1. Introduction
After long-term use, cement concrete structures are susceptible to various external factors. Concrete will shrink and creep, incur aging deformation or corrosion, and other issues, leading to structure surface damage, load-bearing capacity decline, and unreliable safety performance [
1,
2]. Cement-based materials have problems with high shrinkage and low flexibility [
3]. At the same time, they will cause environmental pollution during the production and construction process, which is not in line with the design concept of modern civil engineering structures [
1,
2]. Therefore, the search for new building materials with excellent performance to supplement and replace the traditional cement material, to effectively improve the load-bearing capacity of the structure through scientific means, and to extend the service life of the building structure has become a hotspot for scholars’ research in recent years.
Polyurethane is a polymer containing urethane groups in its molecular structure. It is a formed polymer composite material. With excellent properties, such as high wear resistance, good overall mechanical strength, and ease of use [
4], polyurethane can be traced back as far as 1837 when Professor Bayer’s research group obtained it using the preparation of 1,6-hexane diisocyanate and 1,4-butanedioic acid [
5]. Polyurethane is widely used in electromechanical, shipbuilding, aviation, civil construction, textile, and other departments. The variety of products is increasing yearly, and it occupies a very important position in the material industry [
6]. In 2012, the worldwide sales value of polyurethane materials reached about 43.2 billion pounds, with an annual growth rate of nearly 7.4%. The sale of polyurethane products can be predicted to rise to about 88.2 billion pounds in 2022 [
7].
Many scholars at home and abroad have carried out a series of studies on the excellent properties of polyurethane in civil engineering materials. (1) Mixing polyurethane with other materials can significantly improve the ductility and flexibility of the raw material [
8]. Due to the environment, concrete building materials undergo carbonation and need to be repaired or reconstructed [
9]; when reinforced concrete surfaces are coated with a thin layer of polyurethane protection, they are protected from corrosive environments and improve structural ductility, flexibility, and energy absorption [
10,
11]. (2) Polyurethane has stable chemical properties and good corrosion resistance and can be used as a surface protective coating for metal structures.
A study by Johns [
12] showed that a 6 mm thick polyurethane lining could provide a service life of 20 years, while a steel pipe without a lining wears out within 12 weeks. This study demonstrates that the polyurethane coating can protect the metal structure well. (3) Polyurethane materials have the potential for self-healing and can repair themselves without external interference [
13]. Polyurethane, as a healing agent, can restore the strength and stiffness of cementitious materials, reduce water permeability, and extend the service life of the material [
14]. (4) Polyurethane also has good thermal stability and is a good refractory material. Modifying polyurethane and adding composite flame retardants can offer good flame retardant and smoke suppression effects on the outer walls of high-rise buildings and improve the safety performance of building structures [
15,
16]. (5) Polyurethane is a polymer material that contains a significant amount of isocyanates, which can react with aromatic compounds in asphalt in an additional reaction to achieve a modified treatment of asphalt materials [
17].
Polyurethane-modified asphalt has excellent resistance to permanent deformation; the modified asphalt is less sensitive to temperature and significantly improves the asphalt’s high-low temperature rheological properties. At the same time, the heteroatomic groups in polyurethane strengthen the polarity and aromaticity of asphalt, thus improving the adsorption capacity of aggregates and the resistance to water damage of polyurethane composite-modified asphalt mixes is also significantly enhanced [
18].
Polyurethane cement composite is a new type of high-strength, and high toughness organic–inorganic composite material by mixing polyurethane glue and cement evenly, and the performance of the composite material can be improved significantly after solidification. It can be applied in rapidly repairing materials in building structures, bridge structure reinforcement [
19], and bridge deck pavement layers. Many researchers have studied and explored the ratio of polyurethane cement. A series of compressive and flexural tests have been carried out to arrive at the most reasonable ratio [
20,
21]. Polyurethane materials contain active cations that can improve the mechanical properties of cementitious materials, and the addition of polyurethane significantly reduces the early brittleness of cementitious materials [
22]. The product obtained by mixing polyurethane and silicate cement is similar to ordinary silicate cement but has much higher plasticity than ordinary silicate cement [
23]. The mechanical properties have been significantly improved and enhanced. A comparative study of the response of polyurethane cement composite and carbon-fiber-reinforced material reinforced RC frames shows that a polyurethane cement composite makes the structure more ductile and has a more straightforward construction method and lower material cost. Polyurethane cement composites enable more accessible, higher quality, and lower-cost structural performance modifications than carbon-fiber-reinforced materials [
24]. The polyurethane and the cement can form a polyurethane cement foam material, which has good mechanical properties, such as lightweight and high strength and is suitable for seismic components of structures. It also has good heat and sound insulation, water vapor permeability, and fire resistance [
25]. In bridge reinforcement, traditional concrete reinforcement methods have the disadvantages of long construction times, complex maintenance, and severe environmental pollution [
26].
