Abstract
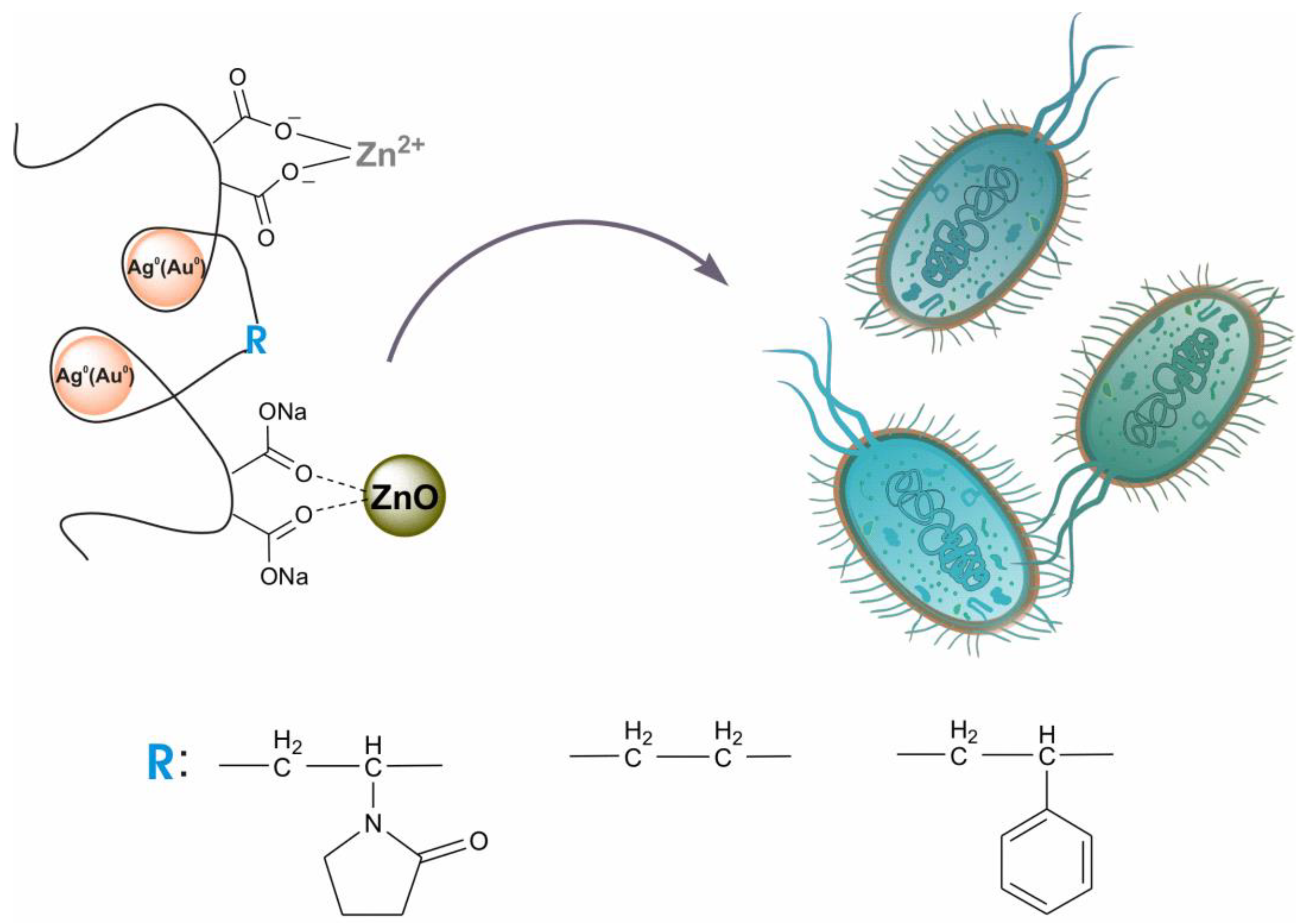
A simple one-pot method is proposed for obtaining the colloidal nanohybrid structures of silver (gold) and zinc oxide as well as nanostructures doped with zinc ions. The copolymers of maleic acid were used for the stabilization of nanoheterostructures. To characterize the preparation, UV–Vis spectroscopy, TEM, FTIR, XPS, and XRD were used. The bactericidal properties of the nanoheterostructures were studied in relation to the fungus C. albicans and the bacteria E. coli and S. aureus, used in planktonic form. In general, the samples containing nanosilver were the most active, and the preparations containing gold nanoparticles were the least active. The minimum inhibitory concentrations (MICs) of the Ag/ZnO samples, based on all copolymers, were in the ranges of 1.4–1.7 μg/mL for C. albicans, 2.9–6.8 μg/mL for E. coli, and 23–27 μg/mL for S. aureus; the MIC values of Au/ZnO samples were 472 μg/mL for S. aureus and 945 μg/mL for C. albicans and E. coli. The additional introduction of zinc cations into heterodimers had practically no effect on the antimicrobial properties of the composites. For all prepared composites and all tested microorganisms, the fractional inhibitory concentration indexes were in the range of 0.5–2.2, which indicates a close-to-additive contribution of the bioactive components in the samples used in the bactericidal process.
1. Introduction
Due to their unique physicochemical properties, nanotechnological products are widely used in biotechnology [1,2,3,4]. In the antibiotics-resistant era, nano-antibiotics are potential nano-weapons in the fight against multidrug-resistant pathogens and even biofilms [5,6,7,8,9]. The results of using metal nanoparticles, their compounds, and their various combinations as antibacterial agents are very encouraging, since such structures are active against protozoa, fungi, bacteria, and viruses. Materials with antiseptic properties containing nanoparticles of silver or cations of copper, zinc, iron, manganese, etc., are known and widely used. Silver nanoparticles (AgNPs), known for their antimicrobial properties, are used as food additives (E 174). Silver, unlike conventional antibiotics, practically does not cause resistance to a wide range of pathogens [10]. Numerous reviews have been published on the production and use of AgNPs [11,12,13,14,15]. As for the mechanism of the toxic effect of AgNPs on microorganisms, a number of researchers believe that this occurs due to the release of silver ions from nanoparticles depositing corresponding cations [16,17]. Silver ions may bind to the phosphorous-containing groups of nucleic acids in microorganism cell nuclei or the sulfhydryl and carboxylic groups of bacterial enzymes. They can produce reactive oxygen species (ROSs) inside the pathogen cell, while disrupting the activity of the electron transport chain inside the cell. These factors are the cause of the suppression of vital activity and the colonization of bacteria [18,19,20]. Unlike silver nanoparticles, gold nanoparticles (AuNPs) are much less often used as bactericides. For example, AuNPs were found to have an almost negligible effect on E. coli, S. aureus, B. subtilis, and A. tumefaciens [21,22]. Greater bactericidal activity was observed for silver nanoparticles than for AuNPs [23,24,25,26,27].
Gold NPs exert their antibacterial activities in two predominant ways: one is to collapse the membrane potential, inhibiting ATPase activities to decrease the ATP level; the other is to inhibit the subunit of ribosome from binding to tRNA. Gold NPs enhance chemotaxis in an early-phase reaction. The actions of gold NPs do not include a reactive oxygen species (ROS)-related mechanism, which is the cause for cellular death induced by most bactericidal antibiotics and nanomaterials [28]. The disruption of the cell membrane of the P. aurigenosa and the uncontrolled leakage of cell DNA was reported after interacting with 4,6-dihydroxyl-2-pyrimidinethiol-functionalized cationic 3 nm AuNPs [29].
Gold nanoparticles were also tested against pathogenic microorganisms in combination with antibiotics. It was shown, for example, that vancomycin hybrid AuNPs showed better antibacterial activity than free vancomycin [30]. As for the toxicity of silver and gold nanoparticles, a comparative assessment was carried out on the adverse bioactivity of virtually equidimensional gold and silver nanoparticles that were administered to rats either intratracheally or intraperitoneally at equal mass doses. It was demonstrated that AgNPs were more toxic than AuNPs, notwithstanding the latter’s higher accumulation in RES-abundant organs [31]. In addition, gold has a low toxicity to biological systems, followed by silver, whether the systems are bacterial, animal, or human [32]. In other words, although silver nanoparticles have been shown to be more toxic than AuNPs, AgNPs have a relatively low toxicity to human cells in comparison with lower forms of life [31,33,34,35].
Zinc oxide NPs (ZnO NPs) and zinc cations are also among the most studied antibacterial agents. It is believed that ZnO NPs are biocompatible and nontoxic [36]. ZnO NPs are more active against S. epidermidis, S. pyogenes, S. aureus, and B. subtilis than MgO, TiO2, Al2O3, CuO, and CeO2 NPs [37]. It should be noted here that ZnO NPs have a minimal impact on human cells and have a highly specific toxicity against planktonic forms of bacteria and biofilms. The mechanism of the bacteriolytic action of ZnO NPs is explained by the formation of ROS, which damages the cell wall of the pathogen [38]. The use of ZnO micro- and nanoparticles in biotechnology, the food industry, and medicine is well known [39,40,41]. For example, they are used as a food additive and packaging component to protect against spoilage [42], in remedies for fungal infections or acne [43], and as a wound-healing stimulant that also keeps wounds moist and clean. Zinc cations are less bactericidal but less toxic compared to Ag cations and are also used in biomedicine [24]. Zinc sulfate and chloride are used in dental materials, such as cements, filling and impression materials, and dentifrice pastes [44]. The combination of zinc ions with a cholera vaccine containing a recombinant cholera toxin subunit had a positive effect on the course of both cholera and diarrhea in children [45]. The mechanism of action of ZnO NPs on microbial cells is similar to that of AgNPs. They can also be considered as a source of metal cations, which can be released under physiological conditions. Zinc ions released from ZnO NPs in the acidic environment of lysosomes interact with biomolecules in the bacterial cell, causing its death. Thus, these cations are bioactive, and the apoptosis of pathogen cells is associated with them [46,47]. Another hypothesis suggests that the death of microbial cells exposed to zinc oxide nanoparticles is due to the breakdown of proteins and lipids by ZnO NPs, which leads to the destruction of bacterial cells due to the leakage of intracellular contents [48].
It is known that zinc ions are essential nutrients for microbial growth [49]. However, under certain conditions, concentrations and combinations with a number of components, zinc cations are able to exhibit an antiviral and antibacterial effect [50,51,52]. Therefore, the combination of pyrithione (PT) and Zn2+ at concentrations of 2 μM Zn2+ and 2 μM PT inhibits the replication of RNA viruses: poliovirus, influenza virus, and SARS-CoV-2 (coronavirus). Zinc complexes with compounds of the fluoroquinolones such as levofloxacin and ciprofloxacin have higher bactericidal activity against K. pneumoniae, S. aureus, E. coli, and B. dysenteriae than original antibiotics [53,54]. Zinc-bound vancomycin implements were found to increase activity against bacteria which were resistant to vancomycin itself [55]. A combination of Ag+ and Zn2+ ions led to a synergistic antibacterial effect against E. faecalis [56].
When combining various antibacterial components, their additive or synergistic complicity is often revealed, which leads to a decrease in the therapeutic dose of the preparation and in some cases, to a cheaper drug due to the introduction of a cheaper bioactive component into the composites [57,58,59,60,61]. Similar effects were observed when using heterostructures, in particular Ag/ZnO composites. Various complexes based on Ag/ZnO have not only bactericidal [62,63,64,65] but also antiviral [66] activity.
As for the ways for obtaining such heterostructures, the one-pot method seems attractive, in which precursors (metal cations) are simultaneously introduced into the reaction system. One-pot syntheses of Au/ZnO hybrids at high temperature in the presence of hexamethylenetetramine, oleylamine, hydrazine hydrate, or in 1-dodecanol have been described previously [67,68,69,70,71].
Thus, the above-mentioned processes for the formation of Ag(Au)/ZnO heterodimer systems are quite laborious: high temperature and pressure, and a long synthesis time are used. As a rule, when using these methods, spontaneous formation of cluster structures occurs and, as a result, water-insoluble composites are obtained. These factors pose serious limitations for the wide application of such composites in biomedicine, laboratory settings, and at industrial scales especially.
