Abstract
Green walnuts—unripe fruits of Juglans regia L. are known for their many biological activities and therapeutic potential. Tinctures based on unripe walnuts (samples 1 and 2) and tinctures with the addition of spices (ginger, cloves, bay leaves, juniper fruit: samples 3–6) were tested for polyphenol profile and antioxidant capacity. The effect on a normal monkey kidney epithelial cell line (VERO) was evaluated. For monitoring the changes in cell proliferation, real-time cell analysis (xCELLigence system) was employed and cell viability was measured by the MTS test. All tinctures showed the presence of polyphenols, mainly phenolic acids, flavonoids, and naphthoquinones, and in the sample with cloves, eugenol was found. Addition of spices increased the antioxidant capacity. Tinctures show a dose-dependent cytotoxic effect. The lowest concentrations (125 µg mL−1) of both tinctures without additives (1 and 2) and with bay leaves (4) did not adversely affect (p > 0.05) and even supported cell proliferation (p < 0.05) in comparison to the control cells without treatment. Viability was lower in all cells except for the cells treated with tincture with cloves addition (p > 0.05). It indicates the beneficial effect of cloves enrichment and supports the assumption that the appropriate dosage of tinctures is necessary to be safe for the consumers.
1. Introduction
Herbal preparations have a long history in many cultures and are widely used in traditional medicine. Due to the richness of bioactive substances, such products are of interest to science. One of the forms of herbal preparations are tinctures which are extracts prepared by maceration or percolation using only ethanol of a suitable concentration for extraction of the herbal drug [1,2].
Green nuts are used in folk medicine and pharmacology as well as in the food industry—for the production of jams and liqueurs [3,4]. Beneficial health effects of unripe Juglans regia L. nuts were described by many authors [5,6,7]. Thanks to this, green nuts have great potential in the production of functional food, especially alcoholic beverages that form an important part of the overall nutrition scheme. Such drinks are traditionally prepared from traditional raw materials such as fruits and vegetables however, the introduction of new, uncommon plant materials such as walnuts at the milk-wax maturity stage is applicable and relevant [4]. Tinctures of unripe walnuts—fruits of Juglans regia L. have been known and used all over the world.
Green nuts and green walnut husks exhibit several biological effects due to their extremely rich phytochemical composition. They are abundant in compounds from the classes of polyphenols, naphthoquinones, steroids, as well as vitamins and minerals [5,8,9,10]. Green nut extracts possess antioxidant, antimicrobial and antiparasitic properties [8,11]. Antiproliferative activity has also been found against HL-60 leukemia cells [6,12].
Green nut liqueurs and tinctures are traditional products, popular especially in Italy, Romania and Slovenia [5,13,14,15,16]. The most famous example of such an alcoholic drink is Nocino, a liqueur made in Italy from green nuts harvested during the time of the feast of St. John the Baptist (24 June). Research has shown that this product is a valuable source of polyphenolic compounds with high antioxidant power, and the influence of various production variables on its quality has been also investigated [13,14]. In Poland, green walnut tinctures are traditionally produced as a remedy against ailments of the digestive system. However, there is no scientific evidence of their therapeutic effect nor healing effects; their use results from the traditions and experiences of ancestors. Various spices known to aid in digestion such as ginger, cloves, cinnamon, and pepper, are usually added to tinctures. In the case of the Nocino liqueur, depending on the recipe, flavor additives such as cloves, cinnamon, coriander, coffee beans, and lemon zest are used [14]. There are also attempts to use innovative additives such as chocolate, fruit, or honey [3].
The addition of spices is intended not only to enhance the flavour aspect but also to introduce additional phytochemical components that affect the biological activity of the final product. For example, cloves are known for their antibacterial and antifungal properties due to the presence of eugenol [17] whereas ginger is rich in bioactive compounds from the phenolics and terpene group which exhibit anti-carcinogenic, anti-diabetic, and anti-tumor activity [18]. Laurus nobilis leaves (bay leaves) contain essential oils, terpenes, flavonoids, alkaloids, tocopherols, and have a broad healing effect [19]. On the other hand, juniper fruits are rich in volatile substances, terpene acids, leucoanthocyanidin, alkaloids, flavonoids, and tannins which are responsible for its diuretic, anti-arthritis, anti-diabetes, and anti-septic properties as well as are responsible for its positive effect for the treatment of gastrointestinal disorders [20].
The purpose of this study was to observe the effect of the addition of spices (ginger, cloves, bay leaves and juniper fruit) on antioxidant properties and the polyphenol profile of unripe green walnut tinctures prepared according to traditional Polish recipes. Moreover, also the effect of tinctures on cells in vitro was assessed, which is the main scientific novelty of the described research. While antitumor activity was described by many authors [6,12,21,22,23] our research is focused on evaluating the effect on normal epithelial kidney cells which can prove the safety of consumption of green walnuts – based beverages.
2. Materials and Methods
2.1. Raw Materials and Preparation of Tinctures
Unripe walnuts of the Czech variety ‘Mars’ were harvested on 5 July 2021 at the walnut plantation in Urzejowice (Podkarpackie, Poland, 50°0′49″ N, 22°27′23″ E). Spices: fresh ginger (Zingiber officinalis), cloves (Syzygium aromaticum), bay leaves (Laurus nobilis) and juniper fruits (Juniperus communis) were purchased at a local market. The fruits were cut into quarters and poured with 40% ethyl alcohol into glass jars. Six versions of the tinctures were prepared (Table 1) and aged for 2 months at room temperature with exposure to sunlight, except for sample No. 2. After this time, they were filtered using filter paper. The tinctures were prepared based on known traditional recipes, as well as on the basis of our previous attempts to modify organoleptic features of the product. The graphical scheme of study approach is presented in Figure 1.