Due to the good tensile properties of polyurethane elastomers, polyurethane cement for reinforcement does not require an internal configuration of reinforcement, and the short curing time of polyurethane cement makes the construction process relatively difficult and easy [
27]. In pavement engineering, the performance of pavement materials depends on the construction quality and later maintenance level of the road [
28,
29]. Current common pavement materials, such as petroleum asphalt and cement concrete, can hardly meet the requirements of some special projects, such as airport runways, tunnel pavements, and coastal highways [
30,
31]. Due to its good properties, polyurethane cement materials have been widely used in pavement engineering. They can partially or entirely replace traditional pavement materials in the pavement layer of roads [
32].
Compared with common cement-based materials in engineering, polyurethane–cement composite materials have the following characteristics: (1) Polyurethane, as a highly adhesive substance, can firmly adsorb cement particles, while cement particles can be uniformly distributed inside the polyurethane colloid so that the internal pores of polyurethane cement composites are filled densely, forming an organic–inorganic composite network structure [
33], which ensures that the properties of the material are the same and have good homogeneity. (2) High toughness is an essential feature of polyurethane composite materials [
13]. High toughness is an essential feature of polyurethane composites. Compared to cementitious materials, polyurethane cement composites, as a homogeneous body made of polymeric elastomer combined with inorganic cement particles, have good tensile and tear strengths, can withstand significant stresses without fracture damage, and have higher flexural strength than ordinary concrete materials [
34]. (3) The density of polyurethane cement composites is about 1.45~1.62 g/cm
3 [
13], while the density of cement concrete is about 2.4 g/cm
3 [
35], which is 1.58~1.65 times the density of polyurethane cement composites.
As a new lightweight construction material, polyurethane cement composites can reduce the stress on the structure caused by the material’s gravity and can be used in building structures with high seismic intensity levels. (4) The chemical properties of the polyurethane composite material are stable, and the chemical substances in the surrounding environment only have a minimal reaction to them. Polyurethane composites have strong anti-corrosion and anti-aging properties. The components or structures of the polyurethane cement composite material can easily adapt to areas with seawater erosion, soil microbial corrosion, or chemical pollution [
36,
37]. (5) There is no hydration reaction process in the preparation process of polyurethane cement composites, which can be solidified and formed in 60 min at room temperature under the action of a catalyst, and the performance requirements for regular use can be achieved within 24 h. The curing and setting times are much less than cement concrete, and no maintenance treatment is required at a later stage, effectively saving construction time and reducing construction costs [
38].
Polyurethane cement is a new organic–inorganic composite material—its performance should meet the requirements of the use of the environment, inevitably affected by the external environment in the actual use of the process, the impact of the external environment on its mechanical properties cannot be ignored. Temperature is an essential factor in the external environment. The properties of materials at different temperatures are different. Therefore, the mechanical properties of polyurethane cement at different temperatures play an essential role in its application in practical engineering.
In this paper, in order to explore the flexural properties of polyurethane cement composites under temperature loads, bending tests were carried out in strain control mode. The samples’ reaction characteristics, stress processes, and failure modes under different temperature loads were obtained. The polyurethane cement composites were analyzed for flexural strength in the actual working environment, further verifying the feasibility of polyurethane cement composites to supplement and replace traditional materials.
