Study on the Mechanical Properties and Hydration Behavior of Steel Slag–Red Mud–Electrolytic Manganese Residue Based Composite Mortar
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials and Analysis
2.2. Experimental Program
2.2.1. Specimens’ Preparation and Test Procedure
2.2.2. Test Procedures
- (1)
- Density and specific surface area test: The density test of raw materials refers to the Chinese National Standard GB/T 208-2014 Cement Density Determination Method [12]. The specific surface area test refers to the Chinese National Standard GB/T 8074-2008 Cement-Specific Surface Area Determination Method: Burr’s Method [13].
- (2)
- Mechanical performance test: Referring to Chinese National Standard GB/T 17671-1999 Test Method For Cementitious Sand Strength (ISO Method) [14], the flexural strength and compressive strength of mortar specimens were tested at 3 d, 7 d, and 28 d.
- (3)
- Isothermal calorimetry: The hydration process of the slurry was monitored using isothermal calorimetry (TAM Air Thermometric, I-CAL4000/8000 made by Calmetrix Corporation, Boston, MA, USA, set at 25 °C). Manual stirring was performed, using a disposable spoon for 1 min to achieve the desired homogeneity. After stirring, the spoon was left in the sample cup to minimize the sample mass loss. Measurements were performed every minute for 72 h.
- (4)
- XRD analysis: The block samples of 3 d and 28 d were dried in a vacuum-drying oven at 40 ± 5 °C and then ground through 200 square mesh hole sieves with agate mortar powder. Next, a certain amount was taken with a medicine spoon, pressed into a mold, and tested using an X-ray diffractometer (PANalytical’s XPert Pro X-ray). The test diffraction angle was 5~80°, the dwell time was 0.5 s, and the scanning speed was 0.02°/min to identify the crystalline phase of the sample.
- (5)
- TG–DTG analysis: The 28 d lump samples were dried in a vacuum oven at 40 °C ± 5 °C and then ground through 200 square mesh hole sieves with agate mortar powder. Next, a certain amount was spooned into a crucible and tested using a thermogravimetric analyzer (Q 5000 IR) with an air atmosphere and a heating rate of 10 °C/min for the analysis of the hydration products of the samples.
- (6)
- Pore structure analysis: After the block sample at 28 d was cut with a precision cutter about a size of 1 cm × 1 cm × 1 cm or so, the sample was dried in a vacuum drying oven at 40 °C ± 5 °C to obtain the final product. The pore structure of the mortar specimen was tested and analyzed by a high-performance automatic mercury pressure instrument.
- (7)
- Calculation method of activity index: Referring to Chinese National Standard GB/T 51003-2014 Technical Specifications For The Application Of Mineral Dopants, [15], the activity index of composite mortar was calculated according to the following formula, using pure cement group as the reference group.where
- A—activity index (%);
- Rt—compressive strength of the examined sand at the corresponding age, in MPa;
- R0—compressive strength of contrasting colluvium at the corresponding age, in MPa.
