Development and Validation of Two Intact Lumbar Spine Finite Element Models for In Silico Investigations: Comparison of the Bone Modelling Approaches
Abstract
:1. Introduction
2. Materials and Methods
2.1. Geometry of the FE Models
2.2. Material Properties
| Model | Material | Element Type | Constitutive Law | Material Properties |
|---|---|---|---|---|
| LBM | Cortical Bone | C3D4 | Linear elastic | E = 10,000 [MPa], ν = 0.3 [34] |
| Trabecular Bone | C3D4 | Linear elastic | E = 100, ν = 0.2 [35] | |
| Posterior Elements | C3D4 | Linear elastic | E = 3500, ν = 0.25 [35] | |
| Bony Endplate | C3D4 | Linear elastic | E = 1200, ν = 0.29 [16] | |
| PSM | Bone | C3D4 | Relationship between HU and E was determined using Equations (1)–(5) ν = 0.3 [37,38,39] | |
| LBM & PSM | Cartilaginous Endplate | C3D4, C3D5 | Linear elastic | E = 23.8, ν = 0.42 [27] |
| Facet Cartilage | C3D6 | Neo-Hooke | C10 = 5.36; D1 = 0.04 [27] | |
| Nucleus Pulposus | C3D8H | Mooney-Rivlin | C10 = 0.12; C01 = 0.03 [45] | |
| Annulus Fibrosus Ground Substance | C3D8H | Mooney-Rivlin | C10 = 0.18; C01 = 0.045 [43] | |
