Bioactive Compounds, Antioxidant Activity, and Mineral Content of Wild Rocket (Diplotaxis tenuifolia L.) Leaves as Affected by Saline Stress and Biostimulant Application
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Settings and Design, Crop Management, and Soil Sampling
2.2. ABTS and Hydrophilic Antioxidant Activity, Total Phenols, and Total Ascorbic Acid Analysis
2.3. Chlorophylls and Carotenoids Analysis
2.4. Mineral Content Analysis
2.5. Statistical Analysis
3. Results and Discussion
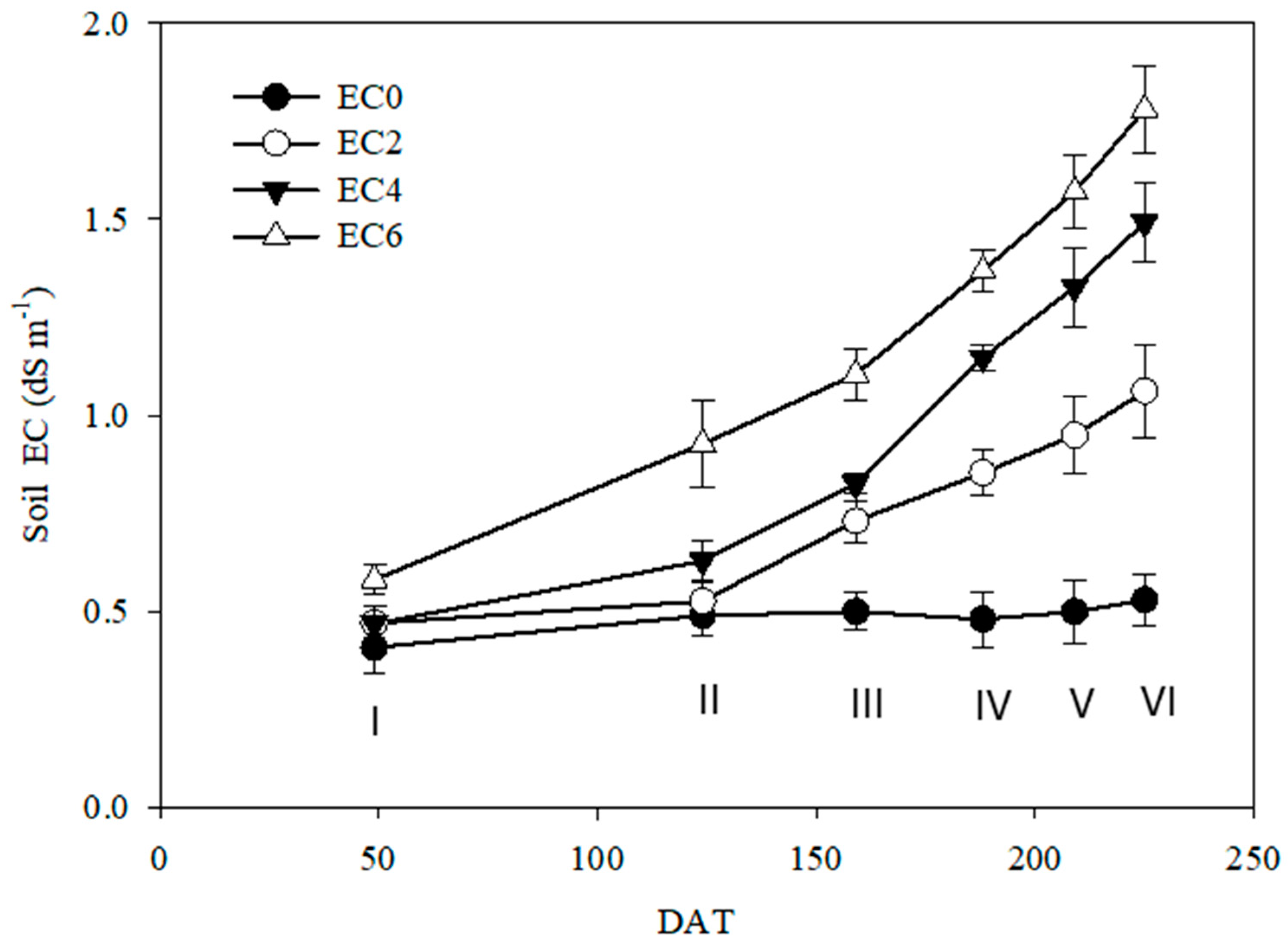
3.1. Soil Electrical Conductivity
3.2. Antioxidant Activity and Compounds
3.3. Chlorophyll and Carotenoid Content
3.4. Leaf Nutrient Composition
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jardim, S.; Sequeira, M.; Capelo, J.; Aguiar, C.; Costa, J.C.; Espírito-Santo, D.; Lousã, M. XXXVI: The vegetation of Madeira: IV—Coastal Vegetation of Porto Santo Island (Archipelag of Madeira). Notas Herbário Estação Florest. Nac. 1994, XVII, 1–5. [Google Scholar]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Machado, R.M.A.; Serralheiro, R.P. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef] [Green Version]
- Shannon, M.C.; Grieve, C.M. Tolerance of vegetable crops to salinity. HortScience 1999, 78, 5–38. [Google Scholar] [CrossRef]
- Rao, N.S.; Shivashankara, K.S.; Laxman, R.H. Abiotic Stress Physiology of Horticultural Crops; Rao, N.S., Shivashankara, K.S., Laxman, R.H., Eds.; Springer: New Delhi, India, 2016; ISBN 9788132227236. [Google Scholar]
- Correa, R.C.G.; Di Gioia, F.; Ferreira, I.; SA, P. Halophytes for future horticulture: The case of small-scale farming in the Mediterranean basin. In Halophytes for Future Horticulture: From Molecules to Ecosystems towards Biosaline Agriculture; Grigore, M.-N., Ed.; Springer Nature: Cham, Switzerland, 2020; pp. 1–28. ISBN 9783030178543. [Google Scholar]
- Hazell, P.; Poulton, C.; Wiggins, S.; Dorward, A. The Future of Small Farms: Synthesis Paper; World Bank: Washington, DC, USA, 2006; pp. 1–51. [Google Scholar]
- Ventura, Y.; Sagi, M. Halophyte crop cultivation: The case for Salicornia and Sarcocornia. Environ. Exp. Bot. 2013, 92, 144–153. [Google Scholar] [CrossRef]
- Mohammed, A.H. The Valuable Impacts of Halophytic Genus Suaeda; Nutritional, Chemical, and Biological Values. Med. Chem. 2020, 16, 1044–1057. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Ali, H.M.; Qureshi, K.A.; Alsharidah, M.; Kandil, Y.I.; Said, R.; Mohammed, S.A.A.; Al-omar, M.S.; Al Rugaie, O.; Abdellatif, A.A.H.; et al. Four Major Medicinal Halophytes from Qassim Flora. Plants 2021, 10, 2208. [Google Scholar] [CrossRef]
- Amin, E.; Abdel-Bakky, M.S.; Mohammed, H.A.; Chigrupati, S.; Qureshi, K.A.; Hassan, M.H.A. Phytochemical Analysis and Evaluation of the Antioxidant and Antimicrobial Activities of Five Halophytes from Qassim Flora. Polish J. Environ. Stud. 2022, 31, 3005–3012. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar] [CrossRef]
- Shahrajabian, M.H.; Chaski, C.; Polyzos, N.; Petropoulos, S.A. Biostimulants Application: A Low Input Cropping Management Tool for Sustainable Farming of Vegetables. Biomolecules 2021, 11, 698. [Google Scholar] [CrossRef] [PubMed]
- Andreotti, C. Management of abiotic stress in horticultural crops: Spotlight on biostimulants. Agronomy 2020, 10, 1514. [Google Scholar] [CrossRef]