3. Results and Discussion
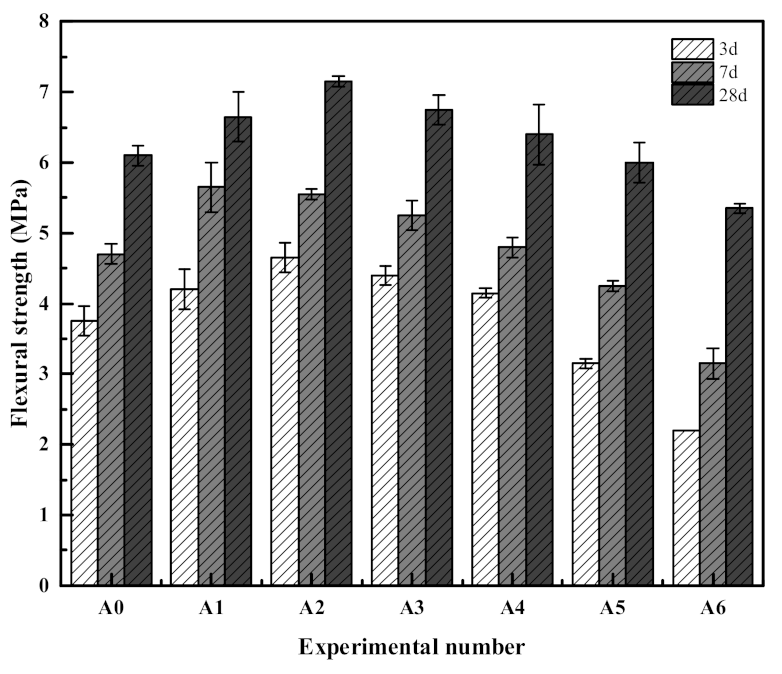
3.1. Mechanical Properties of Composite Mortar
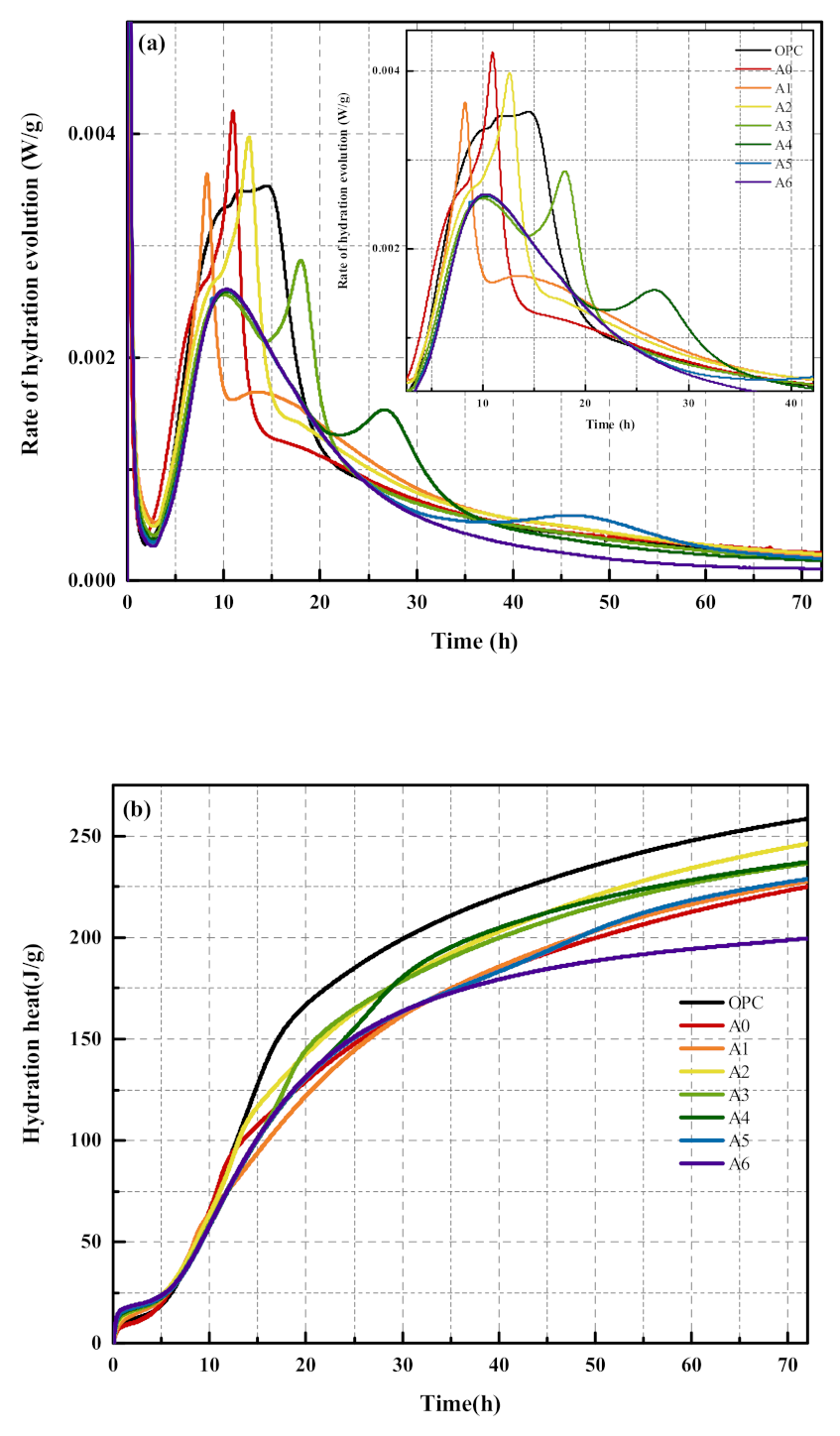
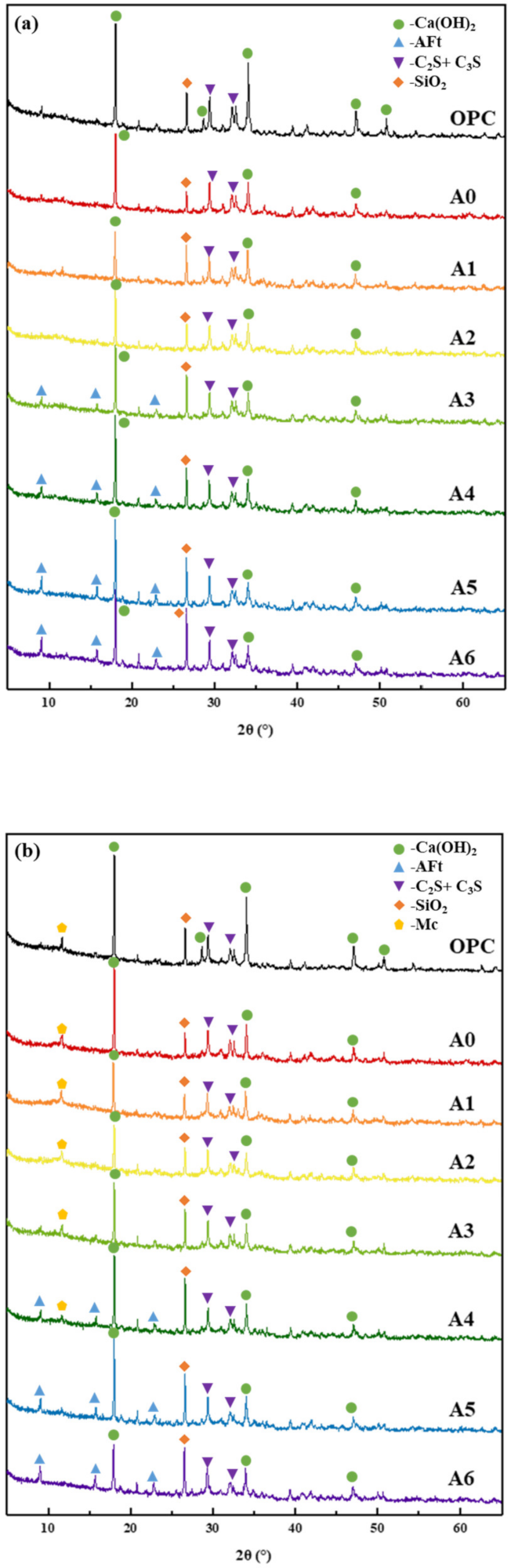
3.2. Analysis of Hydration Characteristics of Composite Mortar
4. Conclusions
- Compared with the OPC group specimens, 30% SS alone will inhibit the hydration reaction of the slurry, resulting in the decrease of the mechanical properties of the mortar, while the compound mortar can improve the mechanical properties with the appropriate amount of RM and EMR. The 28 d flexural and compressive strengths of the composite mortar were the highest when 15% of SS, 12% of RM, and 3% of EMR were mixed, which were 7.2 MPa and 41.4 MPa, respectively. Compared with the test group with 30% SS alone, the 28 d flexural strength increased by 18.0% and the compressive strength increased by 25.5%.