| Annulus Fibrosus Fibres | T3D2 | Nonlinear stress-strain curve; cross-section area values calculated at each layer from AF volume. 23% (outermost), 20%, 17%, 13%, 9%, 5% (innermost) [35,43,46] | ||
| Ligaments | SPRINGA | Nonlinear stress-strain curve [47] | ||
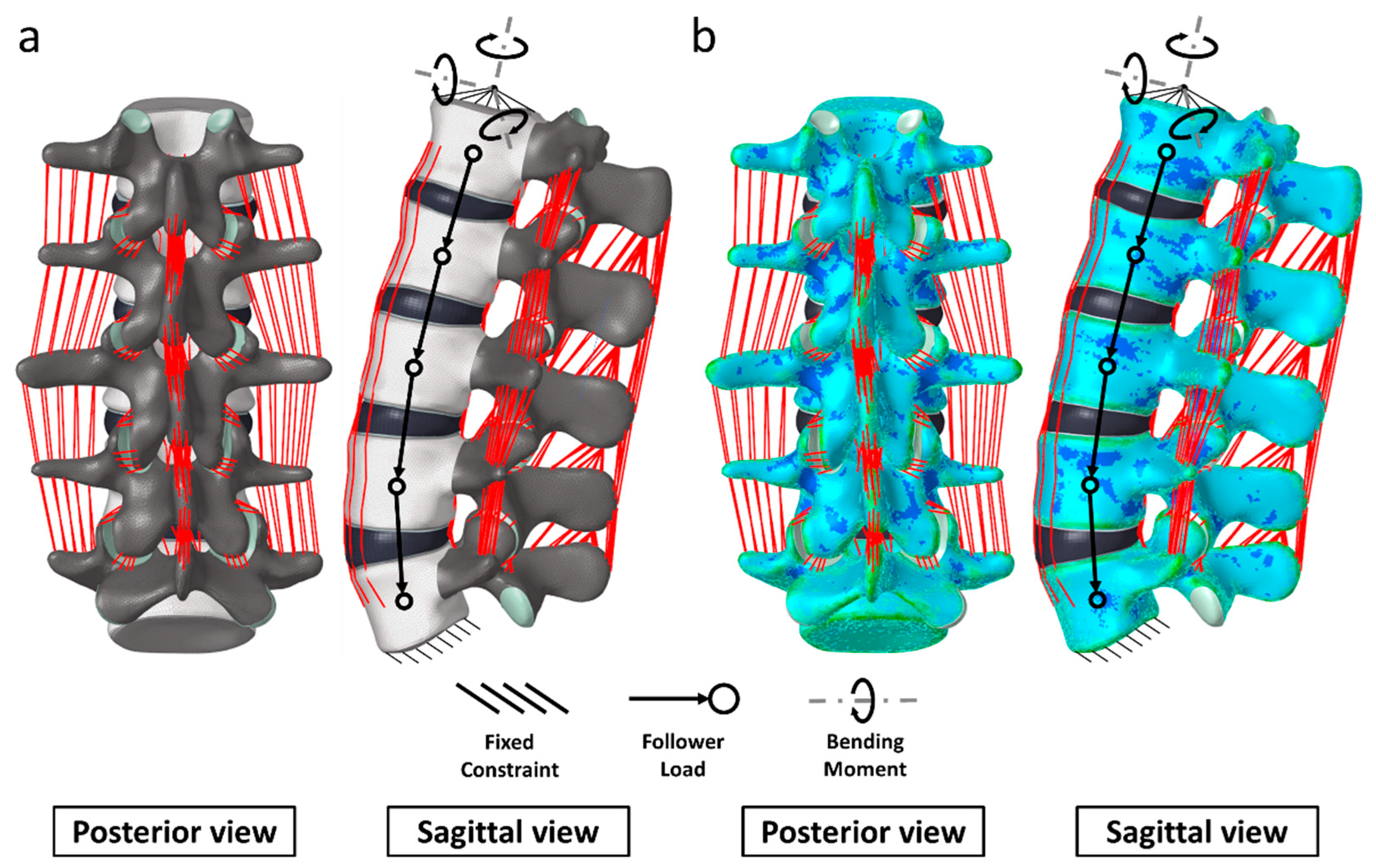
2.3. Loading and Boundary Conditions
2.4. Mesh Convergence
2.5. Validation
2.6. Stress Distribution
3. Results
3.1. Mesh Convergence
3.2. Computational Times
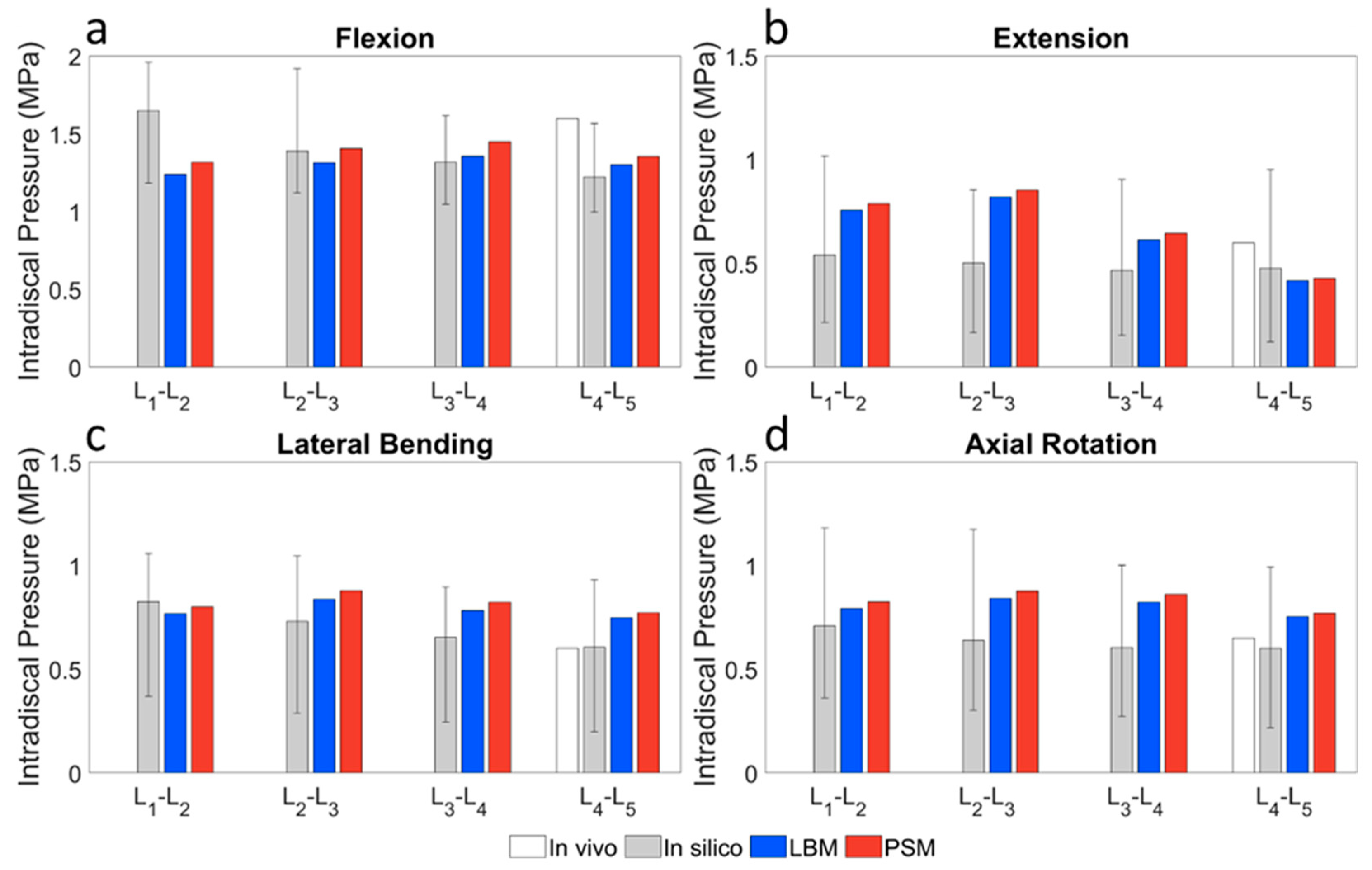
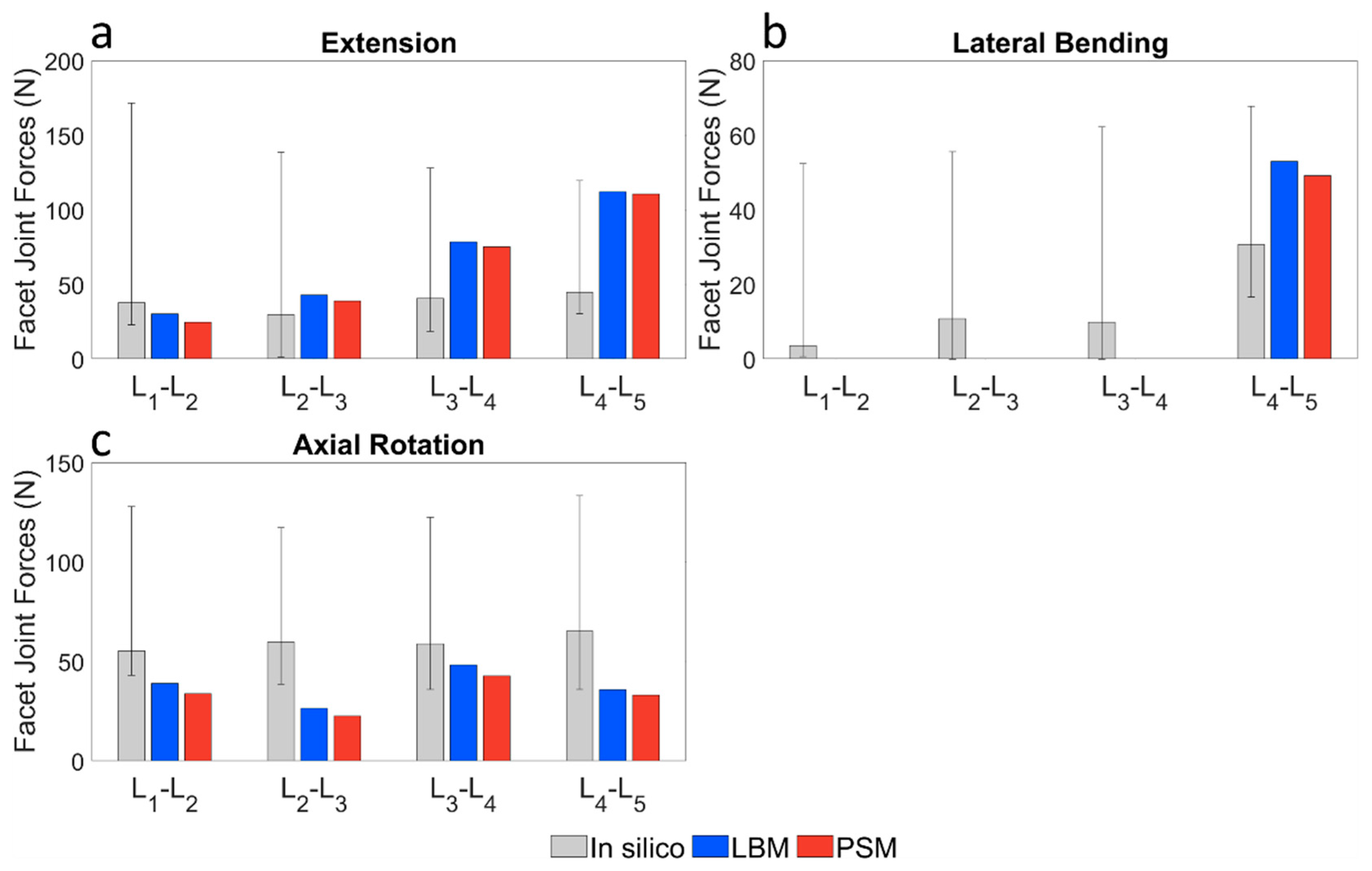
3.3. Validation of the Lumbar Spine Models
3.3.1. Pure Bending Moment Load
3.3.2. Pure Compression Load
3.3.3. Combined Load
3.4. Stress Distributions
4. Discussion
4.1. Locally Defined Material Properties
4.2. Mesh Convergence
4.3. Computational Times
4.4. Model Validation
4.5. Stress Distributions
4.6. Limitations, Significance
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Ligament | No. Elements | Length of 1 Element (mm), Le | Length of 1 Chain (mm), Lchain |
