Porcine Cortical Bone Lamina as a Predictable Technique for Guided Bone Regeneration: Histomorphometric and Radiographic Evaluation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pre-Surgical Phase
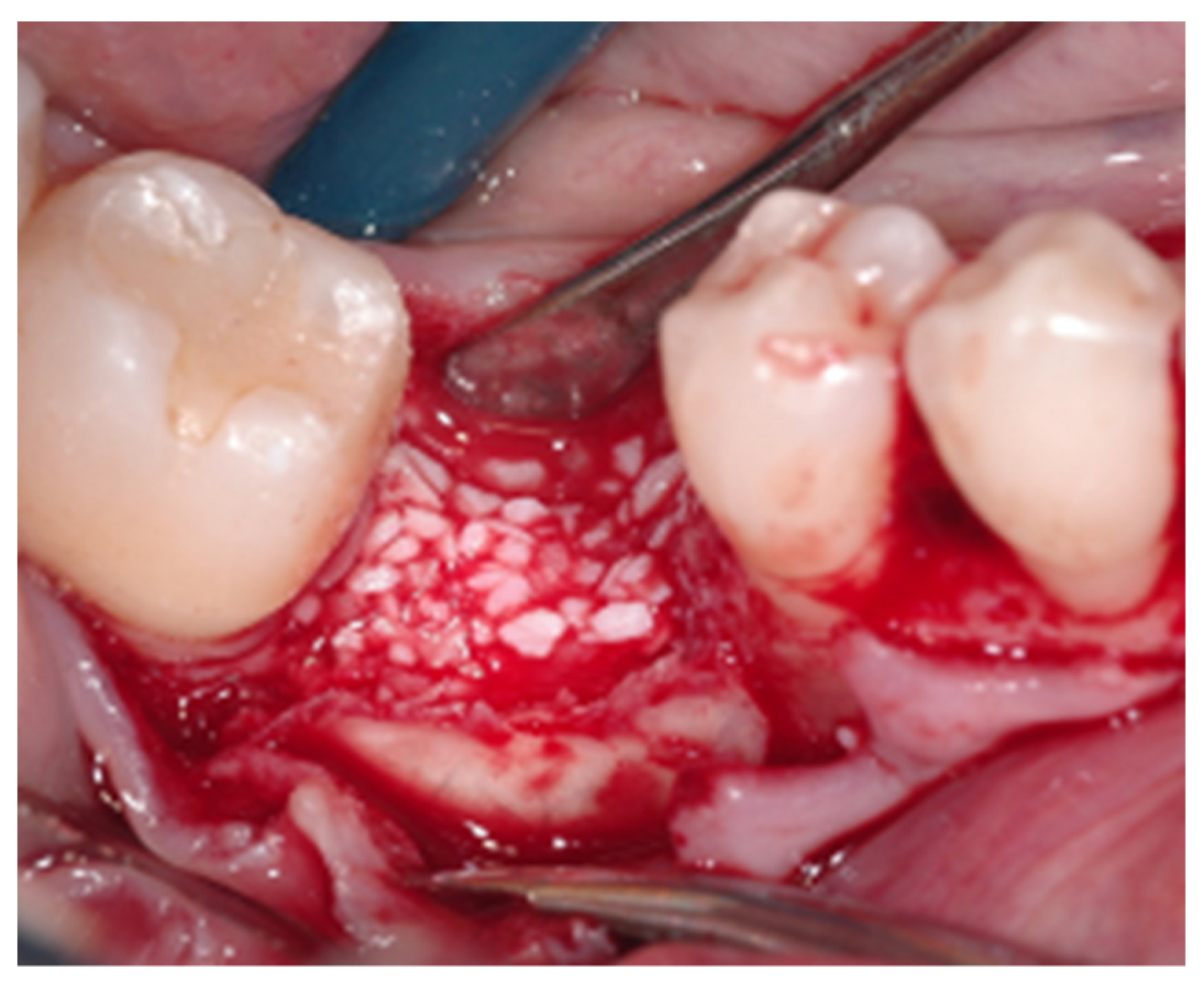
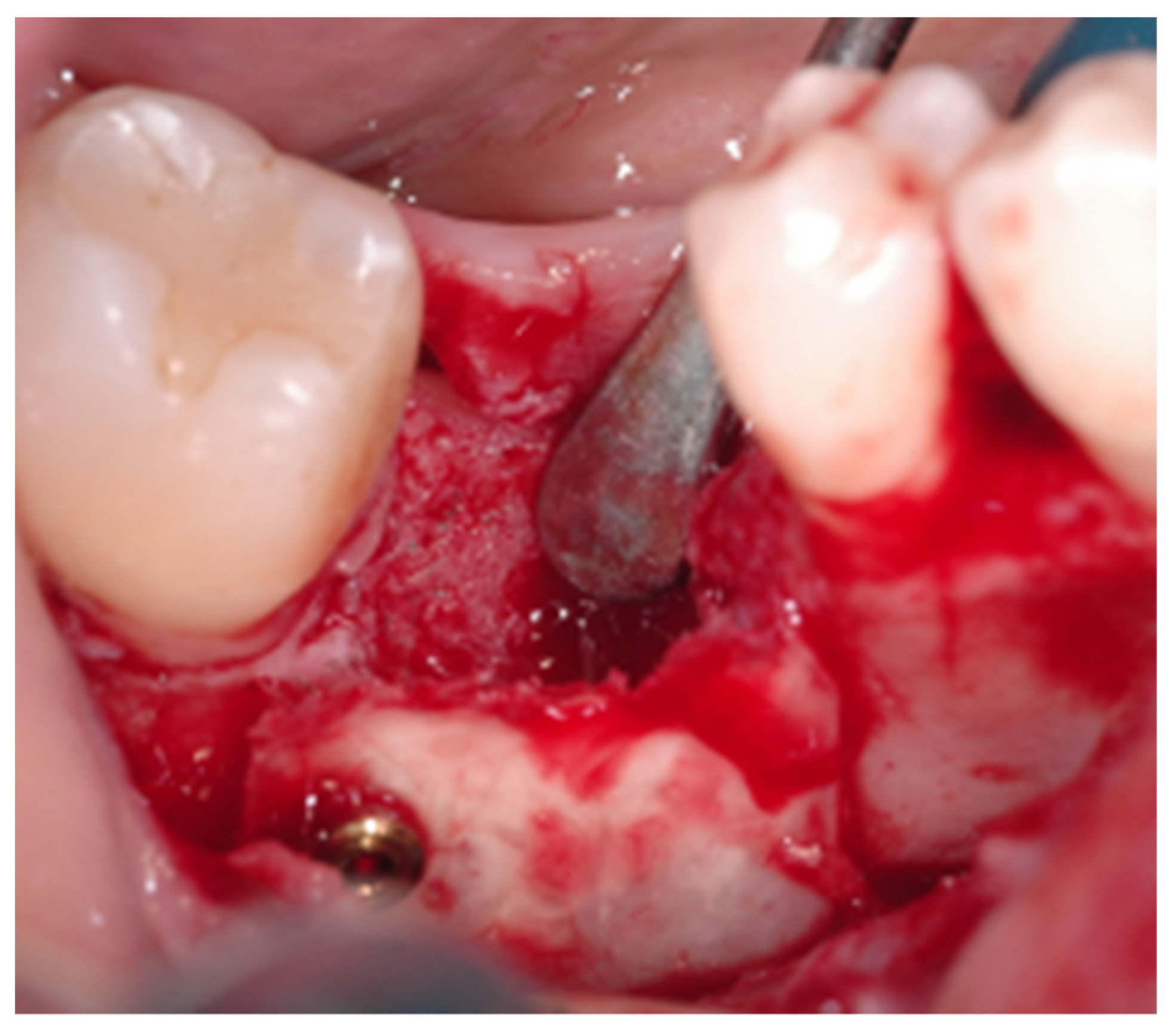
2.2. Surgical Procedure
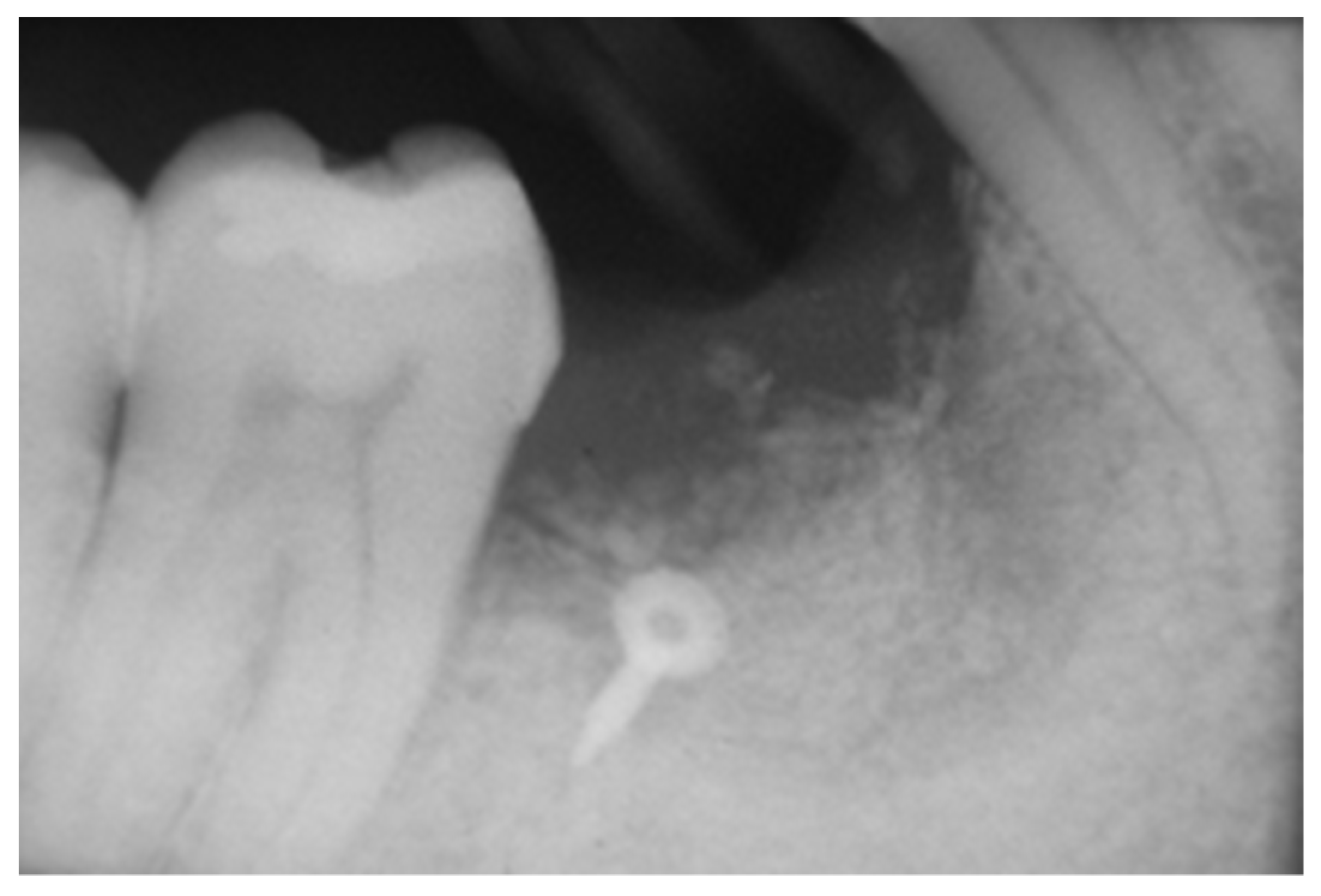
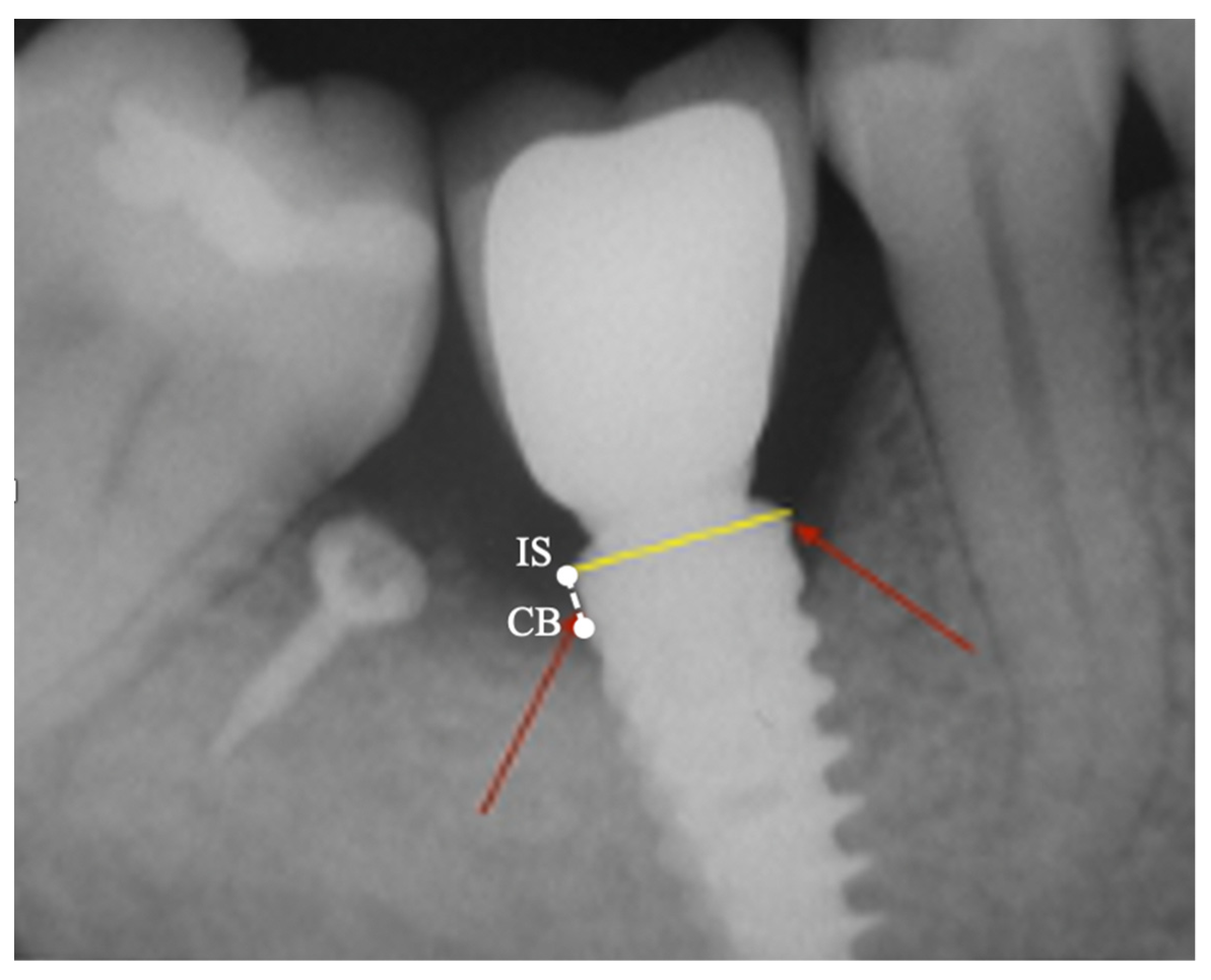
2.3. Peri-Implant Bone Levels Assessment
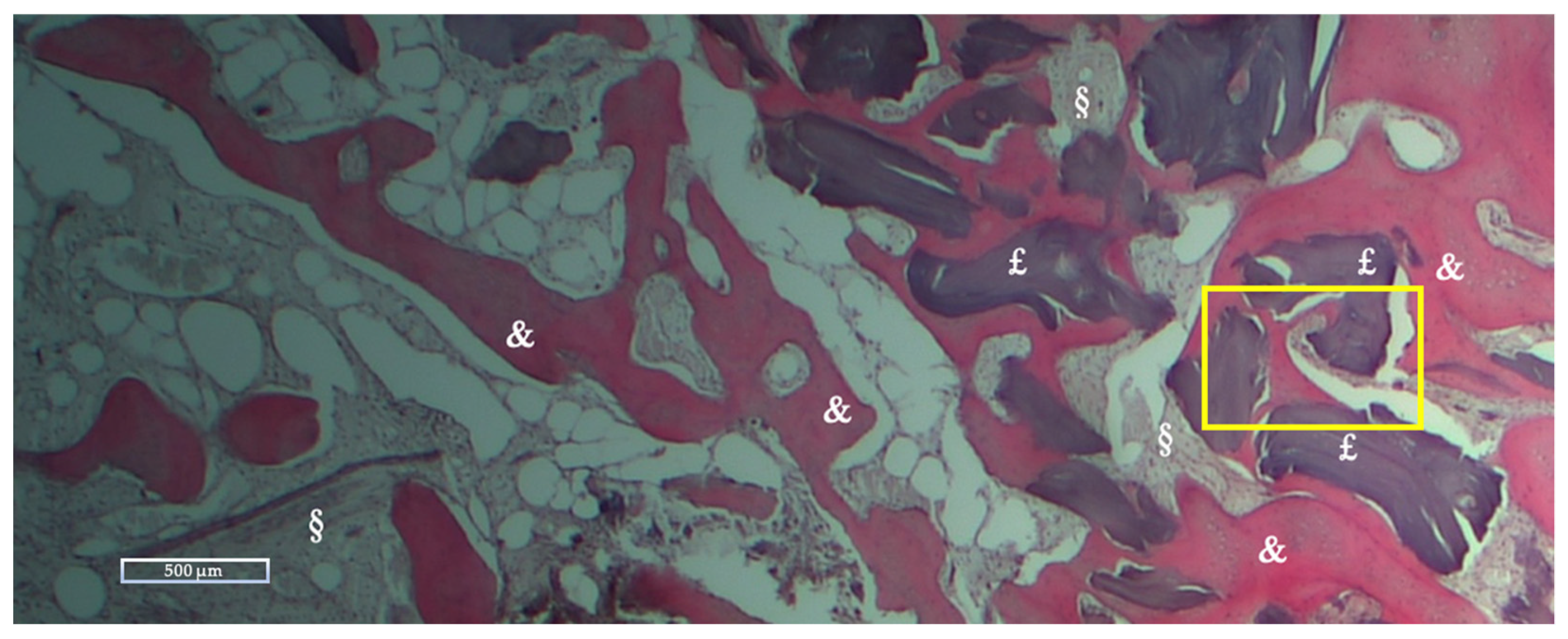
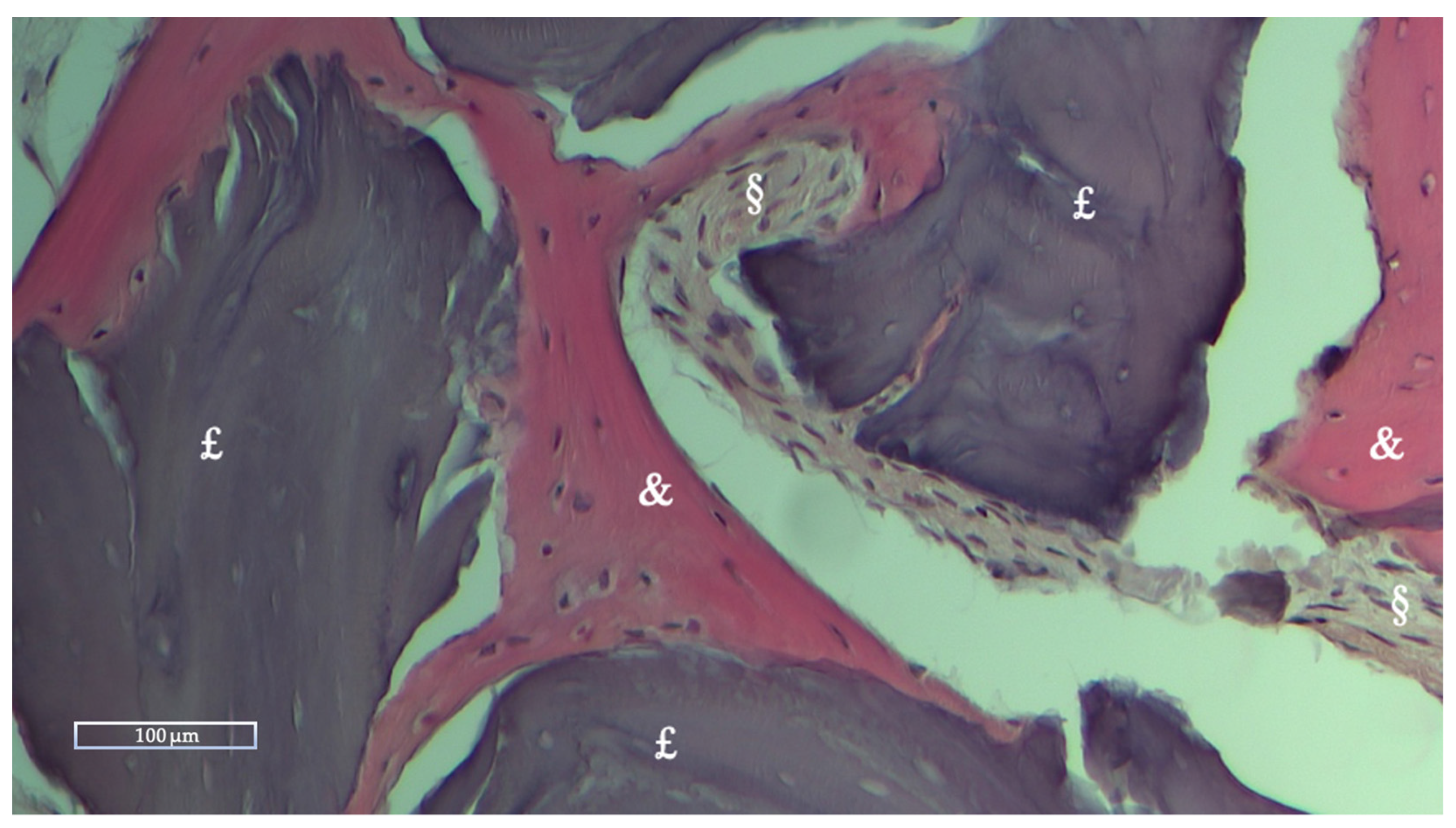
2.4. Histologic and Histomorphometric Analysis
3. Results
Histologic and Histomorphometric Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A

References
- den Hartog, L.; Huddleston Slater, J.J.R.; Vissink, A.; Meijer, H.J.A.; Raghoebar, G.M. Treatment outcome of immediate, early and conventional single-tooth implants in the aesthetic zone: A systematic review to survival, bone level, soft-tissue, aesthetics and patient satisfaction. J. Clin. Periodontol. 2008, 35, 1073–1086. [Google Scholar] [CrossRef] [PubMed]
- Elgali, I.; Omar, O.; Dahlin, C.; Thomsen, P. Guided bone regeneration: Materials and biological mechanisms revisited. Eur. J. Oral Sci. 2017, 125, 315–337. [Google Scholar] [CrossRef] [Green Version]