- Del Buono, D. Can biostimulants be used to mitigate the effect of anthropogenic climate change on agriculture? It is time to respond. Sci. Total Environ. 2021, 751, 141763. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Cozzolino, E.; Ottaiano, L.; Petropoulos, S.A.; Nocerino, S.; Pelosi, M.E.; Rouphael, Y.; Mori, M.; Di Mola, I. Effect of Biostimulant Application on Plant Growth, Chlorophylls and Hydrophilic Antioxidant Activity of Spinach (Spinacia oleracea L.) Grown under Saline Stress. Horticulturae 2022, 8, 971. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Rouphael, Y.; Carillo, P.; Garcia-Perez, P.; Cardarelli, M.; Senizza, B.; Miras-Moreno, B.; Colla, G.; Lucini, L. Plant biostimulants from seaweeds or vegetal proteins enhance the salinity tolerance in greenhouse lettuce by modulating plant metabolism in a distinctive manner. Sci. Hortic. 2022, 305, 111368. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic action of a microbial-based biostimulant and a plant derived-protein hydrolysate enhances lettuce tolerance to alkalinity and salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef] [Green Version]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.J.; Fonseca, J.M.; Choi, J.H.; Kubota, C.; Dae, Y.K. Salt in irrigation water affects the nutritional and visual properties of romaine lettuce (Lactuca sativa L.). J. Agric. Food Chem. 2008, 56, 3772–3776. [Google Scholar] [CrossRef]
- Rouphael, Y.; Petropoulos, S.A.; Cardarelli, M.; Colla, G. Salinity as eustressor for enhancing quality of vegetables. Sci. Hortic. 2018, 234, 361–369. [Google Scholar] [CrossRef]
- Adhikari, B.; Olorunwa, O.J.; Wilson, J.C.; Barickman, T.C. Morphological and Physiological Response of Different Lettuce Genotypes to Salt Stress. Stresses 2021, 1, 285–304. [Google Scholar] [CrossRef]
- Petropoulos, S.; Fernandes, Â.; Karkanis, A.; Ntatsi, G.; Barros, L.; Ferreira, I.C.F.R. Successive harvesting affects yield, chemical composition and antioxidant activity of Cichorium spinosum L. Food Chem. 2017, 237, 83–90. [Google Scholar] [CrossRef]
- Abdalla, M.M. Boosting the growth of rocket plants in response to the application of Moreinga oleifera extracts as a biostimulant. Life Sci. J. 2014, 11, 1113–1121. [Google Scholar]
- Ventura, Y.; Wuddineh, W.A.; Shpigel, M.; Samocha, T.M.; Klim, B.C.; Cohen, S.; Shemer, Z.; Santos, R.; Sagi, M. Effects of day length on flowering and yield production of Salicornia and Sarcocornia species. Sci. Hortic. 2011, 130, 510–516. [Google Scholar] [CrossRef]
- Caruso, G.; El-Nakhel, C.; Rouphael, Y.; Comite, E.; Lombardi, N.; Cuciniello, A.; Woo, S.L. Diplotaxis tenuifolia (L.) DC. yield and quality as influenced by cropping season, protein hydrolysates, and Trichoderma applications. Plants 2020, 9, 697. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Parrella, G.; Giorgini, M.; Nicoletti, R. Crop systems, quality and protection of Diplotaxis tenuifolia. Agriculture 2018, 8, 55. [Google Scholar] [CrossRef] [Green Version]
