The Effect of Customized Insole Pads on Plantar Pressure Distribution in a Diabetic Foot with Neuropathy: Material and Design Study Using Finite Element Analysis Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Foot Model Reconstruction
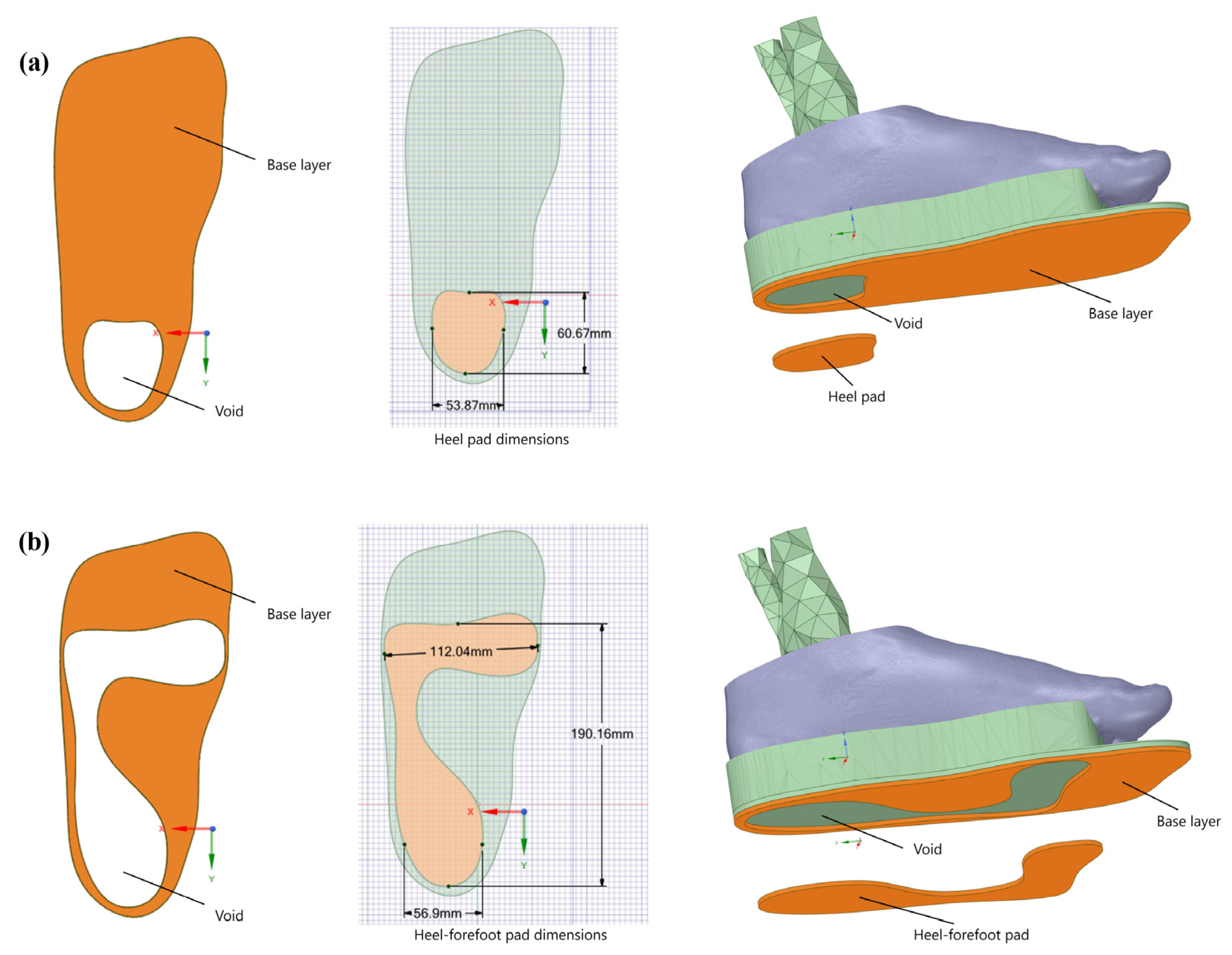
2.2. Pad Designs and Materials
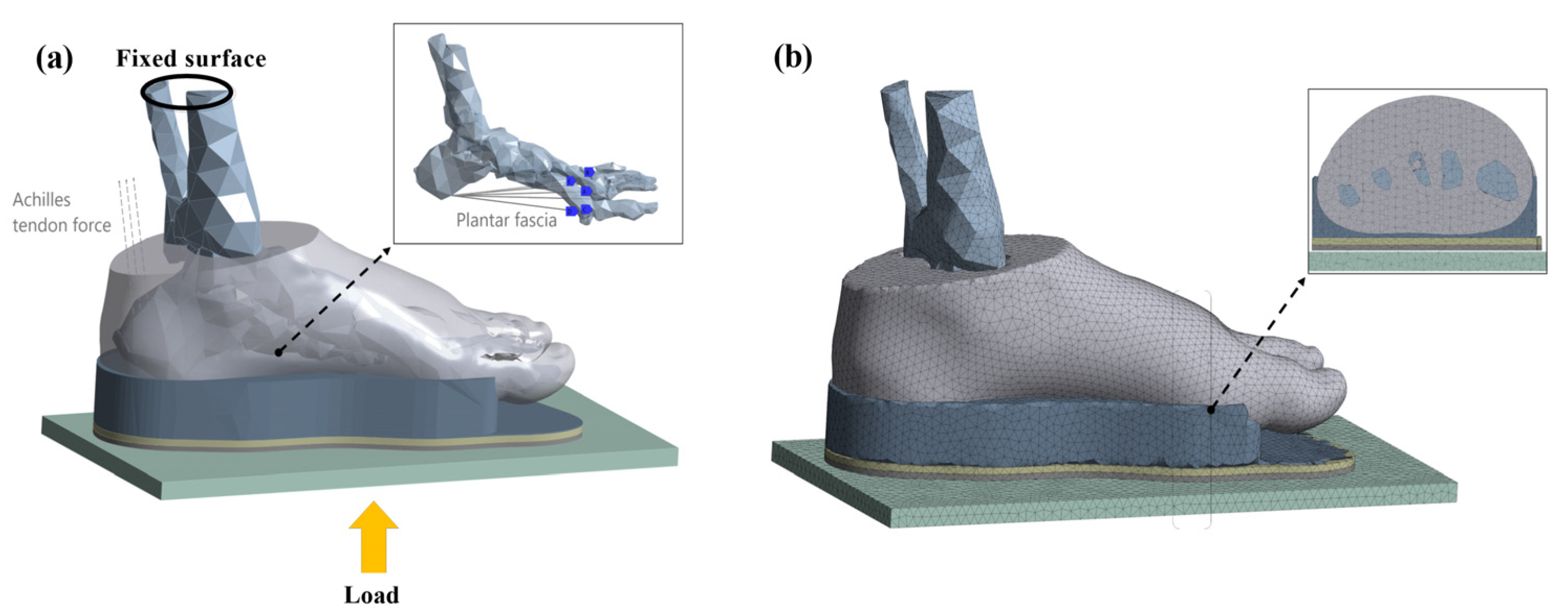
2.3. Boundary and Loading Conditions
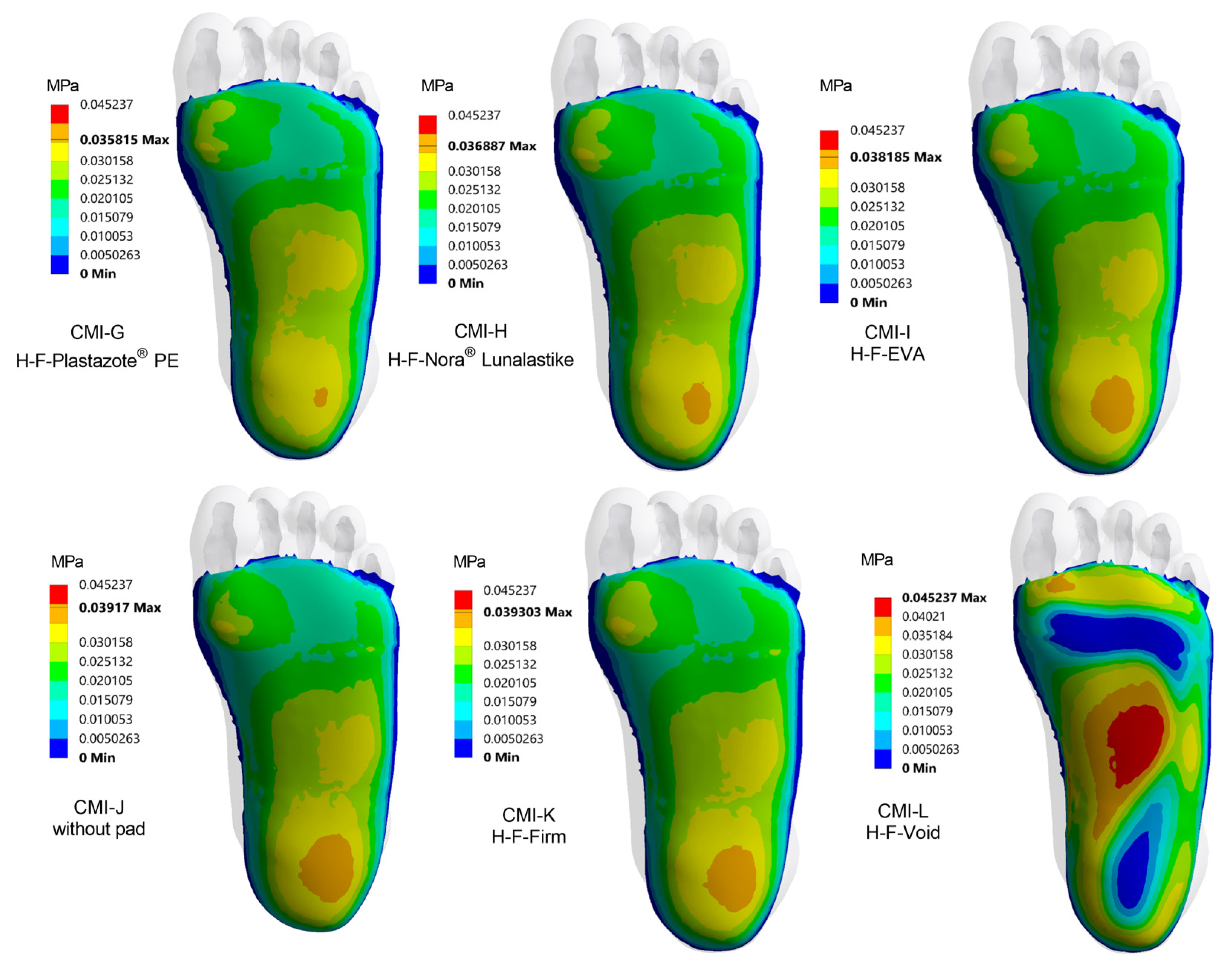
3. Results
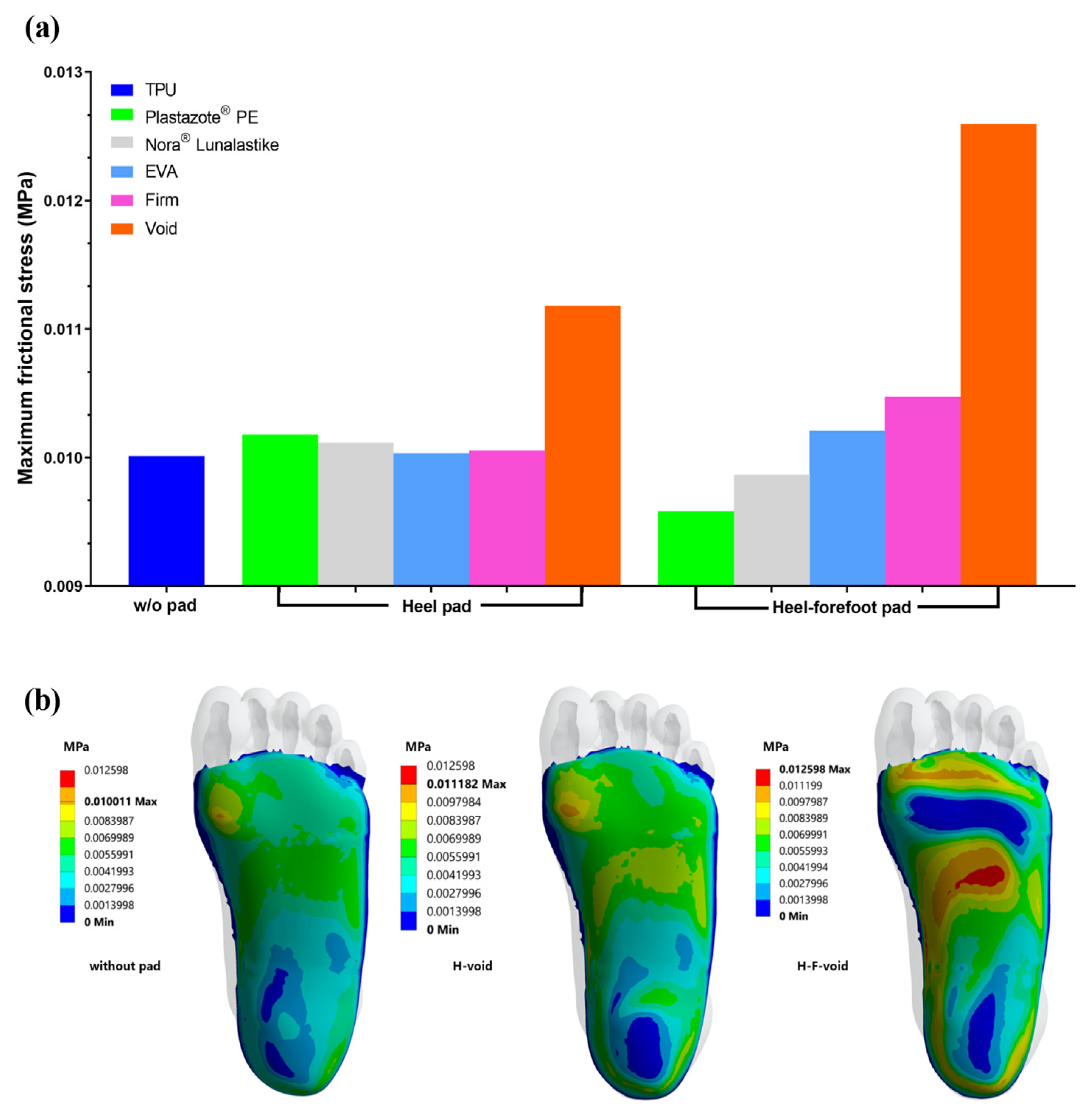
3.1. Frictional Stress
3.2. Plantar Pressure Distribution with Heel Pad
3.3. Plantar Pressure Distribution with Heel–Forefoot Pad
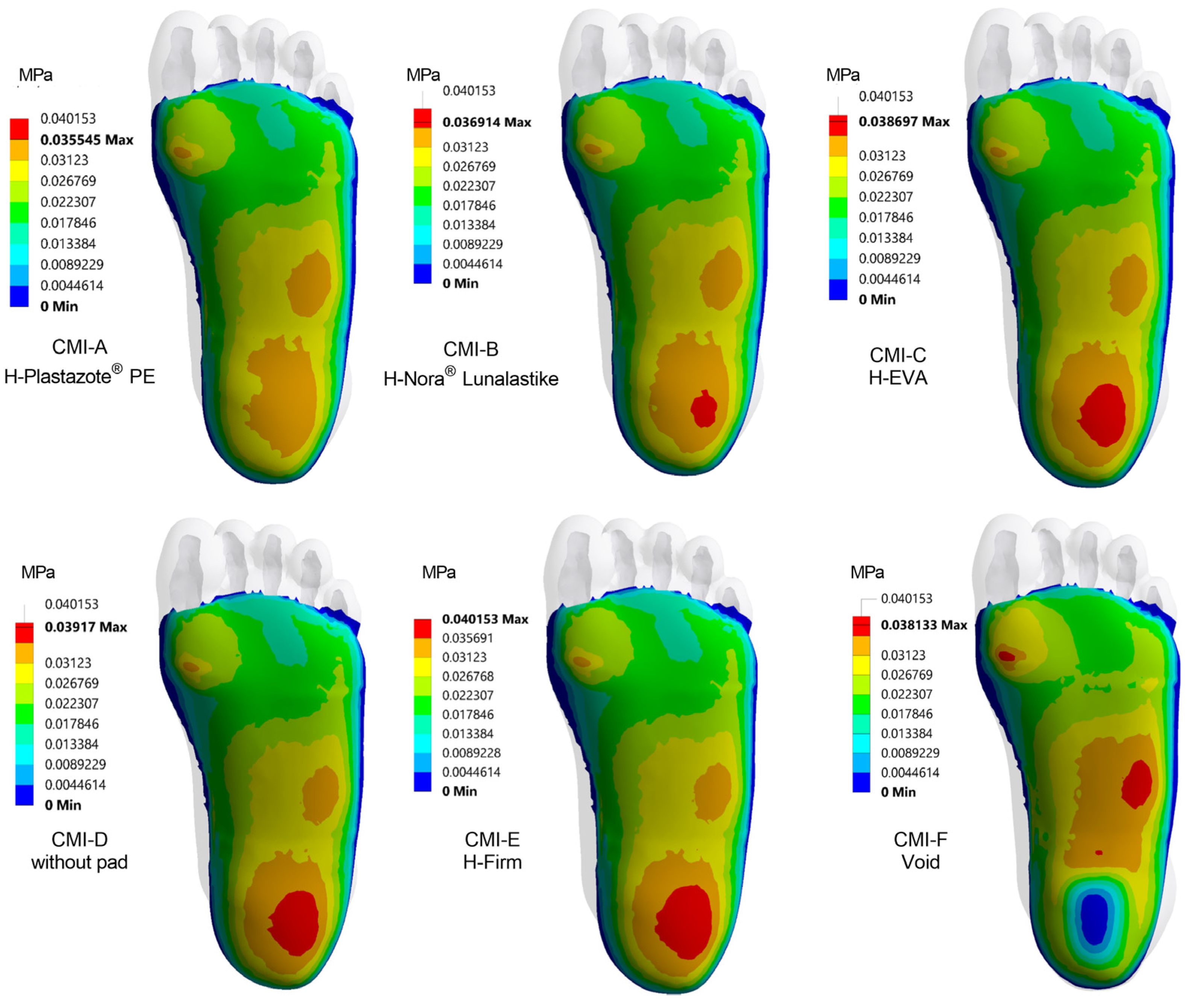
3.4. The Influence of Pad Design on Each Region of the Foot
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Armstrong, D.G.; Boulton, A.J.M.; Bus, S.A. Diabetic Foot Ulcers and Their Recurrence. N. Engl. J. Med. 2017, 376, 2367–2375. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini, P.A.; Pacella, R.E.; Armstrong, D.G.; van Netten, J.J. Diabetes-related lower-extremity complications are a leading cause of the global burden of disability. Diabet. Med. 2018, 35, 1297–1299. [Google Scholar] [CrossRef] [PubMed]
- Lazzarini, P.A.; Hurn, S.E.; Kuys, S.S.; Kamp, M.C.; Ng, V.; Thomas, C.; Jen, S.; Wills, J.; Kinnear, E.M.; d’Emden, M.C.; et al. The silent overall burden of foot disease in a representative hospitalised population. Int. Wound J. 2017, 14, 716–728. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, D.G.; Lavery, L.A. Diabetic foot ulcers: Prevention, diagnosis and classification. Am. Fam. Physician 1998, 57, 1325–1332. [Google Scholar] [PubMed]
- Reiber, G.E.; Pecoraro, R.E.; Koepsell, T.D. Risk factors for amputation in patients with diabetes mellitus. A case-control study. Ann. Intern. Med. 1992, 117, 97–105. [Google Scholar] [CrossRef] [PubMed]