- Compared with the OPC group specimens, 30% SS alone reduced the hydration reaction rate and accumulated heat release of the slurry, while the compounding of appropriate amounts of RM and EMR can improve the hydration reaction rate and accumulated heat release of the slurry. The accumulated heat release of the slurry was 246.2 J/g when 15% of SS, 12% of RM, and 3% of EMR were mixed.
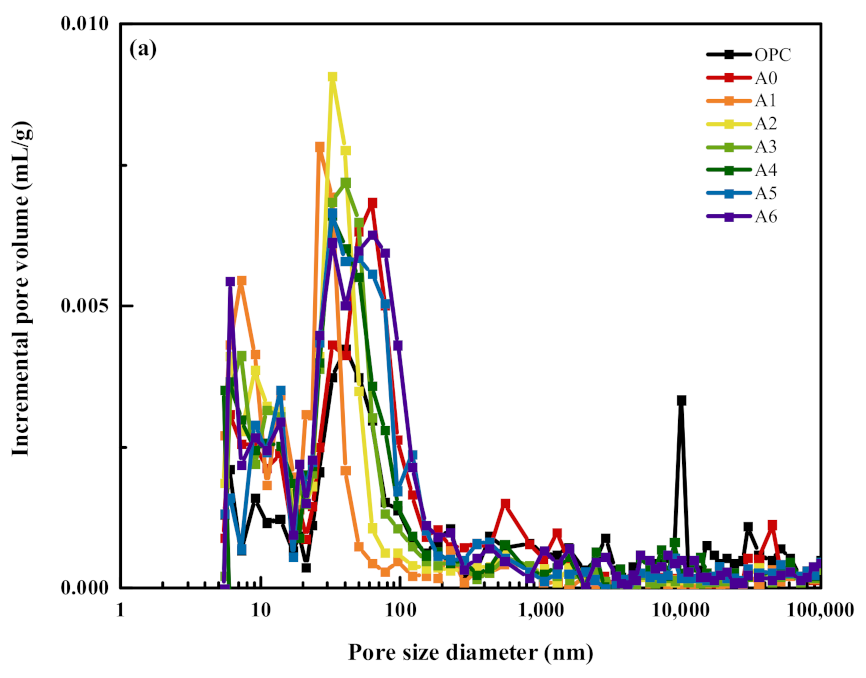
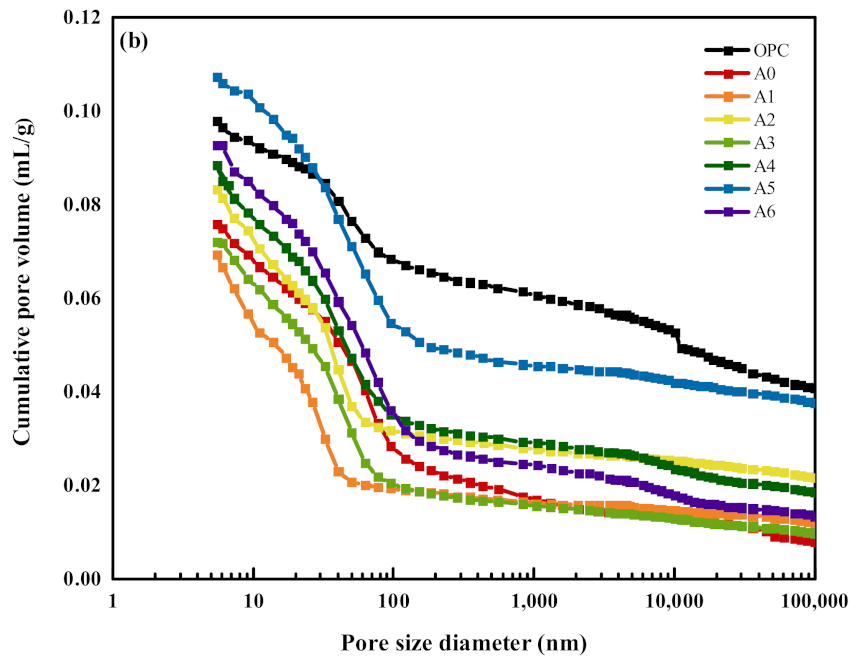
- Compared with the OPC group specimens, 30% SS alone reduced the content of hydration products Ca(OH)2, Aft, and C–S–H gels, while compounding with appropriate amounts of RM and EMR can promote the slurry to produce more AFt and C–S–H gels, which are favorable for microstructure development and form a more dense hardened body structure. Compared with the specimens of 30% SS group, when 15% of SS, 12% of RM, and 3% of EMR are mixed, it can promote the generation of hydration products AFt and C–S–H gel and form more dense hardened body structure, which makes the overall decrease of harmless and harmful pores 26.35% and the overall increase of harmless and less harmful pores 43.57% in the composite mortar specimens.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Statistical Bureau of the People‘s Republic of China. China Statistical Yearbook; China Statistics Bureau Press: Beijing, China, 2021. [Google Scholar]
- Pang, L.; Liao, S.C.; Wang, D.Q.; An, M.Z. Influence of steel slag fineness on the hydration of cement-steel slag composite pastes. J. Build. Eng. 2022, 57, 104866. [Google Scholar] [CrossRef]
- Zhuang, S.Y.; Wang, Q. Inhibition mechanisms of steel slag on the early-age hydration of cement. Cem. Concr. Res. 2021, 140, 106283. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Z.Q.; Hou, G.H.; Yan, P.Y. Preparation of sustainable and green cement-based composite binders with high-volume steel slag powder and ultrafine blast furnace slag powder. J. Clean. Prod. 2021, 289, 125133. [Google Scholar] [CrossRef]
