AS-OCT and Ocular Hygrometer as Innovative Tools in Dry Eye Disease Diagnosis
Abstract
:1. Introduction
2. Current Methods of Diagnosis
3. Anterior Segment Optical Coherence Tomography
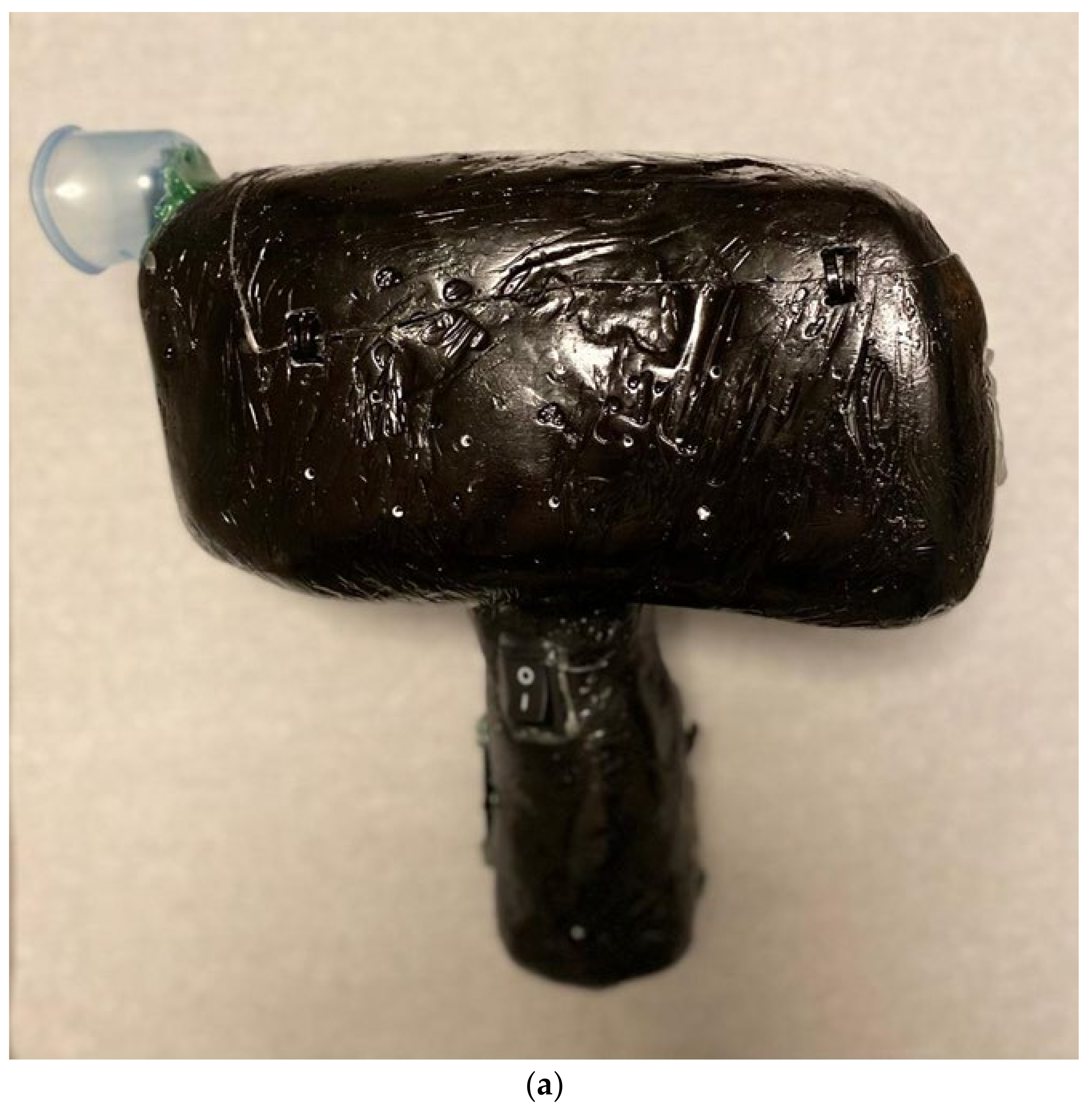
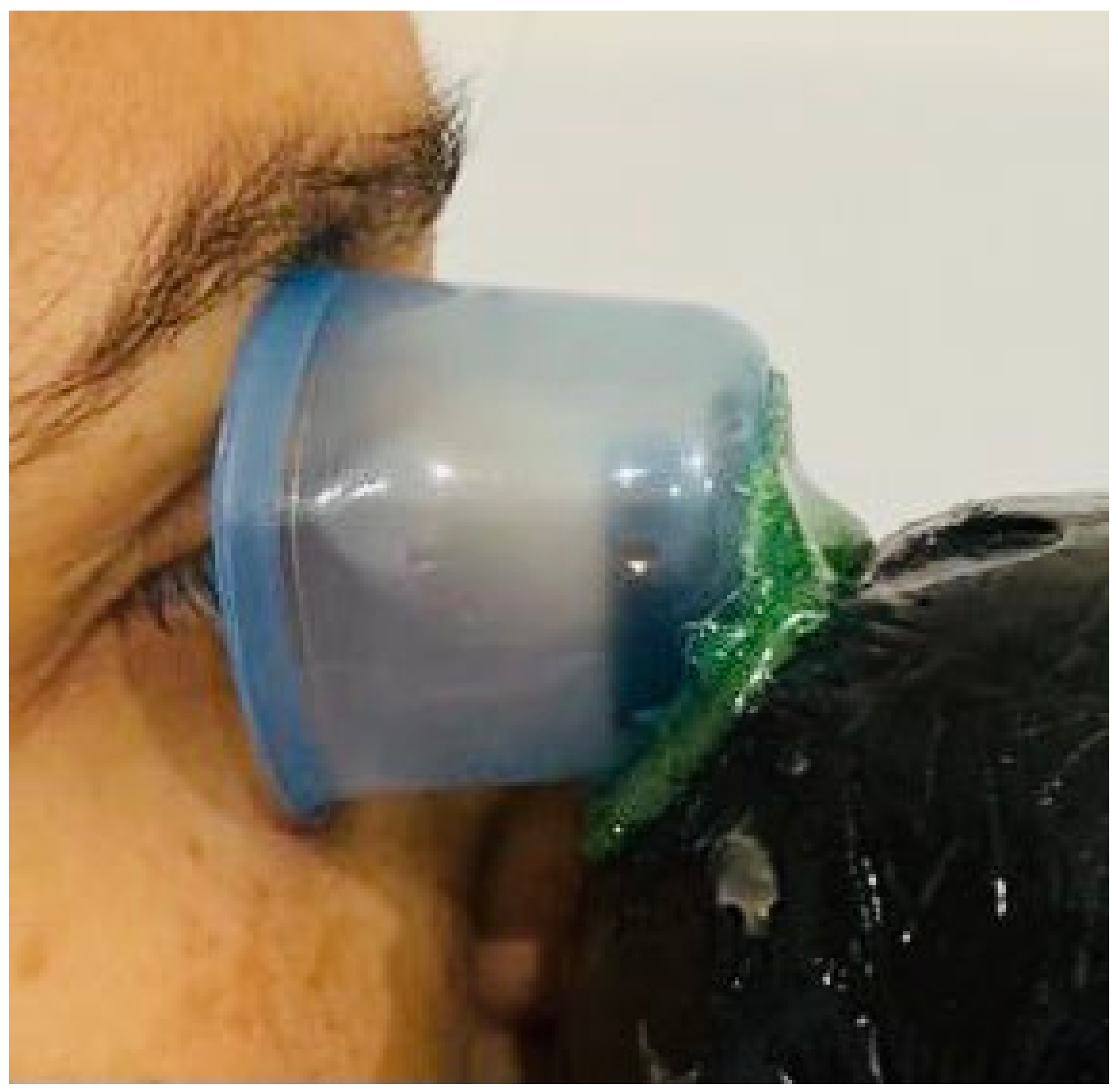
4. Innovative Device
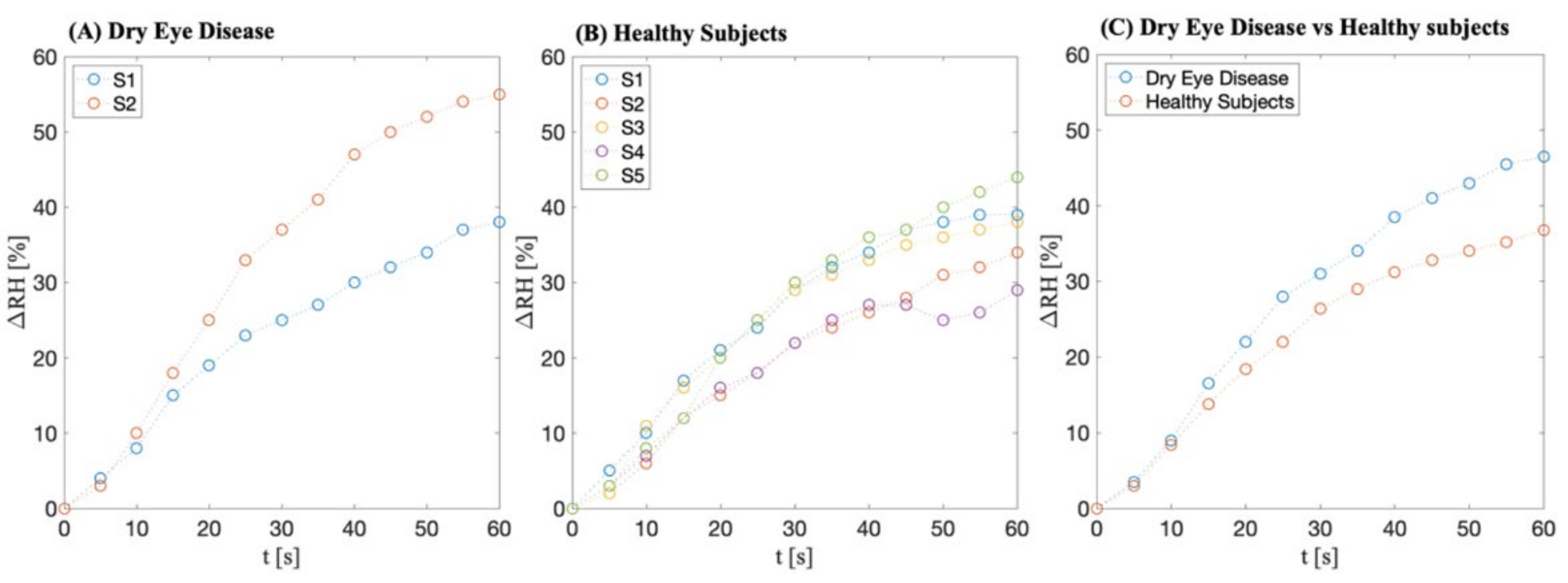
Preliminary Data
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Craig, J.P.; Nichols, K.K.; Akpek, E.K.; Caffery, B.; Dua, H.S.; Joo, C.K.; Liu, Z.; Nelson, J.D.; Nichols, J.J.; Tsubota, K.; et al. TFOS DEWS II Definition and Classification Report. Ocul. Surf. 2017, 15, 276–283. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, F.; Alves, M.; Bunya, V.Y.; Jalbert, I.; Lekhanont, K.; Malet, F.; Na, K.-S.; Schaumberg, D.; Uchino, M.; Vehof, J.; et al. TFOS DEWS II Epidemiology Report. Ocul. Surf. 2017, 15, 334–365. [Google Scholar] [CrossRef]
- Dana, R.; Bradley, J.L.; Guerin, A.; Pivneva, I.; Stillman, I.Ö.; Evans, A.M.; Schaumberg, D.A. Estimated Prevalence and Incidence of Dry Eye Disease Based on Coding Analysis of a Large, All-age United States Health Care System. Am. J. Ophthalmol. 2019, 202, 47–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulsen, A.J.; Cruickshanks, K.J.; Fischer, M.E.; Huang, G.-H.; Klein, B.E.K.; Klein, R.; Dalton, D.S. Dry eye in the beaver dam offspring study: Prevalence, risk factors, and health-related quality of life. Am. J. Ophthalmol. 2014, 157, 799–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrand, K.F.; Fridman, M.; Stillman, I.Ö.; Schaumberg, D.A. Prevalence of Diagnosed Dry Eye Disease in the United States Among Adults Aged 18 Years and Older. Am. J. Ophthalmol. 