- Weightman, R.M.; Huckle, A.J.; Roques, S.E.; Ginsburg, D.; Dyer, C.J. Factors influencing tissue nitrate concentration in field-grown wild rocket (Diplotaxis tenuifolia) in southern England. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2012, 29, 1425–1435. [Google Scholar] [CrossRef] [PubMed]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; El-Nakhel, C.; Rippa, M.; Mormile, P.; Corrado, G.; Rouphael, Y.; Mori, M. Assessment of Yield and Nitrate Content of Wall Rocket Grown under Diffuse-Light-or Clear-Plastic Films and Subjected to Different Nitrogen Fertilization Levels and Biostimulant Application. Horticulturae 2022, 8, 138. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Chatzieustratiou, E.; Constantopoulou, E.; Kapotis, G. Yield and quality of lettuce and rocket grown in floating culture system. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 10611. [Google Scholar] [CrossRef] [Green Version]
- Alexopoulos, A.A.; Marandos, E.; Assimakopoulou, A.; Vidalis, N.; Petropoulos, S.A.; Karapanos, I.C. Effect of Nutrient Solution pH on the Growth, Yield and Quality of Taraxacum officinale and Reichardia picroides in a Floating Hydroponic System. Agronomy 2021, 11, 1118. [Google Scholar] [CrossRef]
- Kristensen, H.L.; Stavridou, E. Deep root growth and nitrogen uptake by rocket (Diplotaxis tenuifolia L.) as affected by nitrogen fertilizer, plant density and leaf harvesting on a coarse sandy soil. Soil Use Manag. 2017, 33, 62–71. [Google Scholar] [CrossRef]
- Petropoulos, S.A.; Constantopoulou, E.; Karapanos, I.; Akoumianakis, C.A.; Passam, H.C. Diurnal variation in the nitrate content of parsley foliage. Int. J. Plant Prod. 2011, 5, 431–438. [Google Scholar]
- Caruso, G.; De Pascale, S.; Cozzolino, E.; Giordano, M.; El-Nakhel, C.; Cuciniello, A.; Cenvinzo, V.; Colla, G.; Rouphael, Y. Protein Hydrolysate or Plant Extract-based Biostimulants Enhanced Yield and Quality Performances of Greenhouse Perennial Wall Rocket Grown in Different Seasons. Plants 2019, 8, 208. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.C.; Bonini, P.; Colla, G. Plant-and seaweed-based extracts increase yield but differentially modulate nutritional quality of greenhouse spinach through biostimulant action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef]
- Vernieri, P.; Borghesi, E. Application of biostimulants in floating system for improving rocket quality. J. Food 2005, 3, 86–88. [Google Scholar]
- Hargreaves, G.; Samani, Z. Reference crop evapotranspiration from temperature. Appl. Eng. Agric. 1985, 1, 96–99. [Google Scholar] [CrossRef]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Senatore, M.; Giordano, M.; El-Nakhel, C.; Sacco, A.; Rouphael, Y.; Colla, G. Plant-Based Biostimulants Influence the Agronomical, Physiological, and Qualitative Responses of Baby Rocket Leaves under Diverse Nitrogen Conditions. Plants 2019, 8, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Fogliano, V.; Verde, V.; Randazzo, G.; Ritieni, A. Method for measuring antioxidant activity and its application to monitoring the antioxidant capacity of wines. J. Agric. Food Chem. 1999, 47, 1035–1040. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Montagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Singleton, V.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteau Reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophyls a and b, as well as tottal carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Graziani, G.; Ritieni, A.; Cardarelli, M.; De Pascale, S. Phenolic composition, antioxidant activity and mineral profile in two seed-propagated artichoke cultivars as affected by microbial inoculants and planting time. Food Chem. 2017, 234, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.; Di Mola, I.; Chiarandà, F.Q. Salt stress and transplant time in snap bean: Growth and productive behaviour. Int. J. Plant Prod. 2011, 5, 49–64. [Google Scholar]