- Hao, X.S.; Liu, X.M.; Zhang, Z.Q.; Zhang, W.; Lu, Y.; Wang, Y.G.; Yang, T.Y. In-depth insight into the cementitious synergistic effect of steel slag and red mud on the properties of composite cementitious materials. J. Build. Eng. 2022, 52, 104449. [Google Scholar] [CrossRef]
- Yi, L.S.; Wen, J. Research status and progress of steel slag activity excitation technology. Silic. Bull. 2013, 32, 2057–2062. [Google Scholar]
- Xu, Y.T.; Liu, X.M.; Zhang, Y.L.; Tang, B.W.; Mukiza, E. Investigation on sulfate activation of electrolytic manganese residue on early activity of blast furnace slag in cement-based cementitious material. Constr. Build. Mater. 2019, 229, 116831. [Google Scholar] [CrossRef]
- Zhang, W.; Hao, X.S.; Wei, C.; Zeng, Q.S.; Ma, S.L.; Liu, X.M.; Zhang, Z.Q.; Webeck, E. Synergistic enhancement of converter steelmaking slag, blast furnace slag, Bayer red mud in cementitious materials: Strength, phase composition, and microstructure. J. Build. Eng. 2022, 60, 105177. [Google Scholar] [CrossRef]
- Wang, Z.W.; Chen, P.; Zhou, H.Y.; Zhao, Y.R.; Liu, R.J.; Wei, J.X. Study on the heat of hydration of steel slag electrolytic manganese residue red mud composite cementitious material. Nonmet. Min. 2019, 42, 88–90. [Google Scholar]
- Palankar, N.; Ravi Shankar, A.U.; Mithun, B.M. Investigations on Alkali-Activated Slag/Fly Ash Concrete with steel slag coarse aggregate for pavement structures. Int. J. Pavement Eng. 2017, 18, 500–512. [Google Scholar] [CrossRef]
- Guo, X.; Shi, H.; Wu, K. Effects of Steel Slag Powder on Workability and Durability of Concrete. J. Wuhan Univ. Technol. 2014, 29, 733–739. [Google Scholar] [CrossRef]
- GB/T 208-2014; Cement Density Determination Method. General Administration of Quality Supervision, Inspection and Quarantine: Beijing, China, 2014; pp. 1–3.
