Poly(lactic acid)-Based Blends: A Comprehensive Review
Abstract
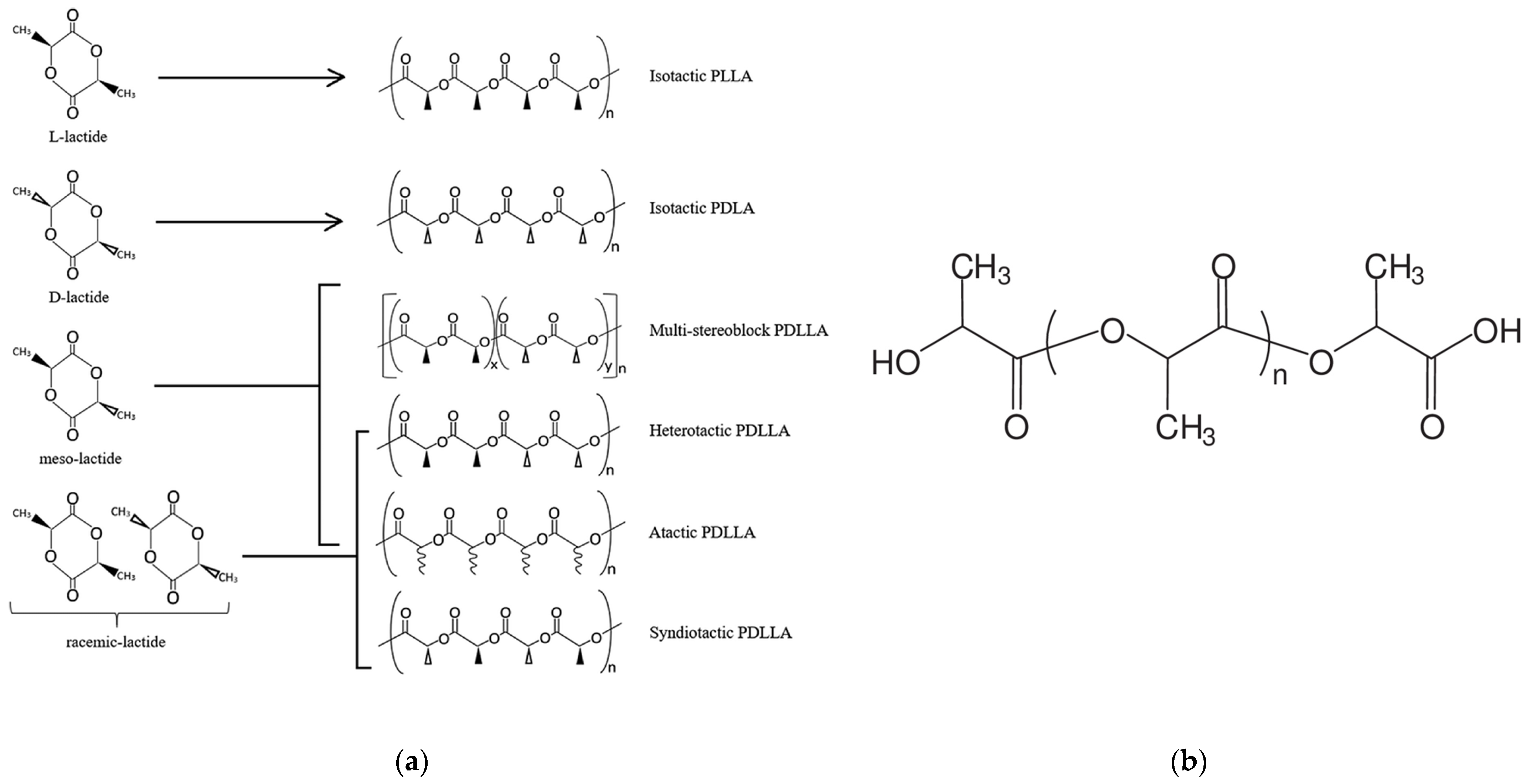