- Schiattone, M.I.; Candido, V.; Cantore, V.; Montesano, F.F.; Boari, F. Water use and crop performance of two wild rocket genotypes under salinity conditions. Agric. Water Manag. 2017, 194, 214–221. [Google Scholar] [CrossRef]
- Feng, G.; Zhang, Z.; Wan, C.; Lu, P.; Bakour, A. Effects of saline water irrigation on soil salinity and yield of summer maize (Zea mays L.) in subsurface drainage system. Agric. Water Manag. 2017, 193, 205–213. [Google Scholar] [CrossRef]
- Chen, W.; Jin, M.; Ferré, T.P.A.; Liu, Y.; Xian, Y.; Shan, T.; Ping, X. Spatial distribution of soil moisture, soil salinity, and root density beneath a cotton field under mulched drip irrigation with brackish and fresh water. Field Crops Res. 2018, 215, 207–221. [Google Scholar] [CrossRef]
- Schiattone, M.I.; Boari, F.; Cantore, V.; Castronuovo, D.; Denora, M.; Di Venere, D.; Perniola, M.; Renna, M.; Sergio, L.; Candido, V. Effects of nitrogen, azoxystrobin and a biostimulant based on brown algae and yeast on wild rocket features at harvest and during storage. Agronomy 2021, 11, 2326. [Google Scholar] [CrossRef]
- Candido, V.; Boari, F.; Cantore, V.; Castronuovo, D.; Di Venere, D.; Perniola, M.; Sergio, L.; Viggiani, R.; Schiattone, M.I. Interactive Effect of Nitrogen and Azoxystrobin on Yield, Quality, Nitrogen and Water Use Efficiency of Wild Rocket in Southern Italy. Agronomy 2020, 10, 849. [Google Scholar] [CrossRef]
- Giordano, M.; El-Nakhel, C.; Caruso, G.; Cozzolino, E.; De Pascale, S.; Kyriacou, M.C.; Colla, G.; Rouphael, Y. Stand-alone and combinatorial effects of plant-based biostimulants on the production and leaf quality of perennial wall rocket. Plants 2020, 9, 922. [Google Scholar] [CrossRef]
- Carillo, P.; Giordano, M.; Raimondi, G.; Napolitano, F.; Di Stasio, E.; Kyriacou, M.C.; Sifola, M.I.; Rouphael, Y. Physiological and nutraceutical quality of green and red pigmented lettuce in response to NaCl concentration in two successive harvests. Agronomy 2020, 10, 1358. [Google Scholar] [CrossRef]
- Corrado, G.; Chiaiese, P.; Lucini, L.; Miras-Moreno, B.; Colla, G.; Rouphael, Y. Successive harvests affect yield, quality and metabolic profile of sweet basil (Ocimum basilicum L.). Agronomy 2020, 10, 830. [Google Scholar] [CrossRef]
- Ciriello, M.; Formisano, L.; El-Nakhel, C.; Kyriacou, M.C.; Soteriou, G.A.; Pizzolongo, F.; Romano, R.; De Pascale, S.; Rouphael, Y. Genotype and successive harvests interaction affects phenolic acids and aroma profile of Genovese basil for pesto sauce production. Foods 2021, 10, 278. [Google Scholar] [CrossRef]
- Di Mola, I.; Rouphael, Y.; Colla, G.; Fagnano, M.; Paradiso, R.; Mori, M. Morphophysiological traits and nitrate content of greenhouse lettuce as affected by irrigation with saline water. HortScience 2017, 52, 1716–1721. [Google Scholar] [CrossRef] [Green Version]