For practical applications as bactericidal and antibiotic drugs, the simplicity of their preparation and their ability to dissolve in water are important properties. Thus, taking into account the potential use of Ag(Au)/Zn complexes as bactericides, a facile efficient and inexpensive route for obtaining such colloidal water-dispersed nanoheterostructures is still required. Nanoparticles of metals, their oxides, and complexes have a natural tendency to agglomerate in colloidal solutions. Surface protection of nanoparticles using various stabilizers increases their kinetic stability [72]. Polymers are most often used to stabilize nanoparticles. In particular, organic polymers can act as stabilizers of bioactive nanoparticles, preventing their aggregation by reducing their surface energy [59]. Water-soluble polymers can promote the binding of metal nanoparticles to the pathogen cell due to highly active potential interactions between repeating polymer units and multivalent surface properties of bacteria [73]. Previously, polyvinylpyrrolidone was used to obtain heterostructures, but through a two-step process [74]. This polymer was used to promote the deposition of Zn oxide onto AuNPs.
Growing oxide shells on seed nanoparticles requires the control of several processes: (a) the nucleation and growth of the shell material; (b) the “wetting” of the shell material on the seeds; and (c) the aggregation of nanoparticles [75]. Synthesis of a ZnO-Ag heterostructure was carried out by a precipitation method with cellulose nanofibers as a stabilizer (with the formation of Ag nanoparticles on ZnO particles) [76]. Using dextran as a reducing agent and stabilizer, ZnO-Au NPs composites with petal-like morphology were synthesized [77].
In comparison with a number of natural and synthetic polymers, maleic acid (anhydride) copolymers have a complex of positive properties. Maleic acid residues contained copolymers have a regular structure; they are water soluble, amphiphilic, available, and nontoxic. Previously, such copolymers were used to stabilize silver nanoparticles [78]. It was shown that such nanocomplexes can be used as antimicrobial agents in combination with traditional antibiotics [57] or silver cations [79]. AgNPs have also been incorporated in a phenol-containing polymer [60] and into preparations containing specific lectin markers on the surface of pathogen cells [80].
The purpose of this study was to develop a convenient synthesis of silver (gold)/ZnO colloidal nanostructure which are stabilized by maleic acid copolymers, and to dope these nanostructures with zinc ions for use as antimicrobials.
2. Materials and Methods
2.1. Materials
Poly(N-vinyl-pyrrolidone-alt-maleic anhydride) (VM-anh.) M = 40,000 was synthesized according to [81]. Poly(ethylene-alt-maleic anhydride) (EM-anh.) M = 25,000 (Monsanto, Saint Louis, MO, USA) and poly(styrene-alt-maleic anhydride) (SM-anh.) M = 50,000 (Sterlitamak chemical plant, Sterlitamak, Russia) were purchased. The copolymers of maleic acid were obtained after hydrolysis of copolymers of maleic anhydride and designated VM, EM, and SM, respectively. NaBH4, HAuCl4·3H2O, and ZnSO4·7H2O were the products of Sigma Aldrich; and AgNO3, NaOH are Reahim products. All reagents were of an analytical grade and were used without purification. The tested microorganisms were as follows (“BD MicrotrolTM”, Becton Dickinson, Diagnostic Systems Product Catalog for Industrial Microbiology, Heidelberg, Germany): Escherichia coli (E. coli) NCTC 11954/ATCC 35218—Gram-negative, facultative anaerobic bacteria; Staphylococcus aureus MRSA (S. aureus MRSA) NCTC 12973/ATCC 43300—Gram-positive bacteria, resistant to oxacillin; Candida albicans (C. albicans) NCPF3255/ATCC 10231—yeast-like fungi.
2.2. Instrumentation
Bench meters (Hanna, Vöhringen, Germany) HI 2211 were used for pH and temperature registration. UV-Vis spectra were measured with Hitachi U-5100 spectrophotometer (Japan). LEO 912 AB microscope (Omega, Karl Zeiss, Jena, Germany) operated at voltage of 100 kV was used to record transmission electron microscopy (TEM) micrographs. To obtain a TEM micrograph, a drop of the investigated solution was evaporated on Formvar-coated copper grid. Fourier transform infrared spectra (FTIR) of the solid samples (KBr) were recorded using a Magna IR-720 Fourier spectrometer Magna IR-720 (Nicolet, Parsons W118, USA). Compositions of the preparations were determined in the laboratory for microanalysis of INEOS RAS. Powder X-ray diffractometry (XRD) phase analysis of the samples placed on flat holders was carried out with a diffractometer D8 Advance (Bruker AXS, San Jose, CA, USA) in the Bragg–Brentano focusing geometry using CuKα radiation, the scan rate was 0.5° min−1 and angular step was 0.02°. Powder patterns were processed with DIFFRACplus EVA (Bruker AXS GmbH DIFFRAC.EVA, Bruker AXS GmbH, Karlsruhe, Germany, 2011), and Bruker AXS software version number 6.0.0.6; the search/match procedure was implemented. The Lvol-IB formalism was used for evaluating the values of mean crystalline size [82]. The content of crystal phases of the heterodimers was calculated using DIFFRAC TOPAZ software (Coelho, A. TOPIC 5.0, Bruker AXS GmbH, Karlsruhe, Germany, 2012; Bruker AXS uses Rietveld refinement of collected X-ray data).
The samples in powder form were characterized by X-ray photoelectron spectroscopy (XPS) in an Axis Ultra DLD (Kratos Analytical, Manchester, UK) spectrometer with monochromatic Al Kα (hν = 1486.6 eV) radiation source operated at 150 W at room temperature under ultrahigh vacuum conditions (<10−8 Torr). High-resolution spectra were measured with 40.0 eV pass energy and 0.1 eV step. The analysis area was 300 μm × 700 μm. The energy scale of the spectrometer was calibrated using the following values: Au 4f7/2–83.96 eV, Ag 3d5/2–368.21 eV, and Cu 2p3/2–932.62 eV. An electron neutralizer was used to compensate for the surface charging. For an internal charge reference, the C 1s spectra were fitted to identify the C–C/C–H state which was assigned a binding energy of 285.0 eV.
2.3. Methods
2.3.1. Preparation of Polymer-Stabilized AgNPs, AuNPs, and Heterodimers
Preparation of VM/Ag0, EM/Ag0, and SM/Ag0
Synthesis of nanosized silver was described elsewhere [78]. Briefly: To the solution of copolymer of maleic acid (0.01 M, here the molar concentration of copolymer refers to the maleic acid units) in deionized (DI) water at pH 7 (titration with NaOH solution, 5%), a freshly prepared solution of AgNO3 (0.1 M) (for the ratio of reagents 1:1 (mol.)) was added with vigorous stirring. Then, after about 5 min, a freshly prepared solution of NaBH4 (0.1 M, 2-fold molar excess with respect to silver ions) in DI water was added to the reaction solution with vigorous stirring. The reaction mixture was then stirred for 24 h at room temperature. The samples of polymer stabilized silver nanoparticles were dried using lyophilization (0.05 mbar, −55 °C) after ultrafiltration (YM5 membrane (“DIAFLO”, AMICON CORPORATION, Palo Alto, CA, USA).
Preparation of VM/Au0
Nano-sized gold was synthesized by the reduction of gold ions at ~20 °C with NaBH4 in the presence of copolymer of maleic acid following the procedure [83]. Briefly: To the solution of the VM (0.007 M) in DI water at pH 6 (titration with water solution of NaOH, 5%), the freshly prepared solution of HAuCl4·3H2O (0.01 M) (for the ratio 1:5 of reagents, respectively) was added with vigorous stirring. Then, after about ten minutes, a freshly prepared solution of NaBH4 in DI water (0.1 M, in 3-fold molar excess with respect to metal ions) was added to the reaction solution with vigorous stirring. The reaction mixture was then stirred for 24 h at room temperature. The dried sample was obtained using lyophilization (0.05 mbar, −55 °C) after ultrafiltration (or dialysis) of the reaction solution.
Synthesis of VM/Au0/ZnO
A measure of 20 mL of VM solution (0.007 M, pH 8), 0.322 mL ZnSO4·7H2O (0.1 M), 2.1 mL HAuCl4·3H2O (0.01 M) in DI water, and 0.4 mL NaOH (1 M) were mixed in glass vial with a screw-caps tightly closed. The pH of the reaction was 12. Then, the test vial was kept in a boiling water bath for 3 h. The molar ratio of units of maleic acid residues of copolymer to gold cations and zinc cations was 1/0.15/0.23. After cooling to room temperature, the solution was left overnight at this temperature and then was dialyzed against DI water and lyophilized (0.05 mbar, −55 °C).
Synthesis of VM (EM, SM)/Ag0/ZnO
The solutions of ZnSO4·7H2O (0.262 mL, 0.1 M), VM (25 mL, 0.007 M), AgNO3 (0.52 mL, 0.1 M), all in DI water, and NaOH (0.325 mL, 1 M) were mixed in glass vial with a screw-caps tightly closed. After shaking (the pH of solution was 12), the test vial was kept in a boiling water bath for 6 h. The molar ratio of units of maleic acid residues of copolymer to silver cations and zinc cations was 1/0.3/0.15. The samples EM/Ag0/ZnO and SM/Ag0/ZnO were synthesized at the same temperature and at the same molar ratio of reagents. The reaction time for these preparations was 7 h. Samples containing zinc ions were prepared with the addition of a zinc salt to the copolymer- or heterodimer-contained copolymer. The samples were dialyzed against DI water or ultrafiltered (YM5 membrane, “DIAFLO”, Amicron Corporation, Palo Alto, CA, USA) and then lyophilized (–55 °C, 0.05 mbar).
2.3.2. Antimicrobial Activity Tests
The determination of the minimum inhibitory concentrations (MICs) of the preparations in relation to the strain of microorganisms used was carried out using a serial microdilution method in accordance with the standard procedure [84]. Initial concentrations of VM/Ag0, SM/Ag0, and EM/Ag0 were 1.35, 1.35, and 1.5 mg/mL, respectively; VM/Ag0/ZnO, SM/Ag0/ZnO, and EM/Ag0/ZnO were 1.9, 1.9, and 1.4 mg/mL, respectively; and VM/Au0 and VM/Au0/ZnO were 1.7 and 1.8 mg/mL, respectively. The description of the experiment is given in the protocol published elsewhere [79]. Briefly: The tests were carried out in 0.2 mL volume using sterile 96-well standard polystyrene plates. The final concentration of the investigated microorganism is 105 CFU/mL. Trypticase-soy broth (Bio-Rad Laboratories, Hercules, CA, USA) was poured into 0.1 mL in each well of the plate. The number of wells was determined by the necessary dilution range, the last well was left for setting negative control. The solutions of studied composites in fourfold ratio to the above-mentioned concentrations in the volume of 0.1 mL were pipetted into the first well containing 0.1 mL of broth.
After mixing, 0.1 mL of this mixture was pipetted to the second well, also initially containing 0.1 mL of broth and so on. The procedure was repeated until a sufficient number of dilutions were made. For inoculation, a microbial suspension of test microorganisms prepared on 0.45% saline solution was used. A measure of 0.1 mL of the inoculum was introduced into testing wells with the appropriate dilution of the composite, as well as in the last well with a nutrient broth without the test preparation (positive control). The plates covered with a sterile film were incubated at 35 °C overnight (48 h for C. albicans). The culture growth in the presence of the composites was compared with the reference wells without the composites (positive control) under visual control (wells in plates were viewed in transmitted light). The MICs of the samples were determined by the lowest concentrations of preparations in the well, which suppress the apparent growth of the microorganisms used. Statistical processing of data based on the results of 3 measurements was carried out.