|---|---|---|---|
| ALL | 5 parallel, 4 in series | 10.36 | 41.44 |
| ISL | 24 in parallel | 6.86 | 6.86 |
| CL | 20 in parallel | 6.54 | 6.54 |
| ITL | 20 in parallel | 22.50 | 22.50 |
| LF | 7 in parallel | 14.08 | 14.08 |
| PLL | 5 parallel, 4 in series | 8.56 | 34.24 |
| SSL | 6 in parallel | 15.14 | 15.14 |
| Ligament | K1 (N/mm) | ε1 (-) | K2 (N/mm) | ε2 (-) | K3 (N/mm) |
|---|---|---|---|---|---|
| ALL | 347 | 0.122 | 787 | 0.203 | 1864 |
| ISL | 1.4 | 0.139 | 1.5 | 0.200 | 14.7 |
| CL | 36 | 0.250 | 159 | 0.300 | 384 |
| ITL | 0.3 | 0.182 | 1.8 | 0.233 | 10.7 |
| LF | 7.7 | 0.059 | 9.6 | 0.490 | 58.2 |
| PLL | 29.5 | 0.111 | 61.7 | 0.230 | 236 |
| SSL | 2.5 | 0.200 | 5.3 | 0.250 | 34 |
- —force (N)
- —number of parallel chains (-)
- —number of elements in a chain (-)
- —stiffness (N/mm)
- —strain (-)
- —elongation (mm)
- —average length of one element (mm)

Appendix B


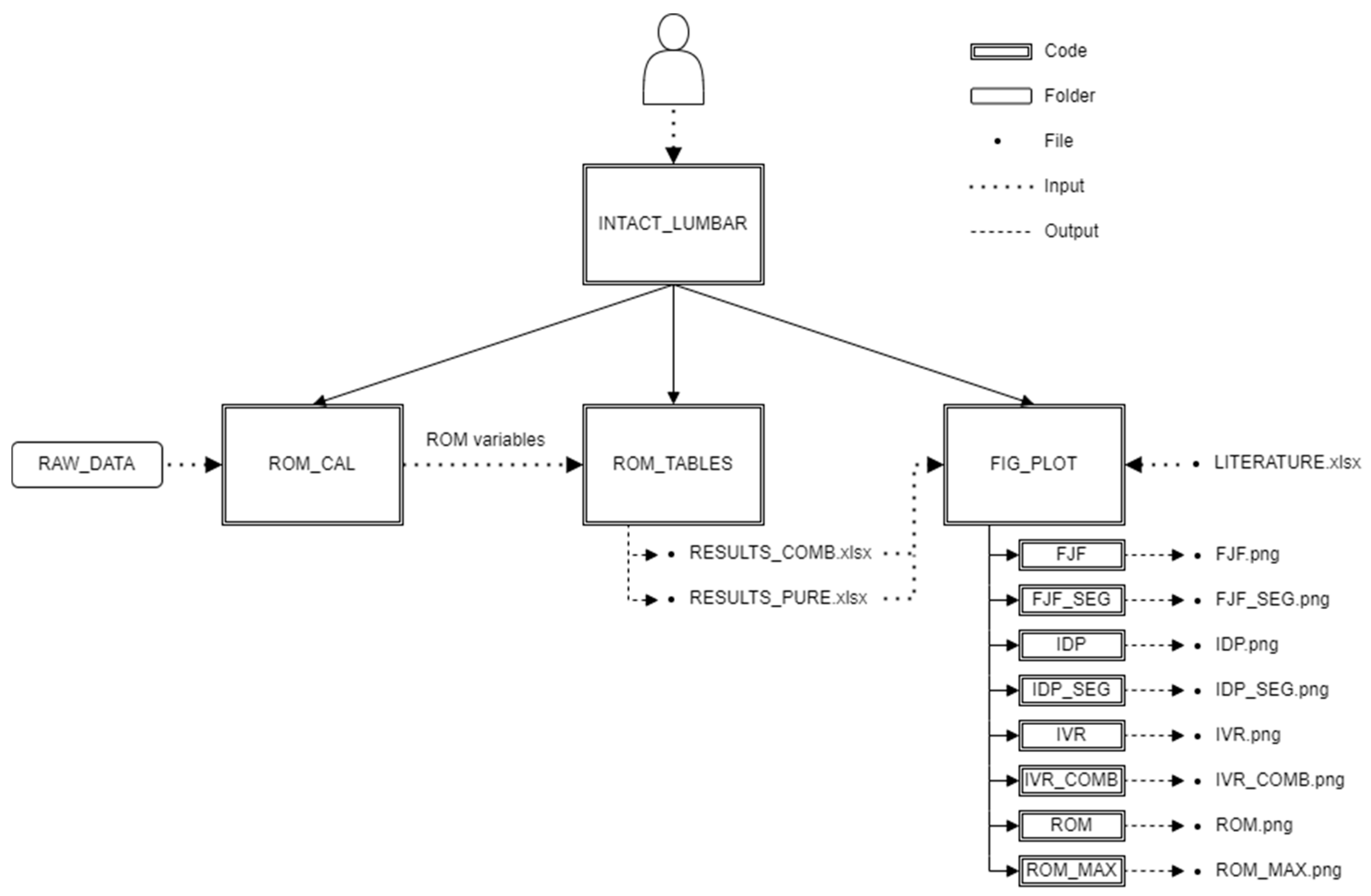
- DIAG_TABLES: contains 3 tables: LITERATURE, RESULTS_PURE and RESULTS_COMB which contain data necessary for the plotting of the diagrams.