- Bonasia, A.; Lazzizera, C.; Elia, A.; Conversa, G. Nutritional, biophysical and physiological characteristics of wild rocket genotypes as affected by soilless cultivation system, salinity level of nutrient solution and growing period. Front. Plant Sci. 2017, 8, 300. [Google Scholar] [CrossRef] [Green Version]
- Hamilton, J.M.; Fonseca, J.M. Effect of saline irrigation water on antioxidants in three hydroponically grown leafy vegetables: Diplotaxis tenuifolia, eruca sativa, and lepidium sativum. HortScience 2010, 45, 546–552. [Google Scholar] [CrossRef]
- Corrado, G.; Vitaglione, P.; Soteriou, G.A.; Kyriacou, M.C.; Rouphael, Y. Configuration by osmotic eustress agents of the morphometric characteristics and the polyphenolic content of differently pigmented baby lettuce varieties in two successive harvests. Horticulturae 2021, 7, 264. [Google Scholar] [CrossRef]
- Franzoni, G.; Cocetta, G.; Trivellini, A.; Ferrante, A. Transcriptional regulation in rocket leaves as affected by salinity. Plants 2020, 9, 20. [Google Scholar] [CrossRef] [Green Version]
- Chaski, C.; Petropoulos, S.A. The Effects of Biostimulant Application on Growth Parameters of Lettuce Plants Grown under Deficit Irrigation Conditions. Horticulturae 2022, 8, 4. [Google Scholar] [CrossRef]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Ferrante, A. Borage extracts affect wild rocket quality and influence nitrate and carbon metabolism. Physiol. Mol. Biol. Plants 2020, 26, 649–660. [Google Scholar] [CrossRef]
- Grattan, S.R.; Grieve, C.M. Salinity-mineral nutrient relations in horticultural crops. Sci. Hortic. 1998, 78, 127–157. [Google Scholar] [CrossRef]
- Chung, J.B.; Jin, S.J.; Cho, H.J. Low water potential in saline soils enhances nitrate accumulation of lettuce. Commun. Soil Sci. Plant Anal. 2005, 36, 1773–1785. [Google Scholar] [CrossRef]
- Caparrotta, S.; Masi, E.; Atzori, G.; Diamanti, I.; Azzarello, E.; Mancuso, S.; Pandolfi, C. Growing spinach (Spinacia oleracea) with different seawater concentrations: Effects on fresh, boiled and steamed leaves. Sci. Hortic. 2019, 256, 108540. [Google Scholar] [CrossRef]
- Malécange, M.; Pérez-Garcia, M.D.; Citerne, S.; Sergheraert, R.; Lalande, J.; Teulat, B.; Mounier, E.; Sakr, S.; Lothier, J. Leafamine®, a Free Amino Acid-Rich Biostimulant, Promotes Growth Performance of Deficit-Irrigated Lettuce. Int. J. Mol. Sci. 2022, 23, 7338. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Al-Omar, M.S.; Mohammed, S.A.A.; Alhowail, A.H.; Eldeeb, H.M.; Sajid, M.S.M.; Abd-Elmoniem, E.M.; Alghulayqeh, O.A.; Kandil, Y.I.; Khan, R.A. Phytochemical analysis, pharmacological and safety evaluations of halophytic plant, Salsola cyclophylla. Molecules 2021, 26, 2384. [Google Scholar] [CrossRef]
- Chang, A.C.; Yang, T.Y.; Riskowski, G.L. Changes in nitrate and nitrite concentrations over 24 h for sweet basil and scallions. Food Chem. 2013, 136, 955–960. [Google Scholar] [CrossRef]
- Bantis, F.; Kaponas, C.; Charalambous, C.; Koukounaras, A. Strategic successive harvesting of rocket and spinach baby leaves enhanced their quality and production efficiency. Agriculture 2021, 11, 465. [Google Scholar] [CrossRef]


| Parameters | Unit | Mean Value |
|---|---|---|
| Sand | % | 91.0 |
| Silt | % | 4.5 |
| Clay | % | 4.5 |
| N—total (Kjeldahl method) | % | 0.101 |
| P2O5 (Olsen method) | mg kg−1 | 253.0 |