2017, 182, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Geerling, G.; Tauber, J.; Baudouin, C.; Goto, E.; Matsumoto, Y.; O’Brien, T.; Rolando, M.; Tsubota, K.; Nichols, K.K. The International Workshop on Meibomian Gland Dysfunction: Report of the Subcommittee on Management and Treatment of Meibomian Gland Dysfunction. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2050–2064. [Google Scholar] [CrossRef] [Green Version]
- Bron, A.J.; de Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef]
- Willcox, M.D.P.; Argüeso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, D.A.; Rocha, E.M.; Aragona, P.; Clayton, J.A.; Ding, J.; Golebiowski, B.; Hampel, U.; McDermott, A.M.; Schaumberg, D.A.; Srinivasan, S.; et al. TFOS DEWS II Sex, Gender, and Hormones Report. Ocul. Surf. 2017, 15, 284–333. [Google Scholar] [CrossRef]
- Antonini, M.; Gaudenzi, D.; Spelta, S.; Sborgia, G.; Poddi, M.; Micera, A.; Sgrulletta, R.; Coassin, M.; Di Zazzo, A. Ocular Surface Failure in Urban Syndrome. J. Clin. Med. 2021, 10, 3048. [Google Scholar] [CrossRef]
- Grasso, A.; Di Zazzo, A.; Giannaccare, G.; Sung, J.; Inomata, T.; Shih, K.C.; Micera, A.; Gaudenzi, D.; Spelta, S.; Romeo, M.A.; et al. Sex Hormones Related Ocular Dryness in Breast Cancer Women. J. Clin. Med. 2021, 10, 2620. [Google Scholar] [CrossRef] [PubMed]
- Micera, A.; Di Zazzo, A.; Esposito, G.; Longo, R.; Foulsham, W.; Sacco, R.; Sgrulletta, R.; Bonini, S. Age-related changes to human tear composition. