Body Weight Distribution and Body Sway in Healthy Female Adults Aged between 51 and 60 Years in Germany—Standard Values
Abstract
1. Introduction
2. Methods
2.1. Subjects
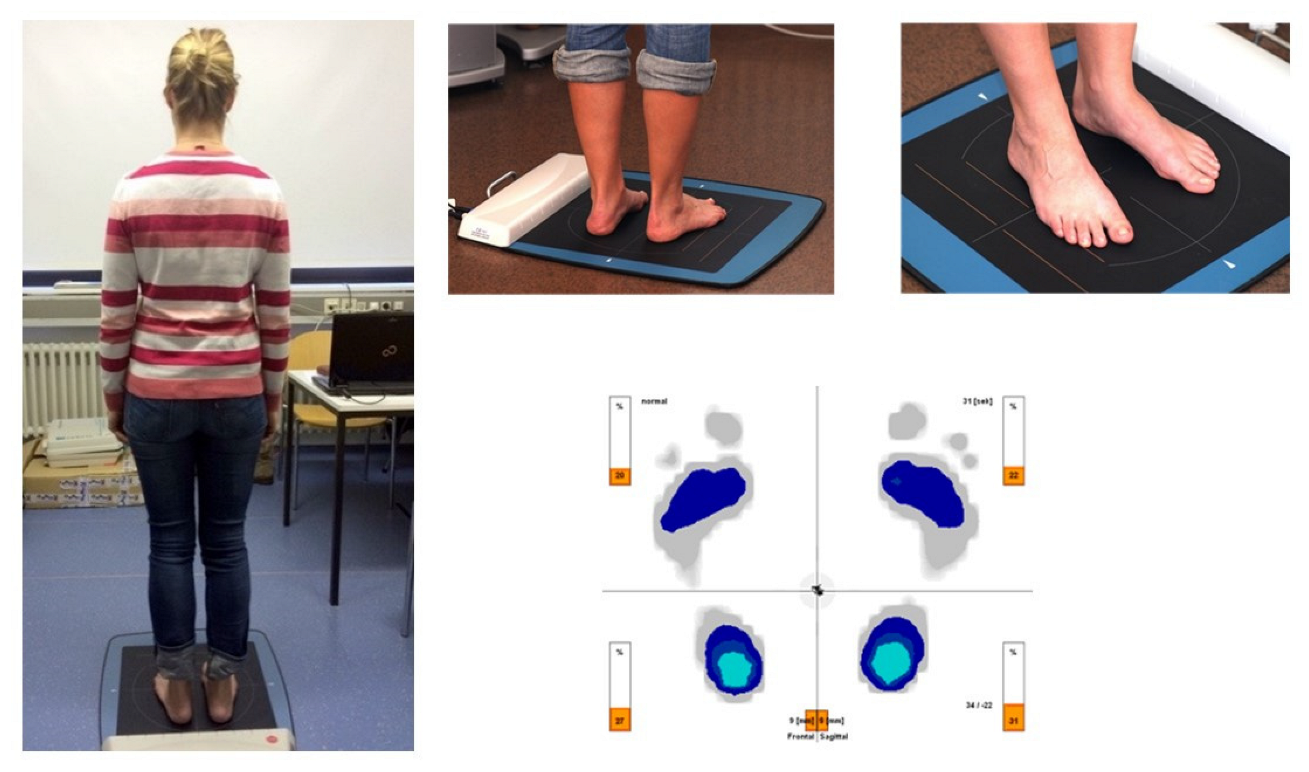
2.2. Pressure Measurement Plate including Evaluation Parameters
2.3. Statistical Evaluation Procedures
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BMI | Body Mass Index |
| WHO | World Health Organization |
| FS | Frontal Sway |
| SS | Sagittal Sway |
References
- WHOEBMI-B. Available online: https://www.euro.who.int/en/health-topics/disease-prevention/nutrition/a-healthy-lifestyle/body-mass-index-bmi?source=post_page&msclkid=d769a880d03211eca638246b855e3c4a (accessed on 10 May 2022).