| K2O (tetraphenylborate method) | mg kg−1 | 490.0 |
| Organic matter | % | 2.5 |
| Electrical conductivity | dS m−1 | |
| pH | 7.4 |
| Treatment | HAA mmol Ascorbic Acid equ. 100 g−1 dw | ABTS AA mmol Trolox equ. 100 g−1 dw | Total Phenols mg Aallic Acid g−1 dw | TAA Total Ascorbic Acid mg 100 g−1 fw | |
|---|---|---|---|---|---|
| EC0 | BC | 10.17 ± 0.44 a | 10.09 ± 0.62 d | 2.29 ± 0.08 ab | 32.88 ± 7.05 a |
| BA | 9.75 ± 0.30 a–c | 11.49 ± 0.35 b–d | 2.19 ± 0.08 a–c | 30.27 ± 8.00 ab | |
| BT | 9.70 ± 0.45 a–c | 12.34 ± 0.57 a–c | 2.37 ± 0.09 a | 27.29 ± 5.42 a–c | |
| EC2 | BC | 8.52 ± 0.32 d–f | 11.61 ± 0.70 b–d | 2.31 ± 0.12 a | 26.31 ± 5.40 a–c |
| BA | 9.89 ± 0.32 ab | 10.86 ± 0.56 cd | 2.32 ± 0.09 a | 31.88 ± 5.75 a | |
| BT | 9.11 ± 0.25 b–d | 12.32 ± 0.51 a–c | 2.40 ± 0.07 a | 32.37 ± 8.23 a | |
| EC4 | BC | 7.95 ± 0.40 f | 13.88 ± 0.66 a | 2.41 ± 0.11 a | 22.85 ± 4.75 bc |
| BA | 8.93 ± 0.31 c–e | 12.69 ± 0.36 ab | 2.07 ± 0.11 bc | 26.74 ± 4.53 a–c | |
| BT | 8.59 ± 0.28 d–f | 12.14 ± 0.41 a–c | 2.27 ± 0.10 ab | 30.63 ± 4.60 ab | |
| EC6 | BC | 8.10 ± 0.36 ef | 11.73 ± 0.67 b–d | 2.28 ± 0.05 ab | 23.52 ± 2.52 bc |
| BA | 8.62 ± 0.38 d–f | 13.19 ± 0.77 ab | 2.21 ± 0.12 a–c | 22.57 ± 4.47 bc | |
| BT | 8.21 ± 0.35 ef | 12.55 ± 0.43 a–c | 2.03 ± 0.08 c | 19.75 ± 2.82 c | |
| Treatment | HAA mmol Ascorbic Acid equ. 100 g−1 dw | ABTS AA mmol Trolox equ. 100 g−1 dw | TAA Total Ascorbic Acid mg 100 g−1 fw | |
|---|---|---|---|---|
| EC0 | I | 8.00 ± 0.34 ik | 13.33 ± 0.56 a–c | 86.76 ± 7.68 a |
| II | 9.54 ± 0.37 d–f | 12.78 ± 0.95 b–e | 33.41 ± 2.24 d | |
| III | 9.90 ± 0.22 c–e | 11.38 ± 0.58 e–h | 19.64 ± 1.44 e | |
| IV | 12.61 ± 0.57 a | 11.00 ± 0.58 g–i | 18.83 ± 4.01 e–g | |
| V | 9.74 ± 0.17 c–e | 9.93 ± 0.64 ij | 11.37 ± 1.95 hi | |
| VI | 9.44 ± 0.17 ef | 9.43 ± 0.64 j | 10.87 ± 1.93 i | |
| EC2 | I | 9.26 ± 0.40 e–g | 9.83 ± 1.24 ij | 85.80 ± 6.49 a |
| II | 10.78 ± 0.39 b | 12.30 ± 0.66 b–g | 30.89 ± 3.01 d | |
| III | 8.97 ± 0.42 f–h | 12.94 ± 0.40 a–c | 18.76 ± 1.85 e–g | |
| IV | 8.51 ± 0.40 h–j | 12.85 ± 0.69 b–d | 17.00 ± 3.00 e–i | |
| V | 8.93 ± 0.38 f–h | 11.09 ± 0.75 f–i | 14.58 ± 1.82 e–i | |
| VI | 8.63 ± 0.38 g–i | 10.59 ± 0.75 h–j | 14.08 ± 1.84 e–i | |
| EC4 | I | 8.02 ± 0.22 i–k | 12.31 ± 0.62 b–g | 60.86 ± 6.95 b |
| II | 10.18 ± 0.18 b–d | 13.01 ± 0.82 a–c | 26.93 ± 2.76 d | |
| III | 8.09 ± 0.37 i–k | 13.65 ± 1.02 ab | 18.12 ± 1.08 e–g | |
| IV | 8.51 ± 0.64 h–j | 13.08 ± 0.64 a–c | 17.65 ± 4.00 e–h | |
| V | 8.22 ± 0.47 ij | 12.94 ± 0.65 a–c | 18.69 ± 3.56 e–g | |
| VI | 7.92 ± 0.47 jk | 12.44 ± 0.65 b–f | 18.19 ± 3.58 e–g | |
| EC6 | I | 8.13 ± 0.55 i–k | 12.18 ± 0.79 c–g | 45.37 ± 5.32 c |
| II | 10.30 ± 0.31 bc | 14.28 ± 1.16 a | 27.01 ± 1.68 d | |
| III | 8.53 ± 0.42 h–j | 12.53 ± 0.98 b–e | 19.63 ± 2.49 ef | |
| IV | 8.30 ± 0.55 h–j | 12.47 ± 0.49 b–f | 12.90 ± 1.73 g–i | |
| V | 7.45 ± 0.17 kl | 12.00 ± 0.94 c–g | 13.65 ± 1.79 e–i | |
| VI | 7.15 ± 0.17 l | 11.50 ± 0.94 d–h | 13.15 ± 1.77 f–i | |
| Treatment | Chlorophyll a mg g−1 fw | Chlorophyll b mg g−1 fw | Total Chlorophyll mg g−1 fw | Carotenoids mg g−1 fw |
|---|---|---|---|---|
| EC0 | 1.03 ± 0.01 | 0.57 ± 0.02 a | 1.61 ± 0.03 a | 0.323 ± 0.012 b |
| EC2 | 1.01 ± 0.01 | 0.52 ± 0.01 ab | 1.52 ± 0.02 b | 0.348 ± 0.006 a |
| EC4 | 1.02 ± 0.01 | 0.53 ± 0.01 ab | 1.58 ± 0.04 ab | 0.329 ± 0.009 ab |