- van Netten, J.J.; Lazzarini, P.A.; Armstrong, D.G.; Bus, S.A.; Fitridge, R.; Harding, K.; Kinnear, E.; Malone, M.; Menz, H.B.; Perrin, B.M.; et al. Diabetic Foot Australia guideline on footwear for people with diabetes. J. Foot Ankle Res. 2018, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Shaw, J.E.; Sicree, R.A.; Zimmet, P.Z. Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res. Clin. Pract. 2010, 87, 4–14. [Google Scholar] [CrossRef]
- Pecoraro, R.E.; Reiber, G.E.; Burgess, E.M. Pathways to diabetic limb amputation. Basis for prevention. Diabetes Care 1990, 13, 513–521. [Google Scholar] [CrossRef]
- Woolard, R.H.; Carty, K.; Wirtz, P.; Longabaugh, R.; Nirenberg, T.D.; Minugh, P.A.; Becker, B.; Clifford, P.R. Research fundamentals: Follow-up of subjects in clinical trials: Addressing subject attrition. Acad. Emerg. Med. 2004, 11, 859–866. [Google Scholar] [CrossRef]
- Wang, D.; Cai, P. Finite Element Analysis of the Expression of Plantar Pressure Distribution in the Injury of the Lateral Ligament of the Ankle. Nano Biomed. Eng. 2019, 11, 290–296. [Google Scholar] [CrossRef]
- Ghassemi, A.; Mossayebi, A.R.; Jamshidi, N.; Naemi, R.; Karimi, M.T. Manufacturing and finite element assessment of a novel pressure reducing insole for Diabetic Neuropathic patients. Australas. Phys. Eng. Sci. Med. 2015, 38, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Cheung, J.T.; Zhang, M. A 3-dimensional finite element model of the human foot and ankle for insole design. Arch. Phys. Med. Rehabil. 2005, 86, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Cavanagh, P.R. Therapeutic footwear for people with diabetes. Diabetes Metab. Res. Rev. 2004, 20 (Suppl. S1), S51–S55. [Google Scholar] [CrossRef] [PubMed]
- Bus, S.A.; Maas, J.C.; Otterman, N.M. Lower-extremity dynamics of walking in neuropathic diabetic patients who wear a forefoot-offloading shoe. Clin. Biomech. 2017, 50, 21–26. [Google Scholar] [CrossRef]
- Anderson, J.; Williams, A.E.; Nester, C. Development and evaluation of a dual density insole for people standing for long periods of time at work. J. Foot Ankle Res. 2020, 13, 42. [Google Scholar] [CrossRef]
