Biocompatibility and Antioxidant Capabilities of Carbon Dots Obtained from Tomato (Solanum lycopersicum)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Instruments and Solutions
2.2. Carbon Dot Synthesis
2.3. Luminescence Experiments
2.4. Evaluation of the Antioxidant Capacity
2.5. In Vitro Studies
2.6. Cytotoxicity Assay
3. Results and Discussion
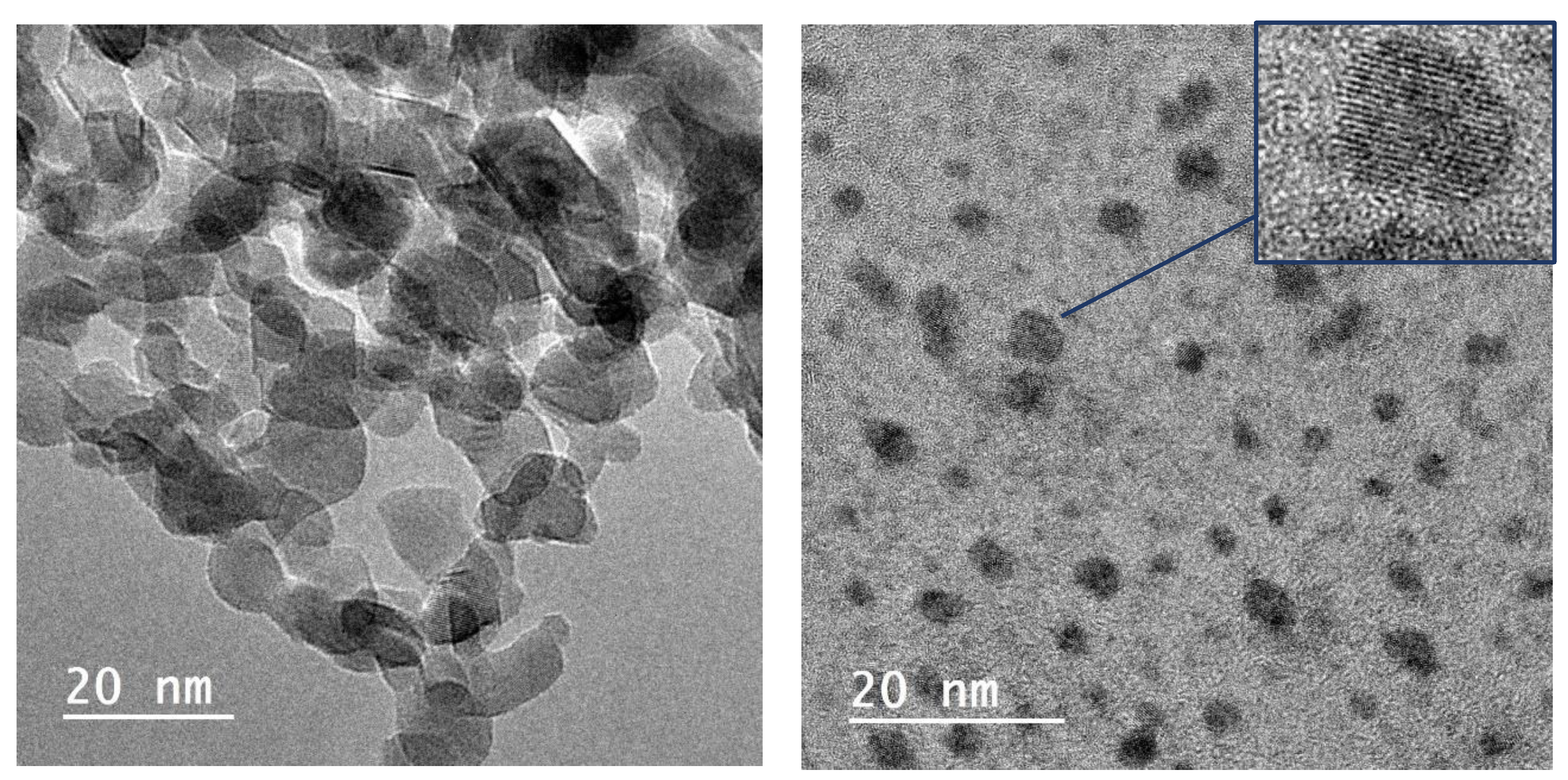
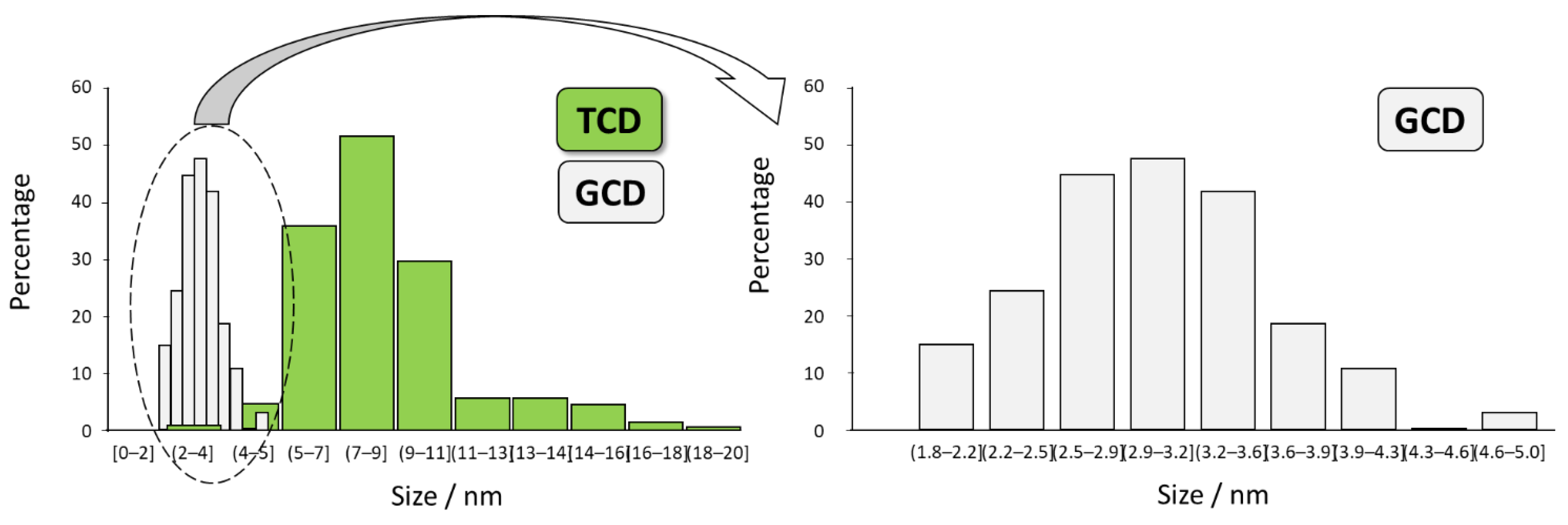
3.1. Structural and Morphological Characterization
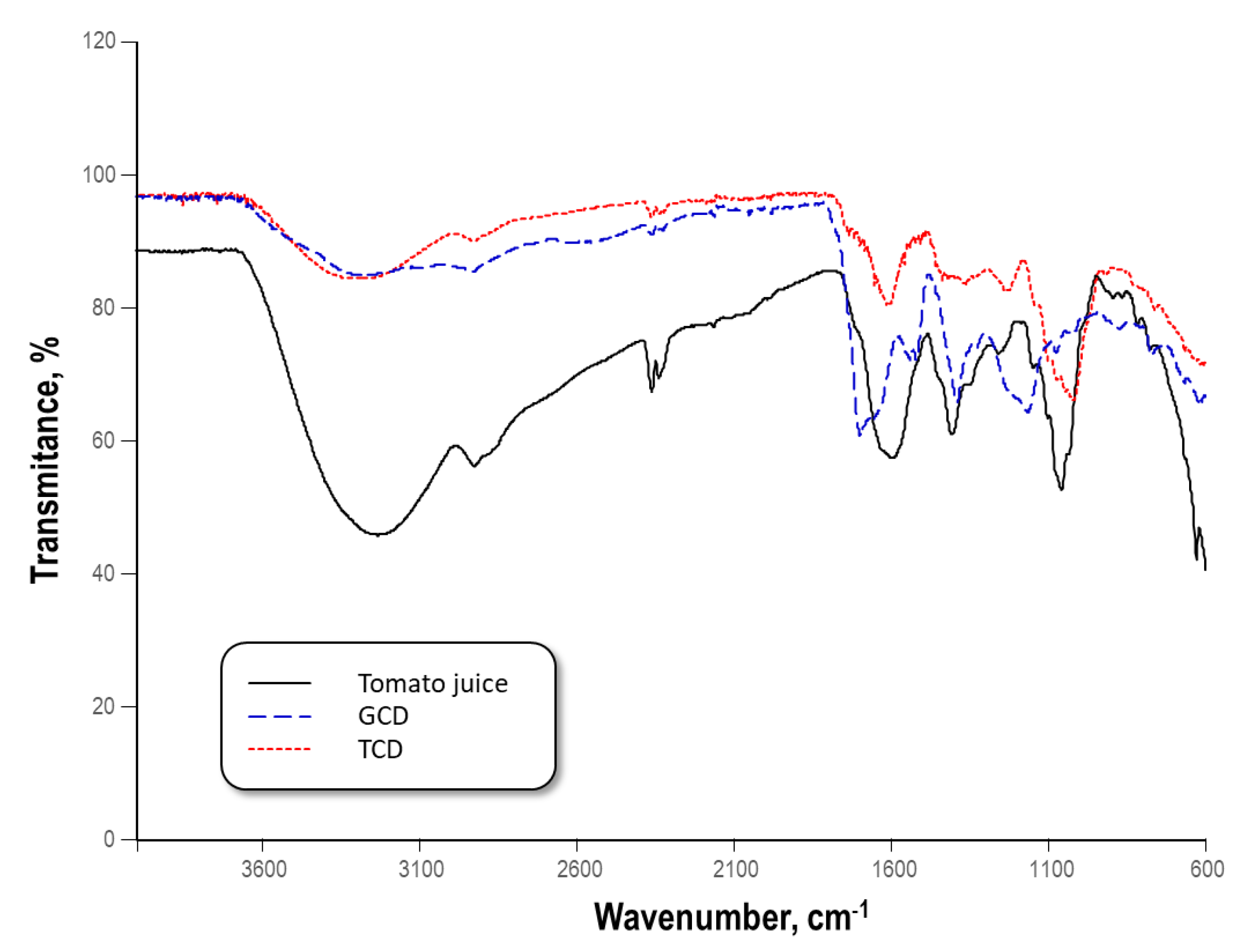
3.2. Surface Characterization
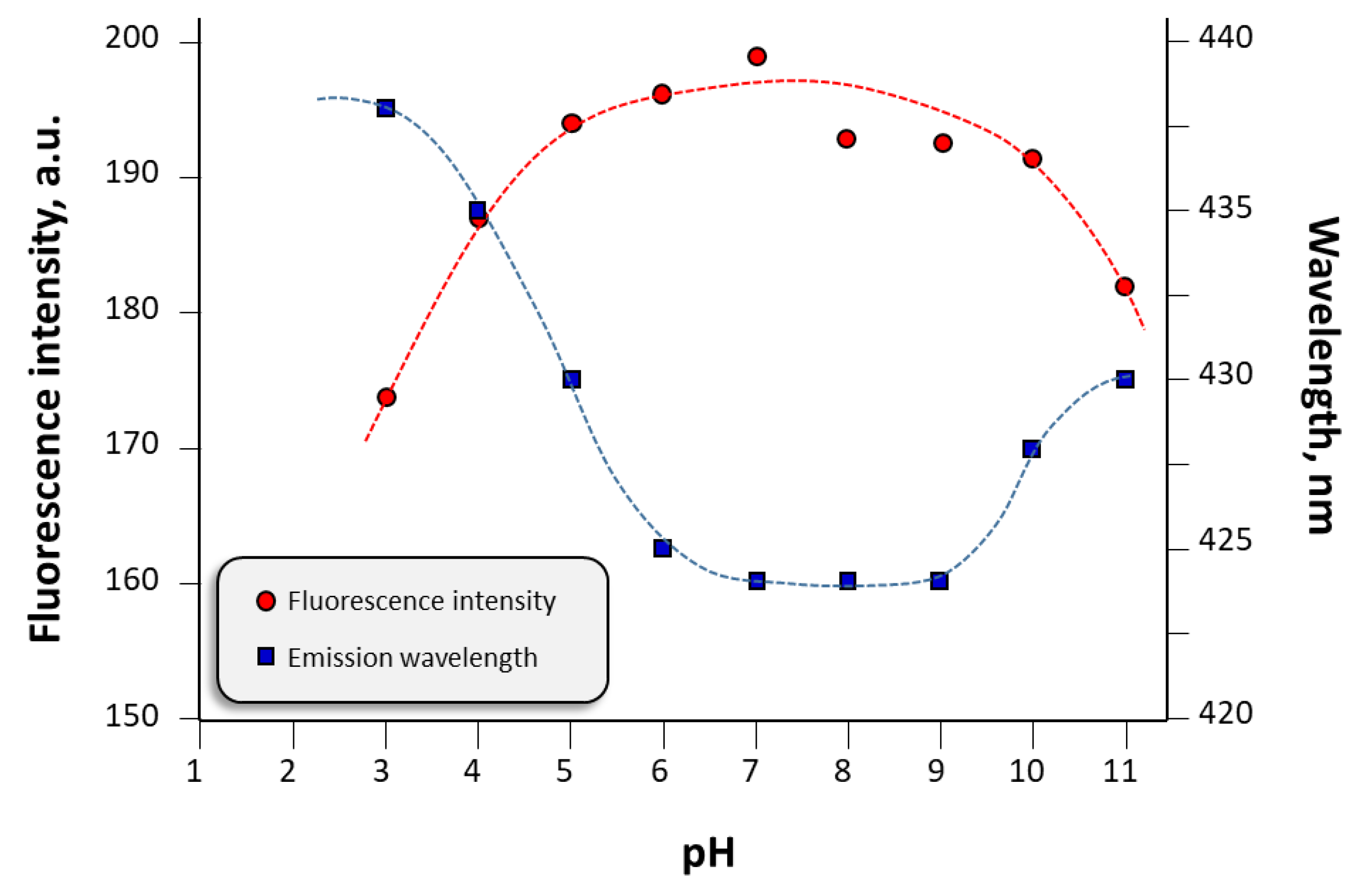
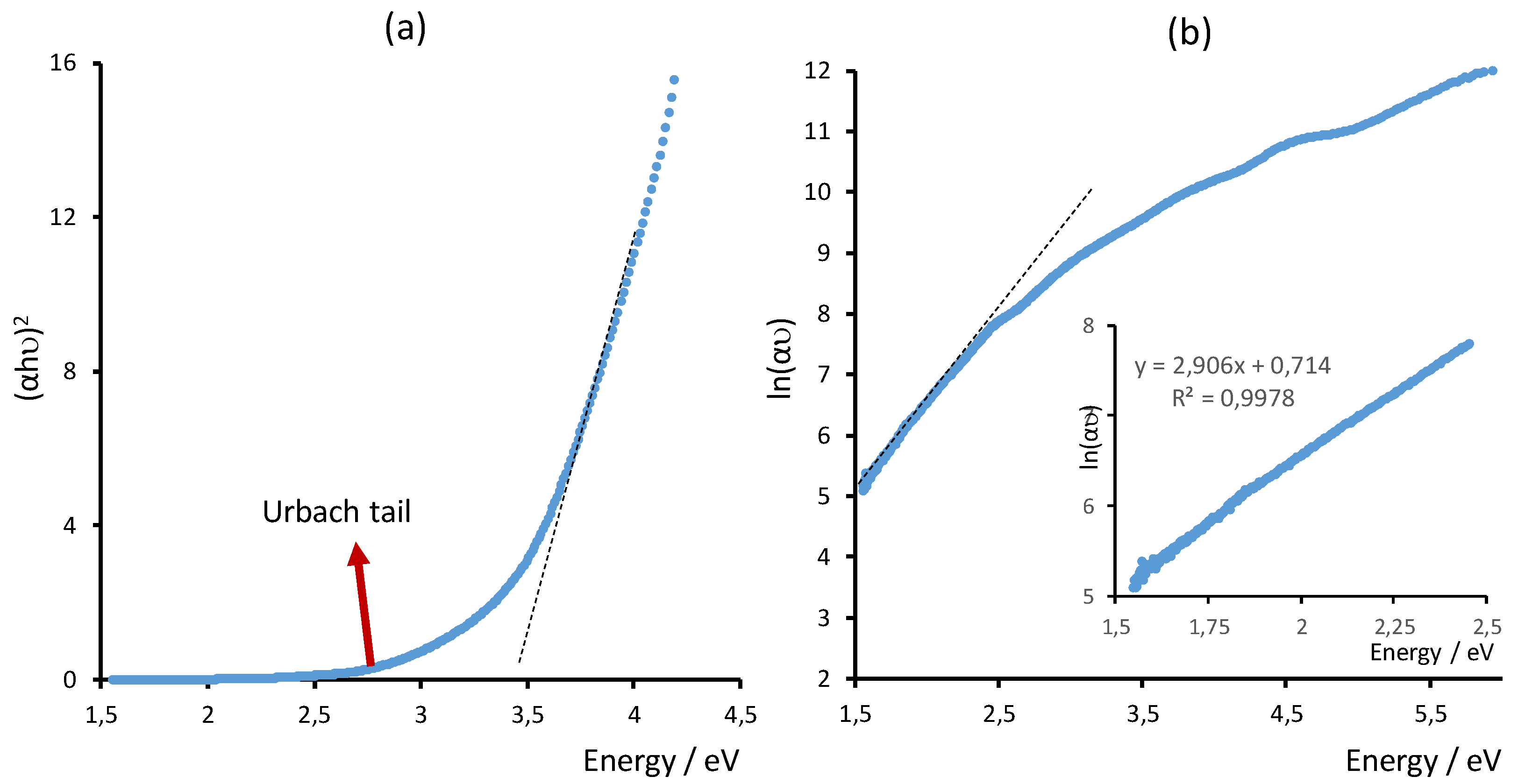
3.3. Spectroscopic Characterization
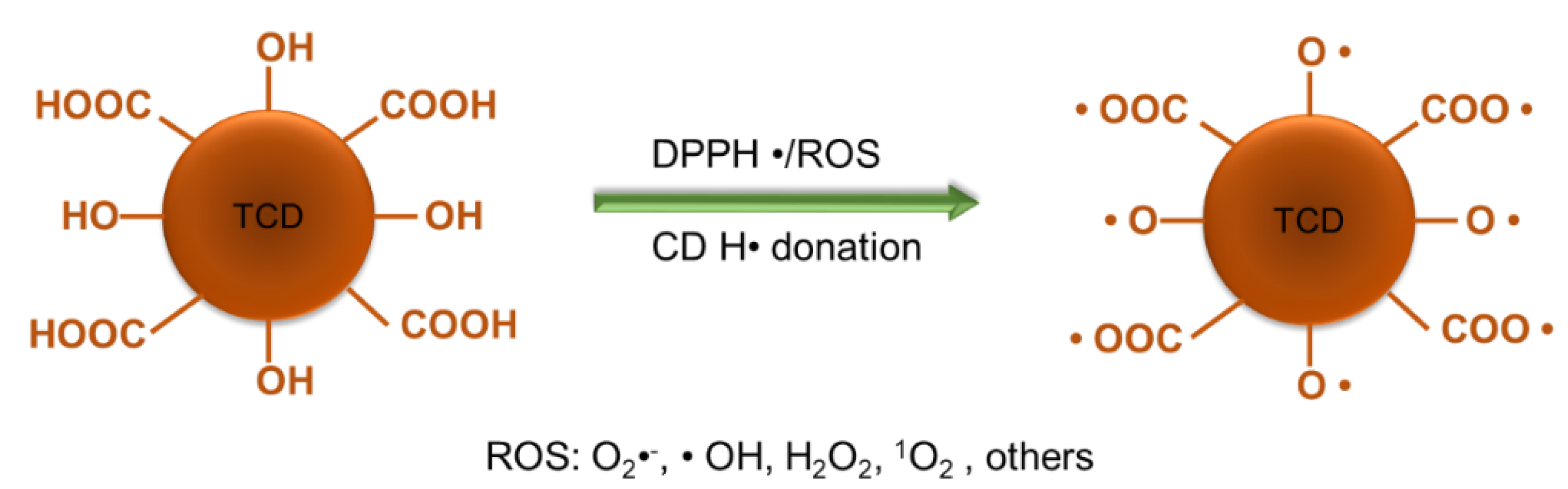
3.4. Antioxidant Capabilities of TCDs
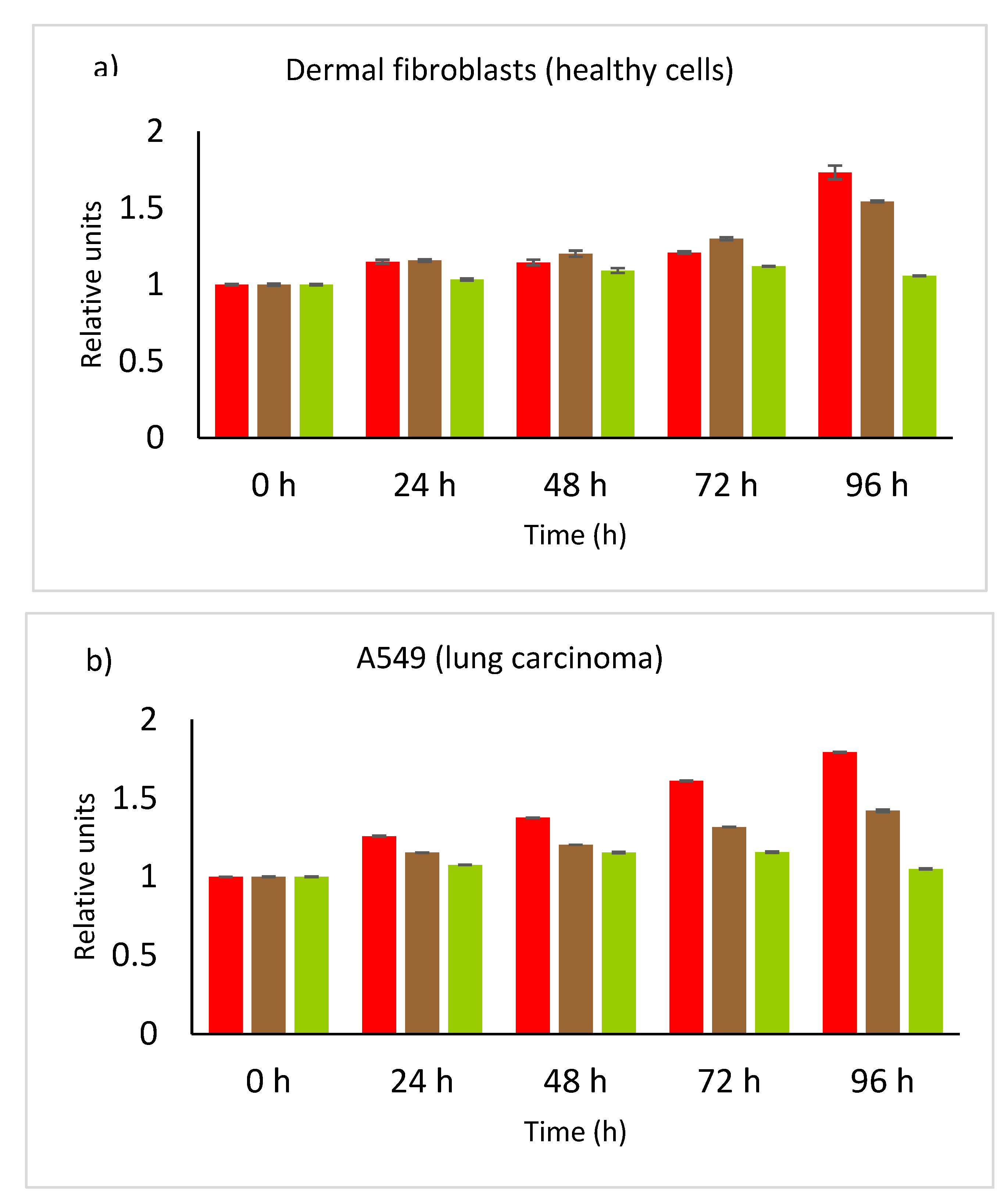
3.5. Cell Viability—Toxicity Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic Analysis and Purification of Fluorescent Single-Walled Carbon Nanotube Fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef]