- Pape, H.-C.K.A.; Silbernagel, S. Physiologie, 9th ed.; Georg Thieme Velag: Stuttgart, Germany, 2019. [Google Scholar]
- Schmidt, R.F.L.F.; Brandes, R. Physiologie des Menschen: Mit Pathophysiologie, 32nd ed.; Springer Medizin: Berlin, Germany, 2019. [Google Scholar]
- Laube, W.; von Heymann, W. Das sensomotorische System und die Auswirkungen der Physiologie des Alterungsprozesses. Man. Medizin. 2012, 50, 223–234. [Google Scholar] [CrossRef]
- Hüther-Becker, A. (Ed.) Das neue Denkmodell in der Physiotherapie Band 2: Bewegungsentwicklung und Bewegungskontrolle, 1st ed.; Thieme Georg: Stuttgart, Germany, 2005; No. 2. [Google Scholar]
- Laube, W. Sensomotorisches System: Physiologisches Detailwissen für Physiotherapeuten, 1st ed.; Thieme: Stuttgart, Germany, 2009. [Google Scholar]
- Silbernagel, S.D.A.; Draguhn, A. Taschenatlas Physiologie, 9th ed.; Georg Thieme: Stuttgart, Germany, 2018. [Google Scholar]
- Schünke, M. Topographie und Funktion des Bewegungssystems, 3rd ed.; Georg Thieme: Stuttgart, Germany, 2018. [Google Scholar]
- Myers, T.W. Anatomy Trains Myofasziale Leitbahnen für Manual- und Bewegungstherapeuten, 3rd ed.; Elsevier: Berlin, Germany, 2015. [Google Scholar]
- Wanke, E.M.; Mill, H.; Groneberg, D.A. Ballet as high-performance activity: Health risks exemplified by acute injuries in dance students. Sportverletz Sportschaden. 2012, 26, 164–170. [Google Scholar] [PubMed]
- Schiebler, T.H.; Korf, H.W. Anatomie: Histologie, Entwicklungsgeschichte, Makroskopische und Mikroskopische Anatomie, Topographie; Steinkopff: Heidelberg, Germany, 2007. [Google Scholar]
- Tsikopoulos, K.; Sidiropoulos, K.; Kitridis, D.; Cain Atc, S.M.; Metaxiotis, D.; Ali, A. Do External Supports Improve Dynamic Balance in Patients with Chronic Ankle Instability? A Network Meta-analysis. Clin. Orthop. Relat. Res. 2020, 478, 359–377. [Google Scholar] [CrossRef] [PubMed]
- Pomarino, D.; Pomarino, A. Plantar Static Pressure Distribution in Healthy Individuals: Percentiles for the Evaluation of Forefoot Loading. Foot Ankle Spec. 2014, 7, 293–297. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.A.; Pasinato, F.; Correa, E.C.; da Silva, A.M. Global body posture and plantar pressure distribution in individuals with and without temporomandibular disorder: A preliminary study. J. Manip. Physiol. Ther. 2014, 37, 407–414. [Google Scholar] [CrossRef]
- Syed, N.; Karvannan, H.; Maiya, A.G.; Binukumar, B.; Prem, V.; Chakravarty, R.D. Plantar pressure distribution among asymptomatic individuals: A cross-sectional study. Foot Ankle Spec. 2012, 5, 102–106. [Google Scholar] [CrossRef]
- Ohlendorf, D.; Kerth, K.; Osiander, W.; Holzgreve, F.; Fraeulin, L.; Ackermann, H.; Groneberg, D.A. Standard reference values of weight and maximum pressure distribution in healthy adults aged 18-65 years in Germany. J. Physiol. Anthropol. 2020, 39, 39. [Google Scholar] [CrossRef]