- Ahmed, S.; Barwick, A.; Butterworth, P.; Nancarrow, S. Footwear and insole design features that reduce neuropathic plantar forefoot ulcer risk in people with diabetes: A systematic literature review. J. Foot Ankle Res. 2020, 13, 30. [Google Scholar] [CrossRef]
- Goske, S.; Erdemir, A.; Petre, M.; Budhabhatti, S.; Cavanagh, P.R. Reduction of plantar heel pressures: Insole design using finite element analysis. J. Biomech. 2006, 39, 2363–2370. [Google Scholar] [CrossRef]
- Wibowo, D.B.; Widodo, A.; Haryadi, G.D.; Caesarendra, W.; Harahap, R. Effect of in-shoe foot orthosis contours on heel pain due to calcaneal spurs. Appl. Sci. 2019, 9, 495. [Google Scholar] [CrossRef] [Green Version]
- Collings, R.; Freeman, J.; Latour, J.M.; Paton, J. Footwear and insole design features for offloading the diabetic at risk foot-A systematic review and meta-analyses. Endocrinol. Diabetes Metab. 2021, 4, e00132. [Google Scholar] [CrossRef] [Green Version]
- Raspovic, A.; Landorf, K.B.; Gazarek, J.; Stark, M. Reduction of peak plantar pressure in people with diabetes-related peripheral neuropathy: An evaluation of the DH Pressure Relief Shoe™. J. Foot Ankle Res. 2012, 5, 25. [Google Scholar] [CrossRef]
- Shi, Q.Q.; Li, P.L.; Yick, K.-L.; Li, N.-W.; Jiao, J. Effects of contoured insoles with different materials on plantar pressure offloading in diabetic elderly during gait. Sci. Rep. 2022, 12, 15395. [Google Scholar] [CrossRef] [PubMed]
- Chatzistergos, P.E.; Naemi, R.; Healy, A.; Gerth, P.; Chockalingam, N. Subject specific optimisation of the stiffness of footwear material for maximum plantar pressure reduction. Ann. Biomed. Eng. 2017, 45, 1929–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohuchi, H.; Chavez, J.S.; Alvarez, C.A.D. Changes in calcaneal pitch and heel fat pad thickness in static weight bearing radiographs while wearing shoes with arch support and heel cup orthotics. Asia-Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2019, 17, 21–24. [Google Scholar] [CrossRef] [PubMed]
