Development of a Novel De-NOx Technology for the Aftertreatment of Ship Exhaust Gases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Technical Specifications of MS Sapphire
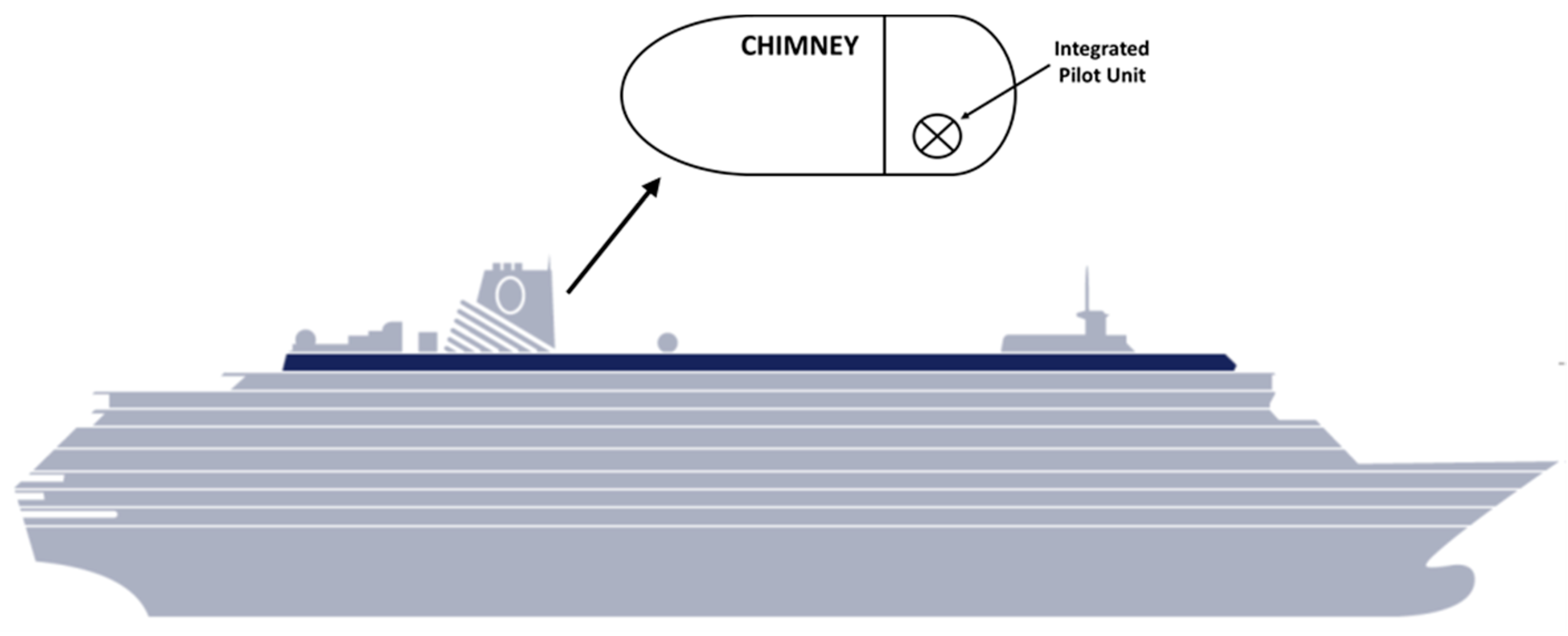
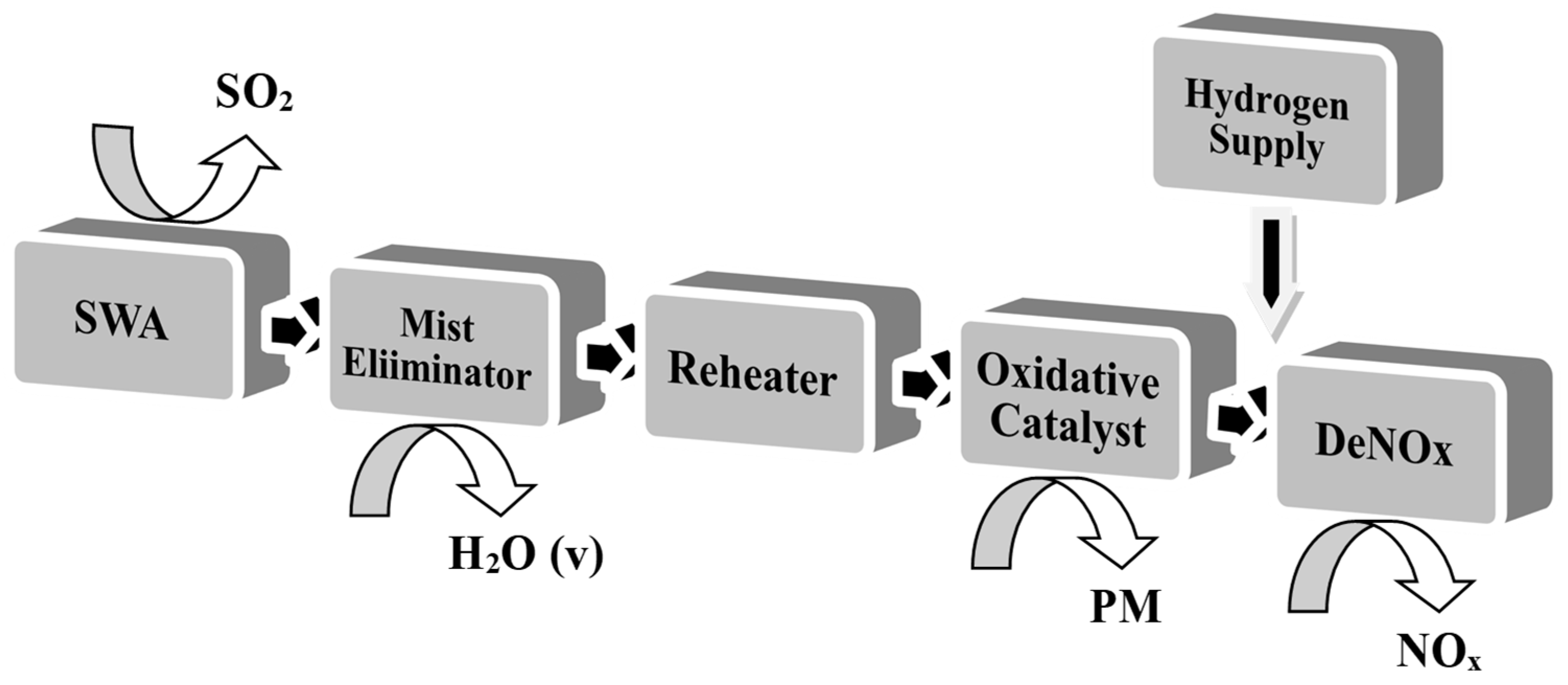
2.2. Pilot Unit Description
2.3. H2-SCR Catalyst Synthesis
3. Results and Discussion
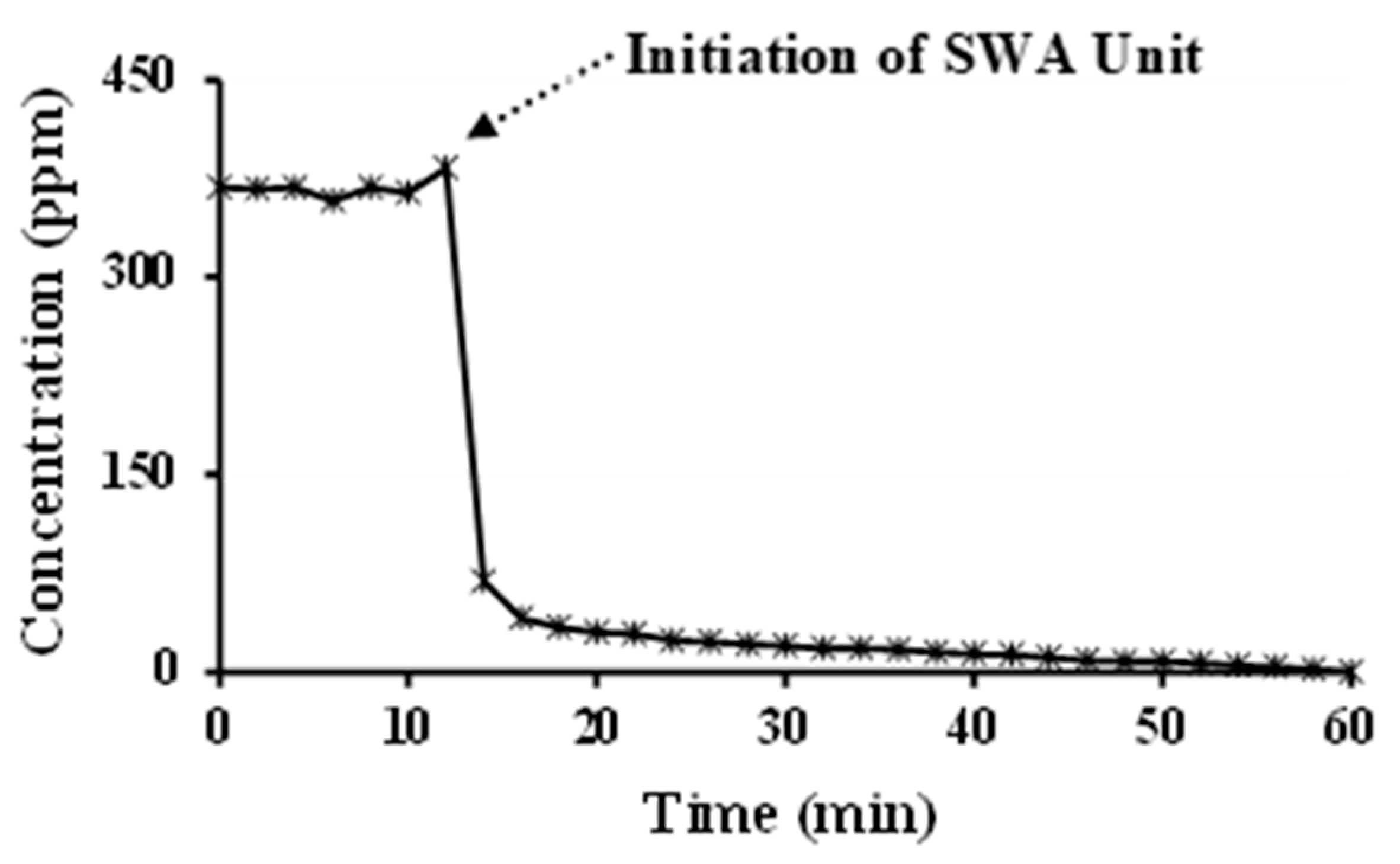
3.1. Operation of Sea Water Absorption Unit
3.2. Operation of Catalytic Oxidation Unit
3.3. Operation of Selective Catalytic Reduction Unit
4. Conclusions
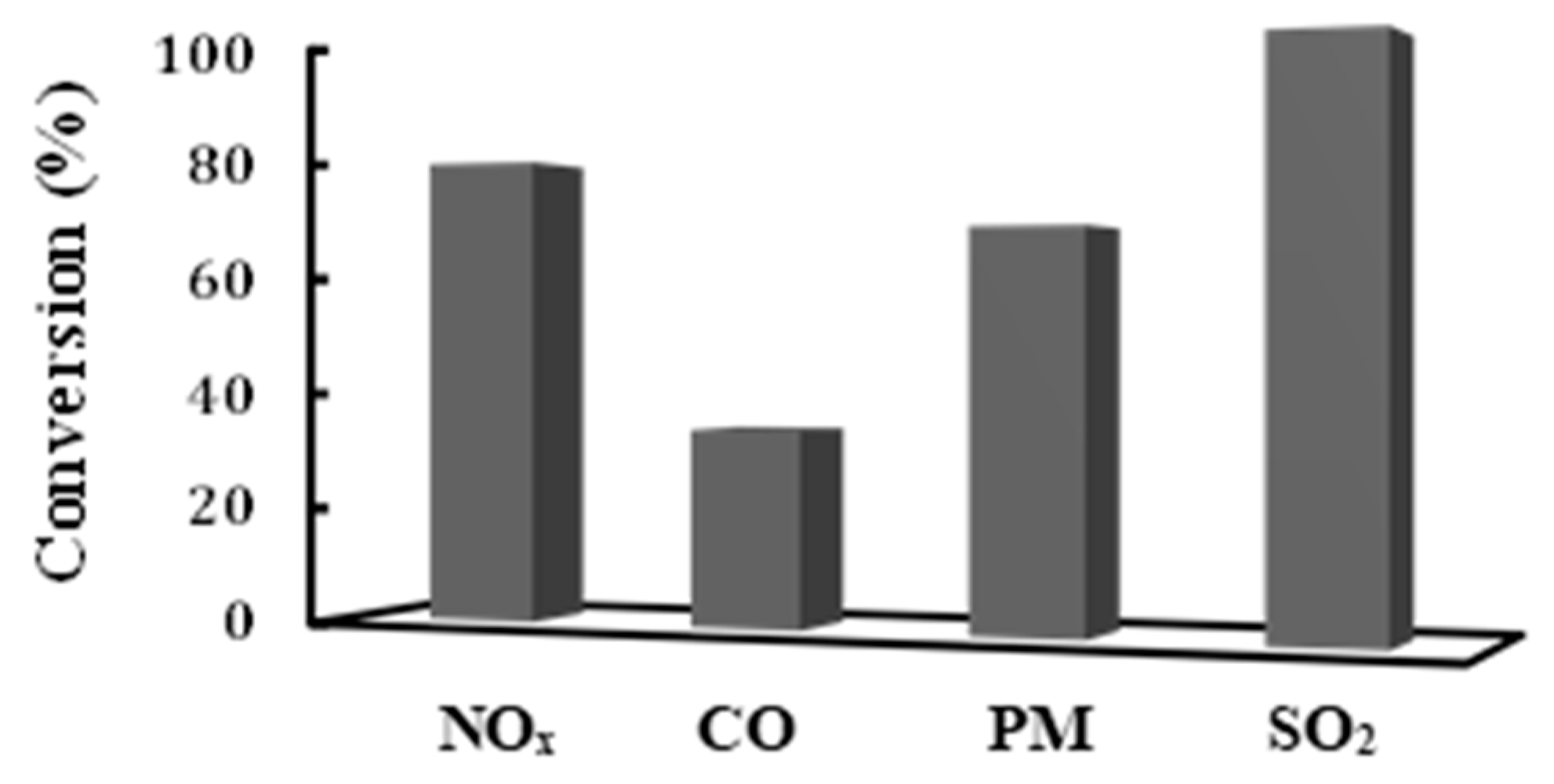