- Cavanagh, P.R.; Rodgers, M.M.; Iiboshi, A. Pressure distribution under symptom-free feet during barefoot standing. Foot Ankle 1987, 7, 262–276. [Google Scholar] [CrossRef]
- Ohlendorf, D.; Doerry, C.; Fisch, V.; Schamberger, S.; Erbe, C.; Wanke, E.M.; Groneberg, D.A. Standard reference values of the postural control in healthy young female adults in Germany: An observational study. BMJ Open 2019, 9, e026833. [Google Scholar] [CrossRef]
- Ohlendorf, D.; Pflaum, J.; Wischnewski, C.; Schamberger, S.; Erbe, C.; Wanke, E.M.; Holzgreve, F.; Groneberg, D.A. Standard reference values of the postural control in healthy female adults aged between 31 and 40 years in Germany: An observational study. J. Physiol. Anthropol. 2020, 39, 27. [Google Scholar] [CrossRef]
- Ohlendorf, D.; Fay, V.; Avaniadi, I.; Erbe, C.; Wanke, E.M.; Groneberg, D.A. Association between constitution, axiography, analysis of dental casts, and postural control in women aged between 41 and 50 years. Clin. Oral Investig. 2021, 25, 2595–2607. [Google Scholar] [CrossRef] [PubMed]
- Pomarino, D.N.A.; Beyer, J. Altersabhängige Messungen zur posturalen Stabilität gesunder Probanden. OUP 2013, 2, 420–425. [Google Scholar]
- Patti, A.; Bianco, A.; Şahin, N.; Sekulic, D.; Paoli, A.; Iovane, A.; Messina, G.; Gagey, P.M.; Palma, A. Postural control and balance in a cohort of healthy people living in Europe: An observational study. Medicine 2018, 97, e13835. [Google Scholar] [CrossRef] [PubMed]
- Scharnweber, B.; Adjami, F.; Schuster, G.; Kopp, S.; Natrup, J.; Erbe, C.; Ohlendorf, D. Influence of dental occlusion on postural control and plantar pressure distribution. Cranio J. Craniomandib. Pract. 2017, 35, 358–366. [Google Scholar] [CrossRef]
- Wanke, E.M.; Arendt, M.; Mill, H.; Koch, F.; Wanke, A.; Groneberg, D.A. Traumatic injuries in revue dancers. J. Danc. Med. Sci. Off. Publ. Int. Assoc. Danc. Med. Sci. 2014, 18, 22–28. [Google Scholar] [CrossRef]
- Wanke, E.M.; Schmidt, M.; Leslie-Spinks, J.; Fischer, A.; Groneberg, D.A. Physical and mental workloads in professional dance teachers. Med. Probl. Perform. Artist. 2015, 30, 54–60. [Google Scholar] [CrossRef]
- Ohlendorf, D.; Krüger, D.; Christian, W.; Ackermann, H.; Keil, F.; Oremek, G.; Maurer-Grubinger, C.; Groneberg, D.A. Standard reference values of the upper body posture in healthy male adults aged between 51 and 60 years in Germany. Sci. Rep. 2022, 12, 6961. [Google Scholar] [CrossRef]
- Ohlendorf, D.; Mickel, C.; Filmann, N.; Wanke, E.M.; Groneberg, D.A. Standard values of the upper body posture and postural control: A study protocol. J. Occup. Med. Toxicol. 2016, 11, 34. [Google Scholar] [CrossRef]
- Maurer-Grubinger, C.; Adjami, F.; Avaniadi, I.; Christian, W.; Doerry, C.; Fay, V.; Fisch, V.; Gerez, A.; Goecke, J.; Kaya, U.; et al. Symmetrical dental occlusion blocking—Changes of body sway and weight distribution in healthy subjects across 4 age decades. J. Occup. Med. Toxicol. 2021, 16, 7. [Google Scholar] [CrossRef]