| EC6 | 1.00 ± 0.01 | 0.49 ± 0.02 b | 1.50 ± 0.03 b | 0.328 ± 0.005 ab |
| BC | 1.00 ± 0.01 b | 0.50 ± 0.01 b | 1.52 ± 0.03 b | 0.326 ± 0.010 |
| BA | 1.01 ± 0.01 b | 0.51 ± 0.01 b | 1.52 ± 0.02 b | 0.340 ± 0.006 |
| BT | 1.05 ± 0.01 a | 0.57 ± 0.02 a | 1.62 ± 0.02 a | 0.329 ± 0.005 |
| I | 1.04 ± 0.02 a | 0.57 ± 0.02 a | 1.60 ± 0.03 ab | 0.348 ± 0.005 b |
| II | 1.00 ± 0.01 ab | 0.57 ± 0.02 a | 1.54 ± 0.03 a–c | 0.397 ± 0.005 a |
| III | 1.04 ± 0.01 a | 0.55 ± 0.02 ab | 1.59 ± 0.03 ab | 0.342 ± 0.003 b |
| IV | 0.98 ± 0.01 b | 0.52 ± 0.02 a–c | 1.47 ± 0.03 c | 0.334 ± 0.004 b |
| V | 1.03 ± 0.01 ab | 0.50 ± 0.01 bc | 1.63 ± 0.05 a | 0.296 ± 0.010 c |
| VI | 1.02 ± 0.01 ab | 0.47 ± 0.01 c | 1.49 ± 0.02 bc | 0.276 ± 0.010 c |
| Treatment | Mineral Composition (g kg−1 dw) | Nitrate (mg kg−1 fw) | ||||||
|---|---|---|---|---|---|---|---|---|
| Na | K | Ca | Mg | Cl | P | |||
| EC0 | BC | 5.14 ± 0.31 e | 60.06 ± 2.31 a | 22.50 ± 0.98 bc | 5.09 ± 0.13 a | 25.75 ± 1.57 de | 2.66 ± 0.11 ab | 3791.5 ± 391.0 de |
| BA | 4.81 ± 0.18 e | 57.16 ± 1.50 ab | 22.00 ± 0.69 c–e | 4.79 ± 0.12 ab | 20.47 ± 1.00 ef | 2.81 ± 0.08 a | 5403.9 ± 320.2 a | |
| BT | 4.72 ± 0.23 e | 53.66 ± 1.44 bc | 24.50 ± 0.96 a | 5.12 ± 0.16 a | 19.20 ± 0.63 f | 2.68 ± 0.09 ab | 5799.0 ± 365.8 a | |
| EC2 | BC | 10.39 ± 0.64 d | 55.41 ± 1.58 a–c | 22.24 ± 0.70 b–d | 4.63 ± 0.10 b | 37.57 ± 2.41 c | 2.60 ± 0.08 ac | 3286.7 ± 279.5 f |
| BA | 8.45 ± 0.55 d | 54.35 ± 2.63 a–c | 24.07 ± 0.51 ab | 4.86 ± 0.12 ab | 28.23 ± 1.52 d | 2.49 ± 0.07 b–d | 4569.8 ± 245.8 bc | |
| BT | 9.43 ± 0.54 d | 55.62 ± 1.32 a–c | 22.90 ± 0.56 a–c | 5.09 ± 0.08 a | 29.83 ± 1.69 d | 2.69 ± 0.09 ab | 4810.2 ± 210.0 b | |
| EC4 | BC | 14.83 ± 1.26 ab | 52.00 ± 1.07 bc | 21.08 ± 0.99 c–f | 4.86 ± 0.09 ab | 50.81 ± 3.85 a | 2.60 ± 0.07 a–c | 2746.0 ± 396.1 g |
| BA | 10.88 ± 0.82 cd | 55.46 ± 1.38 a–c | 19.91 ± 0.65 f | 4.62 ± 0.09 b | 37.05 ± 2.03 c | 2.69 ± 0.08 ab | 4230.9 ± 228.1 cd | |
| BT | 14.17 ± 1.11 ab | 53.89 ± 2.00 bc | 20.53 ± 0.56 d–f | 4.85 ± 0.10 ab | 44.84 ± 2.33 b | 2.81 ± 0.10 a | 3962.6 ± 227.6 d | |
| EC6 | BC | 13.86 ± 1.02 bc | 51.22 ± 1.40 bc | 19.88 ± 0.86 f | 4.66 ± 0.11 b | 49.91 ± 2.91 ab | 2.19 ± 0.08 d | 2422.4 ± 453.5 g |
| BA | 17.06 ± 1.29 a | 52.07 ± 1.82 bc | 19.99 ± 0.77 f | 4.76 ± 0.09 ab | 50.34 ± 3.16 ab | 2.42 ± 0.08 b–d | 3471.0 ± 285.5 ef | |
| BT | 15.95 ± 1.27 ab | 50.55 ± 1.38 c | 20.18 ± 0.78 ef | 4.59 ± 0.11 b | 49.14 ± 2.98 ab | 2.32 ± 0.07 cd | 3740.4 ± 254.5 d–f | |
| Treatment | Mineral Composition (g kg−1 dw) | Nitrate (mg kg−1 fw) | |||||
|---|---|---|---|---|---|---|---|
| Na | Ca | Mg | Cl | S | |||
| EC0 | I | 3.65 ± 0.36 m | 20.42 ± 0.86 fgh | 4.29 ± 0.13 ij | 18.61 ± 1.16 h | 7.52 ± 0.34 b–e | 4386.2 ± 338.4 ef |
| II | 5.23 ± 0.21 lm | 28.97 ± 0.67 a | 5.73 ± 0.16 a | 18.86 ± 1.17 h | 7.44 ± 0.42 b–f | 5159.8 ± 238.9 d | |
| III | 5.44 ± 0.31 lm | 23.89 ± 1.23 cd | 5.46 ± 0.11 a | 22.19 ± 1.78 f–h | 5.18 ± 0.55 i | 6729.8 ± 487.5 a | |
| IV | 4.50 ± 0.20 lm | 22.58 ± 0.99 d–f | 4.81 ± 0.14 c–h | 21.09 ± 1.59 gh | 3.73 ± 0.46 j | 4780.7 ± 534.5 cd | |
| V | 4.87 ± 0.37 lm | 21.22 ± 0.47 fg | 4.89 ± 0.13 b–g | 24.28 ± 1.90 fg | 5.14 ± 0.94 i | 4738.7 ± 164.4 d | |