6. Results and Discussion
Indicators of flexural properties include tensile strength at break, tensile strain at break, and modulus of stiffness. The flexural tensile strength, failure strain, and stiffness modulus are not only affected by the test temperature but also by the loading speed. This paper mainly studies the effect of temperature on the bending properties of polyurethane cement. Therefore, in the test groups of different temperatures, the loading speed is the same, and the vertical loading rate is 1 mm/min to carry out the bending test of polyurethane cement trabeculae. According to the test results, the flexural tensile strength R
b, the flexural tensile strain ε
b, and the stiffness modulus S
b are calculated. The calculation equations are as follows:
In the formula, Rb is the flexural tensile strength of the specimen at failure (MPa); εb is the maximum bending and tensile strain when the specimen fails (με); Sb is the modulus of bending stiffness when the specimen fails (MPa); b is the width of the cross-interruption interview (mm); h is the height of the cross-interruption interview piece (mm); L is the calculated span of the specimen (mm); Pb is the maximum load when the specimen fails (N); and d is the mid-span deflection when the specimen fails (mm).
The width b of the polyurethane cement small beam specimen is 30 mm, the height is 35 mm, the calculated span L is 200 mm, and the bending test results under different temperature levels are listed in
Table 8.
As can be seen from
Table 8, with the increase in the test temperature, the maximum deflection in the span of the polyurethane cement composite at the time of damage is increasing, and the change process can be divided into three stages. The first stage is from −50 °C to 30 °C, the maximum deflection in the span increases slowly, from 1.365 mm to 4.661 mm, and the growth rate is 0.412 mm/(10 °C). The second stage is from 30 °C to 40 °C, the maximum deflection in the span increases sharply from 4.661 mm to 22.925 mm, with a growth rate of 18.264 mm/(10 °C). The third stage is from 40 °C to 50 °C, the maximum deflection in the span increases slowly from 22.925 mm to 24.476 mm, with a growth rate of 1.551 mm/(10 °C). The rate of change of deflection in the first and third stages was slight and the deflection changed slowly, indicating that the flexural properties of polyurethane cement changed gradually with the change of temperature, but the rate of change of deflection in the second stage was very large, 44.33 times and 11.77 times of the rate of change in the first and third stages, and the deflection changed rapidly, indicating that in the temperature range of 30 °C to 40 °C, the flexural performance underwent a sudden change.
The flexural–tensile strength, flexural–tensile strain at failure, modulus of failure stiffness, load–displacement curve, and failure mode of polyurethane–cement composites at different temperature levels will be analyzed in detail below.
6.1. Flexural Strength
Figure 20 shows the bending and tensile strength of the polyurethane cement composites at different temperature levels. It can be seen from
Figure 20 and
Table 8 that the flexural tensile strength of the polyurethane cement composites showed a trend of decreasing, then increasing and then decreasing with increasing temperature during the increase of temperature T from −50 °C to 50 °C. There are two extreme points of bending tensile strength with the change of temperature–first a minimal value point (temperature of −30 °C) and then a multiple value point (temperature of 0 °C), which indicates that the polyurethane cement composites have a turning point in the growth law of bending tensile strength of polyurethane cement due to the change of internal properties of polyurethane cement material in the range around the temperature of these two extreme points.
The bending and tensile strength of polyurethane cement specimens gradually decrease during the increase of temperature T from −50 °C to −30 °C, gradually increases during the increase of temperature T from −30 °C to 0 °C, and gradually decreases during the change of temperature T from 0 °C to 50 °C. However, the decreasing bending and tensile strength speed are very fast during the change of temperature T from 20 °C to 30 °C. In changing temperature T from −50 °C to 0 °C, the bending and tensile strength of polyurethane cement composites show the change law of decreasing and then increasing with the increase of temperature. The changing trend shows a “V” shape. While changing temperature T from 0 °C to 50 °C, the bending and tensile strength of polyurethane cement composites show a general trend of decreasing with temperature increase. In the process of temperature T changing from 0 °C to 50 °C, the bending and tensile strength of polyurethane cement composites show a trend of gradually decreasing with the increase in temperature. The front and back part of the curve is small. The middle part of the curve decreases sharply, and the shape of the curve is “S”.