- Chiapasco, M.; Casentini, P. Horizontal bone-augmentation procedures in implant dentistry: Prosthetically guided regeneration. Periodontol. 2000 2018, 77, 213–240. [Google Scholar] [CrossRef]
- Block, M.S.; Kent, J.N. Sinus augmentation for dental implants: The use of autogenous bone. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 1997, 55, 1281–1286. [Google Scholar] [CrossRef]
- Christensen, D.K.; Karoussis, I.K.; Joss, A.; Hämmerle, C.H.F.; Lang, N.P. Simultaneous or staged installation with guided bone augmentation of transmucosal titanium implants. A 3-year prospective cohort study. Clin. Oral Implants Res. 2003, 14, 680–686. [Google Scholar] [CrossRef]
- Carini, F.; Longoni, S.; Amosso, E.; Paleari, J.; Carini, S.; Porcaro, G. Bone augmentation with TiMesh. autologous bone versus autologous bone and bone substitutes. A systematic review. Ann. Stomatol. 2014, 5, 27–36. [Google Scholar]
- Elian, N.; Jalbout, Z.; Ehrlich, B.; Classi, A.; Cho, S.-C.; Al-Kahtani, F.; Froum, S.; Tarnow, D.P. A Two-Stage Full-Arch Ridge Expansion Technique: Review of the Literature and Clinical Guidelines. Implant Dent. 2008, 17, 16–23. [Google Scholar] [CrossRef]