- Seligman, D.A.; Dawson, D.R. Customized heel pads and soft orthotics to treat heel pain and plantar fasciitis. Arch. Phy. Med. Rehabil. 2003, 84, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Hastings, M.K.; Mueller, M.J.; Pilgram, T.K.; Lott, D.J.; Commean, P.K.; Johnson, J.E. Effect of metatarsal pad placement on plantar pressure in people with diabetes mellitus and peripheral neuropathy. Foot Ankle Int. 2007, 28, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Chatzistergos, P.E.; Naemi, R.; Chockalingam, N. A method for subject-specific modelling and optimisation of the cushioning properties of insole materials used in diabetic footwear. Med. Eng. Phys. 2015, 37, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Chatzistergos, P.E.; Gatt, A.; Formosa, C.; Farrugia, K.; Chockalingam, N. Optimised cushioning in diabetic footwear can significantly enhance their capacity to reduce plantar pressure. Gait Posture 2020, 79, 244–250. [Google Scholar] [CrossRef]
- Nouman, M.; Dissaneewate, T.; Chong, D.Y.R.; Chatpun, S. Effects of custom-made insole materials on frictional stress and contact pressure in diabetic foot with neuropathy: Results from a finite element analysis. Appl. Sci. 2021, 11, 3412. [Google Scholar] [CrossRef]
- Nouman, M.; Chong, D.Y.R.; Chatpun, S. Finite Element Approach Towards Selection of Appropriate Materials to Redistribute Peak Plantar Pressure in Diabetic Foot with Neuropathy: Selecting Appropriate Materials for Diabetic Foot. PSU Med. J. 2021, 1, 43–54. [Google Scholar] [CrossRef]
- Brilakis, E.; Kaselouris, E.; Xypnitos, F.; Provatidis, C.G.; Efstathopoulos, N. Effects of foot posture on fifth metatarsal fracture healing: A finite element study. J. Foot Ankle Surg. 2012, 51, 720–728. [Google Scholar] [CrossRef]
- Chen, W.P.; Ju, C.W.; Tang, F.T. Effects of total contact insoles on the plantar stress redistribution: A finite element analysis. Clin. Biomech. 2003, 18, S17–S24. [Google Scholar] [CrossRef] [PubMed]