- GB/T 8074-2008; Cement-Specific Surface Area Determination Method: Burr’s Method. General Administration of Quality Supervision, Inspection and Quarantine: Beijing, China, 2008; pp. 1–4.
- GB/T 17671-1999; Test Method for Cementitious Sand Strength (ISO Method). The State Bureau of Quality and Technical Supervision: Beijing, China, 1999; p. 9.
- GB/T 51003-2014; Technical Specifications for the Application of Mineral Dopants. General Administration of Quality Supervision, Inspection and Quarantine: Beijing, China, 2014; pp. 24–27.
- Jennings, H.M.; Johnson, S.K. Simulation of microstructure development during the hydration of a cement compound. J. Am. Ceram. Soc. 1986, 69, 790–795. [Google Scholar] [CrossRef]
- Wu, W.J. Study on the Hydration Process and Performance of Steel Slag-Cement Composite Cementitious Materials. Master’s Thesis, Beijing University of Chemical Technology, Beijing, China, 2016. [Google Scholar]
- Zhang, S.F.; Niu, D.T. Hydration and mechanical properties of cement-steel slag system incorporating different activators. Constr. Build. Mater. 2023, 363, 129981. [Google Scholar] [CrossRef]
- Zhou, X.; Du, L.; Li, F.Y.; Zhou, S.F.; Shao, S.; Zhang, X.; Peng, J. Study on the effect of clinker fineness of silicate cement on hydration behavior and strength of phosphorus building gypsum. New Build. Mater. 2022, 49, 129–132. [Google Scholar]
- Huang, S.Y.; Huo, B.B.; Chen, C.; Zhang, Y.M. Effect of metakaolin on the hydration of steel slag cement-based composite systems under steaming conditions. Mater. Guide 2022, 36, 69–74. [Google Scholar]
- Boháč, M.; Palou, M.; Novotný, R.; Másilko, J.; Všianský, D.; Staněk, T. Investigation on early hydration of ternary Portland cement-blast-furnace slag metakaolin blends. Constr. Build. Mater. 2014, 46, 333–341. [Google Scholar] [CrossRef]