- MATLAB: contains the main code INTACT_LUMBAR and folders below:
- ○
- ROM: contains the calculating and exporting codes, which use tables from RAW_DATA folder as input and write sheets in tables in DIAG_TABLES folder as output
- ○
- PLOT: contains the plotting codes
- PICTURES: contains the figures of the investigated variables
- RAW_DATA: contains tables of the raw data of the investigated variables
- ○
- COMBINED_LOADS: contains tables in the case of combined loads
- ▪
- LBM: contains data of the literature-based model
- IDP_DATA: contains rpt files exported from Abaqus for IDP calculation
- ▪
- PSM: contains data of the patient-specific model
- IDP_DATA: contains rpt files exported from Abaqus for IDP calculation
- ○
- PURE_LOADS: tables in the case of pure loads
- ▪
- LBM: contains data of the literature-based model
- IDP_DATA: contains rpt files exported from Abaqus for IDP calculation
- ▪
- PSM: contains data of the patient-specific model
- IDP_DATA: contains rpt files exported from Abaqus for IDP calculation
- INTACT_LUMBAR: main code, handle data input and runs the three other main functions,
- ROM_CAL: function for rotation calculation. Input: tables from RAW_DATA, Output: internal ROM variables,
- ROM_TABLES: function for data arrangement and export. Input: internal ROM variables, Output: result tables,
- FIG_PLOT: function for visualisation of the results. Input: result tables and a table containing literature results, Output: figures of the investigated variables.
References
- Schmidt, H.; Galbusera, F.; Rohlmann, A.; Shirazi-Adl, A. What Have We Learned from Finite Element Model Studies of Lumbar Intervertebral Discs in the Past Four Decades? J. Biomech. 2013, 46, 2342–2355. [Google Scholar] [CrossRef]
- Dreischarf, M.; Zander, T.; Shirazi-adl, A.; Puttlitz, C.M.; Adam, C.J.; Chen, C.S.; Goel, V.K.; Kiapour, A.; Kim, Y.H.; Labus, K.M.; et al. Comparison of Eight Published Static Finite Element Models of the Intact Lumbar Spine: Predictive Power of Models Improves When Combined Together. J. Biomech. 2014, 47, 1757–1766. [Google Scholar] [CrossRef] [Green Version]
- Fagan, M.J.; Julian, S.; Mohsen, A.M. Finite Element Analysis in Spine Research. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2002, 216, 281–298. [Google Scholar] [CrossRef]
- Little, J.P.; Adam, C.J. Geometric Sensitivity of Patient-Specific Finite Element Models of the Spine to Variability in User-Selected Anatomical Landmarks. Comput. Methods Biomech. Biomed. Eng. 2013, 18, 676–688. [Google Scholar] [CrossRef]
- Viceconti, M.; Pappalardo, F.; Rodriguez, B.; Horner, M.; Bischoff, J.; Tshinanu, F.M. In Silico Trials: Verification, Validation and Uncertainty Quantification of Predictive Models Used in the Regulatory Evaluation of Biomedical Products. Methods 2021, 185, 120–127. [Google Scholar] [CrossRef]
- Viceconti, M.; Henney, A.; Morley-Fletcher, E. In Silico Clinical Trials: How Computer Simulation Will Transform the Biomedical Industry. Int. J. Clin. Trials 2016, 3, 37. [Google Scholar] [CrossRef] [Green Version]
- Pappalardo, F.; Russo, G.; Tshinanu, F.M.; Viceconti, M. In Silico Clinical Trials: Concepts and Early Adoptions. Brief. Bioinform. 2019, 20, 1699–1708. [Google Scholar] [CrossRef]
- La Barbera, L.; Larson, A.N.; Rawlinson, J.; Aubin, C.-E. In Silico Patient-Specific Optimization of Correction Strategies for Thoracic Adolescent Idiopathic Scoliosis. Clin. Biomech. 2021, 81, 105200. [Google Scholar] [CrossRef]
- Agarwal, A.; Agarwal, A.K.; Jayaswal, A.; Goel, V.K. Outcomes of Optimal Distraction Forces and Frequencies in Growth Rod Surgery for Different Types of Scoliotic Curves: An in Silico and in Vitro Study. Spine Deform. 2017, 5, 18–26. [Google Scholar] [CrossRef]
- Vergari, C.; Gaume, M.; Persohn, S.; Miladi, L.; Skalli, W. From in Vitro Evaluation of a Finite Element Model of the Spine to in Silico Comparison of Spine Instrumentations. J. Mech. Behav. Biomed. Mater. 2021, 123, 104797. [Google Scholar] [CrossRef]
- Costa, M.C.; Eltes, P.; Lazary, A.; Varga, P.P.; Viceconti, M.; Dall’Ara, E. Biomechanical Assessment of Vertebrae with Lytic Metastases with Subject-Specific Finite Element Models. J. Mech. Behav. Biomed. Mater. 2019, 98, 268–290. [Google Scholar] [CrossRef]
- Xiao, Z.; Wang, L.; Gong, H.; Zhu, D.; Zhang, X. A Non-Linear Finite Element Model of Human L4-L5 Lumbar Spinal Segment with Three-Dimensional Solid Element Ligaments. Theor. Appl. Mech. Lett. 2011, 1, 064001. [Google Scholar] [CrossRef] [Green Version]
- Shirazi-Adl, A. Biomechanics of the Lumbar Spine in Sagittal/Lateral Moments. Spine 1994, 19, 2407–2414. [Google Scholar] [CrossRef]