| VI | 5.63 ± 0.16 l | 20.93 ± 0.81 fg | 4.80 ± 0.07 c–h | 25.82 ± 2.27 f | 7.00 ± 0.96 c–f | 4139.5 ± 390.1 fg | |
| EC2 | I | 5.25 ± 0.23 lm | 24.89 ± 0.71 bc | 4.63 ± 0.15 gh | 19.59 ± 1.52 h | 7.69 ± 0.31 b–d | 5136.5 ± 3510.5 b |
| II | 8.61 ± 0.38 hi | 26.14 ± 0.56 b | 5.02 ± 0.15 b–d | 25.53 ± 1.41 f | 6.39 ± 0.45 f–h | 4017.4 ± 144.5 gh | |
| III | 11.34 ± 0.43 g | 21.86 ± 0.74 ef | 5.14 ± 0.16 b | 36.05 ± 2.38 e | 5.32 ± 0.43 hi | 5077.9 ± 235.6 bc | |
| IV | 10.68 ± 0.36 g | 21.85 ± 0.81 ef | 4.97 ± 0.14 b–e | 36.20 ± 2.27 e | 6.83 ± 0.62 d–f | 3915.4 ± 313.6 g–i | |
| V | 10.17 ± 0.80 gh | 21.66 ± 0.59 ef | 4.72 ± 0.13 d–h | 36.01 ± 2.11 e | 7.77 ± 0.70 b–d | 3825.5 ± 276.2 h–j | |
| VI | 10.49 ± 0.68 gh | 22.02 ± 0.52 ef | 4.70 ± 0.15 e–h | 37.87 ± 2.12 e | 9.31 ± 0.26 a | 3360.5 ± 149.1 k–m | |
| EC4 | I | 6.14 ± 0.28 jk | 21.92 ± 0.79 ef | 4.57 ± 0.14 hi | 25.55 ± 1.23 f | 8.01 ± 0.24 bc | 4526.7 ± 224.6 de |
| II | 11.69 ± 0.41 fg | 25.06 ± 0.51 bc | 5.04 ± 0.11 bc | 37.95 ± 2.04 e | 5.62 ± 0.24 g–i | 3277.7 ± 214.5 mn | |
| III | 13.32 ± 0.46 ef | 18.59 ± 0.63 ij | 4.83 ± 0.08 b–h | 47.24 ± 2.88 d | 4.51 ± 0.48 ij | 3904.6 ± 334.0 g–i | |
| IV | 16.70 ± 1.45 ac | 18.81 ± 0.98 hi | 4.93 ± 0.20 b–f | 50.66 ± 3.46 cd | 5.31 ± 0.80 hi | 3642.0 ± 296.3 ij | |
| V | 16.31 ± 1.35 bc | 18.90 ± 0.83 hi | 4.69 ± 0.09 e–h | 51.23 ± 4.31 cd | 6.76 ± 0.69 d–g | 3568.3 ± 287.3 j–l | |
| VI | 15.59 ± 1.67 cd | 19.76 ± 0.69 g–i | 4.60 ± 0.10 g–i | 52.78 ± 3.58 bc | 8.50 ± 0.76 ab | 2959.8 ± 346.8 o | |
| EC6 | I | 7.79 ± 0.23 ij | 23.02 ± 0.57 de | 4.59 ± 0.11 g–i | 25.87 ± 0.91 f | 8.08 ± 0.22 bc | 4773.5 ± 489.4 cd |
| II | 14.02 ± 0.64 de | 24.94 ± 0.68 bc | 5.00 ± 0.12 be | 47.26 ± 2.26 d | 6.47 ± 0.24 e–h | 2431.0 ± 103.4 p | |
| III | 17.53 ± 1.02 ab | 18.10 ± 0.51 ij | 4.74 ± 0.11 c–h | 54.99 ± 1.45 a–c | 5.47 ± 0.39 hi | 3098.8 ± 290.7 m–o | |
| IV | 18.28 ± 0.97 a | 18.37 ± 0.33 ij | 4.75 ± 0.07 c–h | 56.14 ± 1.17 ab | 4.64 ± 0.51 ij | 3204.1 ± 355.5 m–o | |
| V | 18.21 ± 1.33 ab | 18.07 ± 0.58 ij | 4.64 ± 0.14 f–h | 57.21 ± 2.65 a | 6.77 ± 0.71 d–g | 3364.1 ± 318.0 mn | |
| VI | 17.90 ± 2.15 ab | 17.61 ± 0.76 j | 4.26 ± 0.21 j | 57.30 ± 2.32 a | 7.75 ± 0.82 b–d | 2396.3 ± 229.9 p | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Mola, I.; Petropoulos, S.A.; Ottaiano, L.; Cozzolino, E.; El-Nakhel, C.; Rouphael, Y.; Mori, M. Bioactive Compounds, Antioxidant Activity, and Mineral Content of Wild Rocket (Diplotaxis tenuifolia L.) Leaves as Affected by Saline Stress and Biostimulant Application. Appl. Sci. 2023, 13, 1569. https://doi.org/10.3390/app13031569
Di Mola I, Petropoulos SA, Ottaiano L, Cozzolino E, El-Nakhel C, Rouphael Y, Mori M. Bioactive Compounds, Antioxidant Activity, and Mineral Content of Wild Rocket (Diplotaxis tenuifolia L.) Leaves as Affected by Saline Stress and Biostimulant Application. Applied Sciences. 2023; 13(3):1569. https://doi.org/10.3390/app13031569
Chicago/Turabian StyleDi Mola, Ida, Spyridon A. Petropoulos, Lucia Ottaiano, Eugenio Cozzolino, Christophe El-Nakhel, Youssef Rouphael, and Mauro Mori. 2023. "Bioactive Compounds, Antioxidant Activity, and Mineral Content of Wild Rocket (Diplotaxis tenuifolia L.) Leaves as Affected by Saline Stress and Biostimulant Application" Applied Sciences 13, no. 3: 1569. https://doi.org/10.3390/app13031569