- The Sea Water Absorption technology was found to be capable of practically eliminating sulphur dioxide from the exhaust gas stream. SO2 was almost completely removed from the ships exhaust stream (more than 99% conversion), which is in full accordance with the new IMO MARPOL regulations [2].
- Moreover, a significant reduction in PM was also achieved with the use of an oxidizing catalytic converter. In the framework of the present study, it has been found that more than 70% of the PM in the exhaust gas stream of the ship was successfully removed with the use a commercial oxidation catalyst.
- Most importantly, within the present work, the H2-SCR de-NOx technology was examined, for the first time ever, in a real application, as a candidate technology for the reduction of NOx present in ships exhaust gasses. It was proven that the Selective Catalytic Reduction of NOx with the use of H2 as a reducing agent in combination with a suitable catalyst, can be considered an effective NOx-pollution control technology for ships. In particular, it was found that more than 80% of NOx can be successfully reduced to N2 with the use of the novel Pt/MgO-CeO2 supported catalyst.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- International Maritime Organization (IMO). International Shipping Facts and Figures—Information Resources on Trade, Safety, Security, Environment, 2nd ed.; International Maritime Organization (IMO): London, UK, 2009; Available online: https://wwwcdn.imo.org/localresources/en/OurWork/Environment/Documents/SecondIMOGHGStudy2009.pdf (accessed on 20 September 2023).
- International Maritime Organization (IMO). Air Pollution and Greenhouse Gas (GHG) Emissions from International Shipping, (Marpol Annex VI (SOx, NOx, ODS, VOC)/Greenhouse Gas (CO2) and Climate Change); International Maritime Organization (IMO): London, UK, 2011; Available online: http://www.cleanshipping.org/download/111128_Air%20pollution%20from%20ships_New_Nov-11.pdf (accessed on 20 September 2023).