- Wright, D.G.; Rennels, D.C. A study of the elastic properties of plantar fascia. J. Bone Joint Surg. Am. 1964, 46, 482–492. [Google Scholar] [CrossRef] [PubMed]
- Lo, W.T.; Yick, K.; Ng, Z.; Yip, J. Numerical simulation of orthotic insole deformation for diabetic foot. J. Fiber Bioeng. Inform. 2015, 8, 401–411. [Google Scholar] [CrossRef] [Green Version]
- Lewis, G. Finite element analysis of a model of a therapeutic shoe: Effect of material selection for the outsole. Biomed. Mater. Eng. 2003, 13, 75–81. [Google Scholar] [PubMed]
- Frick, A.; Rochman, A. Characterization of TPU-elastomers by thermal analysis (DSC). Polym. Test. 2004, 23, 413–417. [Google Scholar] [CrossRef]
- Su, S.; Mo, Z.; Guo, J.; Fan, Y. The effect of arch height and material hardness of personalized insole on correction and tissues of flatfoot. J. Healthc. Eng. 2017, 8614341. [Google Scholar] [CrossRef] [Green Version]
- Mueller, M.J.; Lott, D.J.; Hastings, M.K.; Commean, P.K.; Smith, K.E.; Pilgram, T.K. Efficacy and mechanism of orthotic devices to unload metatarsal heads in people with diabetes and a history of plantar ulcers. Phys. Ther. 2006, 86, 833–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bus, S.A.; Ulbrecht, J.S.; Cavanagh, P.R. Pressure relief and load redistribution by custom-made insoles in diabetic patients with neuropathy and foot deformity. Clin. Biomech. 2004, 19, 629–638. [Google Scholar] [CrossRef]
- Ashry, H.R.; Lavery, L.A.; Murdoch, D.P.; Frolich, M.; Lavery, D.C. Effectiveness of diabetic insoles to reduce foot pressures. J. Foot Ankle Surg. 1997, 36, 268–271. [Google Scholar] [CrossRef]
- Tsung, B.Y.; Zhang, M.; Mak, A.F.; Wong, M.W. Effectiveness of insoles on plantar pressure redistribution. J. Rehabil. Res. Dev. 2004, 41, 767–774. [Google Scholar] [CrossRef]