When the temperature is −30 °C, the bending and tensile strength of polyurethane cement composites appear to have a minimal value, and then the bending and tensile strength increases, showing stress hardening characteristics; when the temperature is 0 °C, the bending and tensile strength of polyurethane cement composites appear to have a considerable value, and then the bending and tensile strength decreases, showing apparent stress softening characteristics. The two extreme points of bending and tensile strength and stress hardening and softening of polyurethane cement are that polyurethane is a material susceptible to temperature [
50]. The change in temperature leads to the change of mechanical properties of polyurethane composing polyurethane cement composites. At the same time, the polyurethane content of polyurethane cement is significant, with 50% by weight, which leads to the bending and tensile strength of polyurethane cement composites also being susceptible to the change of temperature-sensitive temperature changes [
51].
During the change of temperature from −50 °C to 50 °C, the mechanical properties of the polyurethane cement composites undergo two essential changes. The first manifested as a change from brittle to viscoelastic with a cut-off point temperature of −30 °C, and the second manifested as a change from viscoelastic to plastic with a cut-off point temperature of 30 °C. Since the bending and tensile strength of the polyurethane cement composite undergoes a sharp decrease in the process of increasing from 20 °C to 30 °C, it can be considered that a specific temperature of increasing from 20 °C to 30 °C, recorded as (20 °C, 30 °C), is the demarcation point between viscoelasticity and plasticity of the polyurethane cement composite. It can be seen from
Figure 20 that the load–displacement curve shows a linear growth trend when the temperature is lower than 20 °C. The polyurethane cement beam undergoes brittle damage when the ultimate load is reached. The load–displacement curve does not show a plateau period and a slow decreasing section. However, it shows a sharp decrease, indicating that the polyurethane cement composite material mainly shows brittle characteristics when the temperature is lower, and the bending and tensile deformation ability is inferior. At this time, the breaking load and bending and pulling strength of polyurethane cement beams are more significant; however, the bending and pulling strength increases after the temperature is lower than −30 °C, which is because of the brittleness of polyurethane cement composites is very large at this time. As the temperature decreases, the breaking load increases, and the displacement decreases so that the bending and pulling strength increases. When the temperature is higher than 20 °C, the polyurethane cement composites mainly show viscous characteristics, the ability to bend and tensile deformation is perfect, brittle damage will not occur. The form of damage is bending plastic damage with good ductility, which shows that the load–displacement relationship curve at 30 °C, 40 °C, and 50 °C is not linearly changing. A plateau period will appear before reaching the ultimate load. After the ultimate load, the load–displacement curve decreases slowly, an apparent decreasing section appears, and the bending and tensile strength of polyurethane cement composites at this stage is minimal, and the resistance to deformation is significant.
Polyurethane cement, as an organic–inorganic composite, has the properties of both organic polyurethane colloid and inorganic cement particles. The cement is mainly a crystalline structure formed by the coalescence of particles such as C
2S and C
3S, with a reticulated internal distribution, which mainly provides compressive strength. Polyurethane colloid is made of polyether polyol soft chain segments and isocyanate hard chain segments polymerized into a polymeric substance with long molecular chains and interwoven distribution of soft and hard chain segments, which has good tensile properties and directly determines the flexural strength of polyurethane cement. The principle of bending strength of polyurethane cement is explained from the microscopic molecular point of view. The soft chain segment of polyurethane colloid presents a rubbery state, which provides low-temperature performance; the hard chain segment presents a glassy or semi-crystalline state, which provides high-temperature performance [
52]. Reflecting the temperature performance of polyurethane is mainly expressed in the glass transition temperature T
g, the magnitude of T
g depends on the binding force between macromolecules and the length of molecular chains, so the slender and soft chain segments have a more obvious effect on the T
g of polyurethane cement [
53].