- Kurian, M.; Paul, A. Recent trends in the use of green sources for carbon dot synthesis—A short review. Carbon Trends 2021, 3, 100032. [Google Scholar] [CrossRef]
- Ridha, A.A.; Pakravan, P.; Azandaryani, A.H.; Zhaleh, H. Carbon dots; the smallest photoresponsive structure of carbon in advanced drug targeting. J. Drug Deliv. Sci. Technol. 2020, 55, 101408. [Google Scholar] [CrossRef]
- Ma, Y.S.; Xu, G.H.; Wei, F.D.; Cen, Y.; Song, Y.Y.; Ma, Y.J.; Xu, X.M.; Shi, M.L.; Sohail, M.; Hu, Q. Carbon dots based immunosorbent assay for the determination of GFAP in human serum. Nanotechnology 2018, 29, 145501. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.M.; Li, G.L.; Lv, Z.X.; Qiu, N.N.; Kong, W.H.; Gong, P.W.; Chen, G.; Xia, L.; Guo, X.X.; You, J.M.; et al. Facile and ultrasensitive fluorescence sensor platform for tumor invasive biomaker beta-glucuronidase detection and inhibitor evaluation with carbon quantum dots based on inner-filter effect. Biosens. Bioelectron. 2016, 85, 358–362. [Google Scholar] [CrossRef] [PubMed]