- Park, W.M.; Kim, K.; Kim, Y.H. Effects of Degenerated Intervertebral Discs on Intersegmental Rotations, Intradiscal Pressures, and Facet Joint Forces of the Whole Lumbar Spine. Comput. Biol. Med. 2013, 43, 1234–1240. [Google Scholar] [CrossRef]
- Schmidt, H.; Galbusera, F.; Rohlmann, A.; Zander, T.; Wilke, H.J. Effect of Multilevel Lumbar Disc Arthroplasty on Spine Kinematics and Facet Joint Loads in Flexion and Extension: A Finite Element Analysis. Eur. Spine J. 2012, 21, 663–674. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Shang, J.; Zhou, Y.; Li, C.; Liu, H. Finite Element Analysis of a New Pedicle Screw-Plate System for Minimally Invasive Transforaminal Lumbar Interbody Fusion. PLoS ONE 2015, 10, e0144637. [Google Scholar] [CrossRef] [Green Version]
- Faulkner, K.G.; Cann, C.E.; Hasegawa, B.H. Effect of Bone Distribution on Vertebral Strength: Assessment with Patient-Specific Nonlinear Finite Element Analysis. Radiology 1991, 179, 669–674. [Google Scholar] [CrossRef]
- Crawford, R.P.; Cann, C.E.; Keaveny, T.M. Finite Element Models Predict in Vitro Vertebral Body Compressive Strength Better than Quantitative Computed Tomography. Bone 2003, 33, 744–750. [Google Scholar] [CrossRef]
- Groenen, K.H.J.; Bitter, T.; van Veluwen, T.C.G.; van der Linden, Y.M.; Verdonschot, N.; Tanck, E.; Janssen, D. Case-Specific Non-Linear Finite Element Models to Predict Failure Behavior in Two Functional Spinal Units. J. Orthop. Res. 2018, 36, 3208–3218. [Google Scholar] [CrossRef] [Green Version]
- O’Reilly, M.A.; Whyne, C.M. Comparison of Computed Tomography Based Parametric and Patient-Specific Finite Element Models of the Healthy and Metastatic Spine Using a Mesh-Morphing Algorithm. Spine 2008, 33, 1876–1881. [Google Scholar] [CrossRef]
- Sapin-De Brosses, E.; Jolivet, E.; Travert, C.; Mitton, D.; Skalli, W. Prediction of the Vertebral Strength Using a Finite Element Model Derived from Low-Dose Biplanar Imaging: Benefits of Subject-Specific Material Properties. Spine 2012, 37, E156–E162. [Google Scholar] [CrossRef] [PubMed]
- Van Rijsbergen, M.; Van Rietbergen, B.; Barthelemy, V.; Eltes, P.; Lazáry, Á.; Lacroix, D.; Noailly, J.; Tho, M.C.H.B.; Wilson, W.; Ito, K. Comparison of Patient-Specific Computational Models vs. Clinical Follow-up, for Adjacent Segment Disc Degeneration and Bone Remodelling after Spinal Fusion. PLoS ONE 2018, 13, e0200899. [Google Scholar] [CrossRef] [Green Version]
- Aryanto, K.Y.E.; Oudkerk, M.; van Ooijen, P.M.A. Free DICOM De-Identification Tools in Clinical Research: Functioning and Safety of Patient Privacy. Eur. Radiol. 2015, 25, 3685–3695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bereczki, F.; Turbucz, M.; Kiss, R.; Eltes, P.E.; Lazary, A. Stability Evaluation of Different Oblique Lumbar Interbody Fusion Constructs in Normal and Osteoporotic Condition—A Finite Element Based Study. Front. Bioeng. Biotechnol. 2021, 9, 749914. [Google Scholar] [CrossRef] [PubMed]
- Shirazi-Adl, S.A.; Shrivastava, S.C.; Ahmed, A.M. Stress Analysis of the Lumbar Disc-Body Unit in Compression. A Three-Dimensional Nonlinear Finite Element Study. Spine 1984, 9, 120–134. [Google Scholar] [CrossRef]
- Baroud, G.; Nemes, J.; Heini, P.; Steffen, T. Load Shift of the Intervertebral Disc after a Vertebroplasty: A Finite-Element Study. Eur. Spine J. 2003, 12, 421–426. [Google Scholar] [CrossRef] [Green Version]
- Finley, S.M.; Brodke, D.S.; Spina, N.T.; DeDen, C.A.; Ellis, B.J.; Finley, S.M.; Brodke, D.S.; Spina, N.T.; DeDen, C.A. FEBio Finite Element Models of the Human Lumbar Spine. Comput. Methods Biomech. Biomed. Eng. 2018, 21, 444–452. [Google Scholar] [CrossRef]
- Zeng, Z.L.; Zhu, R.; Wu, Y.C.; Zuo, W.; Yu, Y.; Wang, J.J.; Cheng, L.M. Effect of Graded Facetectomy on Lumbar Biomechanics. J. Healthc. Eng. 2017, 2017, 7981513. [Google Scholar] [CrossRef] [Green Version]
- Remus, R.; Lipphaus, A.; Neumann, M.; Bender, B. Calibration and Validation of a Novel Hybrid Model of the Lumbosacral Spine in ArtiSynth–The Passive Structures. PLoS ONE 2021, 16, e0250456. [Google Scholar] [CrossRef]
- Panjabi, M.M.; White, A.A. Clinical Biomechanics of the Spine; Lippincott: Philadelphia, PA, USA, 1990; ISBN 9780323026222. [Google Scholar]
- Noailly, J.; Wilke, H.J.; Planell, J.A.; Lacroix, D. How Does the Geometry Affect the Internal Biomechanics of a Lumbar Spine Bi-Segment Finite Element Model? Consequences on the Validation Process. J. Biomech. 2007, 40, 2414–2425. [Google Scholar] [CrossRef]
- Rohlmann, A.; Burra, N.K.; Zander, T.; Bergmann, G. Comparison of the Effects of Bilateral Posterior Dynamic and Rigid Fixation Devices on the Loads in the Lumbar Spine: A Finite Element Analysis. Eur. Spine J. 2007, 16, 1223–1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.M.; Hutton, W.C.; Gharpuray, V.M. Do Bending, Twisting, and Diurnal Fluid Changes in the Disc Affect the Propensity to Prolapse? A Viscoelastic Finite Element Model. Spine 1996, 21, 2570–2579. [Google Scholar] [CrossRef] [PubMed]