Table 9 lists the T
g of several common types of polyurethane colloids [
54]. From the table, it can be seen that the T
g of MDI-PPG polyurethane colloid is −32 °C. The glass transition of polyurethane will occur when the temperature increases to −32 °C, which is consistent with the results shown in
Figure 20, where the first inflection point occurs near −30 °C. When the temperature is lower than −30 °C, the polyurethane tends to a flexible rubbery state with an increase in bending strength, corresponding to the change in the interval from −50 °C to −30 °C in
Figure 20. When the temperature is higher than −30°C, the molecules inside the polyurethane continuously absorb heat from the outside as the temperature rises, and the hydrogen bonding between molecules gradually strengthens, leading to another increase in bending strength [
55]. This change is consistent with the results shown in the −30 °C to 0 °C interval in
Figure 20. However, the change in bending strength with increasing temperature is not continuous. Due to the poor heat resistance of polyurethane, when the temperature is too high, the spacing between the soft and hard chain segments becomes smaller and smaller, limiting the intermolecular movement, and the polyurethane tends to soften, leading to a continuous decrease in bending strength. This is consistent with the results shown in the last interval in
Figure 20.
6.2. Analysis of Failure Strain Test Results
Figure 18 shows the breaking strains of the polyurethane cement composites at different temperature levels. It can be seen from
Figure 21 and
Table 8 that the breaking strain of the polyurethane cement composites increases with the increase in temperature. The reason for this is that at temperatures below and including 30 °C, polyurethane mainly exhibits brittleness and viscoelasticity [
56], resulting in polyurethane cement exhibiting more pronounced elasticity, and the lower the temperature, the more significant the elastic characteristics of polyurethane cement composites are, and the smaller the strain in bending damage of polyurethane cement specimens. A local analysis of the disruption strain in the temperature range of −50 °C change to 30 °C shows that the disruption strain is gradually increasing; in the process of increasing from −50 °C to 30 °C, the temperature-destructive strain curve can be divided into three stages as the temperature continues to increase, the first stage is the increase from −50 °C to −30 °C, the deadly strain grows slowly, the increase rate is low and approximates a straight line, the second stage is −30 °C to 0 °C, the damaging strain proliferates, the increase rate is more significant, and it is approximately a straight line. The third stage is 0 °C to 30 °C; the damaging strain proliferates, the increase rate is low at the beginning and then increases rapidly, and it is approximately a concave curve. The higher the temperature is, the more pronounced the viscoelastic characteristics of polyurethane cement composites are. The greater the strain when the polyurethane cement specimen is bent and damaged, the greater the change of damage strain rate is with the change of temperature.
In the process of increasing from −50 °C to 50 °C, changing the damaging strain of polyurethane cement composites mainly shows a trend of two stages of change. The first stage is in the temperature range of −50 °C to 30 °C, the damaging strain of polyurethane cement composites is minor and reaches a significant value at 30 °C, and the damaging strain reaches 114,048 με; the second stage is in the temperature range of 30 °C to 50 °C, the damaging strain suddenly increases a lot at 40 °C, the damaging strain is 2,759,124 με at this time, which is 30 °C 24.2 times, indicating that in the range of 30 °C to 40 °C, the polyurethane cement composites transformed into plasticity and the elastic characteristics decreased, which showed an obvious turning point in the damage strain curve. The shape of the damage strain curve in the temperature range of −50 °C to 50 °C is approximately L-shaped, and the damage curve in the temperature range of −50 °C to 30 °C is similar to the flat side of the “L” shape. The damage curve in the temperature range of 30 °C to 50 °C is similar to the “L” shape. The damage curve is similar to the vertical side of the “L” shape.
Polyurethane is a polymer with a large number of polar bonds in the system, which can be divided into hard chain segments, hydrogen bonds between soft chain segments, and intra-molecular hydrogen bonds. When the temperature rises, the thermal movement of molecular chains intensifies, and the hydrogen bonds between soft and hard chain segments absorb a significant of energy and break gradually, forming internal molecular hydrogen bonds continuously [
57,
58]. At this time, the spacing between the polyurethane molecular chains keeps decreasing, and the polyurethane colloid tends to curl, which is macroscopically realized as the effective crosslink density of polyurethane cement composites decreases, the strain keeps increasing, and the elastic modulus gradually decreases. This is consistent with the trend of the increasing strain of damage with increasing temperature and decreasing modulus of elasticity expressed in
Figure 21 and
Figure 22.