- Dhenadhayalan, N.; Lin, K.C.; Saleh, T.A. Recent Advances in Functionalized Carbon Dots toward the Design of Efficient Materials for Sensing and Catalysis Applications. Small 2020, 16, 1905767. [Google Scholar] [CrossRef] [PubMed]
- Atabaev, T.S. Doped Carbon Dots for Sensing and Bioimaging Applications: A Minireview. Nanomaterials 2018, 8, 342. [Google Scholar] [CrossRef] [Green Version]
- Fernando, K.A.S.; Sahu, S.; Liu, Y.M.; Leiws, W.K.; Guliants, E.A.; Jafariyan, A.; Wang, P.; Bunker, C.E.; Sun, Y.P. Carbon Quantum Dots and Applications in Photocatalytic Energy Conversion. ACS App. Mat. Inter. 2015, 7, 8363–8376. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998; Volume 30. [Google Scholar]
- OECD, Sustainable chemistry, Retrieved 14 December 2021. Available online: http://www.oecd.org/env/ehs/risk-management/sustainablechemistry.htm (accessed on 11 December 2021).
- Hsu, P.-C.; Chang, H.-T. Synthesis of high-quality carbon nanodots from hydrophilic compounds: Role of functional groups. Chem. Comm. 2012, 48, 3984–3986. [Google Scholar] [CrossRef]