- Milinkovic, I.; Cordaro, L. Are there specific indications for the different alveolar bone augmentation procedures for implant placement? A systematic review. Int. J. Oral Maxillofac. Surg. 2014, 43, 606–625. [Google Scholar] [CrossRef] [PubMed]
- Kanno, T.; Mitsugi, M.; Furuki, Y.; Akamatsu, H.; Natsumi, Y.; Tanimoto, Y. Alveolar Distraction for Post-traumatic Atrophic Alveolar Ridge: Evaluation of Bone Height. Asian J. Oral Maxillofac. Surg. 2006, 18, 202–207. [Google Scholar] [CrossRef]
- Kahnberg, K.E.; Nystrom, E.; Bartholdsson, L. Combined use of bone grafts and Brånemark fixtures in the treatment of severely resorbed maxillae. Int. J. Oral Maxillofac. Implants 1989, 4, 297–304. [Google Scholar]
- Browaeys, H.; Bouvry, P.; De Bruyn, H. A literature review on biomaterials in sinus augmentation procedures. Clin. Implant Dent. Relat. Res. 2007, 9, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.L. Sinus augmentation for dental implants: The use of alloplastic materials. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 1997, 55, 1287–1293. [Google Scholar] [CrossRef]
- Barone, A.; Covani, U. Maxillary alveolar ridge reconstruction with nonvascularized autogenous block bone: Clinical results. J. Oral Maxillofac. Surg. Off. J. Am. Assoc. Oral Maxillofac. Surg. 2007, 65, 2039–2046. [Google Scholar] [CrossRef]