3. Results
Colloidal nanoheterostructures containing silver (or gold) and zinc oxide were prepared using the one-pot method and were used as potential bactericidal agents (Scheme 1). Maleic-acid-containing alternating copolymers with N-vinyl-2-pyrrolidone (VM), ethylene (EM), and styrene (SM) were used as matrices for binding precursor metal ions and stabilizing the resulting nanoparticles. The transformation of metal cations into nanoparticles was carried out by the hydrothermal method in an alkaline medium. For the production ZnO/Ag0 and ZnO/Au0 composites, the numbers of entered metal cations in terms of each maleic acid residue in the copolymers were practically equivalent: Zn2+/Ag+ = 0.15/0.3 and Zn2+/Au3+ = 0.23/0.15 (mol/mol).
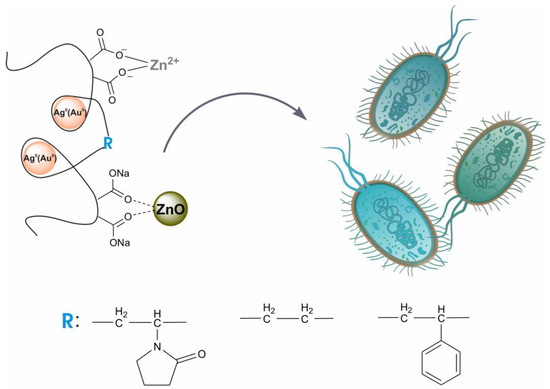
Scheme 1.
Maleic acid copolymers stabilized nanoheterostructures containing Ag(Au) NPs, ZnO NPs, and Zn2+ ions as antimicrobials.
The amount of zinc cations introduced into the composite was limited by the solubility of the polymer salt obtained and depends on the structure of the copolymer used. In addition, this limit turned out to be different for the initial copolymers and polymer complexes containing AgNPs. In the first case, the limit of salt stability is reached when 0.25 mol of zinc cations is introduced onto the copolymer link. When zinc cations are introduced into a solution of silver-containing nanocomplexes, this limit increases, and the ratio reaches 0.5 for VM and SM, and 1.0 for EM. This is due to the different conformation of the initial copolymers and nanocomplexes.
In our previous study [85], using the EM copolymer, we showed by diffuse light scattering (DLS) that this copolymer was characterized by a wide and bimodal particle size distribution (hydrodynamic radius, Rh = 50–300 nm, depending on ionic strength and pH) and an intermolecular association. In the study of the complex of this copolymer with EM/Ag0 silver nanoparticles by DLS, well-formed and narrowly dispersed (Rh = 105 nm) micelles were detected [85].
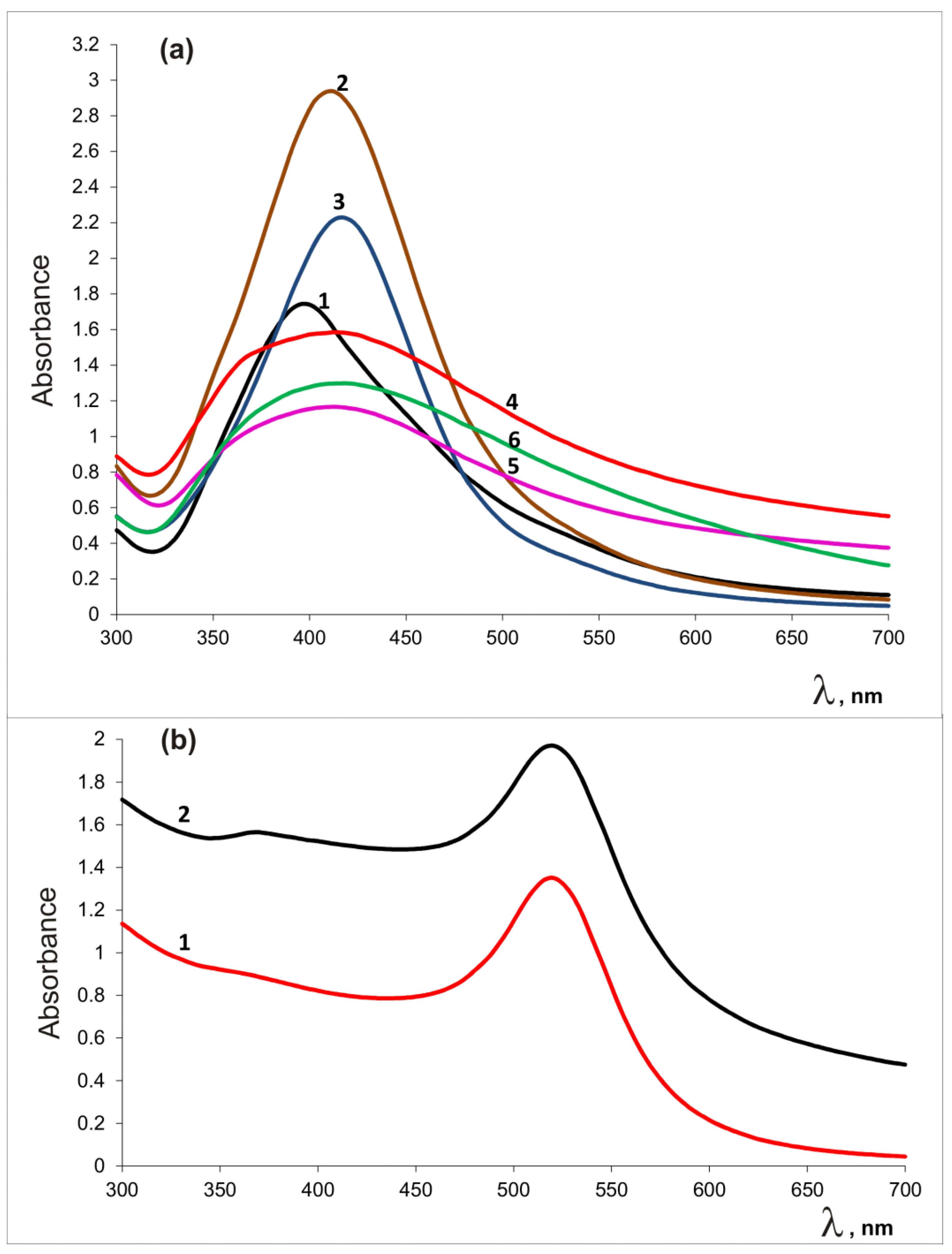
Simultaneous production of silver (gold) and zinc oxide nanoparticles was recorded by heating an alkaline solution containing noble metal and zinc salts. The course of conversion of metal cations to reaction products was monitored by UV-visible spectra. Metal oxide and noble metal nanoparticles have specific absorption bands in the visible region [86,87,88]. Figure 1 shows UV-Vis spectra of reaction products. The broad absorption band at 365–375 nm indicates the presence of ZnO; the peak at 406 nm and the band at 520 nm appeared due to surface plasmon resonance corresponding to the Ag0 and Au0 states, respectively.
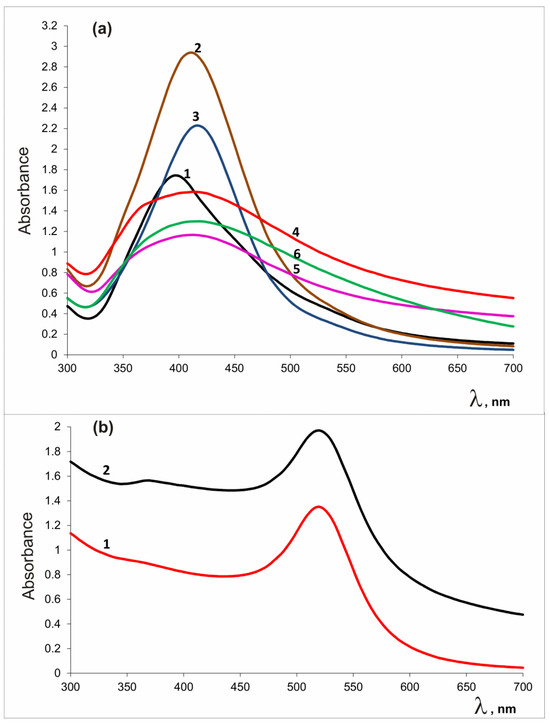
Figure 1.
UV-Vis spectra of AgNPs-containing preparations (a): VM/Ag0 (1), EM/Ag0 (2), SM/Ag0 (3), VM/Ag0/ZnO (4), EM/Ag0/ZnO (5), SM/Ag0/ZnO (6), and (b) gold-containing preparations: VM/Au0 (1), VM/Au0/ZnO (2).
In the presence of copolymer stabilizer VM, Au, and Zn cations under the conditions used, the formation of nanoparticles was almost complete after 2 h. In the case of use of Ag and Zn cations, and the VM copolymer, the reaction was practically completed in 6 h; for EM and SM, the reaction was practically completed after 7 h. Figure S1a–c (Supplementary Materials) shows FTIR spectra of polymer-stabilized heterostructures in comparison with those of the initial copolymers. This method allows you to make sure qualitatively that the preparations contain zinc oxide. The Zn-O stretching mode was observed in the 400–700 cm−1 range for ZnO crystals [87,89,90,91,92], so the region of 400–800 cm−1 is presented in detail. The bands at 625, 624, 551, and 544 cm−1 are stretching vibrations of Zn–O and Ag–ZnO bonds [87,89,90,91,92].
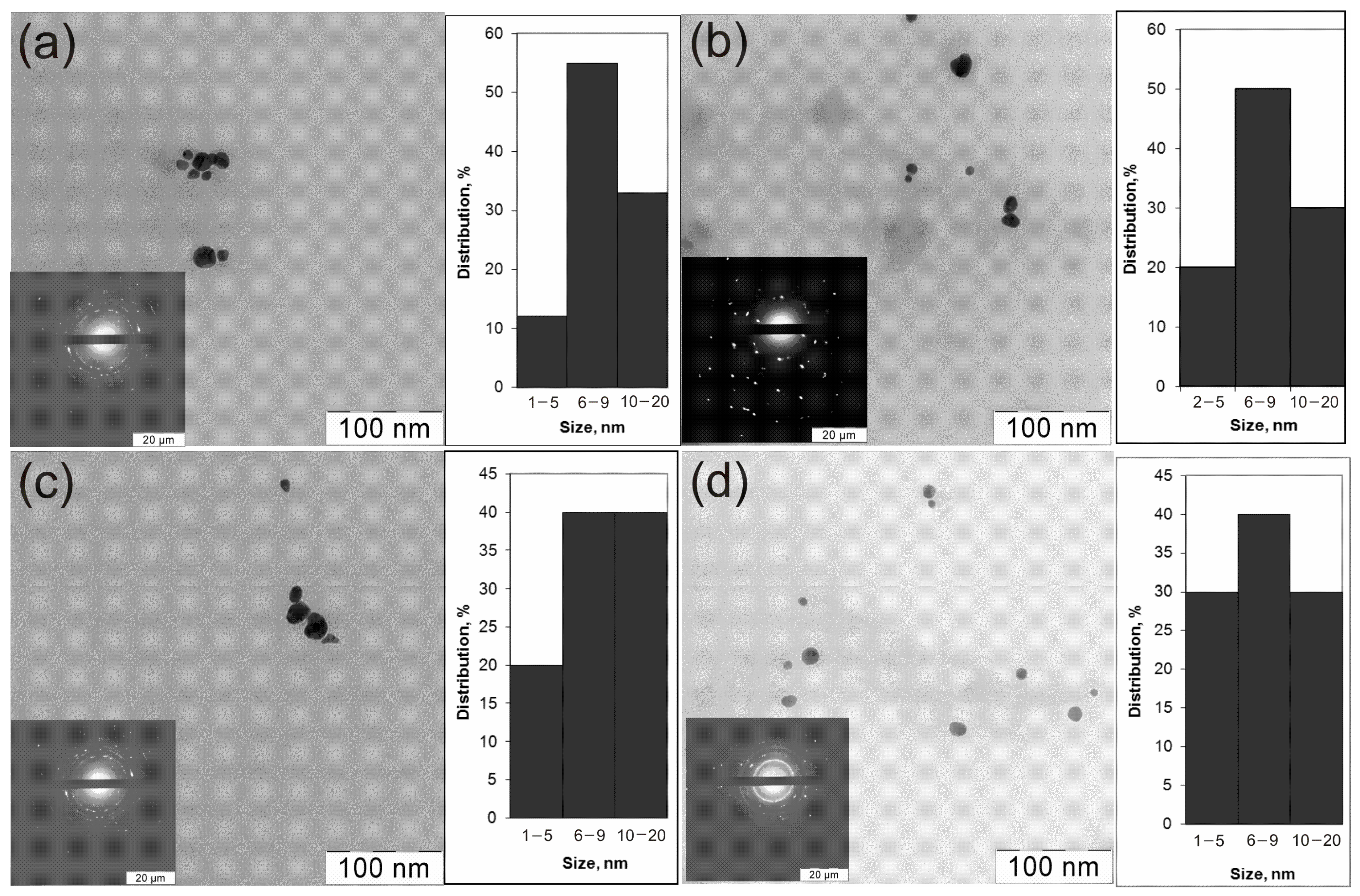
TEM micrographs of colloidal solutions of all prepared Au0/ZnO and Ag0/ZnO composites show relatively monodispersed nanoparticles, predominantly 6–20 nm in size with a shape close to a sphere (Figure 2). The size and shape of the particles shown in the micrographs did not depend on the nature of the polymers used as stabilizers of nanoparticles.