- Sahu, S.; Behera, B.; Maiti, T.K.; Mohapatra, S. Simple one-step synthesis of highly luminescent carbon dots from orange juice: Application as excellent bio-imaging agents. Chem. Comm. 2012, 48, 8835–8837. [Google Scholar] [CrossRef]
- Scherlinger, M.; Tsokos, G.C. Reactive oxygen species: The Yin and Yang in (auto-)immunity. Autoimmun. Rev. 2021, 20, 102869. [Google Scholar] [CrossRef] [PubMed]
- Galadari, S.; Rahman, A.; Pallichankandy, S.; Thayyullathil, F. Reactive oxygen species and cancer paradox: To promote or to suppress? Free Radic. Biol. Med. 2017, 104, 144–164. [Google Scholar] [CrossRef]
- Erge, H.S.; Karadeniz, F. Bioactive compounds and antioxidant activity of tomato cultivars. Int. J. Food Prop. 2011, 14, 968–977. [Google Scholar] [CrossRef]
- Liu, W.; Li, C.; Sun, X.; Pan, W.; Yu, G.; Wang, J. Highly crystalline carbon dots from fresh tomato: UV emission and quantum confinement. Nanotechnology 2017, 28, 485705. [Google Scholar] [CrossRef]
- Lai, Z.; Guo, X.; Cheng, Z.; Ruan, G.; Du, F. Green Synthesis of Fluorescent Carbon Dots from Cherry Tomatoes for Highly Effective Detection of Trifluralin Herbicide in Soil Samples. Chem. Sel. 2020, 5, 1956–1960. [Google Scholar] [CrossRef]
- Murru, C.; Badía-Laíño, R.; Díaz-García, M.E. Synthesis and Characterization of Green Carbon Dots for Scavenging Radical Oxygen Species in Aqueous and Oil Samples. Antioxidants 2020, 9, 1147. [Google Scholar] [CrossRef]
- Chattopadhyay, S.; Mehrotra, N.; Jain, S.; Singh, H. Development of novel blue emissive carbon dots for sensitive detection of dual metal ions and their potential applications in bioimaging and chelation therapy. Microchem. J. 2021, 170, 106706. [Google Scholar] [CrossRef]
- Bayda, S.; Amadio, E.; Cailotto, S.; Frión-Herrera, Y.; Perosa, A.; Rizzolio, F. Carbon dots for cancer nanomedicine: A bright future. Nanoscale Adv. 2021, 3, 5183. [Google Scholar] [CrossRef]
- Hong, W.T.; Yang, H.K. Anti-counterfeiting application of fluorescent carbon dots derived from wasted coffee grounds. Optik 2021, 241, 166449. [Google Scholar] [CrossRef]