- Fon, G.T.; Pitt, M.J.; Thies, A.C., Jr. Thoracic kyphosis: Range in normal subjects. Am. J. Roentgenol. 1980, 134, 979–983. [Google Scholar] [CrossRef]
- Kopp, S. Okklusale und Klinisch Funktionelle Befunde im Cranio-Mandibulären System (CMS) bei Kindern und Jugendlichen; Friedrich-Schiller-Universität: Jena, Germany, 2005. [Google Scholar]
- Perry, J. Ganganalyse. Norm und Pathologie des Gehens, 1st ed.; Urban & Fischer/Elsevier GmbH: Munich, Germany, 2003. [Google Scholar]
- Missalla, S.; Schulze, J.; Bille, J.; Maltry, L.; Ohlendorf, D. Auswirkungen von Kniebeschwerden auf die posturale Kontrolle unter Ausschluss der Muskulatur des kraniomandibulären Systems. Orthopäde 2020, 49, 510–521. [Google Scholar] [CrossRef] [PubMed]
- Steib, S.; Pfeifer, K. Beeinträchtigungen der sensomotorischen Kontrolle bei funktioneller Sprunggelenkinstabilität. Z. Orthop Unfall. 2015, 153, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Vaz, M.M.; Costa, G.C.; Reis, J.G.; Junior, W.M.; Albuquerque de Paula, F.J.; Abreu, D.C. Postural control and functional strength in patients with type 2 diabetes mellitus with and without peripheral neuropathy. Arch. Phys. Med. Rehabil. 2013, 94, 2465–2470. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, J.A.; Silva, C.C.; dos Santos, H.H.; de Almeida Ferreira, J.J.; de Andrade, P.R. A preliminary study of static and dynamic balance in sedentary obese young adults: The relationship between BMI, posture and postural balance. Clin. Obes. 2017, 7, 377–383. [Google Scholar] [CrossRef]
- Obens, T. Belastungs-/Druckverteilung unter dem Fuß in Statik und Dynamik. BMJ Open 2019, 9, e026833. [Google Scholar]
- Lalande, X.; Vie, B.; Weber, J.P.; Jammes, Y. Normal Values of Pressures and Foot Areas Measured in the Static Condition. J. Am. Podiatr. Med. Assoc. 2016, 106, 265–272. [Google Scholar] [CrossRef][Green Version]
- Aagaard, P.; Suetta, C.; Caserotti, P.; Magnusson, S.P.; Kjær, M. Role of the nervous system in sarcopenia and muscle atrophy with aging: Strength training as a countermeasure. Scand. J. Med. Sci. Sports 2010, 20, 49–64. [Google Scholar] [CrossRef]
- Kitabayashi, T.; Demura, S.; Kawabata, H.; Uchiyama, M.; Demura, T. Comparison of the body-sway characteristics of young adults compared to healthy elderly and elderly with equilibrium disorder. Percept. Mot. Ski. 2011, 113, 547–556. [Google Scholar] [CrossRef]
- Singh, N.B.; Taylor, W.R.; Madigan, M.L.; Nussbaum, M.A. The spectral content of postural sway during quiet stance: Influences of age, vision and somatosensory inputs. J. Electromyogr. Kinesiol. Off. J. Int. Soc. Electrophysiol. Kinesiol. 2012, 22, 131–136. [Google Scholar] [CrossRef]
- Ebenbichler, G.R.; Kerschan-Schindl, K. Sicher Bewegen im Alter: Veränderungen der sensomotorischen Fähigkeiten. Phys. Med. Rehabil. Kurortmed. 2009, 19, 44–58. [Google Scholar] [CrossRef]
- Espenschade, A.S.E.H.M. Motor Development. Merill: Indianapolis, IN, USA, 1967. [Google Scholar]