6.3. Analysis of Failure Stiffness Modulus Test Results
Figure 19 shows the modulus of damage stiffness of the polyurethane cement composites at different temperature levels. From
Figure 22 and
Table 8, it can be seen that the flexural modulus of damage modulus of polyurethane cement composites decreases continuously with the increase in temperature. When the temperature is lower than 20 °C (including 20 °C), the changing relationship of the bending modulus of the polyurethane cement composites is divided into two stages. The first stage is characterized by a nearly linear decrease (−50 °C~−20 °C), and the second stage is characterized by a concave curve decrease (−20 °C~20 °C). The average rate of change in the first half of the second stage is larger than the average rate of change in the second half. When the temperature is higher than 20 °C, the modulus of damage strength of polyurethane cement composites decreases sharply with the temperature increase. A temperature of 20 °C is the folding point of the change of modulus of damage strength of polyurethane cement composites, mainly because the mechanical properties of polyurethane cement composites change considerably at 20 °C; when the temperature is lower than 20 °C, polyurethane cement mainly shows the elastic characteristics, and as the temperature decreases, the elastic characteristics; when the temperature is lower than 20 °C, the polyurethane cement mainly exhibits elastic characteristics, and as the temperature decreases, the elastic characteristics become more apparent, the deformation capacity becomes smaller, and the modulus of damage strength becomes larger; when the temperature is higher than 20 °C, the polyurethane cement composites mainly exhibit viscous characteristics, and as the temperature increases, the viscous characteristics become more apparent, the deformation capacity becomes more extensive, and the modulus of damage strength becomes smaller.
The temperature dramatically influences the failure stiffness modulus of polyurethane cement composites. During the process of increasing from −50 °C to 50 °C, the failure stiffness modulus of polyurethane cement decreases from 4198.3 MPa to 1.3 MPa, and the reduction range is extensive, decreasing by about 3228 times; the temperature change curve of the failure stiffness modulus maintains a continuous downward trend, and is generally a concave curve, the slope of each point is negative, the failure stiffness modulus and temperature performance is a negative relationship. The modulus of breaking strength is very small at 40 °C and 50 °C. It can be understood from the above analysis of breaking strain that the breaking strain of polyurethane cement composites is tremendous under these two temperature conditions, mainly because the adhesion of polyurethane is excellent at higher temperatures, which leads to the polyurethane cement composites mainly presenting as sticky. From a macroscopic point of view, polyurethane cement is softer and better able to withstand changes and has better ductility.
6.4. Load-Displacement Curve Analysis
Figure 20 shows the load–displacement curves of the polyurethane cement composites at different temperature levels. The slope of the curves decreases as the temperature increases. When the temperature is lower than 20 °C (including 20 °C), the load–displacement curve of the polyurethane cement beamlet shows an increasing linear trend. When the maximum load is reached, the specimen breaks at the mid-span position, and the load suddenly decreases. The curve shows an obvious turning point, which shows typical brittle damage, and the displacements of the specimens are all less than 5 mm at the time of damage, and the damage load is more significant. When the temperature is higher than 20 °C, the displacement of polyurethane cement beams increases with the load, showing a typical nonlinear increase. The displacement is still increasing when the load is close to the maximum value, an apparent plateau appears, the maximum displacement is close to 25 mm, and the damage form is bending ductile damage.
In order to analyze the load–displacement relationship between the polyurethane cement specimens more conveniently,
Figure 23a is decomposed into
Figure 23b,c and the splitting limit is chosen as 20 °C. From
Figure 23b, it can be seen that the damage load shows a trend of decreasing, then increasing, and then decreasing with the increase in temperature. However, the damage displacement shows a trend of increasing with increasing temperature.
Figure 23c shows that the load–displacement curve appears during a plateau period after the temperature increases, and the damage load keeps decreasing. The damage displacement keeps increasing as the temperature increases, and the load–displacement curve of the plateau period keeps extending.