- Nannmark, U.; Sennerby, L. The bone tissue responses to prehydrated and collagenated cortico-cancellous porcine bone grafts: A study in rabbit maxillary defects. Clin. Implant Dent. Relat. Res. 2008, 10, 264–270. [Google Scholar] [CrossRef]
- Mellonig, J.T.; Nevins, M.; Sanchez, R. Evaluation of a bioabsorbable physical barrier for guided bone regeneration. Part II. Material and a bone replacement graft. Int. J. Periodontics Restorative Dent. 1998, 18, 129–137. [Google Scholar]
- Lopez, M.A.; Andreasi Bassi, M.; Confalone, L.; Carinci, F.; Ormianer, Z.; Lauritano, D. The use of resorbable cortical lamina and micronized collagenated bone in the regeneration of atrophic crestal ridges: A surgical technique. Case series. J. Biol. Regul. Homeost. Agents 2016, 30, 81–85. [Google Scholar]
- Rossi, R.; Foce, E.; Scolavino, S. The Cortical Lamina technique: A new option for alveolar ridge augmentation procedure. J. Leb. Dent. Ass. 2017, 52, 35–41. [Google Scholar]
- Heitz-Mayfield, L.J.; Aaboe, M.; Araujo, M.; Carrión, J.B.; Cavalcanti, R.; Cionca, N.; Cochran, D.; Darby, I.; Funakoshi, E.; Gierthmuehlen, P.C.; et al. Group 4 ITI Consensus Report: Risks and biologic complications associated with implant dentistry. Clin. Oral Implants Res. 2018, 29, 351–358. [Google Scholar] [CrossRef] [Green Version]