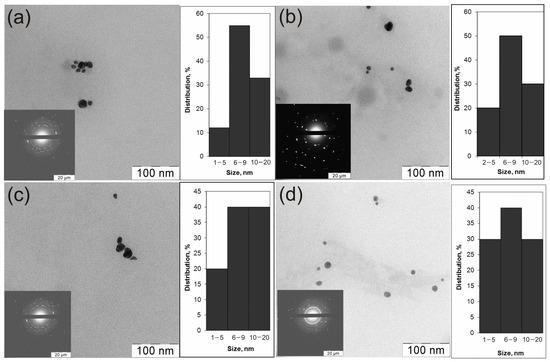
Figure 2.
TEM micrographs of the preparations: VM/Ag0/ZnO (a), EM/Ag0/ZnO (b), SM/Ag0/ZnO (c), and VM/Au0/ZnO (d).
Previously, similar images were recorded for heterodimers obtained in a different way [76]. In addition to the TEM, the XRD gives a more detailed qualitative and quantitative composition of the crystal phases of the heterodimers. SAED patterns demonstrate concentric rings with intermittent dots, indicating that these nanoparticles are crystalline in nature. Table 1 contains the XRD characteristics of the composites. The crystal structures of metal-containing components in the obtained noble metal–zinc oxide heterostructures stabilized by a copolymer were studied by powder XRD (Table 1, Figure S2).

Table 1.
Composition of the nanoheterodimers.
The X-ray examination of heterodimer samples showed the presence of the following crystalline phases: the cubic phase related to either silver or gold and the hexagonal phase of zincite (ZnO) with the average sizes of 6–9 and 16–20 nm (Table 1), respectively. The mean crystallite sizes of the nanoparticles were calculated from the broadening of the peaks using the Scherrer formula (Supplementary Materials).
The peaks corresponding to the Ag crystal phase are at ~38.1 and 44.2°; those corresponding to the Au crystal phase are at ~38.2 and 44.3°; those corresponding to the ZnO (zincite) phase are at ~31.8, 34.4, 36.1, 47.5, and 56.7. Their positions correspond to the Miller indexes: 111 and 002 for Ag(Au), and 100, 002, 101, 102, and 110 for ZnO (Figure S2).
The content of AgNPs in the crystalline phase of the corresponding samples VM/Ag0/ZnO, EM/Ag0/ZnO, and SM/Ag0/ZnO was 80–91% and zinc oxide was 8–20%. The gold-containing composite VM/Au0/ZnO contained 77.5% AuNPs and 22.5% zincite.
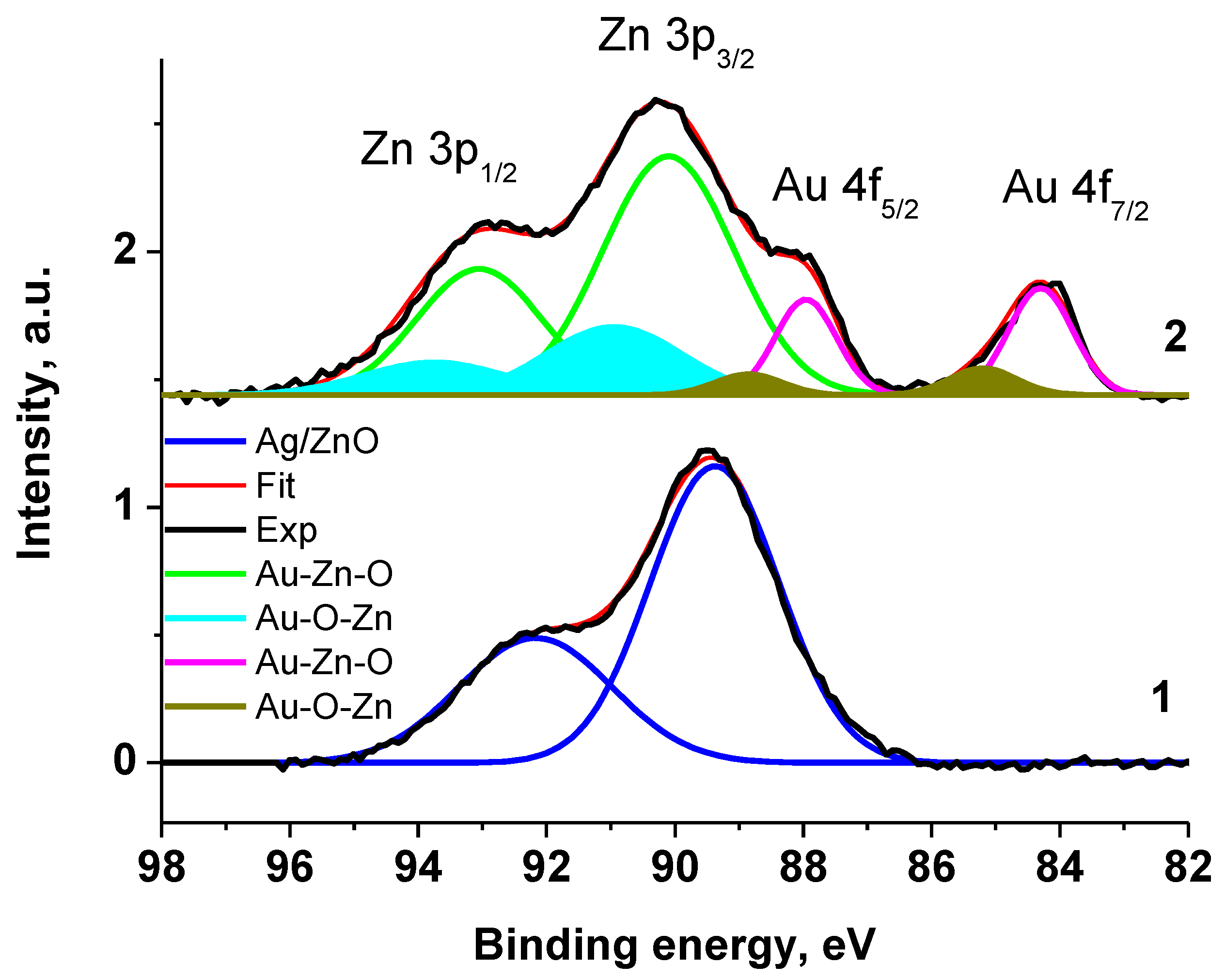
The chemical compositions of the samples VM/Ag0/ZnO and VM/Au0/ZnO were studied by XPS. The Au 4f7/2, Zn 2p3/2 a, and Zn 3p spectra are given in Figure 3 and Figure 4. In view of the overlap of the photoelectron spectra of Zn 3p and Au 4f core levels in the spectrum of the VM/Au0/ZnO composite and the presence of a satellite peak characteristic of the Zn 3p spectrum, the analysis of the spectra was started from the Zn 3p core-level of Ag0/ZnO nanoheterodimer stabilized with VM. The spectrum is presented as a sum of two Gaussian peaks at 89.4 and 92.25 eV, which are related to 3p3/2 and 3p1/2 sublevels with the 3p3/2/3p1/2 branching ratio of 0.5 and the satellite peak at 86.39 eV (subtracted for clarity). The latter describes a low-energy region and may be assigned to a specific energy loss [93,94,95]. The corresponding characteristics of the photoelectron spectrum given in Table S1 were used for approximation of the Zn 3p-Au 4f energy region of the VM/Au0/ZnO sample. For better perception, the satellite peaks associated with the energy losses of photoelectrons have been subtracted in Figure 3. The spectrum of Zn 3p core-level spectrum of the VM stabilized Au0/ZnO nanoheterodimer was approximated with two spin–orbit doublets and with the same constraints.
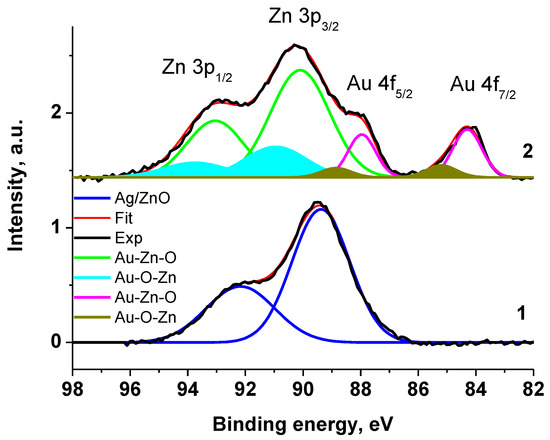
Figure 3.
The Zn 3p and Au 4f XPS spectra of Ag0/ZnO (1) and Au0/ZnO (2) structures stabilized with VM.