- Lu, Q.; Zhang, Y.; Liu, S. Graphene quantum dots enhanced photocatalytic activity of zinc porphyrin toward the degradation of methylene blue under visible-light irradiation. Nanoscale 2014, 6, 1890–1895. [Google Scholar] [CrossRef]
- Díaz-Faes López, T.; Fernández-González, A.; Díaz-García, M.E.; Badía-Laíño, R. Highly efficient Förster resonance energy transfer between carbon nanoparticles and europium–tetracycline complex. Carbon 2015, 94, 142–151. [Google Scholar] [CrossRef]
- Chhabra, V.A.; Kaur, R.; Kumar, N.; Deep, A.; Rajesh, C.; Kim, K.-H. Synthesis and spectroscopic studies of functionalized graphene quantum dots with diverse fluorescence characteristics. RSC Adv. 2018, 8, 11446. [Google Scholar] [CrossRef] [Green Version]
- Wen, Z.-H.; Yin, X.-B. Excitation-Independent Carbon Dots, from Photoluminescence Mechanism to Single-Color Application. RSC Adv. 2016, 6, 27829–27835. [Google Scholar] [CrossRef]
- Du, J.; Cullen, J.J.; Buettner, G.R. Ascorbic acid: Chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta 2012, 1826, 443–457. [Google Scholar] [CrossRef] [Green Version]