- Rohlmann, A.; Zander, T.; Rao, M.; Bergmann, G. Realistic Loading Conditions for Upper Body Bending. J. Biomech. 2009, 42, 884–890. [Google Scholar] [CrossRef] [PubMed]
- Shirazi-Adl, A.; Ahmed, A.M.; Shrivastava, S.C. Mechanical Response of a Lumbar Motion Segment in Axial Torque Alone and Combined with Compression. Spine 1986, 11, 914–927. [Google Scholar] [CrossRef] [PubMed]
- Tawara, D.; Sakamoto, J.; Murakami, H.; Kawahara, N.; Oda, J.; Tomita, K. Mechanical Evaluation by Patient-Specific Finite Element Analyses Demonstrates Therapeutic Effects for Osteoporotic Vertebrae. J. Mech. Behav. Biomed. Mater. 2010, 3, 31–40. [Google Scholar] [CrossRef]
- Nishiyama, K.K.; Gilchrist, S.; Guy, P.; Cripton, P.; Boyd, S.K. Proximal Femur Bone Strength Estimated by a Computationally Fast Finite Element Analysis in a Sideways Fall Configuration. J. Biomech. 2013, 46, 1231–1236. [Google Scholar] [CrossRef] [PubMed]
- Dragomir-Daescu, D.; Op Den Buijs, J.; McEligot, S.; Dai, Y.; Entwistle, R.C.; Salas, C.; Melton, L.J.; Bennet, K.E.; Khosla, S.; Amin, S. Robust QCT/FEA Models of Proximal Femur Stiffness and Fracture Load During a Sideways Fall on the Hip. Ann. Biomed. Eng. 2011, 39, 742–755. [Google Scholar] [CrossRef] [Green Version]
- Schileo, E.; Dall’Ara, E.; Taddei, F.; Malandrino, A.; Schotkamp, T.; Baleani, M.; Viceconti, M. An Accurate Estimation of Bone Density Improves the Accuracy of Subject-Specific Finite Element Models. J. Biomech. 2008, 41, 2483–2491. [Google Scholar] [CrossRef]
- Morgan, E.F.; Bayraktar, H.H.; Keaveny, T.M. Trabecular Bone Modulus-Density Relationships Depend on Anatomic Site. J. Biomech. 2003, 36, 897–904. [Google Scholar] [CrossRef]
- Mills, M.J.; Sarigul-Klijn, N. Validation of an In Vivo Medical Image-Based Young Human Lumbar Spine Finite Element Model. J. Biomech. Eng. 2019, 141, 031003. [Google Scholar] [CrossRef]
- Rezaei, A.; Carlson, K.D.; Giambini, H.; Javid, S.; Dragomir-Daescu, D. Optimizing Accuracy of Proximal Femur Elastic Modulus Equations. Ann. Biomed. Eng. 2019, 47, 1391–1399. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Heuer, F.; Simon, U.; Kettler, A.; Rohlmann, A.; Claes, L.; Wilke, H.-J. Application of a New Calibration Method for a Three-Dimensional Finite Element Model of a Human Lumbar Annulus Fibrosus. Clin. Biomech. 2006, 21, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Naserkhaki, S.; Arjmand, N.; Shirazi-Adl, A.; Farahmand, F.; El-Rich, M. Effects of Eight Different Ligament Property Datasets on Biomechanics of a Lumbar L4-L5 Finite Element Model. J. Biomech. 2018, 70, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, H.; Heuer, F.; Drumm, J.; Klezl, Z.; Claes, L.; Wilke, H.-J.J. Application of a Calibration Method Provides More Realistic Results for a Finite Element Model of a Lumbar Spinal Segment. Clin. Biomech. 2007, 22, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Rosenau, E.; Paetzold, H.; Klein, A.; Püschel, K.; Morlock, M.M.; Huber, G. Strain Changes on the Cortical Shell of Vertebral Bodies Due to Spine Ageing: A Parametric Study Using a Finite Element Model Evaluated by Strain Measurements. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2013, 227, 1265–1274. [Google Scholar] [CrossRef] [PubMed]
- Rohlmann, A.; Bauer, L.; Zander, T.; Bergmann, G.; Wilke, H.J. Determination of Trunk Muscle Forces for Flexion and Extension by Using a Validated Finite Element Model of the Lumbar Spine and Measured In Vivo Data. J. Biomech. 2006, 39, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Wilke, H.J.; Wenger, K.; Claes, L. Testing Criteria for Spinal Implants: Recommendations for the Standardization of In Vitro Stability Testing of Spinal Implants. Eur. Spine J. 1998, 7, 148–154. [Google Scholar] [CrossRef] [Green Version]
- Dreischarf, M.; Zander, T.; Bergmann, G.; Rohlmann, A. A Non-Optimized Follower Load Path May Cause Considerable Intervertebral Rotations. J. Biomech. 2010, 43, 2625–2628. [Google Scholar] [CrossRef] [PubMed]
- Patwardhan, A.G.; Havey, R.M.; Meade, K.P.; Lee, B.; Dunlap, B. A Follower Load Increases the Load-Carrying Capacity of the Lumbar Spine in Compression. Spine 1999, 24, 1003–1009. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Yang, J.; Lieberman, I.H.; Haddas, R.; Lieberman, I.H.; Haddas, R. Lumbar Spine Finite Element Model for Healthy Subjects: Development and Validation. Comput. Methods Biomech. Biomed. Engin. 2017, 20, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Ayturk, U.M.; Puttlitz, C.M. Parametric Convergence Sensitivity and Validation of a Finite Element Model of the Human Lumbar Spine. Comput. Methods Biomech. Biomed. Eng. 2011, 14, 695–705. [Google Scholar] [CrossRef]