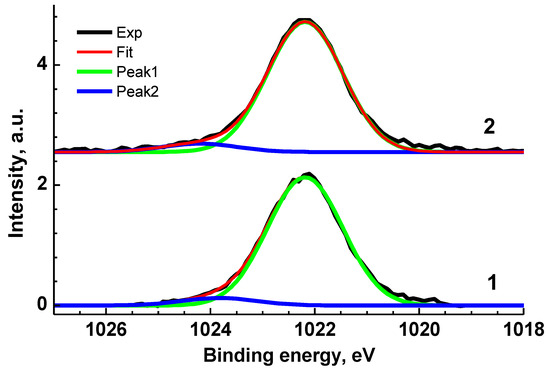
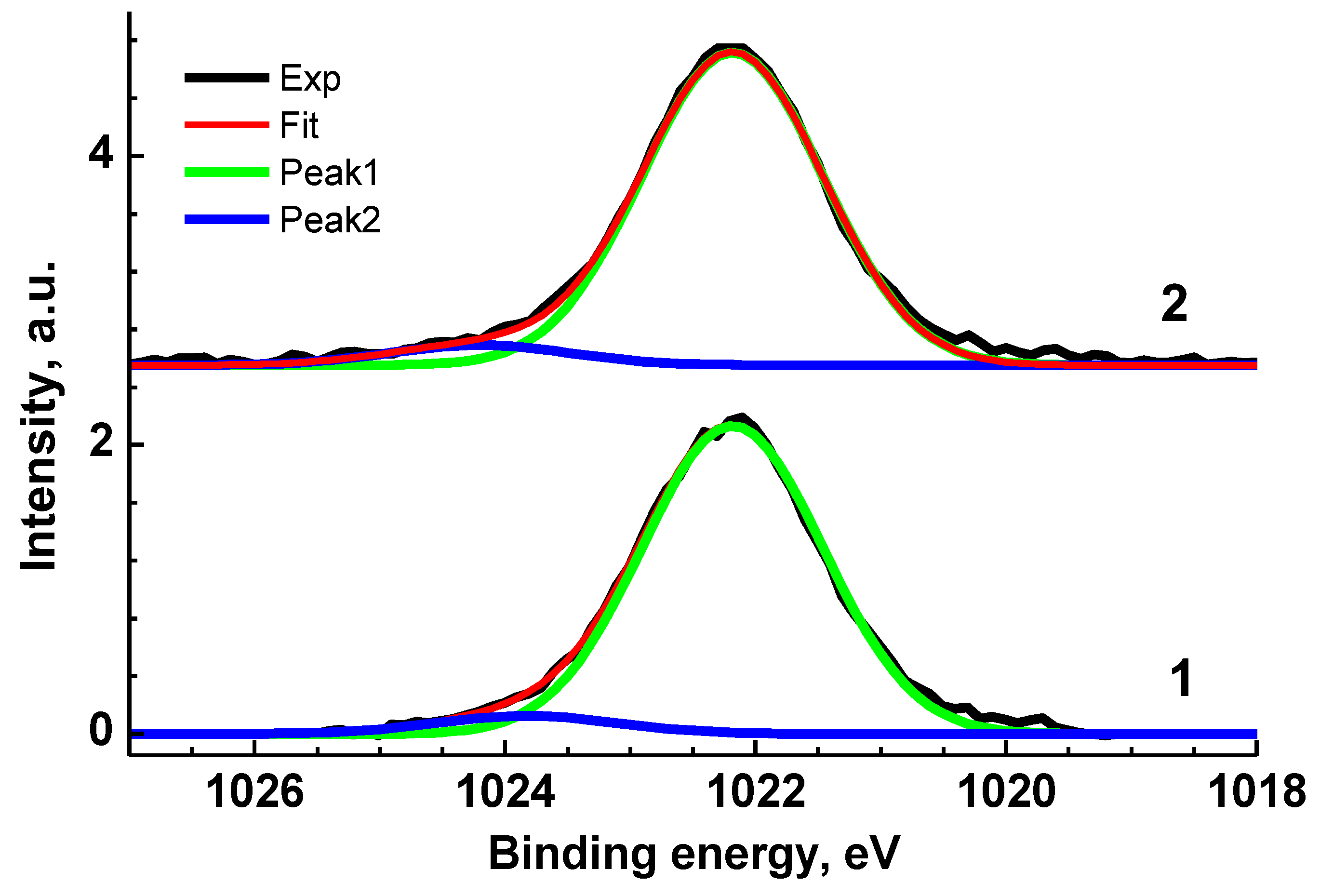
Figure 4.
Zn 2p3/2 XPS spectra of Ag0/ZnO (1) and Au0/ZnO (2) structures stabilized with VM.
The Zn 2p3/2 XPS spectra of VM/Ag0/ZnO and VM/Au0/ZnO samples (Figure 4) are close each other and may be presented as a sum of two Gaussian profiles at 1022.19 and 1024.20 eV, and 1022.20 and 1023.83 eV, respectively, with the same Gaussian width of 1.44 eV. As for the Au 4f spectrum, it was approximated with two 4f7/2–4f5/2 spin–orbit doublets with spin–orbit splitting of 3.65 eV and branching ratio of 1.33. The binding energies of 83.88 and 84.80 eV are attributed to the Au0 and Au+ states related to Au-Zn-O and Au-O-Zn structures, respectively [96,97,98]. Table S1 presents the characteristics of the Zn 3p and Au 4f spectra.
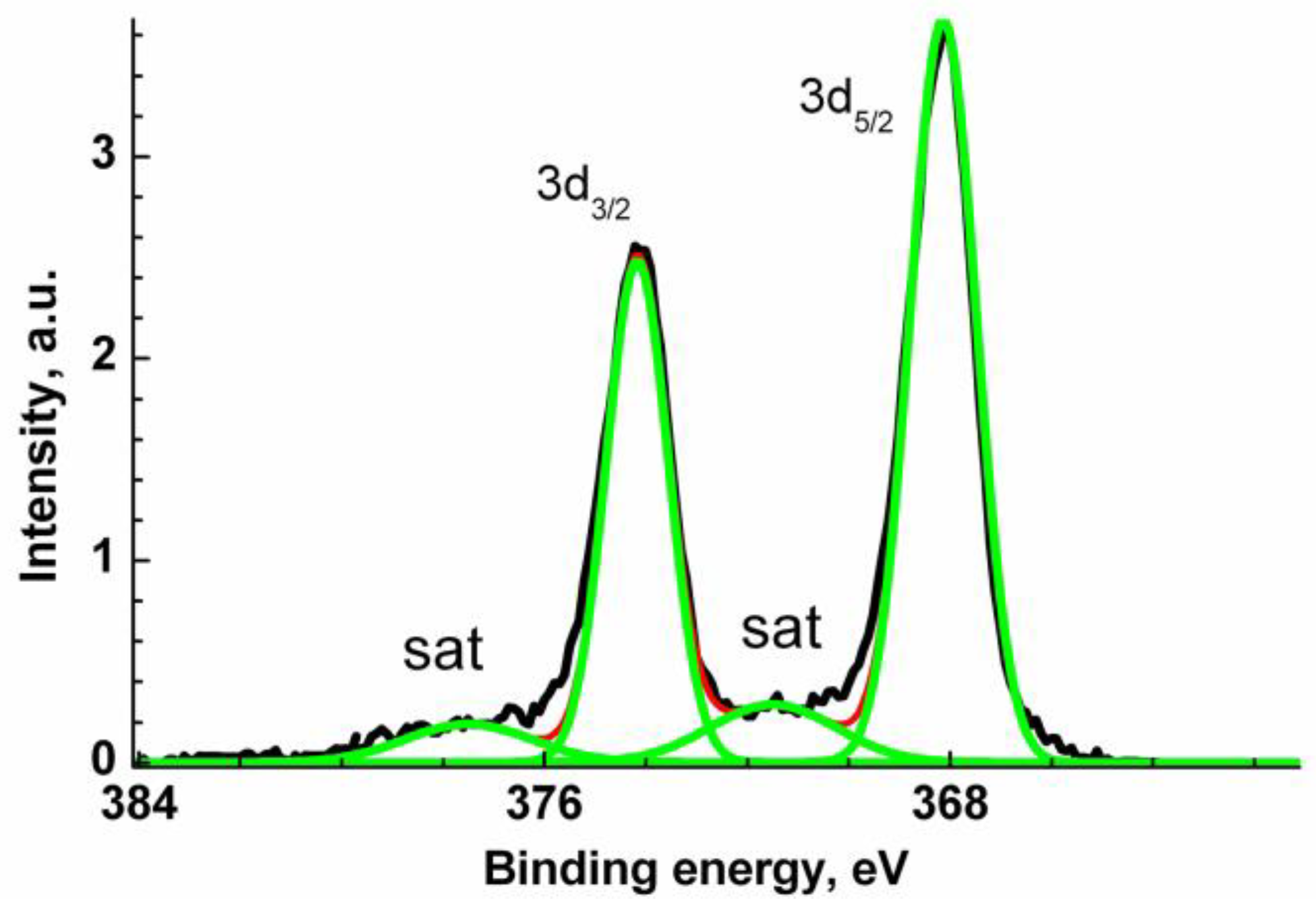
The Ag 3d XPS spectrum (Figure 5) is characterized with two main symmetric Ag 3d5/2 and Ag 3d3/2 peaks at 368.14 and 374.14 eV and two satellite peaks shifted relative to the main peaks by 3.4 eV. The latter are descriptors of Ag0 state. A negative CLS by 0.13 eV relative to the reference value of 368.27 eV may be attributed to Ag-Zn bonds [96]. The binding energies of 367.6 and 373.6 eV recorded for Ag-doped ZnO were assigned to Ag 3d5/2 and Ag 3d3/2 core levels and attributed to Ag-O bonds [93]. The change in the energy interval between the photoelectron peaks of various elements in the spectrum of a compound and elementary solids can serve as a measure of their interaction with each other. Such an interval does not depend on the charge reference and surface charging. Comparing the energy intervals between the Zn 2p3/2 and Ag 3d5/2 peaks for VM/Ag0/ZnO and Ag-doped ZnO [93], which are quite close, 654.2 and 654.1 eV, respectively, one can conclude that the similar chemical states of Ag atoms are similar. The corresponding O 1s, C 1s and N 1s XPS spectra are presented in Supplementary Materials (Figure S3).
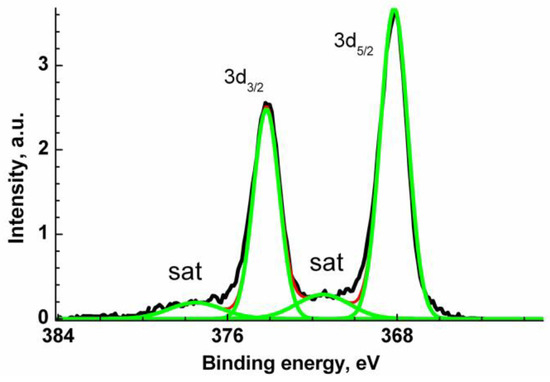
Figure 5.
Ag 3d XPS spectrum of Ag0/ZnO structure stabilized with VM.
Thus, the TEM, XPS, and XRD data indicate the formation of polycrystalline heterodimeric materials with a core–shell structure.
Table 2 presents the bactericidal properties of the obtained heterostructures in relation to Gram-positive S. aureus, Gram-negative E. coli bacteria, and C. albicans fungus used in planktonic form.

Table 2.
Antimicrobial properties of the samples (MIC—minimum inhibitory concentrations; FIC—fractional inhibitory concentration; FICI—fractional inhibitory concentration index).
All the samples obtained were active against the studied microbial strains. The MICs of samples containing AgNPs and zinc oxide for S. aureus were in the range of 44–122, for E. coli they were in the range of 11–31; for C. albicans they were in the range of 2.7–3.8 μg/mL. Thus, all preparations acted better on C. albicans and E. coli but not on S. aureus, as in publication [63], although there is evidence of a reverse trend [64,91]. The differences are associated with different ratios of the metal-containing components in the samples and the nature of the copolymer stabilizers. In general, the samples containing AgNPs were the most active, and the preparations containing gold nanoparticles were the least active. Polymer zinc salts also showed low activity. However, ionic zinc in the copolymer EM turned out to be more active against S. aureus than against E. coli, as it was shown for the complex of these cations with ε-poly-L-lysine [101], and showed almost identical activity in the case of maleic acid copolymers with other co-monomers. Apparently, the low activity of zinc cations is associated with the rapid inactivation of divalent cations when binding to cellular components. The increase in the MIC of heterodimers samples compared to samples containing only AgNPs is explained by a significant decrease in the content of a more expensive component, silver, due to the introduction of cheap and affordable zinc-containing components into its composition. Nevertheless, when studying the bactericidal properties of the obtained heterostructures, it was possible to achieve noticeable activity, especially in the case of using the EM copolymer as a stabilizer of nanoparticles. Moreover, this trend was observed for complex preparations that included zinc oxide in addition to nanometals. For samples containing AgNPs and zinc oxide, the MIC values under using different copolymers-stabilizers were close: 23–27 for S. aureus, 2.9–6.8—for E. coli, and 1.4–1.7 μg/mL—for C. albicans. In addition to the MICs of the samples, the MICs of the bioactive components present in the sample are given in terms of their content in the composite; this makes it possible to account for their mutual influence on the activity of the drug.
The degree of mutual influence of antimicrobial components—nanometals and zinc oxide (X and Y, respectively)—in their conjugates can be estimated by calculations of the fractional inhibitory concentration (FIC) and the fractional inhibitory concentration index (FICI).
where MIC(X,Y) is the MIC for X in combination with Y and MIC(X) is the MIC for X alone.
where MIC(Y,X) is the MIC for Y in combination with X and MIC(Y) is the MIC for Y alone.
For our preparations, FICI values were calculated using the following equation:
or , where
Me0 is Ag0 or Au0
The FICI values are interpreted as follows: if FICI ≤ 0.5, then the interaction of the active components of the system is defined as synergistic; if 0.5 < FICI < 1.0, then the interaction of the active components of the system is defined as additive [102]. According to other interpretation, FICI > 2.0 indicates antagonistic effects, while values from 0.5 to 2.0 characterize additive effects [103].