- Hämmerle, C.H.F.; Jung, R.E.; Feloutzis, A. A systematic review of the survival of implants in bone sites augmented with barrier membranes (guided bone regeneration) in partially edentulous patients. J. Clin. Periodontol. 2002, 29, 226–231. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Pagliani, L.; Andersson, P.; Lanza, M.; Nappo, A.; Verrocchi, D.; Volpe, S.; Sennerby, L. A Collagenated Porcine Bone Substitute for Augmentation at Neoss Implant Sites: A Prospective 1-Year Multicenter Case Series Study with Histology: Porcine Bone for Augmentation at Implant Sites. Clin. Implant Dent. Relat. Res. 2012, 14, 746–758. [Google Scholar] [CrossRef] [PubMed]
- Rossi, R.; Rancitelli, D.; Poli, P.P.; Rasia Dal Polo, M.; Nannmark, U.; Maiorana, C. The use of a collagenated porcine cortical lamina in the reconstruction of alveolar ridge defects. A clinical and histological study. Minerva Stomatol. 2016, 65, 257–268. [Google Scholar]
- Danesh-Sani, S.A.; Tarnow, D.; Yip, J.K.; Mojaver, R. The influence of cortical bone perforation on guided bone regeneration in humans. Int. J. Oral Maxillofac. Surg. 2017, 46, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Rothamel, D.; Schwarz, F.; Sager, M.; Herten, M.; Sculean, A.; Becker, J. Biodegradation of differently cross-linked collagen membranes: An experimental study in the rat. Clin. Oral Implants Res. 2005, 16, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Tatakis, D.N.; Promsudthi, A.; Wikesjö, U.M. Devices for periodontal regeneration. Periodontol. 2000 1999, 19, 59–73. [Google Scholar] [CrossRef]
- Lundgren, D.; Sennerby, L.; Falk, H.; Friberg, B.; Nyman, S. The use of a new bioresorbable barrier for guided bone regeneration in connection with implant installation. Case reports. Clin. Oral Implants Res. 1994, 5, 177–184. [Google Scholar] [CrossRef]
- Simion, M.; Trisi, P.; Piattelli, A. Vertical ridge augmentation using a membrane technique associated with osseointegrated implants. Int. J. Periodontics Restorative Dent. 1994, 14, 496–511. [Google Scholar]
- Simion, M.; Trisi, P.; Piattelli, A. GBR with an e-PTFE membrane associated with DFDBA: Histologic and histochemical analysis in a human implant retrieved after 4 years of loading. Int. J. Periodontics Restorative Dent. 1996, 16, 338–347. [Google Scholar] [CrossRef]