- Pearcy, M.J.; Tibrewal, S.B. Axial Rotation and Lateral Bending in the Normal Lumbar Spine Measured by Three-Dimensional Radiography. Spine 1984, 9, 582–587. [Google Scholar] [CrossRef]
- Pearcy, M.; Portek, I.A.N.; Shepherd, J. Three-Dimensional X-ray Analysis of Normal Movement in the Lumbar Spine. Spine 1984, 9, 294–297. [Google Scholar] [CrossRef] [PubMed]
- Pearcy, M.J. Stereo Radiography of Lumbar Spine Motion. Acta Orthop. Scand. 1985, 56, 1–45. [Google Scholar] [CrossRef]
- Wilke, H.J.; Neef, P.; Hinz, B.; Seidel, H.; Claes, L. Intradiscal Pressure Together with Anthropometric Data—A Data Set for the Validation of Models. Clin. Biomech. 2001, 16, S111–S126. [Google Scholar] [CrossRef]
- Rohlmann, A.; Neller, S.; Claes, L.; Bergmann, G.; Wilke, H. Influence of a Follower Load on Intradiscal Pressure and Intersegmental Rotation of the Lumbar Spine. Spine 2001, 26, 557–561. [Google Scholar] [CrossRef] [PubMed]
- Wilson, D.C.; Niosi, C.A.; Zhu, Q.A.; Oxland, T.R.; Wilson, D.R. Accuracy and Repeatability of a New Method for Measuring Facet Loads in the Lumbar Spine. J. Biomech. 2006, 39, 348–353. [Google Scholar] [CrossRef] [PubMed]
- Brinckmann, P.; Grootenboer, H. Change of Disc Height, Radial Disc Bulge, and Intradiscal Pressure from Discectomy an In Vitro Investigation on Human Lumbar Discs. Spine 1991, 16, 641–646. [Google Scholar] [CrossRef]
- Panjabi, M.M.; Oxland, T.R.; Yamamoto, I.; Crisco, J.J. Mechanical Behavior of the Human Lumbar and Lumbosacral Spine as Shown by Three-Dimensional Load-Displacement Curves. JBJS 1994, 76, 413–424. [Google Scholar] [CrossRef]
- van der Vaart, A.W. Asymptotic Statistics; Cambridge University Press: Cambridge, UK, 1998; p. 443. [Google Scholar]
- Ito, M. Recent Progress in Bone Imaging for Osteoporosis Research. J. Bone Miner. Metab. 2011, 29, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Wüster, C.; Heilmann, R.; Pereira-Lima, J.; Schlegel, J.; Anstätt, K.; Soballa, T. Quantitative Ultrasonometry (QUS) for the Evaluation of Osteoporosis Risk: Reference Data for Various Measurement Sites, Limitations and Application Possibilities. Exp. Clin. Endocrinol. Diabetes 1998, 106, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Rinaldo, N.; Pasini, A.; Donati, R.; Belcastro, M.G.; Gualdi-Russo, E. Quantitative Ultrasonometry for the Diagnosis of Osteoporosis in Human Skeletal Remains: New Methods and Standards. J. Archaeol. Sci. 2018, 99, 153–161. [Google Scholar] [CrossRef]
- Cody, D.D.; Gross, G.J.; Hou, F.J.; Spencer, H.J.; Goldstein, S.A.; Fyhrie, D.P. Femoral Strength Is Better Predicted by Finite Element Models than QCT and DXA. J. Biomech. 1999, 32, 1013–1020. [Google Scholar] [CrossRef]
- Kaneko, T.S.; Bell, J.S.; Pejcic, M.R.; Tehranzadeh, J.; Keyak, J.H. Mechanical Properties, Density and Quantitative CT Scan Data of Trabecular Bone with and without Metastases. J. Biomech. 2004, 37, 523–530. [Google Scholar] [CrossRef] [PubMed]
- Kopperdahl, D.L.; Morgan, E.F.; Keaveny, T.M. Quantitative Computed Tomography Estimates of the Mechanical Properties of Human Vertebral Trabecular Bone. J. Orthop. Res. 2002, 20, 801–805. [Google Scholar] [CrossRef]
- Les, C.M.; Keyak, J.H.; Stover, S.M.; Taylor, K.T.; Kaneps, A.J. Estimation of Material Properties in the Equine Metacarpus with Use of Quantitative Computed Tomography. J. Orthop. Res. 1994, 12, 822–833. [Google Scholar] [CrossRef]
- Carpenter, R.D. Finite Element Analysis of the Hip and Spine Based on Quantitative Computed Tomography. Curr. Osteoporos. Rep. 2013, 11, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Matsuura, Y.; Kuniyoshi, K.; Suzuki, T.; Ogawa, Y.; Sukegawa, K.; Rokkaku, T.; Thoreson, A.R.; An, K.N.; Takahashi, K. Accuracy of Specimen-Specific Nonlinear Finite Element Analysis for Evaluation of Radial Diaphysis Strength in Cadaver Material. Comput. Methods Biomech. Biomed. Eng. 2015, 18, 1811–1817. [Google Scholar] [CrossRef] [Green Version]
- Xin, P.; Nie, P.; Jiang, B.; Deng, S.; Hu, G.; Shen, S.G.F. Material Assignment in Finite Element Modeling: Heterogeneous Properties of the Mandibular Bone. J. Craniofacial Surg. 2013, 24, 405–410. [Google Scholar] [CrossRef]
- Mirzaei, M.; Zeinali, A.; Razmjoo, A.; Nazemi, M. On Prediction of the Strength Levels and Failure Patterns of Human Vertebrae Using Quantitative Computed Tomography (QCT)-Based Finite Element Method. J. Biomech. 2009, 42, 1584–1591. [Google Scholar] [CrossRef]