Table 1.
The composition of the prepared tinctures.
Figure 1.
The scheme of the experiment.
An aliquot of raw tinctures was collected for testing the antioxidant activity and chromatographically analyzed. For cytotoxicity studies, the tinctures were lyophilized for 48 h to a dry extract using the Alpha 1–2 LD plus freeze dryer (Martin Christ, Osterode am Harz, Germany) after alcohol evaporation in the centrifugal evaporator (RVC 2–18 CDPlus, Martin Christ, Osterode am Harz, Germany).
2.2. Total Phenolic and Flavonoid Content and Antioxidant Capacity Measurements
The total content of phenolic compounds was determined according to the Singleton and Rossi (1965) [24] original method modified to enable the measurement using a microplate reader. Briefly, 20 µL of diluted tinctures were pipetted into the microplate wells then, 100 µL of 10× diluted Folin-Ciocalteu reagent was added to the wells and finally, 80 µL of 7.5% Na2CO3 solution was added. The plate was put aside in a dark place for 1 h. After this time, the absorbance was measured using a microplate reader (EPOCH2, Biotek, Winooski, VT, USA) (λ = 760 nm) against the blank (sample replaced with an equal volume of distilled water). Results were expressed as gallic acid equivalents (GAE) from the prepared standard curve in the range of 0–150 µg/mL (y = 0.3364x, r2 = 0.9914).
The total content of flavonoids was determined according to the Biju (2013) [25] method with the modification enabling the measurement using a microplate reader. One hundred microliters of diluted tinctures were poured into the wells, then 100 µL of 2% methanolic AlCl3 solution was added. The plate was placed aside in a dark place for 10 min. After this time, the absorbance was measured using a microplate reader (λ = 415 nm) against the blank (sample replaced with an equal volume of distilled water). Results were expressed in mg as quercetin equivalents (QE) using the prepared standard curve in the range of 0–120 µg/mL (y = 0.0655x, r2 = 0.9999).
The antioxidant activity was measured using FRAP, DPPH, and ABTS methods. Samples measured with the FRAP method were assayed according to Benzie and Strain (1996) [26] with the modification enabling measurement using a microplate reader. Twenty microliters of diluted samples were poured into the wells, 180 µL of FRAP reagent (5 mL of 10 mM 2,4,6-Tris(2-pyridyl)-s-triazine (TPTZ) in 40 mM of hydrochloric acid, 5 mL of 20 mM ferric chloride and 50 mL of acetate buffer (0.3 M; pH = 3.6) was added. Then the plate was incubated for 10 min at 37 °C. The absorbance was measured (λ = 593 nm) against the blank (sample replaced with an equal volume of distilled water). Results are expressed in µg of Trolox equivalents (TE) based on the prepared standard curve in the range of 0–300 µmol/mL (y = 0.1522x, r2 = 0.9989).
The DPPH method was assayed according to Blois (1958) [27] with the modification enabling the measurement using a microplate reader. Twenty microliters of diluted tinctures were poured into the wells then, 180 µL of DPPH reagent diluted with methanol to the absorbance level of ca. 0.9 was added. The plate was placed in a dark place for 30 min. Absorbance was measured at 517 nm against the blank sample (pure methanol) using a Biotek EPOCH2 microplate reader. The percentage of DPPH radical scavenging (DPPH%) was calculated according to the Formula (1):
where AC is the absorbance of the control (sample replaced with an equal volume of methanol) and AS is the absorbance of the sample tested. The obtained results were converted into Trolox equivalents (TE) based on the prepared calibration curve in the range of 0–300 µmol/mL (y = 15.5553x, r2 = 0.9970).
DPPH% = [(AC − AS)/AC] × 100
The ABTS method was carried out according to Re et al. (1999) [28], with the modification enabling the measurement using a microplate reader. Twenty microliters of diluted tinctures were poured into the wells then, 180 µL of ABTS reagent diluted with phosphate buffer (pH 7.4) to the absorbance level of 0.8 was added. The plate was placed in a dark place for 6 min. The absorbance was measured at λ = 734 nm against the blank sample (phosphate buffer) using a Biotek EPOCH2 microplate reader. The percentage of ABTS scavenging (ABTS%) was calculated according to the Formula (2):
where AC is the absorbance of the control (sample replaced with an equal volume of buffer) and AS is the absorbance of the sample tested. The obtained results were converted into Trolox equivalents (TE) based on the prepared calibration curve in the range of 0–300 µmol/mL (y = 9.0447x, r2 = 0.9948).
ABTS% = [(AC − AS)/AC] × 100
2.3. HPLC-DAD Polyphenolic Profile
Chromatographic analysis was performed using the Gilson chromatographic system (Gilson’s Analytical-to-Semipreparative HPLC System, Gilson Inc., Middleton, WI, USA) including a binary gradient pump (Gilson 322), a column thermostat (Knauer, Berlin, Germany), an autosampler with a fraction collector (Liquid Handler GX-271) and a photodiode array detector (DAD, Gilson 172, Gilson, Middleton, WI, USA). The analytical column (Poroshell 120, EC C-18, 4.6 × 150 mm, Agilent Technologies Inc., Santa Clara, CA, USA) was used for chromatographic separation. The column was maintained at 40 °C. The separation was done with the gradient mode of mobile phase: 0.1% (v/v) formic acid in water (phase A) and acetonitrile (phase B). A mobile phase flow of 1 mL/min was used. A following gradient program was applied: 10% B (1.5 min), 10–100% B (1.5–20 min), 100% B (20–25 min), and again 10% B to equilibrate the column. The injection volume of 10-fold diluted tinctures was 10 μL. The chromatograms were recorded at λ = 254, 280, 320, and 360 nm. The phenolic compounds were detected in tinctures by their UV–VIS spectra and by the comparison of their retention time values with the values of analytical standards. Literature data was also used as an identification aid.