- Chen, P.; Hu, C.; Xiang, W.H.; Tian, Y.; Liu, R.J. Research on the structure and properties of steel slag-manganese slag composite cementitious materials. J. Wuhan Univ. Technol. 2019, 41, 1–5. [Google Scholar]
- Zhang, N.; Li, H.X.; Peng, D.D.; Liu, X.M. Properties evaluation of silica-alumina based concrete: Durability and environmental friendly performance. Constr. Build. Mater. 2016, 115, 105–113. [Google Scholar] [CrossRef]
- Wu, C.W. Reflections on the science and technology of concrete. Concr. Cem. Prod. 1988, 6, 4–6. [Google Scholar]
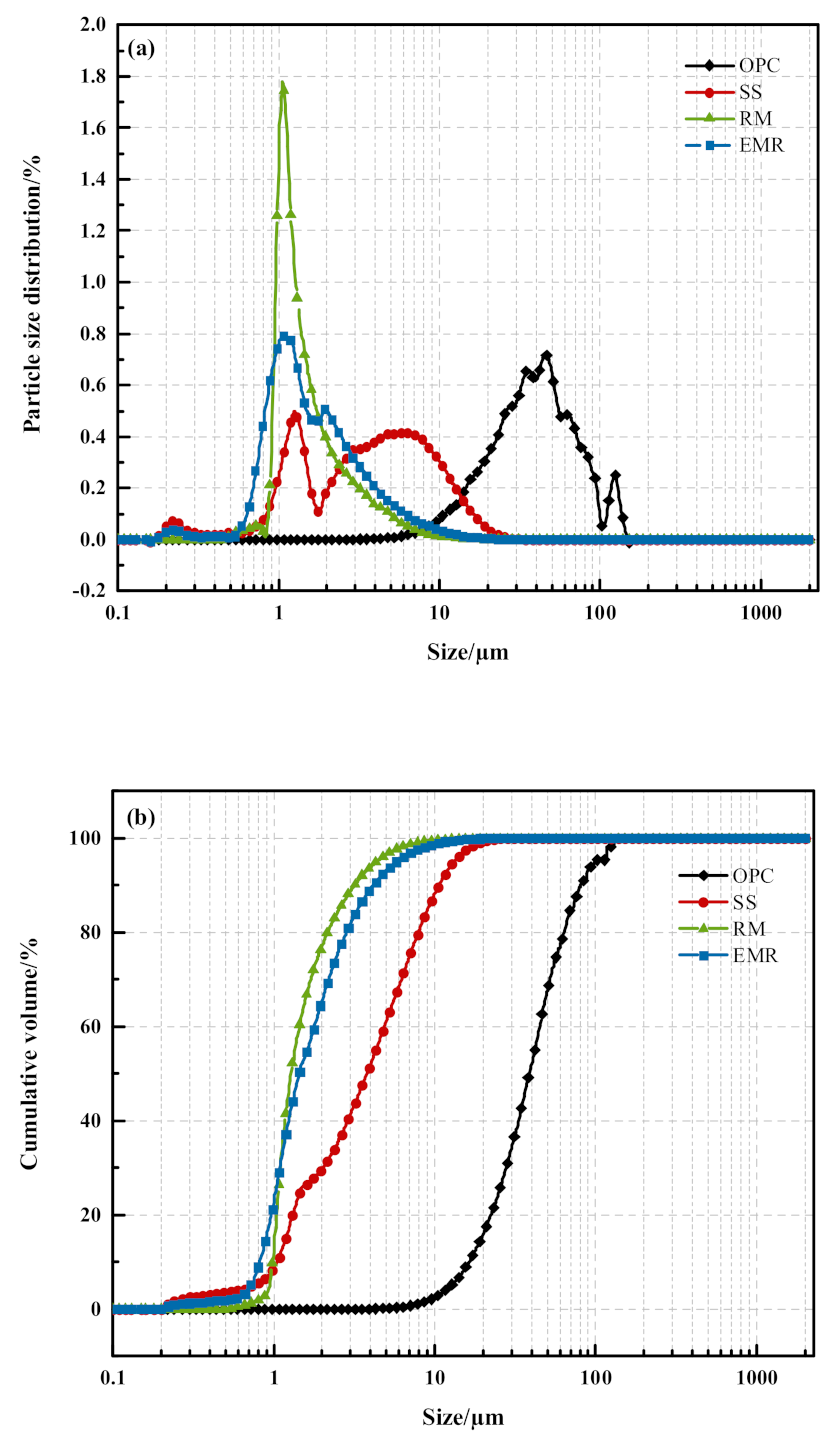



| Density (kg/m3) | Specific Surface Area (m2/kg) | Flexural Strength (MPa) | Compressive Strength (MPa) | |||||
|---|---|---|---|---|---|---|---|---|
| 3 d | 7 d | 28 d | 3 d | 7 d | 28 d | |||
| OPC | 3.15 | 356.50 | 5.2 | 6.4 | 7.2 | 24.2 | 35.6 | 55.1 |
| Fe2O3 | Al2O3 | TiO2 | SiO2 | CaO | MgO | Na2O | K2O | MnO | SO3 | Other | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| OPC | 3.71 | 5.64 | 0.30 | 24.76 | 60.25 | 0.88 | 0.16 | 0.70 | 0.32 | — | 3.28 |
| SS | 24.15 | 5.66 | 0.57 | 26.95 | 29.67 | 5.33 | 0.09 | 0.08 | 5.39 | — | 2.11 |
| RM | 30.58 | 20.39 | 7.80 | 13.49 | 15.82 | 0.33 | 9.15 | 0.19 | 0.14 | — | 2.11 |
| EMR | 5.45 | 7.43 | 0.55 | 28.21 | 18.73 | 3.66 | 0.04 | 2.43 | 4.18 | 28.52 | 0.80 |
| Sample | OPC | SS | RM | EMR | Sand–Binder Ratio | Water–Binder Ratio |
|---|---|---|---|---|---|---|
| A0 | 70 | 30 | 0 | 0 | 1:3 | 0.5 |
| A1 | 70 | 15 | 15 | 0 | ||
| A2 | 70 | 15 | 12 | 3 | ||
| A3 | 70 | 15 | 9 | 6 | ||
| A4 | 70 | 15 | 6 | 9 | ||
| A5 | 70 | 15 | 3 | 12 | ||
| A6 | 70 | 15 | 0 | 15 |
| Sample | Second Exothermic Peak/h | Third Exothermic Peak/h | 72 h Total Heat Release /J·g−1 |
|---|---|---|---|
| OPC | 10.0 | — | 258.5 |
| A0 | 10.9 | — | 225.0 |
| A1 | 8.2 | — | 227.7 |
| A2 | 9.2 | — | 246.2 |
| A3 | 9.5 | 17.9 | 236.6 |
| A4 | 10.0 | 26.2 | 237.3 |
| A5 | 10.1 | 46.1 | 228.9 |
| A6 | 9.9 | — | 199.5 |
| Sample | Mass Loss/% | ||