- Hellstrand Tang, U.; Zügner, R.; Lisovskaja, V.; Karlsson, J.; Hagberg, K.; Tranberg, R. Comparison of plantar pressure in three types of insole given to patients with diabetes at risk of developing foot ulcers—A two-year, randomized trial. J. Clin. Transl. Endocrinol. 2014, 1, 121–132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, G.; Houston, V.L.; Garbarini, M.A.; Beattie, A.C.; Thongpop, C. Finite element analysis of heel pad with insoles. J. Biomech. 2011, 44, 1559–1565. [Google Scholar] [CrossRef] [PubMed]
- Actis, R.L.; Ventura, L.B.; Lott, D.J.; Smith, K.E.; Commean, P.K.; Hastings, M.K.; Mueller, M.J. Multi-plug insole design to reduce peak plantar pressure on the diabetic foot during walking. Med. Biol. Eng. Comput. 2008, 46, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Mündermann, A.; Nigg, B.M.; Humble, R.N.; Stefanyshyn, D.J. Orthotic comfort is related to kinematics, kinetics, and EMG in recreational runners. Med. Sci. Sports Exerc. 2003, 35, 1710–1719. [Google Scholar] [CrossRef]
- Nouman, M.; Dissaneewate, T.; Leelasamran, W.; Chatpun, S. The insole materials influence the plantar pressure distributions in diabetic foot with neuropathy during different walking activities. Gait Posture 2019, 74, 154–161. [Google Scholar] [CrossRef]
- Cheung, J.T.; Zhang, M. Parametric design of pressure-relieving foot orthosis using statistics-based finite element method. Med. Eng. Phys. 2008, 30, 269–277. [Google Scholar] [CrossRef]
- Martinez-Santos, A.; Preece, S.; Nester, C.J. Evaluation of orthotic insoles for people with diabetes who are at-risk of first ulceration. J Foot Ankle Res 2019, 12, 35. [Google Scholar] [CrossRef] [Green Version]
- Healy, A.; Dunning, D.N.; Chockalingam, N. Effect of insole material on lower limb kinematics and plantar pressures during treadmill walking. Prosthetics Orthotics Int. 2012, 36, 53–62. [Google Scholar] [CrossRef]
- Amemiya, A.; Noguchi, H.; Oe, M.; Takehara, K.; Ohashi, Y.; Suzuki, R.; Yamauchi, T.; Kadowaki, T.; Sanada, H.; Mori, T. Shear Stress-Normal Stress (Pressure) Ratio Decides Forming Callus in Patients with Diabetic Neuropathy. J. Diabetes Res. 2016, 2016, 3157123. [Google Scholar] [CrossRef] [Green Version]