The revealed FICI values for all the studied objects and for all the strains used are mainly in the 0.5–2.2 range. This indicates a close-to-additive participation of bioactive components of the systems in the bactericidal process. These circumstances, apparently, can be explained by a similar mechanism of action the bactericides present in the samples on the cells of pathogens. The mechanism of antimicrobial action at the molecular level for composite Ag/ZnO was considered earlier [91].
It should be stressed here that the addition of ZnO NPs into the AgNP-containing system led, in most cases, to better results in comparison with the activity of the AgNPs preparations with added zinc cations (the FICI values were lower in the first case). This might be due to different mechanisms of action of zinc cations and zinc oxide, or the prolongation of the action of cations released from deposited in zinc oxide. Testing was carried out in the dark. It was previously shown that the inactivation of bacteria by zinc oxide follows different mechanisms in the dark and under illumination. In the dark, the action of Zn2+ ions were manifested, while under illumination, mainly the process of photocatalytic injection of electrons took place [104]. For composites containing an EM copolymer, the joint action of the metal-containing components of the system was close to synergistic, and the FICI values were close to 0.5 for all microorganisms used. Apparently, this can be explained by the difference in the structure of the polymer chains of the copolymers used. With the additional introduction of zinc cations into the Ag/ZnO heterostructures, the FICI in terms of Ag/ZnO and Ag/Zn2+ pairs practically did not change, which indicates that there is no expediency of introducing a third bactericidal component into the system.
4. Conclusions
Water-soluble complex nanostructures of silver (gold) with zinc oxide, stabilized by biocompatible nontoxic copolymers of maleic acid, and nanostructures doped with zinc ions were obtained through the use of a one-pot method without using a high-temperature regime. The proposed method is easy to implement; it is carried out in an alkaline environment in the absence of difficult-to-remove organic solvents and additionally introduced reagents. The obtained samples can be stored in dry form. Our preparations turned out to be interesting not only in terms of their formation, but also as antimicrobial agents in relation to representatives of conditionally pathogenic microorganisms. AuNP-containing preparations were noticeably less active than Ag-containing composites. It was possible to achieve noticeable activity with a decrease in the composition of the more expensive component of silver (gold) due to the introduction of nontoxic, affordable zinc derivatives into the composition. The calculated FICI values for all synthesized composites in relation to all tested pathogens were mainly in the range of 0.5–2.2; this indicates additional interactions of metal-containing components in the preparations. For the composites which contained the copolymer of maleic acid with ethylene as stabilized agent, the participation of the metal-containing components of the system was close to synergistic (the FICI values were close to 0.5 for all microorganisms used). This is explained by the specific structure of macromolecules of this copolymer.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app132011121/s1, Figure S1a–c: FTIR spectra of initial copolymers and heterodimers; Figure S2a,b: XRD patterns of heterodimers: (a) VM/Ag0/ZnO (1), EM/Ag0/ZnO (2), SM/Ag0/ZnO (3), (b)VM/Au0/ZnO; Table S1: Characteristics of the Au 4f, Zn 2p, and Zn 3p photoelectron spectra: binding energies (Eb), Gaussian widths (W), and relative intensities (Irel) of photoelectron peaks belonging to different chemical groups in the C 1s and N 1s spectra; Figure S3: The O 1s, C 1s, and N 1s of Ag0/ZnO (1) and Au0/ZnO (2) structures stabilized with VM. References [105,106] are cited in the supplementary materials.
Author Contributions
Conceptualization, N.A.S.; methodology, N.A.S.; validation, N.A.S., A.V.N. and A.A.K.; formal analysis, A.V.N., A.A.K., N.M.A. and D.A.P.; investigation, N.A.S., A.V.N., A.A.K., N.M.A. and M.A.K.; data curation, N.A.S., A.V.N., A.A.K. and M.A.K.; writing—original draft preparation, N.A.S.; writing—review and editing, N.A.S., M.A.K., A.V.N. and D.A.P.; visualization, M.A.K. and A.V.N.; supervision, A.V.N. and D.A.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We are grateful to I. Blagodatskikh and S. Abramchuk for help with TEM, and Z. Klemenkova for help with the FTIR data. This work was supported by the Ministry of Science and Higher Education of the Russian Federation (Contract No. 075-03-2023-642) and was performed employing the equipment of Center for molecular composition studies of INEOS RAS.
Conflicts of Interest
The authors declare no conflict of interests.
References
- Zielińska-Gorska, M.K.; Sawosz, E.; Gorski, K.; Chwalibog, A. Does nanobiotechnology create new tools to combat microorganisms? Nanotechnol. Rev. 2016, 6, 171–189. [Google Scholar] [CrossRef]
- Khuspe, P.; Kokate, K.; Mandhare, T.; Nangre, P.; Rathi, B.A. Comprehensive review on novel pharmaceutical nanotechnology and its applications. IAJPS 2017, 4, 4640–4647. [Google Scholar]
- Beyth, N.; Houri-Haddad, Y.; Domb, A.; Khan, W.; Hazan, R. Alternative Antimicrobial Approach: Nano-Antimicrobial Materials. Evid.-Based Complement. Alternant. Med. 2015, 2015, 246012. [Google Scholar] [CrossRef] [PubMed]
- Shivaramakrishnan, B.; Gurumurthy, B.; Balasubramanian, A. Potential Biomedical Applications of Metallic Nanobiomaterials: A Review. Int. J. Pharm. Sci. Res. 2017, 8, 985–1000. [Google Scholar] [CrossRef]
- Shatila, F.; YALÇIN, H.T.; Yaşa, İ. Insight on Microbial Biofilms and Recent Antibiofilm Approaches. Acta Biol. Turcica 2019, 32, 220–235. [Google Scholar]
- Yah, C.S.; Simate, G.S. Nanoparticles as potential new generation broad spectrum antimicrobial agents. DARU J. Pharm. Sci. 2015, 23, 43. [Google Scholar] [CrossRef]
- Khan, F.; Khan, M.M.; Kim, Y.-M. Recent Progress and Future Perspectives of Antibiofilm Drugs Immobilized on Nanomaterials. Curr. Pharm. Biotechnol. 2018, 19, 631–643. [Google Scholar] [CrossRef]
- Khameneh, B.; Diab, R.; Ghazvini, K.; Bazzaz, B.S.F. Breakthroughs in bacterial resistance mechanisms and the potential ways to combat them. Microb. Pathog. 2016, 95, 32–42. [Google Scholar] [CrossRef]
- Rudramurthy, G.R.; Swamy, M.K.; Sinniah, U.R.; Ghasemzadeh, A. Nanoparticles: Alternatives against Drug-Resistant Pathogenic Microbes. Molecules 2016, 21, 836. [Google Scholar] [CrossRef] [PubMed]
- Percival, S.L.; Bowler, P.G.; Russell, D. Bacterial resistance to silver in wound care. J. Hosp. Infect. 2005, 60, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.U.; Saleh, T.A.; Wahab, A.; Hafeez, M.; Khan, U.; Khan, D.; Khan, W.U.; Rahim, A.; Kamal, S.; Khan, F.U.; et al. Nanosilver: New ageless and versatile biomedical therapeutic scaffold. Int. J. Nanomed. 2018, 13, 733–762. [Google Scholar] [CrossRef]
- Verma, P.; Maheshwari, S.K. Applications of Silver nanoparticles in diverse sectors. Int. J. Nano Dimens. 2019, 10, 18–36. [Google Scholar] [CrossRef]
- Rai, M.; Birla, S.; Ingle, A.P.; Gupta, I.; Gade, A.; Abd-Elsalam, K.; Marcato, P.D.; Duran, N. Nanosilver: An inorganic nanoparticle with myriad potential applications. Nanotechnol. Rev. 2014, 3, 281–309. [Google Scholar] [CrossRef]
- Medici, S.; Peana, M.; Nurchi, V.M.; Zoroddu, M.A. Medical Uses of Silver: History, Myths, and Scientific Evidence. J. Med. Chem. 2019, 62, 5923–5943. [Google Scholar] [CrossRef]
- Al-Hasnawy, H.H. The Therapeutic Potential of Silver Nano Particles. Int. J. Psychosoc. Rehabil. 2020, 24, 4217–4224. [Google Scholar] [CrossRef]
- Feng, Q.L.; Wu, J.; Chen, G.Q.; Cui, F.Z.; Kim, T.N.; Kim, J.O. A mechanistic study of the antibacterial effect of silver ions on Escherichia coli and Staphylococcus aureus. J. Biomed. Mater. Res. 2000, 52, 662–668. [Google Scholar] [CrossRef] [PubMed]
- Xiu, Z.M.; Zhang, Q.B.; Puppala, H.L.; Colvin, V.L.; Alverez, P.J.J. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef]
- Franci, G.; Falanga, A.; Galdiero, S.; Palomba, L.; Rai, M.; Morelli, G.; Galdiero, M. Silver nanoparticles as potential antibacterial agents. Molecules 2015, 20, 8856–8874. [Google Scholar] [CrossRef] [PubMed]
- Raiand, R.V.; Bai, J.A. Science against Microbial Pathogen. In Communicating Current Research and Technological Advances; Méndez-Vilas, A., Ed.; Formatex Research Center: Badajoz, Spain, 2011; Volume 2, pp. 197–209. [Google Scholar]
- Lemire, J.A.; Harrison, J.J.; Turner, R.J. Antimicrobial activity of metals: Mechanisms, molecular targets and applications. Nat. Rev. Microbiol. 2013, 11, 371–384. [Google Scholar] [CrossRef] [PubMed]
- Nyberg, L.; Turco, R.F.; Nies, L. Assessing the impact of nanomaterials on anaerobic microbial communities. Environ. Sci. Technol. 2008, 42, 1938–1943. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Wang, L.; Wang, Y.; Liang, Y.; Zhang, J. Toxicity effects of four typical nanomaterials on the growth of Escherichia coli, Bacillus subtilis and Agrobacterium tumefaciens. Environ. Earth Sci. 2012, 65, 1643–1649. [Google Scholar] [CrossRef]
- Ahmad, T.; Wani, I.A.; Manzoor, N.; Ahmed, J.; Asiri, A.M. Biosynthesis, structural characterization and antimicrobial activity of gold and silver nanoparticles. Colloids Surf. B-Biointerfaces 2013, 107, 227–234. [Google Scholar] [CrossRef]
- Hernandez-Sierra, J.F.; Ruiz, F.; Cruz Pena, D.C.; Martinez-Guttierrez, F.; Martinez, A.E.; De Jesus Pozos Guillen, A.; Tapia-Perez, H.; Martinez Castanon, G. The antimicrobial sensitivity of Streptococcus mutans to nanoparticles of silver, zinc oxide, and gold. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 237–240. [Google Scholar] [CrossRef] [PubMed]