|---|---|---|---|
| 60~300 °C | 400~600 °C | 600~750 °C | |
| OPC | 9.31 | 8.02 | 9.18 |
| A0 | 21.81 | 15.88 | 27.97 |
| A1 | 35.48 | 30.45 | 3.28 |
| A2 | 26.22 | 27.89 | 4.27 |
| A3 | 25.26 | 32.45 | 10.94 |
| A4 | 24.58 | 23.02 | 10.10 |
| A5 | 13.45 | 19.87 | 12.82 |
| A6 | 19.73 | 23.29 | 19.31 |
| Sample | Porosity/% | Pore Size Distribution/% | |||
|---|---|---|---|---|---|
| <20 nm | 20~50 nm | 50~200 nm | >200 nm | ||
| OPC | 18.25 | 9.31 | 8.02 | 9.18 | 73.49 |
| A0 | 15.90 | 21.81 | 15.88 | 27.97 | 34.34 |
| A1 | 14.13 | 35.48 | 30.45 | 3.28 | 30.79 |
| A2 | 16.80 | 26.22 | 27.89 | 4.27 | 41.62 |
| A3 | 14.98 | 25.26 | 32.45 | 10.94 | 31.35 |
| A4 | 18.21 | 24.58 | 23.02 | 10.10 | 42.30 |
| A5 | 21.13 | 13.45 | 19.87 | 12.82 | 53.86 |
| A6 | 19.16 | 19.73 | 23.29 | 19.31 | 37.67 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, L.; Chen, P.; Hu, C.; Xia, H.; Liang, Z. Study on the Mechanical Properties and Hydration Behavior of Steel Slag–Red Mud–Electrolytic Manganese Residue Based Composite Mortar. Appl. Sci. 2023, 13, 5913. https://doi.org/10.3390/app13105913
Zhou L, Chen P, Hu C, Xia H, Liang Z. Study on the Mechanical Properties and Hydration Behavior of Steel Slag–Red Mud–Electrolytic Manganese Residue Based Composite Mortar. Applied Sciences. 2023; 13(10):5913. https://doi.org/10.3390/app13105913
Chicago/Turabian StyleZhou, Libo, Ping Chen, Cheng Hu, Haiyang Xia, and Zhifeng Liang. 2023. "Study on the Mechanical Properties and Hydration Behavior of Steel Slag–Red Mud–Electrolytic Manganese Residue Based Composite Mortar" Applied Sciences 13, no. 10: 5913. https://doi.org/10.3390/app13105913
APA StyleZhou, L., Chen, P., Hu, C., Xia, H., & Liang, Z. (2023). Study on the Mechanical Properties and Hydration Behavior of Steel Slag–Red Mud–Electrolytic Manganese Residue Based Composite Mortar. Applied Sciences, 13(10), 5913. https://doi.org/10.3390/app13105913