- Kerschan-Schindl, K.; Ebenbichler, G.R. Sicher Bewegen im Alter: Optimierung der sensomotorischen Fähigkeiten zur Sturzprävention. Phys. Med. Rehab. Kuror. 2009, 19, 107–118. [Google Scholar] [CrossRef]
- Mayer, F.; Scharhag-Rosenberger, F.; Carlsohn, A.; Cassel, M.; Müller, S.; Scharhag, J. Intensität und Effekte von Krafttraining bei Älteren. Dtsch. Arztebl. Int. 2011, 108, 359–364. [Google Scholar] [PubMed]
- Schöne, D.; Freiberger, E.; Sieber, C.C. Einfluss der Skelettmuskulatur auf das Sturzrisiko im Alter. Internist 2017, 58, 359–370. [Google Scholar] [CrossRef] [PubMed]
- Mensink, G.B.M.; Schienkiewitz, A.; Haftenberger, M.; Lampert, T.; Ziese, T.; Scheidt-Nave, C. Übergewicht und Adipositas in Deutschland. Bundesgesundheitsblatt-Gesundh.-Gesundh. 2013, 56, 786–794. [Google Scholar] [CrossRef] [PubMed]
- Olchowik, G.; Tomaszewski, M.; Olejarz, P.; Warchoł, J.; Różańska-Boczula, M. The effect of height and BMI on computer dynamic posturography parameters in women. Acta Bioeng. Biomech. 2014, 16, 53–58. [Google Scholar]
- Son, S.M. Influence of Obesity on Postural Stability in Young Adults. Osong Public Health Res. Perspect. 2016, 7, 378–381. [Google Scholar] [CrossRef]
- Błaszczyk, J.W.; Cieślinska-Świder, J.; Plewa, M.; Zahorska-Markiewicz, B.; Markiewicz, A. Effects of excessive body weight on postural control. J. Biomech. 2009, 42, 1295–1300. [Google Scholar] [CrossRef]
- Cimolin, V.; Cau, N.; Galli, M.; Capodaglio, P. Balance Control in Obese Subjects during Quiet Stance: A State-of-the Art. Appl. Sci. 2020, 10, 1842. [Google Scholar] [CrossRef]
- Simoneau, M.; Teasdale, N. Balance control impairment in obese individuals is caused by larger balance motor commands variability. Gait Posture 2015, 41, 203–208. [Google Scholar] [CrossRef]
- Runge, C.F.; Shupert, C.L.; Horak, F.B.; Zajac, F.E. Ankle and hip postural strategies defined by joint torques. Gait Posture 1999, 10, 161–170. [Google Scholar] [CrossRef]
- 53. Viebrock, H.; Forst, B. (Eds.) Bobath. In Therapiekonzepte in der Physiotherapie, 1st ed.; Georg Thieme: Stuttgart, Germany, 2007. [Google Scholar]
- Horak, F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing 2006, 35 (Suppl. S2), ii7–ii11. [Google Scholar] [CrossRef] [PubMed]
- Taube, W. Neuronale Mechanismen der posturalen Kontrolle und der Einfluss von Gleichgewichtstraining. Neurol. Neurochir. Psychiatrie. 2013, 14, 55–63. [Google Scholar]
- Eckhardt, G. Posturale Kontrolle und die Bedeutung für das Sturzrisiko bei Patienten nach Schlaganfall. Z. Physiother. 2013, 1. [Google Scholar]
- Hue, O.; Simoneau, M.; Marcotte, J.; Berrigan, F.; Doré, J.; Marceau, P.; Marceau, S.; Tremblay, A.; Teasdale, N. Body weight is a strong predictor of postural stability. Gait Posture 2007, 26, 32–38. [Google Scholar] [CrossRef]