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2024–2031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Zazzo, A.; Micera, A.; Coassin, M.; Varacalli, G.; Foulsham, W.; De Piano, M.; Bonini, S. Inflammaging at ocular surface: Clinical and biomolecular analyses in healthy volunteers. Investig. Ophthalmol. Vis. Sci. 2019, 60, 1769–1775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolffsohn, J.S.; Arita, R.; Chalmers, R.; Djalilian, A.; Dogru, M.; Dumbleton, K.; Gupta, P.K.; Karpecki, P.; Lazreg, S.; Pult, H.; et al. TFOS DEWS II Diagnostic Methodology report. Ocul. Surf. 2017, 15, 539–574. [Google Scholar] [CrossRef]
- Nichols, J.J.; Ziegler, C.; Mitchell, G.L.; Nichols, K.K. Self-reported dry eye disease across refractive modalities. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1911–1914. [Google Scholar] [CrossRef] [Green Version]
- Karakus, S.; Mathews, P.M.; Agrawal, D.; Henrich, C.; Ramulu, P.Y.; Akpek, E.K. Impact of Dry Eye on Prolonged Reading. Optom. Vis. Sci. 2018, 95, 1105–1113. [Google Scholar] [CrossRef]
- Nichols, K.K.; Mitchell, G.L.; Zadnik, K. The repeatability of clinical measurements of dry eye. Cornea 2004, 23, 272–285. [Google Scholar] [CrossRef]
- Nichols, K.K.; Nichols, J.J.; Mitchell, G.L. The lack of association between signs and symptoms in patients with dry eye disease. Cornea 2004, 23, 762–770. [Google Scholar] [CrossRef]
- Sullivan, B.D.; Whitmer, D.; Nichols, K.K.; Tomlinson, A.; Foulks, G.N.; Geerling, G.; Pepose, J.S.; Kosheleff, V.; Porreco, A.; Lemp, M.A. An objective approach to dry eye disease severity. Investig. Ophthalmol. Vis. Sci. 2010, 51, 6125–6130. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, J.D.; Johnston, P.R.; Ousler, G.W.; Smith, L.M.; Abelson, M.B. Automated grading system for evaluation of ocular redness associated with dry eye. Clin. Ophthalmol. 2013, 7, 1197–1204. [Google Scholar] [CrossRef] [Green Version]