- Fontana, F.; Maschera, E.; Rocchietta, I.; Simion, M. Clinical classification of complications in guided bone regeneration procedures by means of a nonresorbable membrane. Int. J. Periodontics Restorative Dent. 2011, 31, 265–273. [Google Scholar] [PubMed]
- Maridati, P.C.; Cremonesi, S.; Fontana, F.; Cicciù, M.; Maiorana, C. Management of d-PTFE Membrane Exposure for Having Final Clinical Success. J. Oral Implantol. 2016, 42, 289–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cucchi, A.; Chierico, A.; Fontana, F.; Mazzocco, F.; Cinquegrana, C.; Belleggia, F.; Rossetti, P.; Soardi, C.M.; Todisco, M.; Luongo, R.; et al. Statements and Recommendations for Guided Bone Regeneration: Consensus Report of the Guided Bone Regeneration Symposium Held in Bologna, October 15 to 16, 2016. Implant Dent. 2019, 28, 388–399. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Tennant, M.; Walsh, L.J.; Kruger, E. Is there a consensus on antibiotic usage for dental implant placement in healthy patients? Aust. Dent. J. 2018, 63, 25–33. [Google Scholar] [CrossRef] [PubMed]

| Variable (mm) | Mean | Std dev | Std Err | 95% CI |
|---|---|---|---|---|
| MBLm | 0.11 | 0.49 | 0.16 | −0.46/0.24 |