- Cruz-Gomez, N.S.; Plascencia, G.; Villanueva-Padron, L.A.; Jauregui-Renaud, K. Influence of obesity and gender on the postural stability during upright stance. Obesity Facts 2011, 4, 212–217. [Google Scholar] [CrossRef]
- Kováčiková, Z.; Svoboda, Z.; Neumannová, K.; Bizovská, L.; Cuberek, R.; Janura, M. Assessment of postural stability in overweight and obese middle-aged women. Acta Gymnica 2014, 44, 149–153. [Google Scholar] [CrossRef]
- Hirjaková, Z.; Šuttová, K.; Kimijanová, J.; Bzdúšková, D.; Hlavačka, F. Postural changes during quiet stance and gait initiation in slightly obese adults. Physiol. Res. 2018, 67, 985–992. [Google Scholar] [CrossRef]
- Ku, P.X.; Abu Osman, N.A.; Yusof, A.; Wan Abas, W.A.B. Biomechanical evaluation of the relationship between postural control and body mass index. J. Biomech. 2012, 45, 1638–1642. [Google Scholar] [CrossRef]
- Deepashini, H.; Omar, B.; Paungmali, A.; Amaramalar, N.; Ohnmar, H.; Leonard, J. An insight into the plantar pressure distribution of the foot in clinical practice: Narrative review. Pol. Ann. Med. 2014, 21, 51–56. [Google Scholar] [CrossRef]
- Lelard, T.; Ahmaidi, S. Effects of physical training on age-related balance and postural control. Neurophysiol. Clin. Clin. Neurophysiol. 2015, 45, 357–369. [Google Scholar] [CrossRef]
- Wydra, G. Bedeutung, Diagnose & Therapie von Gleichgewichtsstörungen. Motorik 1993, 16, 100–107. [Google Scholar]
- Hrysomallis, C. Balance ability and athletic performance. Sports Med. 2011, 41, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Low, D.C.; Walsh, G.S.; Arkesteijn, M. Effectiveness of Exercise Interventions to Improve Postural Control in Older Adults: A Systematic Review and Meta-Analyses of Centre of Pressure Measurements. Sports Med. 2017, 47, 101–112. [Google Scholar] [CrossRef]
- Orr, R.; Raymond, J.; Fiatarone Singh, M. Efficacy of progressive resistance training on balance performance in older adults: A systematic review of randomized controlled trials. Sports Med. 2008, 38, 317–343. [Google Scholar] [CrossRef]
- Huber, M. Selbstwirksamkeit und posturale Kontrolle—YES, I CAN! Physiopraxis 2017, 15, 44–47. [Google Scholar] [CrossRef]

| Median | TR | TR | CI | CI | |
|---|---|---|---|---|---|
| Lower Limit | Upper Limit | Left Limit | Right Limit | ||
| Forefoot left (%) | 19.33 | 8.33 | 34 | 18 | 21 |
| Forefoot right (%) | 13 | 2.4 | 29.33 | 12.67 | 15 |
| Rearfoot left (%) | 32.67 | 17.85 | 48.63 | 31.33 | 34 |
| Rearfoot right (%) | 33.67 | 16.65 | 47.82 | 31 | 35.33 |
| Left foot (%) | 52.33 | 38 | 68.03 | 51 | 53.33 |
| Right foot (%) | 47.67 | 31.97 | 62 | 46.67 | 49 |
| Forefoot (%) | 33.33 | 21.37 | 54.6 | 30.67 | 36 |
| Rearfoot (%) | 66.67 | 45.5 | 78.63 | 64 | 69.33 |
| Frontal sway (mm) | 11 | 5.7 | 26.3 | 10 | 11.67 |
| Sagittal sway (mm) | 16 | 7.37 | 34.32 | 14.67 | 18.67 |
| Ellipse area (cm²) | 1.17 | 0.29 | 4.96 | 0.98 | 1.35 |
| Ellipse width (cm) | 0.91 | 0.42 | 1.9 | 0.84 | 1.02 |
| Ellipse height (cm) | 0.4 | 0.22 | 0.89 | 0.38 | 0.43 |