- Zeev, M.S.B.; Miller, D.D.; Latkany, R. Diagnosis of dry eye disease and emerging technologies. Clin. Ophthalmol. 2014, 8, 581–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veres, A.; Tapasztó, B.; Kosina-Hagyó, K.; Somfai, G.M.; Németh, J. Imaging lid-parallel conjunctival folds with OCT and comparing its grading with the slit lamp classification in dry eye patients and normal subjects. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2945–2951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Németh, J.; Fodor, E.; Lang, Z.; Kosina-Hagyó, K.; Berta, A.; Komár, T.; Petricek, I.; Higazy, M.; Prost, M.; Grupcheva, C.; et al. Lid-parallel conjunctival folds (LIPCOF) and dry eye: A multicentre study. Br. J. Ophthalmol. 2012, 96, 1380–1385. [Google Scholar] [CrossRef] [Green Version]
- Yoon, K.C.; Im, S.K.; Kim, H.G.; You, I.C. Usefulness of double vital staining with 1% fluorescein and 1% lissamine green in patients with dry eye syndrome. Cornea 2011, 30, 972–976. [Google Scholar] [CrossRef]
- Savini, G.; Barboni, P.; Zanini, M. The incidence and risk factors for developing dry eye after myopic LASIK. Am. J. Ophthalmol. 2006, 142, 355–356. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Foulks, G.N. Evaluation of the effect of lissamine green and rose bengal on human corneal epithelial cells. Cornea 1999, 18, 328–332. [Google Scholar] [CrossRef]
- Schein, O.D.; Tielsch, J.M.; Munoz, B.; Bandeen-Roche, K.; West, S. Relation between signs and symptoms of dry eye in the elderly. A population-based perspective. Ophthalmology 1997, 104, 1395–1401. [Google Scholar] [CrossRef]