2.4. Cell Culture and Media
For this in vitro experiment monkey epithelial kidney cells VERO (ATCC-CRL 1586) were employed. The cells were grown in a growth medium (MEM—Minimum Essential Medium Eagle) enriched with 10% fetal bovine serum, 1% glutamine, and 0.1% gentamicin (Sigma Aldrich, Taufkirchen, Germany). Cell cultivation took place in an incubator with a humidified atmosphere of 37 °C and 5% CO2. The cells were subcultured every 3–4 days. The absence of mycoplasma contamination was checked for regularly.
2.5. Real-Time Cell Analysis
Monitoring of the cell response after exposure to tested substances was conducted using the xCELLigence system (Real-Time Cell Analyser—RTCA, Acea Biosciences Inc., San Diego, CA, USA). The system allows label-free monitoring of cell behaviour (adhesion, proliferation, growth, and morphology) during the whole time of treatment. The basic principle is the measurement of the impedance on gold electrodes at the bottom of microplate wells. The larger the number of cells attached, the higher the impedance. The values were expressed in dimensionless cell index (CI) and recorded in curves each hour throughout the experiment [29,30,31]. For this study, the cells were seeded at 5 × 103 cells per well of a three 16-well E-plate (Acea Bioscience, San Diego, CA, USA) and incubated for 20 h. Tested samples were diluted with medium to reach final concentrations of 125–1000 µg mL−1 shortly before addition to the cells. Cells without treatment served as a control and their proliferation was considered as 100%. Percentual change in proliferative activity (PA) was calculated using the Formula (3):
2.6. MTS Test
For the measurement of cell viability or metabolic activity after exposure to tested substances, the MTS test (CellTiter 96® Aqueous One Solution Cell Proliferation Assay, Promega, Madison, WI, USA) was performed. This assay has been conducted at the same time as the RTCA, using the cells from the same passage. The cells were seeded into a 96-well plate (Greiner-Bio-One, Kremsmünster, Austria) with a density of 8.6 × 103 cells/well in a final volume of a 100 μL medium. After 20 h, tested samples were added to the wells to reach the final concentration of 125–1000 µg mL−1 and placed into an incubator for 48 h. Finally, 20 μL of MTS solution was pipetted into each well and after 4 h of incubation, the absorbance (A) was measured at 490 nm (microplate reader Synergy HT; Biotek, Winooski, VT, USA). The cells without treatment were considered as the control with 100% metabolic activity (MA). Change in MA was expressed in % using the Formula (4):
2.7. Statistical Evaluation
Results are presented as the means ± standard deviation (SD; n = 3). Obtained data was statistically evaluated by Graph Pad Prism 8.3.1 (GraphPad Software, San Diego, CA, USA). One-way analysis of variance (ANOVA) followed by Tukey’s multiple comparison test was employed to evaluate the total phenolic content and antioxidant activity of tested samples and Dunnett’s comparison test was used for evaluation of the results obtained in in vitro tests. The significance level was set to p < 0.05. All the experiments were conducted in triplicates.
3. Results and Discussion
3.1. Polyphenol Content and Antioxidant Capacity
The total content of polyphenols and flavonoids was determined in all tinctures. The antioxidant capacity was also measured using the FRAP, DPPH, and ABTS methods. The results are summarized in Table 2.

Table 2.
Polyphenolic and flavonoids contents as well as antioxidant properties of green walnut tinctures.
In the basic control tincture (1) the total phenolic content was at the level of 3.56 mg GAE per mL. Maceration in the darkness resulted in a significant increase of phenolic content, as well as of the antioxidant capacity. The highest polyphenol content was determined in tinctures 2 and 5, prepared in darkness and enriched with bay leaves. Keeping the tincture in the dark may have a beneficial effect on the content of bioactive ingredients that can be degraded when exposed to light. The additives used enhanced the antioxidant activity of tinctures.
The results obtained are comparable with the data available in the literature on traditional green walnut liqueurs (Nocino), which contained from 1.18–3.52 mg of catechin equivalents per mL [13,14]. In commercial tinctures, the variability was greater, from 0.23–3.88 mg of catechin equivalents per mL [13]. According to this data, tannins accounted for most of the polyphenols. Antiradical activity determined by the DPPH method according to the literature was from 0.019–2.38 μmol TE/mL for commercial samples and up to 8.94 μmol TE mL−1 for liqueurs prepared with the infusion method [13,14]. The ABTS method was used to investigate the antioxidant potential of tinctures made from harvests at various stages of maturity (at the beginning and end of July), as well as from green husks and leaves of J. regia. The tinctures of walnuts harvested on 25 July and green husks were the most active [32]. The correlation between the content of total phenolic compounds and flavonoids and the antioxidant capacity measured with three methods was also investigated (Table 3). All calculated Pearson’s correlation coefficients were significant at the level of p = 0.05, indicating that the share of polyphenols, including flavonoids, was key in shaping the antioxidant activity of the tinctures.

Table 3.
Correlation matrix for total phenolic and flavonoid content, and antioxidant capacity.
