. [Google Scholar] [CrossRef]
- Yu, H. Photoresponsive liquid crystalline block copolymers: From photonics to nanotechnology. Prog. Polym. Sci. 2014, 39, 781–815. [Google Scholar] [CrossRef]
- Lv, J.; Liu, Y.; Wei, J.; Chen, E.; Qin, L.; Yu, Y. Photocontrol of fluid slugs in liquid crystal polymer microactuators. Nature 2016, 537, 179–184. [Google Scholar] [CrossRef]
- Priimagi, A.; Barrett, C.J.; Shishido, A. Recent twists in photoactuation and photoalignment control. J. Mater. Chem. C 2014, 35, 7155–7162. [Google Scholar] [CrossRef]
- Fukuhara, K.; Fujii, Y.; Nagashima, Y.; Hara, M.; Nagano, S.; Seki, T. Liquid-Crystalline Polymer and Block Copolymer Domain Alignment Controlled by Free-Surface Segregation. Angew. Chem. Int. Ed. 2013, 52, 5988–5991. [Google Scholar] [CrossRef]
- Komura, M.; Yoshitake, A.; Komiyama, H.; Iyoda, T. Control of Air-Interface-Induced Perpendicular Nanocylinder Orientation in Liquid Crystal Block Copolymer Films by a Surface-Covering Method. Macromolecules 2015, 48, 672–678. [Google Scholar] [CrossRef]
- Xie, H.-L.; Li, X.; Suh, H.S.; Ren, J.-X.; Craig, G.S.W.; Arges, C.G.; Nealey, P.F. Water-soluble top coats for orientation control of liquid crystal-containing block copolymer films. J. Polym. Sci. Part B Polym. Phys. 2017, 55, 1569–1574. [Google Scholar] [CrossRef]
- Tanaka, D.; Nagashima, Y.; Hara, M.; Nagano, S.; Seki, T. Alternation of Side-Chain Mesogen Orientation Caused by the Backbone Structure in Liquid-Crystalline Polymer Thin Films. Langmuir 2015, 31, 11379–11383. [Google Scholar] [CrossRef]
- Fukuhara, K.; Hara, M.; Nagano, S.; Seki, T. Free-surface molecular command systems for photoalignment of liquid crystalline materials. Nat. Commun. 2014, 5, 3320. [Google Scholar] [CrossRef] [PubMed]
- Nakai, T.; Tanaka, D.; Hara, M.; Nagano, S.; Seki, T. Free Surface Command Layer for Photoswitchable Out-of-Plane Alignment Control in Liquid Crystalline Polymer Films. Langmuir 2016, 32, 909–914. [Google Scholar] [PubMed]
- Kawatsuki, N.; Miyake, K.; Kondo, M. Facile Fabrication, Photoinduced Orientation, and Birefringent Pattern Control of Photoalignable Films Comprised of N-Benzylideneaniline Side Groups. ACS Macro Lett. 2015, 4, 764–768. [Google Scholar] [CrossRef]
- Kawatsuki, N.; Inada, S.; Fujii, R.; Kondo, M. Photoinduced Birefringent Pattern and Photoinactivation of Liquid-Crystalline Copolymer Films with Benzoic Acid and Phenylaldehyde Side Groups. Langmuir 2018, 34, 2089–2095. [Google Scholar] [CrossRef] [PubMed]
- Ito, A.; Norisada, Y.; Inada, S.; Kondo, M.; Sasaki, T.; Sakamoto, M.; Ono, H.; Kawatsuki, N. Photoinduced Reorientation and Photofunctional Control of Liquid Crystalline Copolymers with in Situ-Formed N-Benzylideneaniline Derivative Side Groups. Langmuir 2021, 37, 1164–1172. [Google Scholar] [CrossRef]
- Bates, C.M.; Seshimo, T.; Maher, M.J.; Durand, W.J.; Cushen, J.D.; Dean, L.M.; Blachut, G.; Ellison, C.J.; Willson, C.G. Polarity-Switching Top Coats Enable Orientation of Sub–10-nm Block Copolymer Domains. Science 2012, 338, 775–779. [Google Scholar] [CrossRef]