6.5. Destruction Mode
The damage forms of the bending test of polyurethane cement beams at different temperature levels are shown in
Figure 24. The forms of damage sections at different temperatures are different, mainly showing two types of damage sections. One is the damage section at temperatures of −50 °C, −40 °C, −10 °C, 0 °C, 10 °C, and 20 °C, which shows an irregular fold shape; the other is the damage section at temperatures of −30 °C and −20 °C, which shows the regular rectangular shape, and the fracture surface overlaps with the loading axis. In the process of temperature rise from −50 °C to 20 °C, the specimen damage form is brittle damage, and the damage section is in the loading point position of the beam, i.e., the span position. The damage of the specimens at 30 °C was ductile and ductile, and more small vertical cracks appeared at the lower edge near the span section. The damage of the specimens at 40 °C and 50 °C is in the form of flexible damage. The deformation of the specimens is excellent, but the ability to bear the load is inferior, and many folds appear at the lower edge near the span section. No apparent cracks appear when the damage occurs.
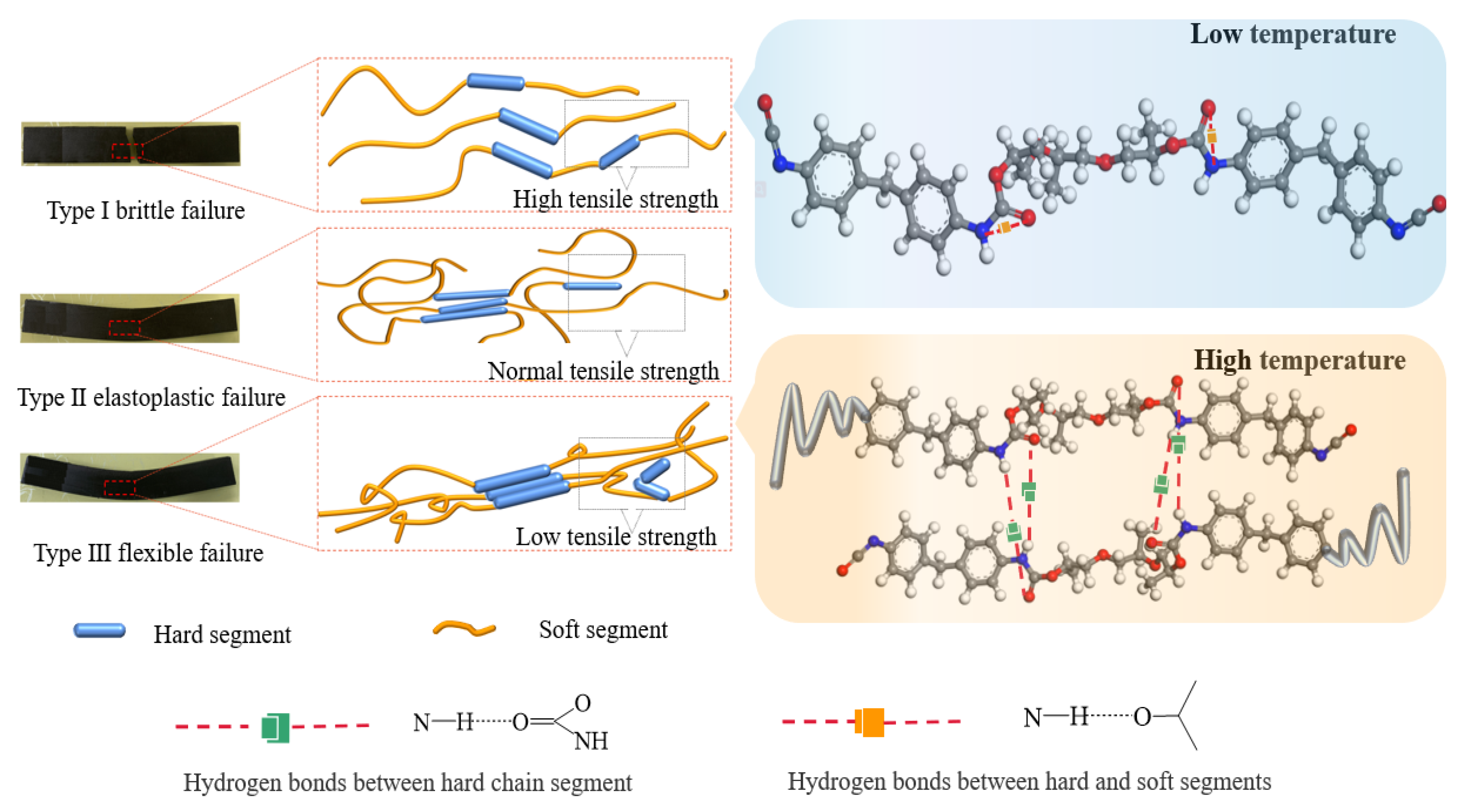
According to the load–displacement curve and damage form of the polyurethane cement beamlet bending test, it can be seen that
Figure 25 represents three damage modes of polyurethane cement at different temperature levels. The first damage mode load–displacement curve shows linear growth before reaching the ultimate load, and then the curve suddenly turns and drops sharply when reaching the ultimate load. The polyurethane cement beamlet instantly loses its bearing capacity and fractures, called Type I brittle damage. The second damage mode load–displacement curve before reaching the ultimate load, first linear growth and then nonlinear growth, the internal reorganization of the material, mechanical properties change, after reaching the ultimate load, there is a process of decline in bearing capacity. Finally, loss of bearing capacity, fracture, and polyurethane cement beams have a certain ductility, known as Type II elastic-plastic damage. In the third damage mode load–displacement curve, before reaching the ultimate load, there is first a tiny section of the curve to maintain a linear growth trend, and then a large section of the nonlinear growth curve, and finally, basically remain as a horizontal line, the bearing capacity no longer increases. However, the deformation is still occurring, and the curve has a prominent platform period, known as Type III flexible damage.
The failure mode of polyurethane cement is explained from the microscopic point of view, and the internal polyurethane stress model under three failure modes is established, as shown in