3.2. Polyphenolic Profile by HPLC-DAD
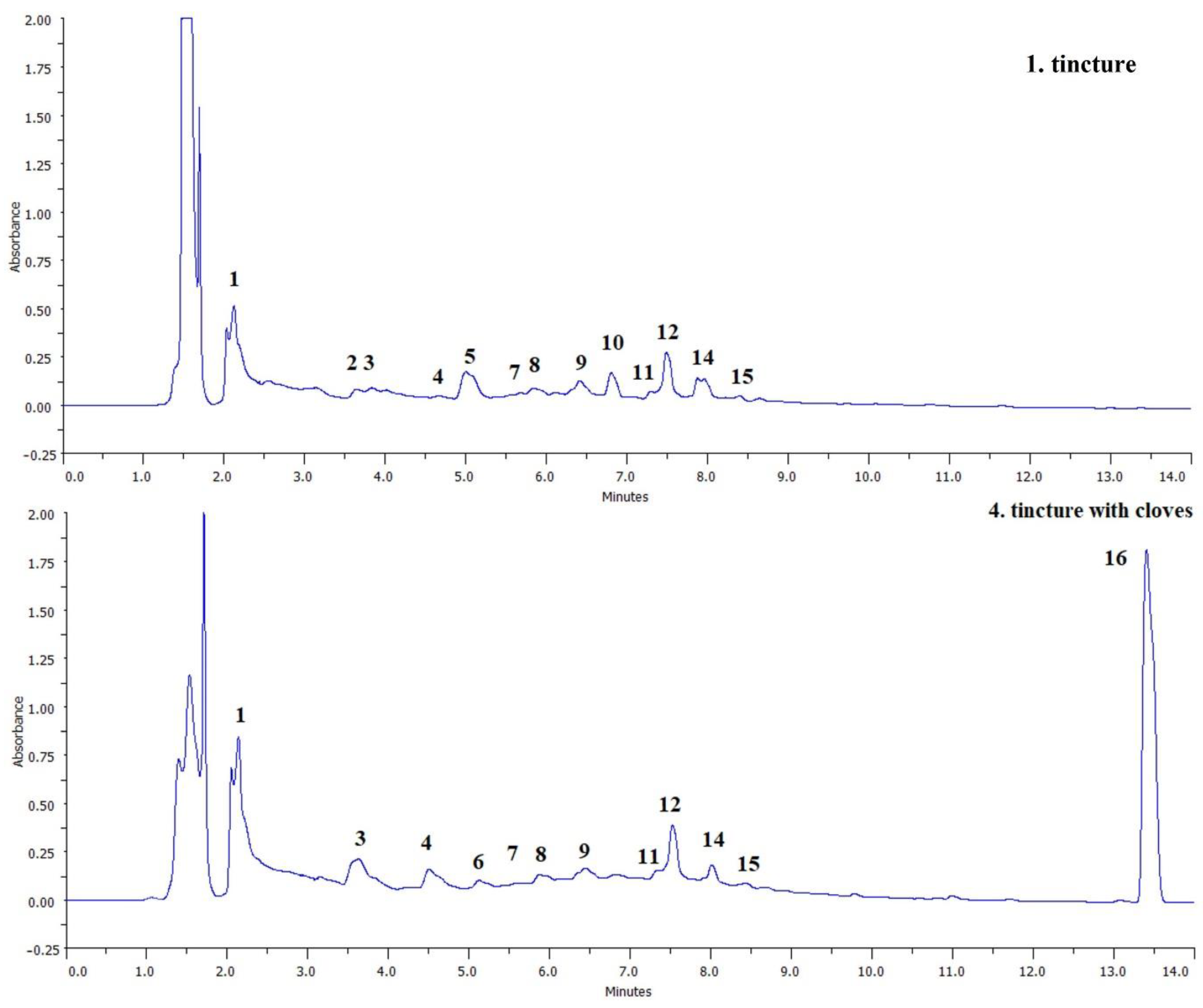
The prepared tinctures were subjected to the qualitative evaluation of the polyphenol profile by the HPLC-DAD method. The compounds identified in individual samples are presented in Table 4 and sample chromatograms recorded at 280 nm (for samples 1 and 4) are shown in Figure 2. All samples are dominated by metabolites typical for green walnuts, belonging to the groups of phenolic acids (gallic acid, syringic acid, and their derivatives), tannins (catechin isomers, and condensed catechins), and flavonoids (rutin and quercitrin). The presence of naphthoquinones was also found, but the juglone, a typical metabolite of J. regia, was not detected. Such compounds were previously reported in tinctures prepared from unripe walnuts. Regardless of the variety and solvent system used, gallic and syringic acid [33], and (+)-catechin, as well as (−)-epicatechin [5] were found to be the dominant compounds. Using the combined LC/MS technique, depsides were also detected—derivatives of caffeic, coumaric, and quinic acids [32]. According to the literature, juglone occurred in tinctures and liqueurs however, its content may be influenced by various factors. It has been shown that juglone content is significantly lower in tinctures than in green husk extracts, and what’s even more important, its content in husks changes seasonally [15,33]. Juglone is also a relatively unstable compound, it can decompose e.g., under the influence of light [34]. The exact identification of the naphthoquinones present in the tinctures was not possible without the available standards.

Table 4.
Polyphenolic profiles of tested green wanut tinctures.
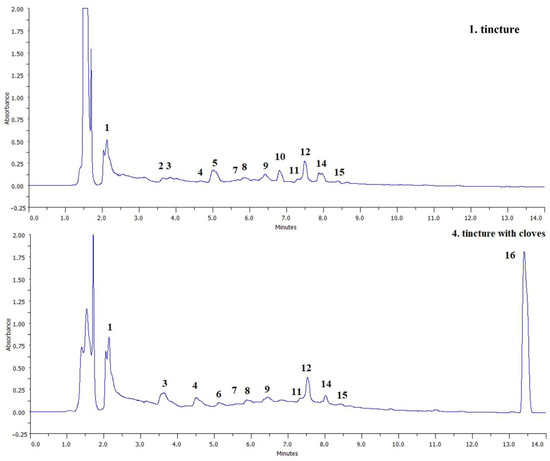
Figure 2.
Example chromatograms of tinctures 1 and 4. Peak numbers correspond to the numbering in Table 4.