- Machado, L.M.; Castro, R.S.; Fontes, B.M. Staining patterns in dry eye syndrome: Rose bengal versus lissamine green. Cornea 2009, 28, 732–734. [Google Scholar] [CrossRef]
- Hamrah, P.; Alipour, F.; Jiang, S.; Sohn, J.H.; Foulks, G.N. Optimizing evaluation of Lissamine Green parameters for ocular surface staining. Eye 2011, 25, 1429–1434. [Google Scholar] [CrossRef]
- Lemp, M.A. Report of the National Eye Institute/Industry workshop on Clinical Trials in Dry Eyes. CLAO J. 1995, 21, 221–232. [Google Scholar]
- Bron, A.J.; Evans, V.E.; Smith, J.A. Grading of corneal and conjunctival staining in the context of other dry eye tests. Cornea 2003, 22, 640–650. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Deng, X.G.; He, M.F. Comparison of the Schirmer I test with and without topical anesthesia for diagnosing dry eye. Int. J. Ophthalmol. 2012, 5, 478–481. [Google Scholar] [CrossRef] [PubMed]
- Kashkouli, M.B.; Pakdel, F.; Amani, A.; Asefi, M.; Aghai, G.H.; Falavarjani, K.G. A modified Schirmer test in dry eye and normal subjects: Open versus closed eye and 1-minute versus 5-minute tests. Cornea 2010, 29, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Ozulken, K.; Aksoy Aydemir, G.; Tekin, K.; Mumcuoğlu, T. Correlation of Non-invasive Tear Break-Up Time with Tear Osmolarity and Other Invasive Tear Function Tests. Semin. Ophthalmol. 2020, 35, 78–85. [Google Scholar] [CrossRef]
- Effect of Tear Film Break-Up on Higher Order Aberrations of the Anterior Cornea in Normal, Dry, and Post-LASIK Eyes—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/16209455 (accessed on 30 December 2021).