- Ichimura, K.; Suzuki, Y.; Seki, T.; Hosoki, A.; Aoki, K. Reversible change in alignment mode of nematic liquid crystals regulated photochemically by command surfaces modified with an azobenzene monolayer. Langmuir 1988, 4, 1214–1216. [Google Scholar] [CrossRef]
- Seki, T.; Sakuragi, M.; Kawanishi, Y.; Suzuki, Y.; Tamaki, T.; Fukuda, R.; Ichimura, K. “Command surfaces” of Langmuir-Blodgett films. Photoregulations of liquid crystal alignment by molecularly tailored surface azobenzene layers. Langmuir 1993, 9, 211–218. [Google Scholar]
- Okano, K. Anisotropic Excluded Volume Effect and Alignment of Nematic Liquid Crystal in a Sandwich Cell. Jpn. J. Appl. Phys. 1983, 22, L343–L344. [Google Scholar] [CrossRef]
- Berreman, D.W. Solid Surface Shape and the Alignment of an Adjacent Nematic Liquid Crystal. Phys. Rev. Lett. 1972, 28, 1683–1686. [Google Scholar] [CrossRef]
- Tanaka, D.; Mizuno, T.; Hara, M.; Nagano, S.; Saito, I.; Yamamoto, K.; Seki, T. Evaluations of Mesogen Orientation in Thin Films of Polyacrylate with Cyanobiphenyl Side Chain. Langmuir 2016, 32, 3737–3745. [Google Scholar] [CrossRef] [PubMed]
- Scaramuzza, N.; Berlic, C.; Barna, E.S.; Strangi, G.; Barna, V.; Ionescu, A.T. Molecular Simulation of the Free Surface Order in NLC Samples. J. Phys. Chem. B 2004, 108, 3207–3210. [Google Scholar] [CrossRef]
- Chen, S.M.; Hsieh, T.C.; Pan, R.P. Magnetic-field-induced Fréedericksz transition and the dynamic response of nematic liquid-crystal films with a free surface. Phys. Rev. A 1991, 43, 2848–2857. [Google Scholar] [CrossRef]
- Canabarro, A.A.; de Oliveira, I.N.; Lyra, M.L. Homeotropic surface anchoring and the layer-thinning transition in free-standing films. Phys. Rev. E 2008, 77, 011704. [Google Scholar] [CrossRef] [PubMed]
- Ocko, B.M.; Braslau, A.; Pershan, P.S.; Als-Nielsen, J.; Deutsch, M. Quantized layer growth at liquid-crystal surfaces. Phys. Rev. Lett. 1986, 57, 94–97. [Google Scholar] [CrossRef]
- Pershan, P.S. Structure of surfaces and interfaces as studied using synchrotron radiation. Liquid surfaces. Faraday Discuss. Chem. Soc. 1990, 89, 231–245. [Google Scholar] [CrossRef]
- Imanishi, R.; Nagashima, Y.; Hara, M.; Nagano, S.; Seki, T. Collective Competition between Two Mesogens showing Opposing Orientational Nature in Side Chain Liquid Crystalline Polymers. Chem. Lett. 2019, 48, 98–101. [Google Scholar] [CrossRef]
- Imanishi, R.; Nagashima, Y.; Takishima, K.; Hara, M.; Nagano, S.; Seki, T. Induction of Highly Ordered Smectic Phases in Side Chain Liquid Crystalline Polymers by Means of Random Copolymerization. Macromolecules 2020, 53, 1942–1949. [Google Scholar] [CrossRef]
- Prucker, O.; Christian, S.; Bock, H.; Ruhe, J.; Frank, C.W.; Knoll, W. On the glass transition in ultrathin polymer films of different molecular architecture. Macromol. Chem. Phys. 1998, 199, 1435–1444. [Google Scholar] [CrossRef]