The most significant effect of the addition of spices to tinctures is the introduction of eugenol in the case of the addition of cloves (tincture 4). In the case of this sample, it is the dominant peak in the chromatogram (Figure 2), which proves its high content as the result of enrichment. Eugenol is the main phytochemical component of cloves, giving them specific sensory properties, and has a strong antioxidant, antibacterial and antifungal effect [17]. No significant influence of other spices on the qualitative composition of polyphenols was observed, perhaps they were used in too low a proportion or the detection method used was insufficiently sensitive.
3.3. The Effect on VERO Cells
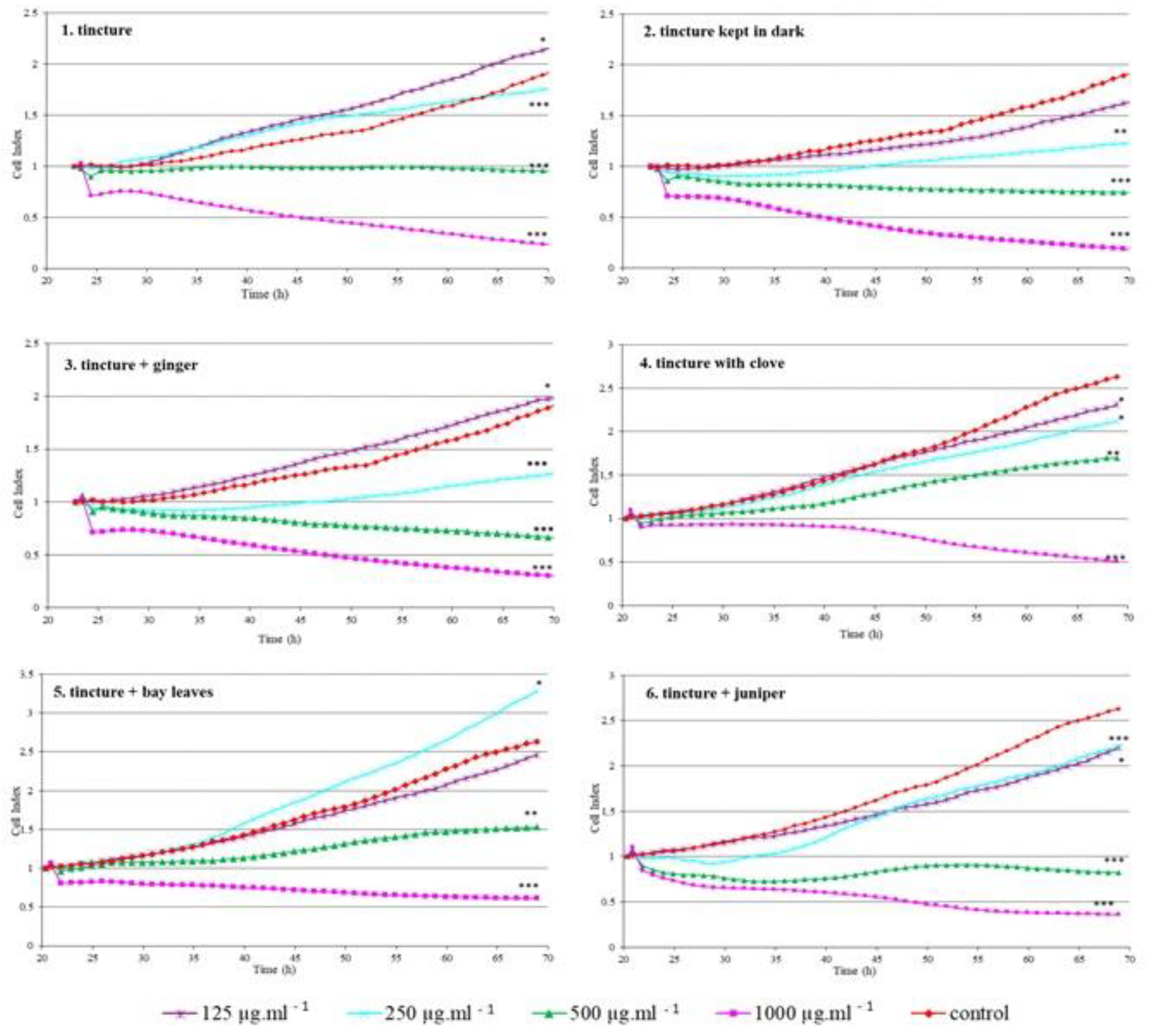
Due to the possibility of the use in the preparation of food and beverages, we focused on evaluating the effect of prepared green walnut tinctures and their combinations with different spices on normal monkey epithelial kidney cells (VERO) as the kidney is the target organ of the elimination of substances from the body. VERO cells are used in testing the toxicity of chemical compounds as well as, to assess the biological safety of new substances used in therapy and in the food industry [35,36]. The effect of tinctures on VERO cells was evaluated in real-time using the xCELLigence system that allows for continuous monitoring of cell status during the entire time of cell exposure to tested substances. The specific RTCA patterns observed in VERO cells exposed to tested tinctures and tinctures with spice additives are presented in Figure 3.
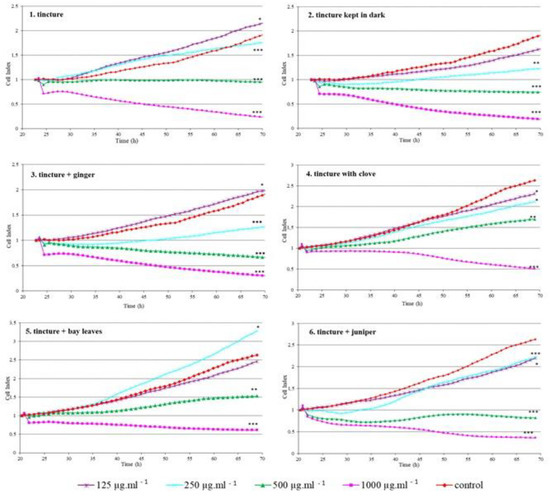
Figure 3.