| Age | BMI | |||
|---|---|---|---|---|
| p-Values | Rho + Effect Size | p-Values | Rho + Effect Size | |
| Forefoot left (%) | 0.2 | −0.13 1 | 0.36 | 0.09 1 |
| Forefoot right (%) | 0.6 | 0.05 1 | 0.11 | 0.16 1 |
| Rearfoot left (%) | 0.5 | 0.07 1 | 0.21 | −0.13 1 |
| Rearfoot right (%) | 0.81 | 0.02 1 | 0.23 | −0.12 1 |
| Left foot (%) | 0.38 | −0.09 1 | 0.86 | −0.02 1 |
| Right foot (%) | 0.38 | 0.09 1 | 0.86 | 0.02 1 |
| Forefoot (%) | 0.79 | −0.03 1 | 0.08 | 0.18 1 |
| Rearfoot (%) | 0.8 | 0.03 1 | 0.08 | −0.17 1 |
| Frontal sway (mm) | 0.06 | 0.19 1 | 0.18 | −0.14 1 |
| Sagittal sway (mm) | 0.04 | 0.21 2 | 0.71 | 0.04 1 |
| Ellipse area (cm2) | 0.04 | 0.21 2 | 0.47 | −0.07 1 |
| Ellipse width (cm) | 0.02 | 0.24 2 | 0.83 | −0.02 1 |
| Ellipse height (cm) | 0.2 | 0.13 1 | 0.16 | −0.14 1 |
| BMI (1) | BMI (2) | BMI (3) | BMI (4) | Conover–Iman test after Bonferroni-Holm Correction | |||||
|---|---|---|---|---|---|---|---|---|---|
| n = 53 | n = 30 | n = 14 | n = 4 | ||||||
| Median | Min./Max. | Median | Min./Max. | Median | Min./Max. | Median | Min./Max. | p-Value | |
| Forefoot left (%) | 18.00 | 8.33/33.33 | 19.17 | 5.67/34.00 | 22.5 | 16.67/34.00 | 24.17 | 18.00/34.33 | 1 vs. 3: 0.05 2 vs. 3: 0.05 |
| Forefoot right (%) | 13.00 | 1.33/32.00 | 16.00 | 3.00/29.33 | 13.84 | 6.67/25.00 | 10.17 | 1.67/19.33 | 0.28 |
| Rearfoot left (%) | 33.33 | 18.33/50.00 | 33.50 | 18.00/48.00 | 30.67 | 17.67/48.33 | 30.17 | 15.00/46.67 | 0.19 |
| Rearfoot right (%) | 35.33 | 6.33/51.67 | 31.84 | 18.00/47.67 | 28.67 | 20.67/41.00 | 34.50 | 23.33/43.67 | 0.23 |
| Left foot (%) | 51.67 | 38.00/74.67 | 52.33 | 31.00/64.00 | 53.84 | 42.67/65.33 | 56.34 | 45.33/64.67 | 0.50 |
| Right foot (%) | 48.44 | 25.33/62.00 | 47.67 | 36.00/69.00 | 46.17 | 34.67/57.33 | 43.67 | 35.33/54.67 | 0.50 |
| Forefoot (%) | 31.67 | 21.00/56.33 | 32.50 | 21.67/62.33 | 40.00 | 24.00/52.00 | 36.34 | 20.00/49.33 | 1 vs. 3: 0.03 |
| Rearfoot (%) | 68.33 | 43.67/79.00 | 67.50 | 37.67/78.33 | 60.00 | 48.00/76.00 | 63.67 | 50.67/80.00 | 1 vs. 3: 0.03 |
| Frontal sway (mm) | 11.33 | 6.67/32.67 | 10.33 | 3.67/17.67 | 10.67 | 5.33/18.67 | 12.00 | 7.00/16.00 | 0.24 |
| Sagittal sway (mm) | 15.67 | 4.00/42.00 | 16.17 | 7.67/36.33 | 19.17 | 8.67/28.67 | 13.50 | 7.00/21.67 | 0.81 |
| Ellipse area (cm²) | 1.19 | 0.25/5.55 | 1.09 | 0.22/3.69 | 1.24 | 0.33/3.30 | 1.04 | 0.48/3.20 | 0.50 |
| Ellipse width (cm) | 0.93 | 0.37/2.82 | 0.90 | 0.40/2.02 | 1.01 | 0.43/1.65 | 0.79 | 0.50/1.55 | 0.88 |
| Ellipse height (cm) | 0.40 | 0.21/0.98 | 0.39 | 0.18/0.71 | 0.37 | 0.22/0.81 | 0.42 | 0.30/0.62 | 0.26 |
| No Sport (n = 26) | 1×/Week Sport (n = 26) | 2×/Week Sport (n = 17) | >2×/Week Sport (n = 32) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Median | Min./Max. | Median | Min./Max. | Median | Min./Max. | Median | Min./Max. | p Values | Eta | |
| Forefoot left (%) | 19.33 | 10.33/ 34.33 | 18.67 | 8.33/ 34.00 | 17.67 | 10.33/ 28.33 | 21.84 | 5.67/ 34.00 | 0.59 | 0.02 1 |