- Cong, Y.; Cheung, J.T.; Leung, A.K.; Zhang, M. Effect of heel height on in-shoe localized triaxial stresses. J. Biomech. 2011, 44, 2267–2272. [Google Scholar] [CrossRef]
- Caselli, A.; Pham, H.; Giurini, J.M.; Armstrong, D.G.; Veves, A. The forefoot-to-rearfoot plantar pressure ratio is increased in severe diabetic neuropathy and can predict foot ulceration. Diabetes Care 2002, 25, 1066–1071. [Google Scholar] [CrossRef] [Green Version]
- Zou, D.; Mueller, M.J.; Lott, D.J. Effect of peak pressure and pressure gradient on subsurface shear stresses in the neuropathic foot. J. Biomech. 2007, 40, 883–890. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.; Doupis, J. Diabetic foot disease: From the evaluation of the "foot at risk" to the novel diabetic ulcer treatment modalities. World J. Diabetes 2016, 7, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Chemello, G.; Salvatori, B.; Morettini, M.; Tura, A. Artificial Intelligence Methodologies Applied to Technologies for Screening, Diagnosis and Care of the Diabetic Foot: A Narrative Review. Biosensors 2022, 12, 985. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Cen, X.; Zhang, Y.; Bíró, I.; Ji, Y.; Gu, Y. Development and validation of a subject-specific coupled model for foot and sports shoe complex: A pilot computational study. Bioengineering 2022, 9, 553. [Google Scholar] [CrossRef] [PubMed]
| Components | Young’s Modulus (MPa) | Poisson’s Ratio v | References |
|---|---|---|---|
| Bone | 7300 | 0.3 | [30] |
| Soft tissue | 0.19 | 0.49 | [31] |
| Plantar fascia | 350 | 0.35 | [32] |
| Plastazote® PE | 0.45 | 0.38 | [33] |
| Nora® Lunalastike | 1.04 | 0.25 | [33] |
| EVA | 5 | 0.40 | [34] |
| TPU | 11 | 0.45 | [35] |
| Firm insole | 1000 | 0.40 | [12] |
| Ground | 21,000,000 | 0.3 | [36] |
| Foot Region | Peak Contact Pressure (kPa) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Plastazote® PE | Nora® Lunalastike | EVA | TPU | Firm | Void | ||||||
| H | H-F | H | H-F | H | H-F | w/o Pad | H | H-F | H | H-F | |