Real-time monitoring of cell response after exposure to tested substances (CI). Significant differences compared to control cells: *—p < 0.05, **—p < 0.001, ***—p < 0.0001.
The proliferation of cells treated with samples 3, 4 and 5 was significantly inhibited with a growing concentration of samples (p < 0.05). The highest concentrations in almost all tested samples caused a decline in CI which indicates that no cells adhered to the well bottom (CI = 0) or that inhibition of cell proliferation occured (CI does not change in time); cells are still alive but do not multiply [37]. There were also cases where the cells treated with the samples showed a higher proliferative activity than the control cells: sample 3 at the lowest concentration, sample 5 at the concentration of 250 µg mL−1. For sample 4, a significant reduction in the proliferation rate was observed even at the lowest tested concentration. Phenolic compounds present in walnuts show the ability to decrease the incidence of tumours which has been reported by many authors [21,22,23,38]. Interestingly, lower concentrations (125 µg mL−1) present in samples 2, and 5 did not affect cell proliferation which may indicate no cytotoxic effect and the potential for safe use of tinctures in beverages.
Using the RTCA, various plant extracts were tested for their effect on cell viability. Various relationships were observed, e.g., for giant goldenrod (Solidago gigantea) extracts, lower concentrations increased the adherence of intestinal porcine epithelial cells IPEC-1, while higher concentrations acted negatively [30]. Viscum album extracts were tested on various tumour cell lines using this method. It was shown that for some cells these extracts are highly cytotoxic while for others, are not [22]. It also happened that the given extract significantly supports cell adherence, as in the case of the acetone dandelion (Taraxacum officinale) flower extract effect applied to RK13 cells [29].
For the evaluation of changes in cell viability by the measurement of metabolic activity, the MTS test was employed. This end-point analysis was conducted after 48 h of cell treatment. Almost all tested samples cause a decrease in metabolic activity, except for sample 4 (125 and 250 µg mL−1) in which the change was not statistically important (p > 0.05). The decrease in metabolic activity exhibits a dose-dependent trend. Changes in metabolic and proliferative activity 48 h after cell treatment are summarized in Table 5.

Table 5.
Values of proliferative and metabolic activity (%) of VERO cells after 48 h of exposure to tinctures (125–1000 µg mL−1) in comparison to control cells without treatment (100%).
The effect of the addition of spices on the metabolic activity was diversified and a clear effect was observed in the case of cloves addition (sample 4). Such a spice reduced the cytotoxicity as compared to the control tinctures (1 and 2) and at lower concentrations, metabolic activity of cells was not significantly different from the value of untreated cells. The weakened cytotoxicity was also observed in samples 5 and 6, however, the changes were less pronounced. The data for metabolic and proliferative activity was correlated at r = 0.639, which indicates a relationship between these parameters in cells exposed to the test samples.
J. regia extracts have been previously shown to be cytotoxic to various types of cells, including cancer cells [39]. Triterpene compounds have been identified as components of green walnut husks responsible for cytotoxic effects. Their structure has an important effect on antitumor activity [23]. Polyphenols exert cytotoxic or antitumor activity through range of mechanisms, e.g., induction of cell apoptosis, changes in the cell cycle or via the modification of signaling pathways [40]. On the other hand, polyphenols belong to phytochemicals that are produced by plants as a part of their defense against environmental stress. They are able to protect cells against oxidative stress induced by toxic substances [41].
The cytotoxic effects of phytochemicals can be a source of side effects when using drugs of natural origin and functional food products. Hence, it is important to test such products for performance within acceptable concentrations and selectivity of action [42,43]. The cytotoxic effect may be beneficial when applied to cancer cells, but a balance between therapeutic effect and safety of healthy cells must be kept [44,45].
4. Conclusions
The addition of spices to tinctures from green walnuts is used to shape the taste and aroma. The conducted research showed that the use of spices enhances the total phenolic content and antioxidant properties of tinctures with the greatest enrichment recorded for the tincture with the addition of cloves, which introduce a high content of eugenol. Studies on VERO cells have shown that excessive consumption of green walnut tinctures can be harmful to the kidneys while the products enriched with spices showed the reduction of cytotoxicity especially in the case of cloves. The results have shown that the addition of spices to the green walnut tincture not only create a pleasant aroma of the product but also enhance its antioxidant properties which in turn increases the health value of the tincture. The information obtained allows us to refine existing recipes for tinctures of green nuts enriched with spices in terms of optimal proportions and processing. The obvious health benefits of this type of product have been proven, along with the limitations of a potential nephrotoxic effect in the case of excessive use. As the dose is important for the safe use of such products, further research is required.
Author Contributions
Conceptualization, M.M.; methodology, M.M. and D.M.; software, M.M. and D.M.; validation, D.M.; formal analysis, M.M. and D.M.; investigation, M.M., D.M., M.K., and D.L.; resources, M.M.; data curation, M.M., M.K., and D.L.; writing—original draft preparation, M.M.; writing—review and editing, M.D. and D.M.; visualization, D.M.; supervision, M.D.; project administration, M.M.; funding acquisition, D.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the grant for young scientists from University of Rzeszow (NIG/29/2021) and National Laboratory for Pesticides at the University of Veterinary Medicine and Pharmacy in Košice.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article.