1. Introduction
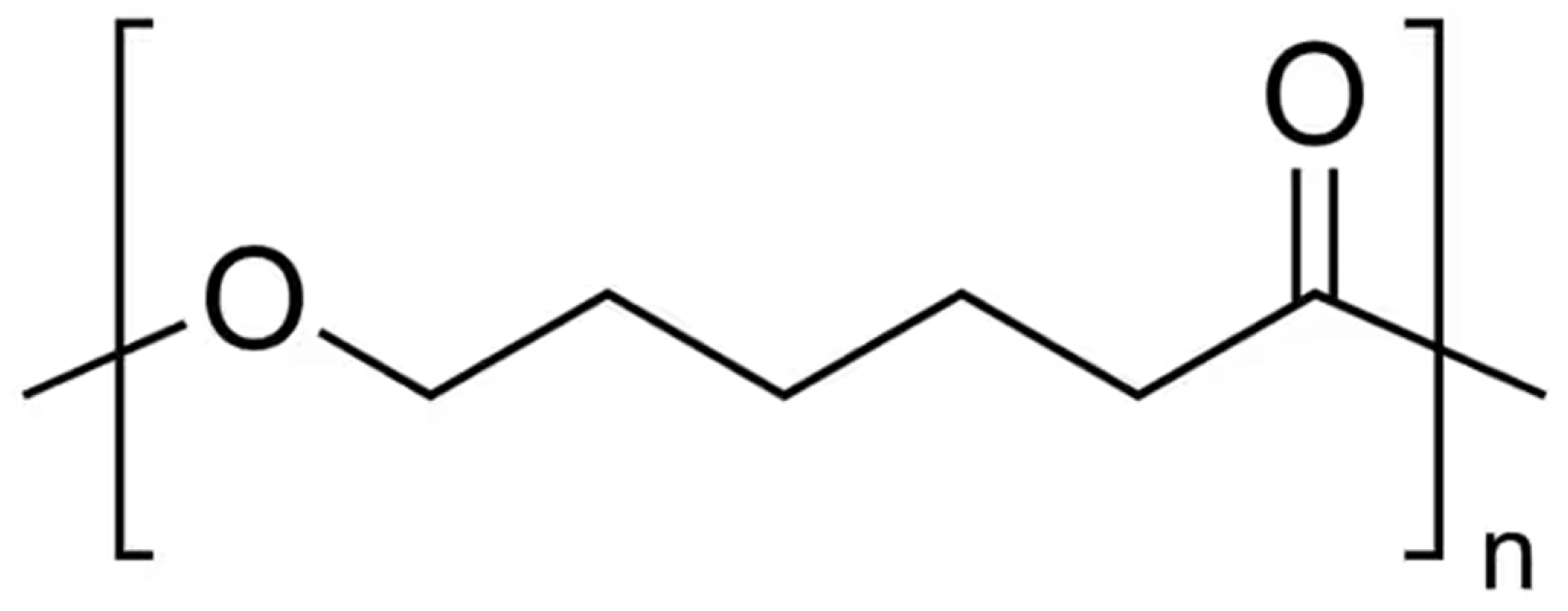
2. Composites of Poly(lactic