- Raczkowska, J.; Stetsyshyn, Y.; Awsiuk, K.; Zemła, J.; Kostruba, A.; Harhay, K.; Marzec, M.; Bernasik, A.; Lishchynskyi, O.; Ohar, O.; et al. Temperature-responsive properties of poly(4-vinylpyridine) coatings: Influence of temperature on the wettability, morphology, and protein adsorption. RSC Adv. 2016, 6, 87469–87477. [Google Scholar]
- van Oss, C.J.; Good, R.J. Surface Tension and the Solubility of Polymers and Biopolymers: The Role of Polar and Apolar Interfacial Free Energies. J. Macromol. Sci. Chem. 1989, A26, 1183–1203. [Google Scholar] [CrossRef]
- Kinose, Y.; Sakakibara, K.; Sato, O.; Tsujii, O. Near-Zero Azimuthal Anchoring of Liquid Crystals Assisted by Viscoelastic Bottlebrush Polymers. ACS Appl. Polym. Mater. 2021, 3, 2618–2625. [Google Scholar] [CrossRef]
- Shimomura, M.; Ando, A.; Kunitake, T. Orientation and Spectral Characteristics of the Azobenzene Chromophore in the Ammonium Bilayer Assembly. Ber. Bunsen-Ges. Phys. Chem. 1983, 87, 1134–1143. [Google Scholar] [CrossRef]
- Uekusa, T.; Nagano, S.; Seki, T. Highly Ordered In-Plane Photoalignment Attained by the Brush Architecture of Liquid Crystalline Azobenzene Polymer. Macromolecules 2009, 42, 312–318. [Google Scholar] [CrossRef]
- Haque, H.A.; Nagano, S.; Seki, T. Lubricant Effect of Flexible Chain in the Photoinduced Motions of Surface-Grafted Liquid Crystalline Azobenzene Polymer Brush. Macromolecules 2012, 45, 6095–6103. [Google Scholar] [CrossRef]
- Mukai, K.; Hara, M.; Nagano, S.; Seki, T. High-Density Liquid-Crystalline Polymer Brushes Formed by Surface Segregation and Self-Assembly. Angew. Chem. Int. Ed. 2016, 55, 14028–14032. [Google Scholar] [CrossRef] [PubMed]





| Polymer | Mn | Mw/Mn | Thermal Transitions (°C) |
|---|---|---|---|
| Paz | 2.2 × 104 | 1.2 | g-43-SmC-100-SmA-120-i 1,2 |
| P(Az-CB) | 1.2 × 104 | 1.1 | g-34-SmA-113-i 1 |
| PBMA | 1.9 × 104 | 1.1 | Tg: 20 |
| P4VP | 6.5 × 104 | 1.1 | Tg: 140 |
| PVP | 1.0 × 106 3 | No datum | Tg: 150 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furuichi, M.; Hara, M.; Nagano, S.; Seki, T. The Effect of a Topcoat with Amorphous Polymer Layers on the Mesogen Orientation and Photoalignment Behavior of Side Chain Liquid Crystalline Polymer Films. Appl. Sci. 2022, 12, 9410. https://doi.org/10.3390/app12199410
Furuichi M, Hara M, Nagano S, Seki T. The Effect of a Topcoat with Amorphous Polymer Layers on the Mesogen Orientation and Photoalignment Behavior of Side Chain Liquid Crystalline Polymer Films. Applied Sciences. 2022; 12(19):9410. https://doi.org/10.3390/app12199410
Chicago/Turabian StyleFuruichi, Mari, Mitsuo Hara, Shusaku Nagano, and Takahiro Seki. 2022. "The Effect of a Topcoat with Amorphous Polymer Layers on the Mesogen Orientation and Photoalignment Behavior of Side Chain Liquid Crystalline Polymer Films" Applied Sciences 12, no. 19: 9410. https://doi.org/10.3390/app12199410
APA StyleFuruichi, M., Hara, M., Nagano, S., & Seki, T. (2022). The Effect of a Topcoat with Amorphous Polymer Layers on the Mesogen Orientation and Photoalignment Behavior of Side Chain Liquid Crystalline Polymer Films. Applied Sciences, 12(19), 9410. https://doi.org/10.3390/app12199410