- Vanley, G.T.; Leopold, I.H.; Gregg, T.H. Interpretation of tear film breakup. Arch. Ophthalmol. 1977, 95, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Szalai, E.; Berta, A.; Szekanecz, Z.; Szûcs, G.; Módis, L. Evaluation of tear osmolarity in non-Sjögren and Sjögren syndrome dry eye patients with the TearLab system. Cornea 2012, 31, 867–871. [Google Scholar] [CrossRef]
- Tomlinson, A.; McCann, L.C.; Pearce, E.I. Comparison of human tear film osmolarity measured by electrical impedance and freezing point depression techniques. Cornea 2010, 29, 1036–1041. [Google Scholar] [CrossRef]
- Tomlinson, A.; Khanal, S.; Ramaesh, K.; Diaper, C.; McFadyen, A. Tear film osmolarity: Determination of a referent for dry eye diagnosis. Investig. Ophthalmol. Vis. Sci. 2006, 47, 4309–4315. [Google Scholar] [CrossRef] [Green Version]
- Versura, P.; Bavelloni, A.; Grillini, M.; Fresina, M.; Campos, E.C. Diagnostic performance of a tear protein panel in early dry eye. Mol. Vis. 2013, 19, 1247. [Google Scholar]
- Ohashi, Y.; Ishida, R.; Kojima, T.; Goto, E.; Matsumoto, Y.; Watanabe, K.; Ishida, N.; Nakata, K.; Takeuchi, T.; Tsubota, K. Abnormal protein profiles in tears with dry eye syndrome. Am. J. Ophthalmol. 2003, 136, 291–299. [Google Scholar] [CrossRef]
- Lee, S.Y.; Han, S.J.; Nam, S.M.; Yoon, S.C.; Ahn, J.M.; Kim, T.I.; Kim, E.K.; Seo, K.Y. Analysis of tear cytokines and clinical correlations in Sjögren syndrome dry eye patients and non-Sjögren syndrome dry eye patients. Am. J. Ophthalmol. 2013, 156, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Itoh, K.; Inoue, K.; Amano, S. Noncontact infrared meibography to document age-related changes of the meibomian glands in a normal population. Ophthalmology 2008, 115, 911–915. [Google Scholar] [CrossRef] [PubMed]
- Nichols, J.J.; Berntsen, D.A.; Mitchell, G.L.; Nichols, K.K. An assessment of grading scales for meibography images. Cornea 2005, 24, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Arita, R.; Morishige, N.; Shirakawa, R.; Sato, Y.; Amano, S. Effects of Eyelid Warming Devices on Tear Film Parameters in Normal Subjects and Patients with Meibomian Gland Dysfunction. Ocul. Surf. 2015, 13, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Gulmez Sevim, D.; Gumus, K.; Unlu, M. Reliable, Noncontact Imaging Tool for the Evaluation of Meibomian Gland Function: Sirius Meibography. Eye Contact Lens 2020, 46 (Suppl. S2), S135–S140. [Google Scholar] [CrossRef]
- Chan, T.C.Y.; Wan, K.H.; Shih, K.C.; Jhanji, V. Advances in dry eye imaging: The present and beyond. Br. J. Ophthalmol. 2018, 102, 295–301. [Google Scholar] [CrossRef]
- Arita, R.; Morishige, N.; Fujii, T.; Fukuoka, S.; Chung, J.L.; Seo, K.Y.; Itoh, K. Tear Interferometric Patterns Reflect Clinical Tear Dynamics in Dry Eye Patients. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3928–3934. [Google Scholar] [CrossRef] [Green Version]
- Finis, D.; Pischel, N.; Schrader, S.; Geerling, G. Evaluation of lipid layer thickness measurement of the tear film as a diagnostic tool for Meibomian gland dysfunction. Cornea 2013, 32, 1549–1553. [Google Scholar] [CrossRef]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical Coherence Tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [Green Version]