acid) with Poly(ε-caprolactone)
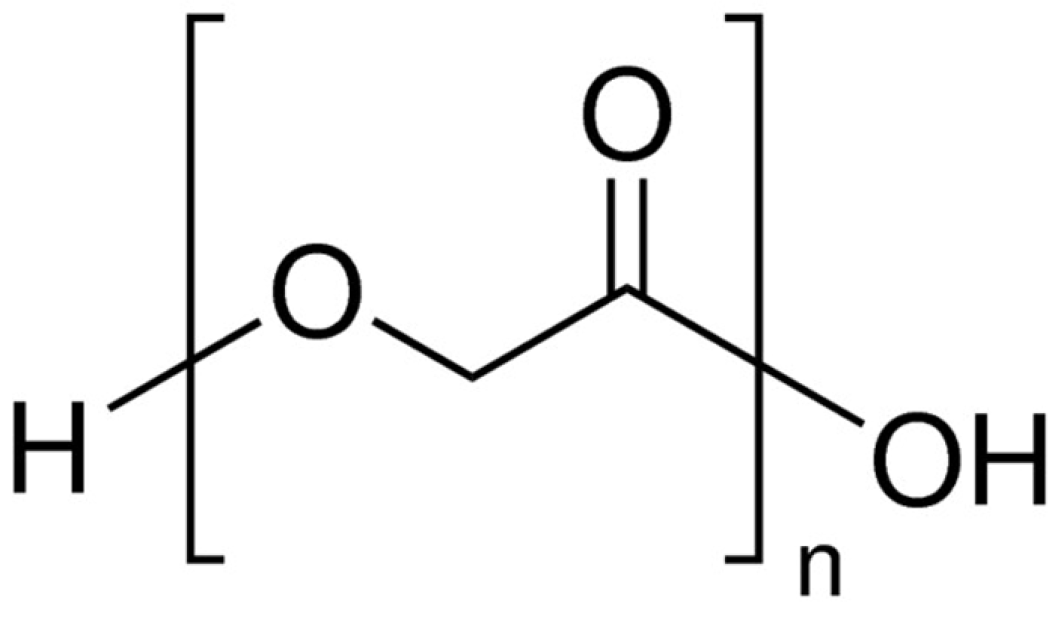
3. Composites of