| Whole foot | 35.5 | 35.8 | 36.9 | 36.9 | 38.7 | 38.2 | 39.2 | 40.2 | 39.3 | 38.1 | 45.2 |
| Forefoot | 34.1 | 31.8 | 33.9 | 32.9 | 33.6 | 34.0 | 33.5 | 33.7 | 34.9 | 38.1 | 38.6 |
| Medial forefoot | 34.1 | 31.8 | 33.9 | 32.9 | 33.6 | 34.0 | 33.5 | 33.7 | 34.9 | 38.1 | 37.4 |
| Central forefoot | 24.6 | 26.4 | 24.5 | 25.9 | 24.5 | 25.7 | 24.0 | 24.4 | 25.9 | 28.0 | 37.9 |
| Lateral forefoot | 26.1 | 26.8 | 26.0 | 26.2 | 25.8 | 26.3 | 25.7 | 26.2 | 26.6 | 28.2 | 38.6 |
| Midfoot | 34.0 | 32.2 | 33.8 | 32.6 | 33.6 | 33.2 | 33.5 | 33.6 | 33.7 | 37.7 | 45.2 |
| Medial midfoot | 32.2 | 31.8 | 32.0 | 30.7 | 31.0 | 30.4 | 31.5 | 31.8 | 30.6 | 34.7 | 44.6 |
| Lateral midfoot | 34.0 | 32.2 | 33.8 | 32.6 | 33.6 | 33.2 | 33.5 | 33.6 | 33.7 | 37.7 | 45.2 |
| Hindfoot | 35.5 | 35.8 | 36.9 | 36.9 | 38.7 | 38.2 | 39.2 | 40.2 | 39.3 | 36.1 | 37.8 |
| Medial hindfoot | 34.2 | 35.1 | 36.4 | 34.7 | 38.3 | 37.0 | 38.0 | 38.8 | 38.5 | 36.1 | 38.2 |
| Lateral hindfoot | 35.5 | 35.8 | 36.9 | 36.9 | 38.7 | 38.2 | 39.2 | 40.2 | 39.3 | 35.9 | 33.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nouman, M.; Chong, D.Y.R.; Srewaradachpisal, S.; Chatpun, S. The Effect of Customized Insole Pads on Plantar Pressure Distribution in a Diabetic Foot with Neuropathy: Material and Design Study Using Finite Element Analysis Approach. Appl. Sci. 2023, 13, 399. https://doi.org/10.3390/app13010399
Nouman M, Chong DYR, Srewaradachpisal S, Chatpun S. The Effect of Customized Insole Pads on Plantar Pressure Distribution in a Diabetic Foot with Neuropathy: Material and Design Study Using Finite Element Analysis Approach. Applied Sciences. 2023; 13(1):399. https://doi.org/10.3390/app13010399
Chicago/Turabian StyleNouman, Muhammad, Desmond Y. R. Chong, Satta Srewaradachpisal, and Surapong Chatpun. 2023. "The Effect of Customized Insole Pads on Plantar Pressure Distribution in a Diabetic Foot with Neuropathy: Material and Design Study Using Finite Element Analysis Approach" Applied Sciences 13, no. 1: 399. https://doi.org/10.3390/app13010399