- Ramos, J.L.B.; Li, Y.; Huang, D. Clinical and research applications of anterior segment optical coherence tomography—A review. Clin. Experiment. Ophthalmol. 2009, 37, 81–89. [Google Scholar] [CrossRef] [Green Version]
- Ibrahim, O.M.A.; Dogru, M.; Takano, Y.; Satake, Y.; Wakamatsu, T.H.; Fukagawa, K.; Tsubota, K.; Fujishima, H. Application of visante optical coherence tomography tear meniscus height measurement in the diagnosis of dry eye disease. Ophthalmology 2010, 117, 1923–1929. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.H.; Zhao, Y.; Tun, T.A.; Tong, L. Repeatability of tear meniscus evaluation using spectral-domain Cirrus® HD-OCT and time-domain Visante® OCT. Cont. Lens Anterior Eye 2015, 38, 368–372. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Gong, L.; Lu, Y.; Jin, H.; Robitaille, M. The diagnostic significance of Fourier-domain optical coherence tomography in Sjögren syndrome, aqueous tear deficiency and lipid tear deficiency patients. Acta Ophthalmol. 2012, 90, e359–e366. [Google Scholar] [CrossRef] [PubMed]
- Szegedi, S.; Scheschy, U.; Schmidl, D.; Aranha Dos Santos, V.; Stegmann, H.; Adzhemian, N.; Fondi, K.; Bata, A.M.; Werkmeister, R.M.; Couderc, C.; et al. Effect of Single Instillation of Two Hyaluronic Acid-Based Topical Lubricants on Tear Film Thickness in Patients with Dry Eye Syndrome. J. Ocul. Pharmacol. Ther. 2018, 34, 605–611. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, R.; Usui, T.; Miyai, T.; Yamagami, S.; Amano, S. Tear meniscus evaluation by anterior segment swept-source optical coherence tomography. Am. J. Ophthalmol. 2013, 155, 620–624. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, R.; Usui, T.; Yamagami, S. Diagnosis of Dry Eye by Tear Meniscus Measurements Using Anterior Segment Swept Source Optical Coherence Tomography. Cornea 2015, 34 (Suppl. S1), S115–S120. [Google Scholar] [CrossRef]
- Villani, E.; Arita, R. Imaging of meibomian glands: From bench to bedside and back. Eye 2019, 33, 695–697. [Google Scholar] [CrossRef]
- Baudouin, C.; Messmer, E.M.; Aragona, P.; Geerling, G.; Akova, Y.A.; Benítez-Del-Castillo, J.; Boboridis, K.G.; Merayo-Lloves, J.; Rolando, M.; Labetoulle, M. Revisiting the vicious circle of dry eye disease: A focus on the pathophysiology of meibomian gland dysfunction. Br. J. Ophthalmol. 2016, 100, 300–306. [Google Scholar] [CrossRef]
- Hwang, H.S.; Shin, J.G.; Lee, B.H.; Eom, T.J.; Joo, C.K. In Vivo 3D Meibography of the Human Eyelid Using Real Time Imaging Fourier-Domain OCT. PLoS ONE 2013, 8, e67143. [Google Scholar] [CrossRef] [Green Version]
- Di Zazzo, A.; Coassin, M.; Varacalli, G.; Galvagno, E.; De Vincentis, A.; Bonini, S. Neurotrophic keratopathy: Pros and cons of current treatments. Ocul. Surf. 2019, 17, 619–623. [Google Scholar] [CrossRef]