- Amin, R.M.; Mohamed, M.B.; Ramadan, M.A.; Verwanger, T.; Krammer, B. Rapid and sensitive microplate assay for screening the effect of silver and gold nanoparticles on bacteria. Nanomedicine 2009, 4, 637–643. [Google Scholar] [CrossRef]
- Zhou, Y.; Kong, Y.; Kundu, S.; Cirillo, J.; Liang, H. Antibacterial activities of gold and silver nanoparticles against Escherichia coli and bacillus Calmette-Guerin. J. Nanobiotechnol. 2012, 10, 19. [Google Scholar] [CrossRef]
- Borah, S.; Tripathy, S.; Bhuyan, B.; Kaishap, P.P.; Sharma, H.K.; Choudhurf, G.; Saikia, L. Metal nanoparticles as potent antimicrobial nanomachetes with an emphasis on nanogold and nanosilver. In Design of Nanostructures for Versatile Therapeutic Applications; Grumezescu, A.M., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018; Chapter 12; pp. 488–524. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, Y.; Tian, Y.; Zhang, W.; Lü, X.; Jiang, X. The molecular mechanism of action of bactericidal gold nanoparticles on Escherichia coli. Biomaterials 2012, 33, 2327–2333. [Google Scholar] [CrossRef]
- Zhao, Y.; Tian, Y.; Cui, Y.; Liu, W.; Ma, W.; Jiang, X. Small Molecule-Capped Gold Nanoparticles as Potent Antibacterial Agents That Target Gram-Negative Bacteria. J. Am. Chem. Soc. 2010, 132, 12349–12356. [Google Scholar] [CrossRef]
- Lai, H.; Chen, W.; Wu, C.; Chen, Y. Potent antibacterial nanoparticles for pathogenic bacteria. ACS Appl. Mater. Interfaces 2015, 7, 2046–2054. [Google Scholar] [CrossRef]
- Katsnelson, B.; Privalova, L.I.; Gurvich, V.B.; Tulakina, L.G. Comparative in Vivo Assessment of Some Adverse Bioeffects of Equidimensional Gold and Silver Nanoparticles and the Attenuation of Nanosilver’s Effects with a Complex of Innocuous Bioprotectors. Int. J. Mol. Sci. 2013, 14, 2449–2483. [Google Scholar] [CrossRef]
- Jin, Y.; Zhao, X. Cytotoxicity of photoactive nanoparticles. In Safety of Nanoparticles: From Manufacturing to Medical Applications; Webster, T., Ed.; Springer Science + Business Media, LLC: New York, NY, USA, 2008; pp. 19–31. [Google Scholar]
- Berger, T.J.; Spadaro, J.A.; Chapin, S.E.; Becker, R.O. Electrically generated silver ions: Quantitative effects on bacterial and mammalian cells. Antimicrob. Agents Chemother. 1976, 9, 357–358. [Google Scholar] [CrossRef]
- Arora, S.; Jain, J.; Rajwade, J.M.; Paknikar, K.M. Cellular Responses Induced by Silver Nanoparticles: In Vitro Studies. Toxicol. Lett. 2008, 179, 93–100. [Google Scholar] [CrossRef]
- Asha Rani, P.V.; Mun, G.L.K.; Hande, M.P.; Valiyaveettil, S. Cytotoxicity and Genotoxicity of Silver Nanoparticles in Human Cells. ACS Nano 2009, 3, 279–290. [Google Scholar] [CrossRef]
- Sharma, P.; Jang, N.-Y.; Lee, J.W.; Park, B.C.; Kim, Y.K.; Cho, N.-H. Application of ZnO-Based Nanocomposites for Vaccines and Cancer Immunotherapy. Pharmaceutics 2019, 11, 493. [Google Scholar] [CrossRef] [PubMed]
- Jones, N.; Ray, B.; Ranjit, K.T.; Manna, A.C. Antibacterial activity of ZnO nanoparticle suspensions on a broad spectrum of microorganisms. FEMS Microbiol. Lett. 2008, 279, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.M.; Feris, K.; Bell, J.; Wingett, D.G.; Hanley, C.; Punnose, A. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl. Phys. Lett. 2007, 90, 213902-1–213902-3. [Google Scholar] [CrossRef] [PubMed]
- Jin, S.-E.; Jin, H.-E. Antimicrobial Activity of Zinc Oxide Nano/Microparticles and Their Combinations against Pathogenic Microorganisms for Biomedical Applications: From Physicochemical Characteristics to Pharmacological Aspects. Nanomaterials 2021, 11, 263. [Google Scholar] [CrossRef] [PubMed]
- Makabenta, J.M.V.; Nabawy, A.; Li, C.-H.; Schmidt-Malan, S.; Patel, R.; Rotello, V.M. Nanomaterial-based therapeutics for antibiotic-resistant bacterial infections. Nature Rev. Microbiol. 2021, 19, 23–36. [Google Scholar] [CrossRef]
- Sánchez-López, E.; Gomes, D.; Esteruelas, G.; Bonilla, L.; Lopez-Machado, A.L.; Galindo, R.; Cano, A.; Espina, M.; Ettcheto, M.; Camins, A.; et al. Metal-Based Nanoparticles as Antimicrobial Agents: An Overview. Nanomaterials 2020, 10, 292. [Google Scholar] [CrossRef]
- Xie, Y.; He, Y.; Irwin, P.L.; Jin, T.; Shi, X. Antibacterial activity and mechanism of action of zinc oxide nanoparticles against Campylobacter jejuni. Appl. Environ. Microbiol. 2011, 77, 2325–2331. [Google Scholar] [CrossRef] [PubMed]
- De Graaf, T.P.; Galley, E.; Butcher, K.E. Use of an Antimicrobial Agent. European Patent EP1079799, 14 July 2004. [Google Scholar]
- Regner, M.; Prendergast, M.J.; Thurlby, O. Oral Zinc Compositions. U.S. Patent 2007, 20070224134, 15 July 2004. [Google Scholar]
- Ishida, T. Zinc Immunology and Zn2+ Ions Binding Anti-Bacterial Vaccine Activity for Bacterial Cell Walls against Gram-Positive and Gram-Negative Bacteria. Arch. Immunol. Allergy 2019, 2, 23–34. [Google Scholar] [CrossRef]
- Stankovic, A.; Dimitrijevic, S.; Uskokovic, D. Influence of size scale and morphology on antibacterial properties of ZnO powders hydrothermally synthesized using different surface stabilizing agents. Colloids Surf. B. Biointerfaces 2013, 102C, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.-E.; Li, Z.-H.; Zheng, W.; Zhao, Y.-F.; Jin, Y.-F.; Tang, Z.-X. Synthesis, antibacterial activity, antibacterial mechanism and food applications of ZnO nanoparticles: A review. Food Addit. Contam. Part A 2014, 31, 173–186. [Google Scholar] [CrossRef] [PubMed]
- Degim, I.T.; Kadioglu, D. Cheap, suitable, predictable, and manageable nanoparticles for drug delivery: Quantum dots. Curr. Drug Deliv. 2013, 10, 32–38. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Chandrangsu, P.; Helmann, T.C.; Romsang, A.; Gaballa, A.; Helmann, D. Bacillithiol is a major buffer of the labile zinc pool in Bacillus subtilis. Molec. Microbiol. 2014, 94, 756–770. [Google Scholar] [CrossRef] [PubMed]
- Velthuis, A.J.W.; Worm, S.H.E.; Sims, A.C.; Baric, R.S.; Snijder, E.J.; Hemert, M.J. Zn2+ Inhibits Coronavirus and Arterivirus RNA Polymerase Activity in Vitro and Zinc Ionophores Block the Replication of These Viruses in Cell Culture. PLoS Pathog. 2010, 6, e1001176. [Google Scholar] [CrossRef] [PubMed]
- Ong, C.-I.Y.; Walker, M.J.; McEwan, A.G. Zinc disrupts central carbon metabolism and capsule biosynthesis in Streptococcus pyogenes. Sci. Rep. 2015, 5, 10799. [Google Scholar] [CrossRef]
- Remy, L.; Carrière, M.; Derré-Bobillot, M.; Martini, C.; Sanguinetti, M.; Borezee-Durant, E. The Staphylococcus aureus Opp1 ABC transporter impairs nickel and cobalt in zinc-depleted conditions and contributes to virulence. Mol. Microbiol. 2013, 87, 730–743. [Google Scholar] [CrossRef] [PubMed]
- Chohan, Z.H.; Supuran, C.T.; Scozzafava, A. Metal binding and antibacterial activity of ciprofloxacin complexes. J. Enzyme Inhib. Med. Chem. 2005, 20, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Uivarosi, V. Metal complexes of quinolone antibiotics and their applications: An update. Molecules 2013, 18, 11153–11197. [Google Scholar] [CrossRef] [PubMed]
- Zarkan, A.; Mackline, H.R.; Chirgadze, D.Y.; Bond, A.D.; Hesketh, A.R.; Hong, H.J. Zn(II) mediates vancomycin polymerization and potentiates its antibiotic activity against resistant bacteria. Sci. Rep. 2017, 7, 4893. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Sun, Q.; Li, Y.; Tay, F.R.; Fan, B. Synergistic mechanism of Ag+–Zn2+ in anti-bacterial activity against Enterococcus faecalis and its application against dentin infection. J. Nanobiotechnol. 2018, 16, 10. [Google Scholar] [CrossRef] [PubMed]
- Samoilova, N.; Krayukhina, M.; Popov, D.; Anuchina, N. Specific effects and features of a combination of amine containing antibacterial agents and silver nanoparticles stabilized by dicarboxylic acid copolymers. Monatsh. Chem. Chem. Mon. 2019, 150, 2071–2080. [Google Scholar] [CrossRef]
- Gupta, A.; Saleh, N.M.; Das, R.; Landis, R.F.; Bigdeli, A.; Motamedchaboki, K.; Campos, A.R.; Pomeroy, K.; Mahmoudi, M.; Rotello, V.M. Synergistic antimicrobial therapy using nanoparticles and antibiotics for the treatment of multidrug-resistant bacterial infection. Nano Futures 2017, 1, 015004. [Google Scholar] [CrossRef]
- Kora, A.J.; Rastogi, L. Enhancement of Antibacterial Activity of Capped Silver Nanoparticles in Combination with Antibiotics, on Model Gram-Negative and Gram-Positive Bacteria. Bioinorg. Chem. Appl. 2013, 2013, 871097. [Google Scholar] [CrossRef]
- Samoilova, N.A.; Krayukhina, M.A.; Anuchina, N.M.; Popov, D.A. Investigation of antimicrobial properties of preparations based on maleic acid copolymers containing silver nanoparticles and phenolic residues. Appl. Biochem. Microbiol. 2021, 57, 377–383. [Google Scholar] [CrossRef]
- Choi, S.-M.; Jang, E.-J.; Cha, J.-D. Synergistic Effect between Fucoidan and Antibiotics against Clinic Methicillin-Resistant Staphylococcus aureus. Adv. Biosci. Biotechnol. 2015, 6, 275–285. [Google Scholar] [CrossRef]
- Leng, D.; Li, Y.; Zhu, J.; Liang, R.; Zhang, C.; Zhou, Y.; Li, M.; Wang, Y.; Rong, D.; Wu, D.; et al. The Antibiofilm Activity and Mechanism of Nanosilver- and Nanozinc-Incorporated Mesoporous Calcium-Silicate Nanoparticles. Int. J. Nanomed. 2020, 15, 3921–3936. [Google Scholar] [CrossRef]
- Bednář, J.; Svoboda, L.; Rybková, Z.; Dvorský, R.; Malachová, K.; Stachurová, T.; Matýsek, D.; Foldyna, V. Antimicrobial Synergistic Effect Between Ag and Zn in Ag-ZnO·mSiO2 Silicate Composite with High Specific Surface Area. Nanomaterials 2019, 9, 1265. [Google Scholar] [CrossRef]
- Naskar, A.; Lee, S.; Kim, K.-S. Easy One-Pot Low-Temperature Synthesized Ag-ZnO Nanoparticles and Their Activity Against Clinical Isolates of Methicillin-Resistant Staphylococcus aureus. Front. Bioeng. Biotechnol. 2020, 8, 216. [Google Scholar] [CrossRef]
- Das, B.; Khan, M.I.; Jayabalan, R.; Behera, S.K.; Yun, S.-I.; Tripathy, S.K.; Mishra, A. Understanding the Antifungal Mechanism of Ag@ZnO Core-shell Nanocomposites against Candida krusei. Sci. Rep. 2016, 6, 36403. [Google Scholar] [CrossRef]