Acknowledgments
Thanks to Anna Jankowska from the walnut plantation in Urzejowice for providing the research material. The authors express their gratitude to Serena Faith Amrit a native speaker from California (USA) for the linguistic revision of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hudz, N.; Makowicz, E.; Shanaida, M.; Białon, M.; Jasicka-Misiak, I.; Yezerska, O.; Svydenko, L.; Wieczorek, P.P. Phytochemical evaluation of tinctures and essential oil obtained from Satureja montana herb. Molecules 2020, 25, 4763. [Google Scholar] [CrossRef]
- Council of Europe. European Pharmacopoeia, 7th ed.; Council of Europe: Strasbourg, France, 2001. [Google Scholar]
- Petrović, M.; Pastor, F.; Đurović, S.; Veljović, S.; Gorjanović, S.; Sredojević, M.; Vukosavljević, P. Evaluation of novel green walnut liqueur as a source of antioxidants: Multi-method approach. J. Food Sci. Technol. 2021, 58, 2160–2169. [Google Scholar] [CrossRef] [PubMed]
- Tiurikova, I.; Peresichnyi, M. Prospects of Using Walnut in Technologies of Drinks. Acta Univ. Cibiniensis Ser. E Food Technol. 2015, 19, 39–50. [Google Scholar] [CrossRef] [Green Version]
- Cosmulescu, S.; Trandafir, I.; Nour, V.; Ionica, M.; Tutulescu, F. Phenolics content, antioxidant activity and color of green walnut extracts for preparing walnut liquor. Not. Bot. Horti Agrobot. Cluj-Napoca 2014, 42, 551–555. [Google Scholar] [CrossRef] [Green Version]
- Soto-Maldonado, C.; Vergara-Castro, M.; Jara-Quezada, J.; Caballero-Valdés, E.; Müller-Pavez, A.; Zúñiga-Hansen, M.E.; Altamirano, C. Polyphenolic extracts of walnut (Juglans regia) green husk containing juglone inhibit the growth of HL-60 cells and induce apoptosis. Electron. J. Biotechnol. 2019, 39, 1–7. [Google Scholar] [CrossRef]
- Pycia, K.; Kapusta, I.; Jaworska, G. Impact of the Degree of Maturity of Walnuts (Juglans regia L.) and Their Variety on the Antioxidant Potential and the Content of Tocopherols and Polyphenols. Molecules 2019, 24, 2936. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Croitoru, A.; Ficai, D.; Craciun, L.; Ficai, A.; Andronescu, E. Evaluation and Exploitation of Bioactive Compounds of Walnut, Juglans regia. Curr. Pharm. Des. 2019, 25, 119–131. [Google Scholar] [CrossRef] [PubMed]
- Vieira, V.; Pereira, C.; Abreu, R.M.V.; Calhelha, R.C.; Alves, M.J.; Coutinho, J.A.P.; Ferreira, O.; Barros, L.; Ferreira, I.C.F.R. Hydroethanolic extract of Juglans regia L. green husks: A source of bioactive phytochemicals. Food Chem. Toxicol. 2020, 137, 111189. [Google Scholar] [CrossRef] [PubMed]
- Nour, V.; Trandafir, I.; Cosmulescu, S. Influence of preparing method on antioxidant activity and polyphenols content of green walnuts comfiture. South-West J. Hortic. Biol. Environ. 2014, 5, 83–94. [Google Scholar]
- Sharma, P.; Ravikumar, G.; Kalaiselvi, M.; Gomathi, D.; Uma, C. In vitro antibacterial and free radical scavenging activity of green hull of Juglans regia. J. Pharm. Anal. 2013, 3, 298–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carvalho, M.; Ferreira, P.J.; Mendes, V.S.; Silva, R.; Pereira, J.A.; Jerónimo, C.; Silva, B.M. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem. Toxicol. 2010, 48, 441–447. [Google Scholar] [CrossRef] [PubMed]
- Alamprese, C.; Pompei, C.; Scaramuzzi, F. Characterization and antioxidant activity of nocino liqueur. Food Chem. 2005, 90, 495–502. [Google Scholar] [CrossRef]
- Alamprese, C.; Pompei, C. Influence of processing variables on some characteristics of nocino liqueur. Food Chem. 2005, 92, 203–209. [Google Scholar] [CrossRef]
- Stampar, F.; Solar, A.; Hudina, M.; Veberic, R.; Colaric, M. Traditional walnut liqueur–cocktail of phenolics. Food Chem. 2006, 95, 627–631. [Google Scholar] [CrossRef]
- Jakopic, J.; Solar, A.; Colaric, M.; Hudina, M.; Veberic, R.; Stampar, F. The influence of ethanol concentration on content of total and individual phenolics in walnut alcoholic drink. Acta Aliment. 2008, 37, 233–239. [Google Scholar] [CrossRef]
- Cortés-Rojas, D.F.; de Souza, C.R.F.; Oliveira, W.P. Clove (Syzygium aromaticum): A precious spice. Asian Pac. J. Trop. Biomed. 2014, 4, 90–96. [Google Scholar] [CrossRef] [Green Version]
- Mele, M.A. Bioactive compounds and biological activity of ginger. J. Multidiscip. Sci. 2019, 1, 1–7. [Google Scholar] [CrossRef]
- Alejo-Armijo, A.; Altarejos, J.; Salido, S. Phytochemicals and biological activities of laurel tree (Laurus nobilis). Nat. Prod. Commun. 2017, 12, 743–757. [Google Scholar] [CrossRef] [Green Version]
- Raina, R.; Verma, P.K.; Peshin, R.; Kour, H. Potential of Juniperus communis L as a nutraceutical in human and veterinary medicine. Heliyon 2019, 5, e02376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karki, N.; Aggarwal, S.; Laine, R.A.; Greenway, F.; Losso, J.N. Cytotoxicity of Juglone and Thymoquinone against Pancreatic Cancer Cells; Elsevier B.V.: Amsterdam, The Netherlands, 2020; Volume 327, ISBN 2255784890. [Google Scholar]