- Sabatino, F.; Di Zazzo, A.; De Simone, L.; Bonini, S. The Intriguing Role of Neuropeptides at the Ocular Surface. Ocul. Surf. 2017, 15, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Di Zazzo, A.; Gaudenzi, D.; Yin, J.; Coassin, M.; Fernandes, M.; Dana, R.; Bonini, S. Corneal angiogenic privilege and its failure. Exp. Eye Res. 2021, 204, 108457. [Google Scholar] [CrossRef] [PubMed]
- Di Zazzo, A.; Yang, W.; Coassin, M.; Micera, A.; Antonini, M.; Piccinni, F.; De Piano, M.; Kohler, I.; Harms, A.C.; Hankemeier, T.; et al. Signaling lipids as diagnostic biomarkers for ocular surface cicatrizing conjunctivitis. J. Mol. Med. 2020, 98, 751–760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Zazzo, A.; Micera, A.; De Piano, M.; Cortes, M.; Bonini, S. Tears and ocular surface disorders: Usefulness of biomarkers. J. Cell. Physiol. 2019, 234, 9982–9993. [Google Scholar] [CrossRef] [PubMed]
- Barabino, S.; Aragona, P.; di Zazzo, A.; Rolando, M.; Berchicci, L.; Bonini, S.; Calabria, G.; Cantera, E.; Gambaro, S.; Leonardi, A.; et al. Updated definition and classification of dry eye disease: Renewed proposals using the nominal group and Delphi techniques. Eur. J. Ophthalmol. 2021, 31, 42–48. [Google Scholar] [CrossRef]
- Alexander, H.; Brown, S.; Danby, S.; Flohr, C. Research Techniques Made Simple: Transepidermal Water Loss Measurement as a Research Tool. J. Investig. Dermatol. 2018, 138, 2295–2300.e1. [Google Scholar] [CrossRef] [Green Version]
- Farahmand, S.; Tien, L.; Hui, X.; Maibach, H.I. Measuring transepidermal water loss: A comparative in vivo study of condenser-chamber, unventilated-chamber and open-chamber systems. Skin Res. Technol. 2009, 15, 392–398. [Google Scholar] [CrossRef]
- Imhof, R.E.; De Jesus, M.E.P.; Xiao, P.; Ciortea, L.I.; Berg, E.P. Closed-chamber transepidermal water loss measurement: Microclimate, calibration and performance. Int. J. Cosmet. Sci. 2009, 31, 97–118. [Google Scholar] [CrossRef]
- Calonge, M.; Labetoulle, M.; Messmer, E.M.; Shah, S.; Akova, Y.A.; Boboridis, K.G.; Merayo-Lloves, J.; Aragona, P.; Benítez-Del-Castillo, J.; Geerling, G.; et al. Controlled Adverse Environment Chambers in Dry Eye Research. Curr. Eye Res. 2018, 43, 445–450. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaudenzi, D.; Mori, T.; Crugliano, S.; Grasso, A.; Frontini, C.; Carducci, A.; Yadav, S.; Sgrulletta, R.; Schena, E.; Coassin, M.; et al. AS-OCT and Ocular Hygrometer as Innovative Tools in Dry Eye Disease Diagnosis. Appl. Sci. 2022, 12, 1647. https://doi.org/10.3390/app12031647
Gaudenzi D, Mori T, Crugliano S, Grasso A, Frontini C, Carducci A, Yadav S, Sgrulletta R, Schena E, Coassin M, et al. AS-OCT and Ocular Hygrometer as Innovative Tools in Dry Eye Disease Diagnosis. Applied Sciences. 2022; 12(3):1647. https://doi.org/10.3390/app12031647
Chicago/Turabian StyleGaudenzi, Daniele, Tommaso Mori, Salvatore Crugliano, Antonella Grasso, Carlo Frontini, Antonella Carducci, Siddharth Yadav, Roberto Sgrulletta, Emiliano Schena, Marco Coassin, and et al. 2022. "AS-OCT and Ocular Hygrometer as Innovative Tools in Dry Eye Disease Diagnosis" Applied Sciences 12, no. 3: 1647. https://doi.org/10.3390/app12031647