- Ibrahim, F. Synthesis of Novel Virus-Like Mesoporous Silica-ZnO-Ag Nanoparticles and Quercetin Synergize with NIR Laser for Omicron Mutated COVID-19 Virus Infectious Diseases Treatment. Adv. Nanopart. 2022, 11, 13–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Mu, J. One-pot synthesis, photoluminescence, and photocatalysis of Ag/ZnO composites. J. Colloid Interface Sci. 2007, 309, 478–484. [Google Scholar] [CrossRef]
- Chen, Y.; Zeng, D.; Zhang, K.; Lu, A.; Wang, L.; Peng, D.-L. Au–ZnO hybrid nanoflowers, nanomultipods and nanopyramids: One-pot reaction synthesis and photocatalytic properties. Nanoscale 2014, 6, 874–881. [Google Scholar] [CrossRef]
- Kantipudi, S.; Sunkara, J.R.; Rallabhandi, M.; Thonangi, C.V.; Cholla, R.D.; Kollu, P.; Parvathaneni, M.K.; Pammi, S.V.N. Enhanced wound healing activity of Ag–ZnO composite NPs in Wistar Albino rats. IET Nanobiotechnol. 2018, 12, 473–478. [Google Scholar] [CrossRef]
- Li, P.; Yu, S.; Zhang, H. Preparation and Performance Analysis of Ag/ZnO Humidity Sensor. Sensors 2021, 21, 857. [Google Scholar] [CrossRef] [PubMed]
- Ganganagappa, N.; Gowda, U.C.; Patil, S.; Prashanth, S.A.; Shastric, M.; Yathisha, K.V.; Anupamad, C.; Rangappa, D. Electrochemical heavy metal detection, photocatalytic, photoluminescence, biodiesel production and antibacterial activities of Ag–ZnO nanomaterial. Mater. Res. Bull. 2017, 94, 54–63. [Google Scholar] [CrossRef]
- Hotze, E.M.; Phenrat, T.; Lowry, G.V. Nanoparticle Aggregation: Challenges to Understanding Transport and Reactivity in the Environment. J. Environ. Qual. 2010, 39, 1909–1924. [Google Scholar] [CrossRef]
- Aguilar, N.M.; Perez-Aguilar, J.M.; González-Coronel, V.J.; Soriano Moro, J.G.; Sanchez-Gaytan, B.L. Polymers as Versatile Players in the Stabilization, Capping, and Design of Inorganic Nanostructures. ACS Omega 2021, 6, 35196–35203. [Google Scholar] [CrossRef]
- Attia, Y.A.; Mohamed, Y.M.A.; Awad, M.M.; Alexeree, S. Ag doped ZnO nanorods catalyzed photo-triggered synthesis of some novel (1H-tetrazol-5-yl)-coumarin hybrids. J. Organomet. Chem. 2020, 919, 121320. [Google Scholar] [CrossRef]
- Sun, H.; He, J.; Wang, J.; Zhang, S.Y.; Liu, C.; Sritharan, T.; Mhaisalkar, S.; Han, M.Y.; Wang, D.; Chen, H. Investigating the Multiple Roles of Polyvinylpyrrolidone for a General Methodology of Oxide Encapsulation. J. Am. Chem. Soc. 2013, 135, 9099–9110. [Google Scholar] [CrossRef]
- Azizi, S.; Ahmad, M.B.H.; Hussein, M.Z.; Ibrahim, N.A. Synthesis, Antibacterial and Thermal Studies of Cellulose Nanocrystal Stabilized ZnO-Ag Heterostructure Nanoparticles. Molecules 2013, 18, 6269–6280. [Google Scholar] [CrossRef] [PubMed]
- Xiang, S.; Mtng, Q.; Zhang, K.; Gu, Y.; Liu, W.; Yang, B. Facile Synthesis of ZnO-Au Nanopetals and Their Application for Biomolecule Determinations. Chem. Res. Chin. Univ. 2019, 35, 924–928. [Google Scholar] [CrossRef]
- Samoilova, N.; Kurskaya, E.; Krayukhina, M.; Askadsky, A.; Yamskov, I. Copolymers of Maleic Acid and Their Amphiphilic Derivatives as Stabilizers of Silver Nanoparticles. J. Phys. Chem. B 2009, 113, 3395–3403. [Google Scholar] [CrossRef] [PubMed]
- Samoilova, N.; Krayukhina, M.; Naumkin, A.; Anuchina, N.; Popov, D. Silver nanoparticles doped with silver cations and stabilized with maleic acid copolymers: Specific structure and antimicrobial properties. New J. Chem. 2021, 45, 14513–14521. [Google Scholar] [CrossRef]
- Samoilova, N.A.; Krayukhina, M.A.; Popov, D.A.; Anuchina, N.M.; Piskarev, V.E. Sialyllactose-decorated Silver Nanoparticles: Lectin Binding and Bactericidal Properties. Biointerface Res. Appl. Chem. 2018, 8, 3095–3099. [Google Scholar]
- Conix, A.; Smets, G. Ring opening in lactam polymers. J. Polym. Sci. 1955, 15, 221–229. [Google Scholar] [CrossRef]
- Leoni, M. Domain Size and Domain-Size Distributions. In International Tables for Crystallography; John Wiley & Sons Ltd.: West Sussex, UK, 2019; Volume H, Chapter 5.1; pp. 524–537. [Google Scholar] [CrossRef]
- Samoilova, N.A.; Krayukhina, M.A.; Klimova, T.P.; Babushkina, T.A.; Vyshivannaya, O.; Blagodatskikh, I.; Yamskov, I.A. Oxidation of glucose to gluconic acid using a colloidal catalyst containing gold nanoparticles and glucose oxidase. Russ. Chem. Bull. 2014, 63, 1009–1016. [Google Scholar] [CrossRef]
- Cockerill, F.R.; Wikler, M.A.; Alder, J.; Dudley, M.N.; Eliopoulos, G.M.; Ferraro, D.J.H.; Hecht, D.W.; Hindler, J.A.; Patel, J.B.; Powell, M.; et al. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard—Ninth Edition. Clin. Lab. Stand. Inst. 2012, 32, M07-A9. [Google Scholar]
- Samoilova, N.A.; Blagodatskikh, I.V.; Kurskaya, E.A.; Krayukhina, M.A.; Vyshivannaya, O.V.; Abramchuk, S.S.; Askadskii, A.A.; Yamskov, I.A. Stabilization of silver nanoparticles with copolymers of maleic acid. Colloid J. 2013, 75, 409–420. [Google Scholar] [CrossRef]
- UV-Vis Spectroscopy Open Source Reference Data Library for Nanoparticles—InstaNANO. Available online: https://instanano.com/characterization/reference/uv-vis-spectroscopy/ (accessed on 14 November 2022).
- Rashid, T.M.; Nayef, U.M.; Jabir, M.S.; Mutlak, F.A.-H. Study of optical and morphological properties for Au-ZnO nanocomposite prepared by Laser ablation in liquid. J. Phys. Conf. Ser. 2021, 1795, 012041. [Google Scholar] [CrossRef]
- Ghasemi, A.; Rabiee, N.; Ahmadi, S.; Hashemzadeh, S.; Lolasi, F.; Bozorgomid, M.; Kalbasi, A.; Nasseri, B.; Dezfuli, A.S.; Aref, A.R.; et al. Optical assays based on colloidal inorganic nanoparticles. Analyst 2018, 143, 3249–3283. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.; Gu, A.; Liang, G.; Yuan, L. New high performance transparent UV-curable poly(methyl methacrylate) grafted ZnO/silicone-acrylate resin composites with simultaneously improved integrated performance. Colloids Surf. A Physicochem. Eng. Asp. 2012, 396, 74–82. [Google Scholar] [CrossRef]
- Djaja, N.F.; Montja, D.A.; Saleh, R. The Effect of Co Incorporation into ZnO Nanoparticles. Adv. Mater. Phys. Chem. 2013, 3, 33–41. [Google Scholar] [CrossRef]
- Matai, I.; Sachdev, A.; Dubey, P.; Kumar, S.U.; Bhushan, B.; Gopinath, P. Antibacterial activity and mechanism of Ag–ZnO nanocomposite on S. aureus and GFP-expressing antibiotic resistant E. coli. Colloids Surf. B Biointerfaces 2013, 115, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Gilja, V.; Vrban, I.; Mandić, V.; Žic, M.; Hrnjak-Murgić, Z. Preparation of a PANI/ZnO Composite for Efficient Photocatalytic Degradation of Acid Blue. Polymers 2018, 10, 940. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, K.; Oba, Y.; Edamoto, K. Oxidation of Cu on ZnO(0001)-Zn: Angle-Resolved Photoelectron Spectroscopy and Low-Energy Electron Diffraction Study. e-J. Surf. Sci. Nanotechnol. 2008, 6, 226–232. [Google Scholar] [CrossRef]
- Haupt, M.; Ladenburger, A.; Sauer, R.; Thonke, K.; Glass, R.; Roos, W.; Spatz, J.P.; Rauscher, H.; Riethmüller, S.; Möller, M. Ultraviolet-emitting ZnO nanowhiskers prepared by a vapor transport process on prestructured surfaces with self-assembled polymers. J. Appl. Phys. 2003, 93, 6252–6257. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, J.; Zhai, M.; Liu, S.; Geng, Y.; Liu, B.; Cao, Q.; Liu, B. Gold-modified ZnO nanocomposites for photo-Fenton-like catalysis of Escherichia coli disinfection. Mater. Lett. 2022, 319, 132275. [Google Scholar] [CrossRef]
- Powell, C.J. Elemental binding energies for X-ray photoelectron spectroscopy. Appl. Surf. Sci. 1995, 89, 141–149. [Google Scholar] [CrossRef]
- Naumkin, A.V.; Kraut-Vass, A.; Gaarenstroom, S.W.; Powell, C.J. NIST X-ray Photoelectron Spectroscopy Database, National Institute of Standards and Technology, Gaithersburg. 2012. Available online: http://srdata.nist.gov/xps/ (accessed on 1 January 2020).
- Moulder, J.F.; Stickle, W.F.; Sobol, P.E.; Bomben, K.D. Handbook of X-ray Photoelectron Spectroscopy; Perkin-Elmer Corporation: Eden Prairie, MN, USA, 1992. [Google Scholar]
- Premanathan, M.; Karthikeyan, K.; Jeyasubramanian, K.; Manivannan, G. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomed. Nanotechnol. Biol. Med. 2011, 7, 184–192. [Google Scholar] [CrossRef]
- Karimiyan, A.; Najafzadeh, H.; Ghorbanpour, M.; Hekmati-Moghaddam, S.H. Antifungal effect of magnesium oxide, zinc oxide, silicon oxide and copper oxide nanoparticles against Candida albicans. Zahedan J. Res. Med. Sci. 2015, 17, 1–10. [Google Scholar] [CrossRef]
- Xie, Y.; Gao, M.; Liu, Y.; Fu, Q.; Wang, G.; Dai, Y.; Jia, S. Interaction of Zn(II) and Cu(II) with ε-poly-L-lysine and properties of the complexes. E3S Web Conf. 2019, 131, 01003. [Google Scholar] [CrossRef]
- Konaté, K.; Mavoungou, J.F.; Lepengué, A.N.; Aworet-Samseny, R.R.; Hilou, A.; Souza, A.; Dicko, M.H.; M’batchi, B. Antibacterial activity against β- lactamase producing Methicillin and Ampicillin-resistants Staphylococcus aureus: Fractional Inhibitory Concentration Index (FICI) determination. Ann. Clin. Microbiol. Antimicrob. 2012, 11, 18. [Google Scholar] [CrossRef]
- Ruden, S.; Hilpert, K.; Berditsch, M.; Wadhwani, P.; Ulrich, A.S. Synergistic interaction between silver nanoparticles and membrane-permeabilizing antimicrobial peptides. Antimicrob. Agents Chemother. 2009, 53, 3538–3540. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, A.; Anceno, A.J.; Baruah, S.; Shipin, O.V.; Dutta, J. Zinc oxide nanorod mediated visible light photoinactivation of model microbes in water. Nanotechnology 2011, 22, 215703. [Google Scholar] [CrossRef]
- Suh, I.-K.; Ohta, H.; Waseda, Y. High-Temperature Thermal Expansion of Six Metallic Elements Measured by Dilatation Method and X-ray Diffraction. J. Mater. Sci. 1988, 23, 757–780. [Google Scholar] [CrossRef]
- Patterson, A.L. The Scherrer Formula for X-ray Particle Size Determination. Phys. Rev. 1939, 56, 978. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).