- Harati, K.; Behr, B.; Daigeler, A.; Hirsch, T.; Jacobsen, F.; Renner, M.; Harati, A.; Wallner, C.; Lehnhardt, M.; Becerikli, M. Curcumin and Viscum album Extract Decrease Proliferation and Cell Viability of Soft-Tissue Sarcoma Cells: An In Vitro Analysis of Eight Cell Lines Using Real-Time Monitoring and Colorimetric Assays. Nutr. Cancer 2017, 69, 340–351. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, B.; Liu, Z.; Jiang, Y.; Liu, Y.; Fu, L.; Wang, X.; Kuang, H. Cytotoxicity of triterpenes from green walnut husks of Juglans mandshurica Maxim in HepG-2 cancer cells. Molecules 2015, 20, 19252–19262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Biju, J.; Reddy, V.; Sulaiman, C.T. Total Phenolics and Flavonoids in Selected Justicia Species. J. Pharmacogn. Phytochem. 2013, 2, 51–52. [Google Scholar]
- Benzie, I.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of ‘‘Antioxidant Power’’: The FRAP Assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Miłek, M.; Marcinčáková, D.; Legáth, J. Polyphenols content, antioxidant activity, and cytotoxicity assessment of Taraxacum officinale extracts prepared through the micelle-mediated extraction method. Molecules 2019, 24, 1025. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sowa, P.; Marcinčáková, D.; Miłek, M.; Sidor, E.; Legáth, J.; Dżugan, M. Analysis of cytotoxicity of selected Asteraceae plant extracts in real time, their antioxidant properties and polyphenolic profile. Molecules 2020, 25, 5517. [Google Scholar] [CrossRef]
- Stefanowicz-Hajduk, J.; Ochocka, J.R. Real-time cell analysis system in cytotoxicity applications: Usefulness and comparison with tetrazolium salt assays. Toxicol. Rep. 2020, 7, 335–344. [Google Scholar] [CrossRef]
- Gawlik-Dziki, U.; Durak, A.; Pecio, Ł.; Kowalska, I. Nutraceutical potential of tinctures from fruits, green husks, and leaves of Juglans regia L. Sci. World J. 2014, 2014, 501392. [Google Scholar] [CrossRef] [Green Version]
- Jakopic, J.; Colaric, M.; Veberic, R.; Hudina, M.; Solar, A.; Stampar, F. How much do cultivar and preparation time influence on phenolics content in walnut liqueur? Food Chem. 2007, 104, 100–105. [Google Scholar] [CrossRef]
- Wright, D.A.; Mitchelmore, C.L.; Dawson, R.; Cutler, H.G. The influence of water quality on the toxicity and degradation of juglone (5-hydroxy 1,4-naphthoquinone). Environ. Technol. 2007, 28, 1091–1101. [Google Scholar] [CrossRef] [PubMed]
- Postnikova, E.; Cong, Y.; DeWald, L.E.; Dyall, J.; Yu, S.; Hart, B.J.; Zhou, H.; Gross, R.; Logue, J.; Cai, Y.; et al. Testing therapeutics in cell-based assays: Factors that influence the apparent potency of drugs. PLoS ONE 2018, 13, e0194880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tam, C.C.; Henderson, T.D.; Stanker, L.H.; Cheng, L.W. Influence of food matrices on the stability and bioavailability of abrin. Toxins 2018, 10, 502. [Google Scholar] [CrossRef] [Green Version]
- Atienzar, F.A.; Tilmant, K.; Gerets, H.H.; Toussaint, G.; Speeckaert, S.; Hanon, E.; Depelchin, O.; Dhalluin, S. The use of real-time cell analyzer technology in drug discovery: Defining optimal cell culture conditions and assay reproducibility with different adherent cellular models. J. Biomol. Screen. 2011, 16, 575–587. [Google Scholar] [CrossRef] [Green Version]
- Erisen, S.; Arasoǧlu, T.; Mansuroglu, B.; Kocacaliskan, I.; Derman, S. Cytotoxic and mutagenic potential of juglone: A comparison of free and nano-encapsulated form. Arh. Hig. Rada Toksikol. 2020, 71, 69–77. [Google Scholar] [CrossRef]
- Shah, U.N.; Mir, J.I.; Ahmed, N.; Jan, S.; Fazili, K.M. Bioefficacy potential of different genotypes of walnut Juglans regia L. J. Food Sci. Technol. 2018, 55, 605–618. [Google Scholar] [CrossRef]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: A narrative review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef] [Green Version]
- Makhafola, T.J.; McGaw, L.J.; Eloff, J.N. In vitro cytotoxicity and genotoxicity of five Ochna species (Ochnaceae) with excellent antibacterial activity. S. Afr. J. Bot. 2014, 91, 9–13. [Google Scholar] [CrossRef] [Green Version]
- Njeru, S.N.; Muema, J.M. In vitro cytotoxicity of Aspilia pluriseta Schweinf. extract fractions. BMC Res. Notes 2021, 14, 57. [Google Scholar] [CrossRef] [PubMed]
- Grover, M.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Virmani, T.; Rachamalla, M.; Farasani, A.; Chigurupati, S.; Alsubayiel, A.M.; et al. In vitro phytochemical screening, cytotoxicity studies of curcuma longa extracts with isolation and characterisation of their isolated compounds. Molecules 2021, 26, 7509. [Google Scholar] [CrossRef] [PubMed]
- See, I.; Ee, G.C.L.; Jong, V.Y.M.; Teh, S.S.; Acuña, C.L.C.; Mah, S.H. Cytotoxic activity of phytochemicals from Garcinia mangostana L. and G. benthamiana (Planch. & Triana) Pipoly against breast cancer cells. Nat. Prod. Res. 2021, 35, 6184–6189. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).