Poly(lactic acid) with Poly(glycolic acid)
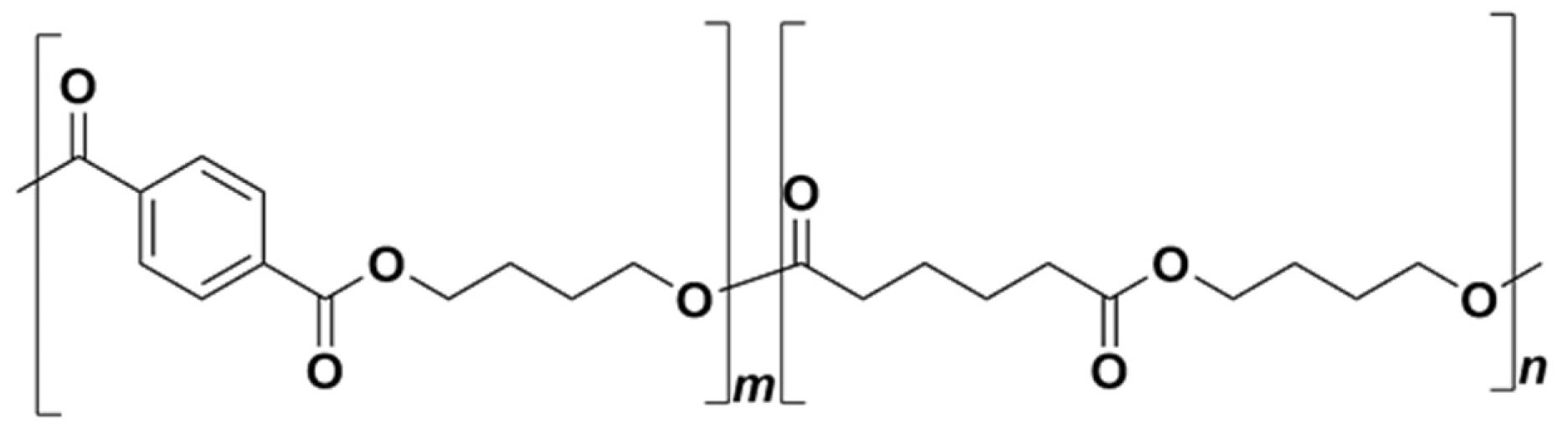