| MBLd | 0.03 | 0.19 | 0.06 | −0.17/0.11 |
| NFB | RB | SF | |
|---|---|---|---|
| Mean % ± Std dev | 28.27 ± 10.62 | 10.39 ± 12.01 | 61.33 ± 12.08 |
| Range % (Min-Max) | 16.51–59.74 | 0–36.89 | 40.26–75.59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez, M.A.; Passarelli, P.C.; Netti, A.; D’Addona, A.; Carinci, F.; Wychowański, P.; Cecchetti, F. Porcine Cortical Bone Lamina as a Predictable Technique for Guided Bone Regeneration: Histomorphometric and Radiographic Evaluation. Appl. Sci. 2022, 12, 10285. https://doi.org/10.3390/app122010285
Lopez MA, Passarelli PC, Netti A, D’Addona A, Carinci F, Wychowański P, Cecchetti F. Porcine Cortical Bone Lamina as a Predictable Technique for Guided Bone Regeneration: Histomorphometric and Radiographic Evaluation. Applied Sciences. 2022; 12(20):10285. https://doi.org/10.3390/app122010285
Chicago/Turabian StyleLopez, Michele Antonio, Pier Carmine Passarelli, Andrea Netti, Antonio D’Addona, Francesco Carinci, Piotr Wychowański, and Francesco Cecchetti. 2022. "Porcine Cortical Bone Lamina as a Predictable Technique for Guided Bone Regeneration: Histomorphometric and Radiographic Evaluation" Applied Sciences 12, no. 20: 10285. https://doi.org/10.3390/app122010285