- Shi, L.; Zhao, B.; Li, X.; Zhang, G.; Zhang, Y.; Dong, C.; Shuang, S. Eco-friendly synthesis of nitrogen-doped carbon nanodots from wool for multicolor cell imaging, patterning, and biosensing. Sens. Actuators B Chem. 2016, 235, 316–324. [Google Scholar] [CrossRef]
- Pan, Y.; Inam, F.; Zhang, M.; Drabold, D.A. Atomistic Origin of Urbach Tails in Amorphous Silicon. Phys. Rev. Lett. 2008, 100, 206403. [Google Scholar] [CrossRef] [Green Version]
- Sun, Y.; Yu, Y.Y.; Cao, S.W. Antioxidant Capacity of Caffeic Acid, Phloretin and Glutathione Mixtures and Formula Optimization. Asn. J. Chem. 2013, 25, 3971–3978. [Google Scholar] [CrossRef]
| [CDs]/ppm | 4 | 8 | 12 | 16 | 20 |
|---|---|---|---|---|---|
| % inhibition TCDs | 54.7% | 59.6% | 61.0% | 63.1% | 63.8% |
| % inhibition GCDs | 21.0% | 39.0% | 46.0% | 53.0% | 60.0% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Varillas, S.; Fontanil, T.; Obaya, Á.J.; Fernández-González, A.; Murru, C.; Badía-Laíño, R. Biocompatibility and Antioxidant Capabilities of Carbon Dots Obtained from Tomato (Solanum lycopersicum). Appl. Sci. 2022, 12, 773. https://doi.org/10.3390/app12020773
Rodríguez-Varillas S, Fontanil T, Obaya ÁJ, Fernández-González A, Murru C, Badía-Laíño R. Biocompatibility and Antioxidant Capabilities of Carbon Dots Obtained from Tomato (Solanum lycopersicum). Applied Sciences. 2022; 12(2):773. https://doi.org/10.3390/app12020773
Chicago/Turabian StyleRodríguez-Varillas, Sandra, Tania Fontanil, Álvaro J. Obaya, Alfonso Fernández-González, Clarissa Murru, and Rosana Badía-Laíño. 2022. "Biocompatibility and Antioxidant Capabilities of Carbon Dots Obtained from Tomato (Solanum lycopersicum)" Applied Sciences 12, no. 2: 773. https://doi.org/10.3390/app12020773
APA StyleRodríguez-Varillas, S., Fontanil, T., Obaya, Á. J., Fernández-González, A., Murru, C., & Badía-Laíño, R. (2022). Biocompatibility and Antioxidant Capabilities of Carbon Dots Obtained from Tomato (Solanum lycopersicum). Applied Sciences, 12(2), 773. https://doi.org/10.3390/app12020773