- Eyring, V.; Köhler, H.W.; Van Aardenne, J.; Lauer, A. Emissions from international shipping: 1. The last 50 years. J. Geophys. Res. D Atmos. 2009, 110, 171–182. [Google Scholar] [CrossRef]
- Stopford, M. Outlook for shipping. Fairplay Magazine, IHS Fairplay, 27 October 2011. [Google Scholar]
- United Nations Conference on Trade and Development (UNCTAD). Review of Maritime Transport; United Nations Publication: Geneva, Switzerland, 2011; pp. 1–233. [Google Scholar]
- Dey, S.; Mehta, N.S. Automobile pollution control using catalysis. Resour. Environ. Sustain. 2020, 2, 100006. [Google Scholar] [CrossRef]
- Patel, K.D.; Subedar, D.; Patel, F. Design and development of automotive catalytic converter using non-noble catalyst for the reduction of exhaust emission: A review. Mater. Today Proc. 2022, 57 Pt 6, 2465–2472. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhou, W.; Xia, C.; Hou, Q. Application and Development of Selective Catalytic Reduction Technology for Marine Low-Speed Diesel Engine: Trade-Off among High Sulfur Fuel, High Thermal Efficiency, and Low Pollution Emission. Atmosphere 2022, 13, 731. [Google Scholar] [CrossRef]
- Koebel, M.; Elsener, M. Kleemann, Urea-SCR: A promising technique to reduce NOx emissions from automotive diesel engines. Catal. Today 2000, 59, 335–345. [Google Scholar] [CrossRef]
- Costa, C.N.; Savva, P.G.; Fierro, G.L.J.; Efstathiou, M.A. Industrial H2-SCR of NO on a novel Pt/MgO–CeO2 catalyst. Appl. Catal. B Environ. 2007, 75, 47–156. [Google Scholar] [CrossRef]
- Dräger MSI GmbHI. Instruction Manual Dräger MSI Compact NT-S/D; Dräger MSI GmbHI: Hagen, Germany, 2011; pp. 1–44. [Google Scholar]
- Balakrishnan, K.; Gonzalez, D.R. Preparation of Pt/Alumina catalysts by the sol-gel method. J. Catal. 1993, 144, 395–413. [Google Scholar] [CrossRef]
- Savva, P.G.; Costa, C.N. Low-temperature H2-SCR of NO on a novel Pt/MgO-CeO2 catalyst. Appl. Catal. B Environ. 2011, 72, 240–252. [Google Scholar]
- Savva, P.G.; Costa, C.N. Hydrogen Lean-DeNOx as an alternative to the ammonia and Hydrocarbon Selective Catalytic Reduction (SCR). Catal. Rev. 2011, 53, 91–151. [Google Scholar] [CrossRef]
- Efstathiou, M.A.; Costa, N.C.; Fierro, G.L.J. Novel Catalyst for the Reduction of NO to N2 with Hydrogen under NOx Oxidation Conditions. U.S. Patent US7105137B2, 28 April 2005. [Google Scholar]
- Costa, C.N.; Efstathiou, A.M. Mechanistic aspects of the H2-SCR of NO on a novel Pt/MgO-CeO2 catalyst. J. Phys. Chem. C 2007, 111, 3010–3020. [Google Scholar] [CrossRef]
- Costa, C.N.; Efstathiou, A.M. Pt/Mg-Ce-O catalyst for NO/H2/O2 lean de-NOx reaction. Environ. Chem. Lett. 2004, 2, 55–58. [Google Scholar] [CrossRef]
- Costa, C.N. Selective Catalytic Reduction of NO with H2 in the Presence of Excess Oxygen (Lean De-NOx) over Pt Supported Catalysts. Ph.D. Thesis, Department of Chemistry, University of Cyprus, Nicosia, Cyprus, 2003. Available online: https://gnosis.library.ucy.ac.cy/handle/7/39485 (accessed on 9 October 2023).