Figure 26. In the bending test, the lower part of the polyurethane cement beam specimen is mainly subjected to tensile stress. As a class of crystalline mixture, the cement particles have good compressive capacity but weak tensile properties, and the polyurethane colloid mainly bear the tensile stress under external loading. Type I brittle damage: In the low-temperature environment, the spacing between soft and hard chain segments inside the polyurethane increases [
59], the molecular chain movement is enhanced, hydrogen bonding exists between the hard and soft chain segments [
60], the polyurethane molecular structure is complete, the mechanical properties of the polyurethane colloid are improved, and the tensile strength provided is very high. The polyurethane does not reach the yield strength under load; instead, the cement particles on the upper part of the beam reach the ultimate compressive strain, the cement is crushed, and the polyurethane cement specimen suddenly fractures. Type III flexible damage: The heat resistance of polyurethane is poor, and the softening temperature is 50 °C~70 °C. Under the high-temperature environment, polyurethane tends to soften, the hydrogen bonds between hard chain segments-soft chain segments break, the hydrogen bonds between hard chain segments are formed, the spacing between internal soft and hard chain segments decreases, and they will be interlaced chaotically [
61], which limits the movement ability of molecular chains, the mechanical properties of polyurethane decreases, and the tensile strength decreases; under the action of load polyurethane soon reaches the yield strength and enters the strengthening stage, the attraction between molecules is difficult to overcome the external; under the action of load, polyurethane soon reaches the yield strength and enters the strengthening stage, and the attraction between molecules is difficult to resist the external action. The internal chemical bonds gradually break, the molecular chains are finally pulled off [
62], and the polyurethane cement specimen is destroyed. Type II elastoplastic damage: at room temperature, the spacing of internal chain segments of polyurethane is standard, and the tensile strength is normal; under load, the polyurethane first reaches yield strength and tends toward the elastic development stage; the crack at the bottom of the beam extends continuously, and as the cement reaches the ultimate compressive strength, the polyurethane cement specimen is destroyed.
The analysis of bending and tensile strength, damage strain, damage stiffness modulus, load–displacement curve, and damage mode of polyurethane cement composites shows that in the temperature range of −20 °C~20 °C, the bending performance of polyurethane cement composites is relatively good. The stiffness modulus is relatively large at low temperatures, which is easy to release brittle damage. The stiffness modulus is relatively small at high temperatures, generally causing flexible damage.