| Forefoot right (%) | 14.50 | 1.33/ 29.33 | 12.17 | 3.00/ 29.33 | 13.00 | 1.67/ 24.33 | 14.67 | 6.00/ 32.00 | 0.33 | 0.03 1 |
| Rearfoot left (%) | 31.33 | 19.00/ 50.00 | 32.67 | 17.67/ 49.00 | 34.67 | 18.00/ 48.00 | 32.50 | 15.00/ 48.33 | 0.68 | 0.02 1 |
| Rearfoot right (%) | 32.33 | 6.33/ 48.00 | 35.00 | 20.33/ 51.67 | 34.67 | 25.00/ 40.67 | 30.83 | 15.00/ 47.67 | 0.58 | 0.02 1 |
| Left foot (%) | 52.17 | 41.67/ 74.67 | 52.50 | 38.00/ 71.33 | 51.00 | 40.67/ 64.67 | 52.83 | 31.00/ 65.33 | 0.95 | 0.003 1 |
| Right foot (%) | 47.84 | 25.33/ 58.33 | 47.50 | 28.67/ 62.00 | 49.00 | 35.33/ 59.33 | 47.17 | 34.67/ 69.00 | 0.95 | 0.003 1 |
| Forefoot (%) | 35.17 | 22.33/ 62.33 | 30.34 | 22.33/ 56.33 | 30.33 | 20.00/ 50.67 | 36.00 | 21.00/ 53.00 | 0.20 | 0.05 1 |
| Rearfoot (%) | 64.84 | 37.67/ 77.67 | 69.67 | 43.67/ 77.67 | 67.67 | 49.33/ 80.00 | 64.00 | 47.00/ 79.00 | 0.20 | 0.05 1 |
| Frontal sway (mm) | 11.00 | 6.33/ 32.67 | 11.34 | 5.33/ 23.00 | 9.00 | 3.67/ 17.00 | 12.00 | 6.00/ 26.67 | 0.14 | 0.05 1 |
| Sagittal sway (mm) | 17.17 | 9.00/ 36.33 | 16.83 | 8.67/ 27.67 | 14.67 | 7.00/ 24.67 | 15.00 | 4.00/ 42.00 | 0.72 | 0.01 1 |
| Ellipse area (cm²) | 1.20 | 0.38/ 5.31 | 1.33 | 0.33/ 4.39 | 1.07 | 0.22/ 2.05 | 1.19 | 0.25/ 5.55 | 0.47 | 0.03 1 |
| Ellipse width (cm) | 0.90 | 0.54/ 2.02 | 0.96 | 0.43/ 1.75 | 0.82 | 0.40/ 1.56 | 0.92 | 0.37/ 2.82 | 0.52 | 0.02 1 |
| Ellipse height (cm) | 0.41 | 0.22/ 0.98 | 0.43 | 0.24/ 0.98 | 0.38 | 0.18/ 0.51 | 0.44 | 0.21/ 0.75 | 0.35 | 0.03 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ohlendorf, D.; Keller, J.; Sosnov, P.; Ackermann, H.; Keil, F.; Maurer-Grubinger, C.; Holzgreve, F.; Oremek, G.; Groneberg, D.A. Body Weight Distribution and Body Sway in Healthy Female Adults Aged between 51 and 60 Years in Germany—Standard Values. Appl. Sci. 2022, 12, 9591. https://doi.org/10.3390/app12199591
Ohlendorf D, Keller J, Sosnov P, Ackermann H, Keil F, Maurer-Grubinger C, Holzgreve F, Oremek G, Groneberg DA. Body Weight Distribution and Body Sway in Healthy Female Adults Aged between 51 and 60 Years in Germany—Standard Values. Applied Sciences. 2022; 12(19):9591. https://doi.org/10.3390/app12199591
Chicago/Turabian StyleOhlendorf, Daniela, Julia Keller, Polyna Sosnov, Hanns Ackermann, Fee Keil, Christian Maurer-Grubinger, Fabian Holzgreve, Gerhard Oremek, and David A. Groneberg. 2022. "Body Weight Distribution and Body Sway in Healthy Female Adults Aged between 51 and 60 Years in Germany—Standard Values" Applied Sciences 12, no. 19: 9591. https://doi.org/10.3390/app12199591
APA StyleOhlendorf, D., Keller, J., Sosnov, P., Ackermann, H., Keil, F., Maurer-Grubinger, C., Holzgreve, F., Oremek, G., & Groneberg, D. A. (2022). Body Weight Distribution and Body Sway in Healthy Female Adults Aged between 51 and 60 Years in Germany—Standard Values. Applied Sciences, 12(19), 9591. https://doi.org/10.3390/app12199591