- Johansen, K. Multi-catalytic soot filtration in automotive and marine applications. Catal. Today 2015, 258, 2–10. [Google Scholar] [CrossRef]
- Abrams, Z.J.; Zaczek, J.S.; Benz, D.A.; Awerbuch, L.; Haidinger, J. Use of seawater in flue gas desulfurization. JAPCA 1988, 38, 969–974. [Google Scholar] [CrossRef]
- Tro, J.N. Chemistry a Molecular Approach, 2nd ed.; Prentice Hall: Hoboken, NJ, USA, 2011. [Google Scholar]
- Flanagan, R.C.; Seinfeld, J.H. Fundamentals of Air Pollution Engineering; Prentice Hall: Hoboken, NJ, USA, 1988; pp. 290–358. [Google Scholar]
- IMO resolution MEPC. Guidelines Addressing Additional Aspects of the NOx Technical Code 2008 with Regard to Particular Requirements Related to Marine Diesel Engines Fitted with Selective Catalytic Reduction (SCR) Systems. 2017. Available online: https://wwwcdn.imo.org/localresources/en/KnowledgeCentre/IndexofIMOResolutions/MEPCDocuments/MEPC.291(71).pdf (accessed on 9 October 2023).
- Herdzik, J. Problems of Nitrogen Oxides Emission Decreasing from Marine Diesel Engines to Fulfil the Limit of Tier 3. Middle Pomeranian Sci. Soc. Environ. Prot. 2019, 21, 659–671. [Google Scholar]
- Kostova, I.; Nikiforov, V.; Pirovsky, C.; Ilieva, G. NOx reduction system selection and energy efficiency impact evaluation for ships sailing in restricted areas. IOP Conf. Ser. Earth Environ. Sci. 2023, 1128, 012026. [Google Scholar] [CrossRef]



| CO2 | NOx | SO2 | PM10 | Fuel Consumption | |
|---|---|---|---|---|---|
| Road Traffic | 4110 (74%) | 27.3 (53%) | 4.3 (26%) | 2.1 (55%) | 1320 (73%) |
| Aviation | 654 (11%) | 2.3 (5%) | 0.15 (1%) | 0.001 (0%) | 207 (16%) |
| Shipping | 812 (15%) | 21.4 (42%) | 12.0 (73%) | 1.7 (45%) | 280 (21%) |
| Total | 5576 | 51 | 16.45 | 3.8 | 1807 |
| Property | Units | Value |
|---|---|---|
| Density @15 °C | kg/m3 | 977.4 |
| Viscosity @ 50 °C | mm2/s | 99.8 |
| Water | vol.% | 0.1 |
| Micro Carbon Residue | wt.% | 11 |
| Sulfur | wt.% | 1.47 |
| Ash | wt.% | 0.05 |
| Vanadium | mg/kg | 77 |
| Sodium | mg/kg | 20 |
| Aluminum | mg/kg | 11 |
| Silicon | mg/kg | 15 |
| Iron | mg/kg | 29 |
| Nickel | mg/kg | 29 |
| Calcium | mg/kg | 17 |
| Magnesium | mg/kg | 4 |
| Lead | mg/kg | <1 |
| Zinc | mg/kg | 1 |
| Flash Point | Deg. C | >70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Savva, P.G.; Fessas, Y.; Efstathiou, A.M.; Costa, C.N. Development of a Novel De-NOx Technology for the Aftertreatment of Ship Exhaust Gases. Appl. Sci. 2023, 13, 11356. https://doi.org/10.3390/app132011356
Savva PG, Fessas Y, Efstathiou AM, Costa CN. Development of a Novel De-NOx Technology for the Aftertreatment of Ship Exhaust Gases. Applied Sciences. 2023; 13(20):11356. https://doi.org/10.3390/app132011356
Chicago/Turabian StyleSavva, Petros G., Yiannis Fessas, Angelos M. Efstathiou, and Costas N. Costa. 2023. "Development of a Novel De-NOx Technology for the Aftertreatment of Ship Exhaust Gases" Applied Sciences 13, no. 20: 11356. https://doi.org/10.3390/app132011356
APA StyleSavva, P. G., Fessas, Y., Efstathiou, A. M., & Costa, C. N. (2023). Development of a Novel De-NOx Technology for the Aftertreatment of Ship Exhaust Gases. Applied Sciences, 13(20), 11356. https://doi.org/10.3390/app132011356