- Lee, C.H.; Landham, P.R.; Eastell, R.; Adams, M.A.; Dolan, P.; Yang, L. Development and Validation of a Subject-Specific Finite Element Model of the Functional Spinal Unit to Predict Vertebral Strength. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2017, 231, 821–830. [Google Scholar] [CrossRef] [PubMed]
- Dall’Ara, E.; Pahr, D.; Varga, P.; Kainberger, F.; Zysset, P. QCT-Based Finite Element Models Predict Human Vertebral Strength in Vitro Significantly Better than Simulated DEXA. Osteoporos. Int. 2012, 23, 563–572. [Google Scholar] [CrossRef]
- Anitha, D.P.; Baum, T.; Kirschke, J.S.; Subburaj, K. Effect of the Intervertebral Disc on Vertebral Bone Strength Prediction: A Finite-Element Study. Spine J. 2020, 20, 665–671. [Google Scholar] [CrossRef]
- Imai, K.; Ohnishi, I.; Yamamoto, S.; Nakamura, K. In Vivo Assessment of Lumbar Vertebral Strength in Elderly Women Using Computed Tomography-Based Nonlinear Finite Element Model. Spine 2008, 33, 27–32. [Google Scholar] [CrossRef] [PubMed]
- Imai, K.; Ohnishi, I.; Matsumoto, T.; Yamamoto, S.; Nakamura, K. Assessment of Vertebral Fracture Risk and Therapeutic Effects of Alendronate in Postmenopausal Women Using a Quantitative Computed Tomography-Based Nonlinear Finite Element Method. Osteoporos. Int. 2009, 20, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Eltes, P.E.; Bartos, M.; Hajnal, B.; Pokorni, A.J.; Kiss, L.; Lacroix, D.; Varga, P.P.; Lazary, A. Development of a Computer-Aided Design and Finite Element Analysis Combined Method for Affordable Spine Surgical Navigation With 3D-Printed Customized Template. Front. Surg. 2021, 7, 176. [Google Scholar] [CrossRef]
- Vena, P.; Franzoso, G.; Gastaldi, D.; Contro, R.; Dallolio, V. A Finite Element Model of the L4-L5 Spinal Motion Segment: Biomechanical Compatibility of an Interspinous Device. Comput. Methods Biomech. Biomed. Eng. 2005, 8, 7–16. [Google Scholar] [CrossRef]
- Woldtvedt, D.J.; Womack, W.; Gadomski, B.C.; Schuldt, D.; Puttlitz, C.M. Finite Element Lumbar Spine Facet Contact Parameter Predictions Are Affected by the Cartilage Thickness Distribution and Initial Joint Gap Size. J. Biomech. Eng. 2011, 133, 061009. [Google Scholar] [CrossRef] [PubMed]
- Noailly, J.; Lacroix, D. Finite Element Modelling of the Spine. In Biomaterials for Spinal Surgery; Elsevier: Amsterdam, The Netherlands, 2012; pp. 144–234. [Google Scholar]
- Dreischarf, M.; Rohlmann, A.; Bergmann, G.; Zander, T. Optimised In Vitro Applicable Loads for the Simulation of Lateral Bending in the Lumbar Spine. Med. Eng. Phys. 2012, 34, 777–780. [Google Scholar] [CrossRef] [PubMed]
- Dreischarf, M.; Rohlmann, A.; Bergmann, G.; Zander, T. Optimised Loads for the Simulation of Axial Rotation in the Lumbar Spine. J. Biomech. 2011, 44, 2323–2327. [Google Scholar] [CrossRef]
- Schmidt, H.; Heuer, F.; Claes, L.; Wilke, H.J. The Relation between the Instantaneous Center of Rotation and Facet Joint Forces—A Finite Element Analysis. Clin. Biomech. 2008, 23, 270–278. [Google Scholar] [CrossRef]
- Mosleh, H.; Rouhi, G.; Ghouchani, A.; Bagheri, N. Prediction of Fracture Risk of a Distal Femur Reconstructed with Bone Cement: QCSRA, FEA, and in-Vitro Cadaver Tests. Phys. Eng. Sci. Med. 2020, 43, 269–277. [Google Scholar] [CrossRef]
- Rezaei, A.; Tilton, M.; Li, Y.; Yaszemski, M.J.; Lu, L. Single-Level Subject-Specific Finite Element Model Can Predict Fracture Outcomes in Three-Level Spine Segments under Different Loading Rates. Comput. Biol. Med. 2021, 137, 104833. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turbucz, M.; Pokorni, A.J.; Szőke, G.; Hoffer, Z.; Kiss, R.M.; Lazary, A.; Eltes, P.E. Development and Validation of Two Intact Lumbar Spine Finite Element Models for In Silico Investigations: Comparison of the Bone Modelling Approaches. Appl. Sci. 2022, 12, 10256. https://doi.org/10.3390/app122010256
Turbucz M, Pokorni AJ, Szőke G, Hoffer Z, Kiss RM, Lazary A, Eltes PE. Development and Validation of Two Intact Lumbar Spine Finite Element Models for In Silico Investigations: Comparison of the Bone Modelling Approaches. Applied Sciences. 2022; 12(20):10256. https://doi.org/10.3390/app122010256
Chicago/Turabian StyleTurbucz, Mate, Agoston Jakab Pokorni, György Szőke, Zoltan Hoffer, Rita Maria Kiss, Aron Lazary, and Peter Endre Eltes. 2022. "Development and Validation of Two Intact Lumbar Spine Finite Element Models for In Silico Investigations: Comparison of the Bone Modelling Approaches" Applied Sciences 12, no. 20: 10256. https://doi.org/10.3390/app122010256
APA StyleTurbucz, M., Pokorni, A. J., Szőke, G., Hoffer, Z., Kiss, R. M., Lazary, A., & Eltes, P. E. (2022). Development and Validation of Two Intact Lumbar Spine Finite Element Models for In Silico Investigations: Comparison of the Bone Modelling Approaches. Applied Sciences, 12(20), 10256. https://doi.org/10.3390/app122010256