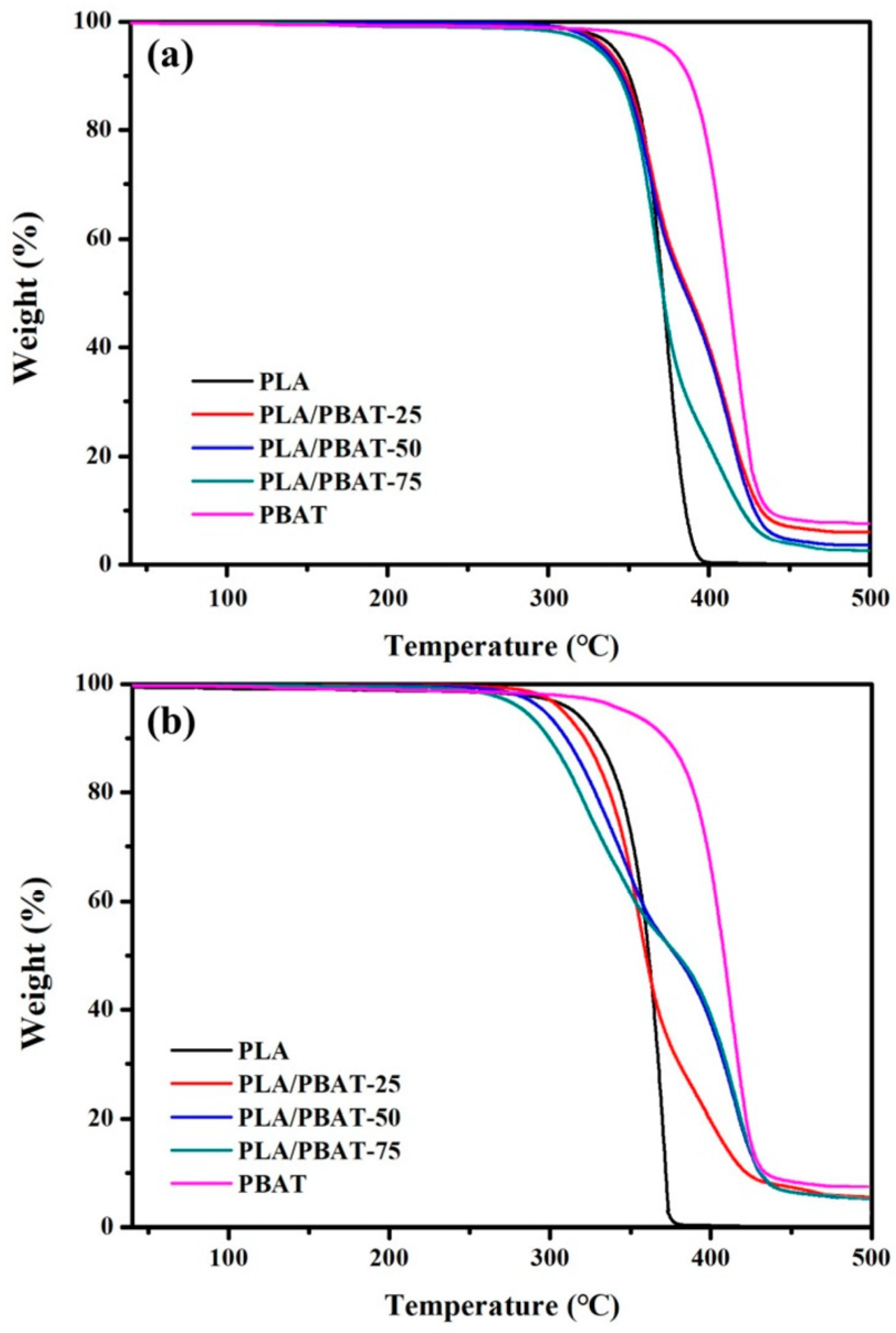
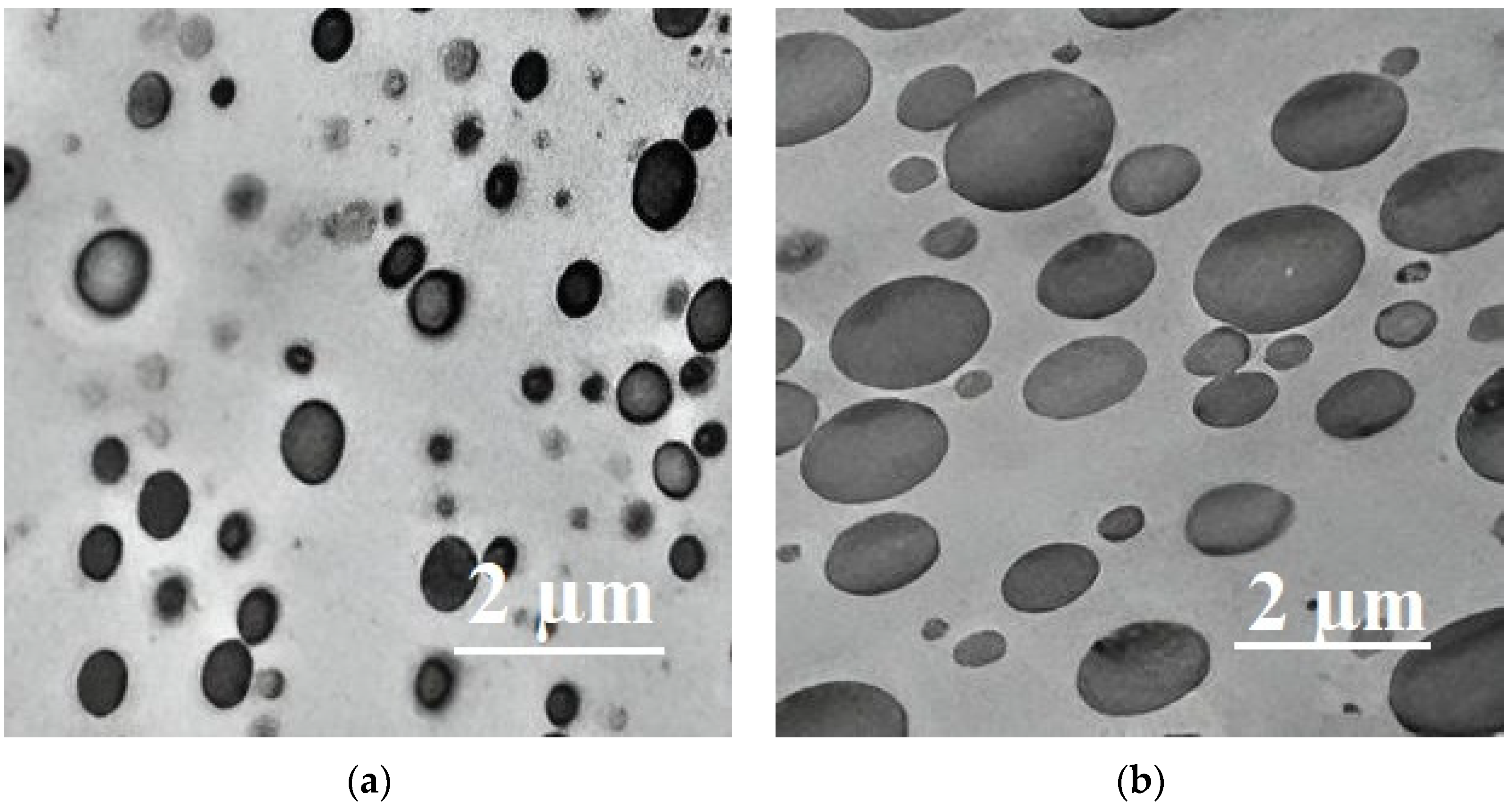
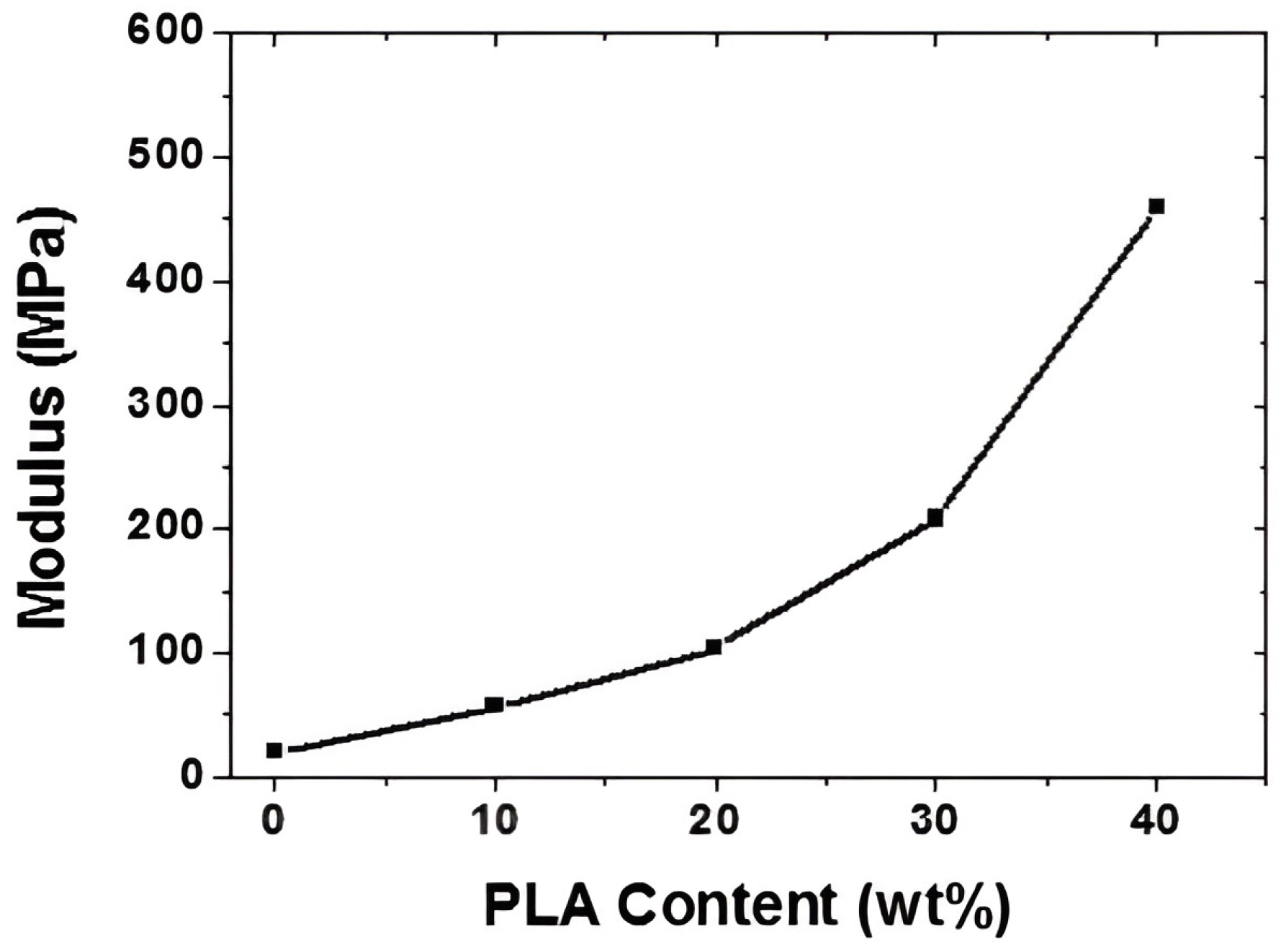
4. Composites of Poly(lactic acid) with Poly(butylene adipate-co-terephthalate)
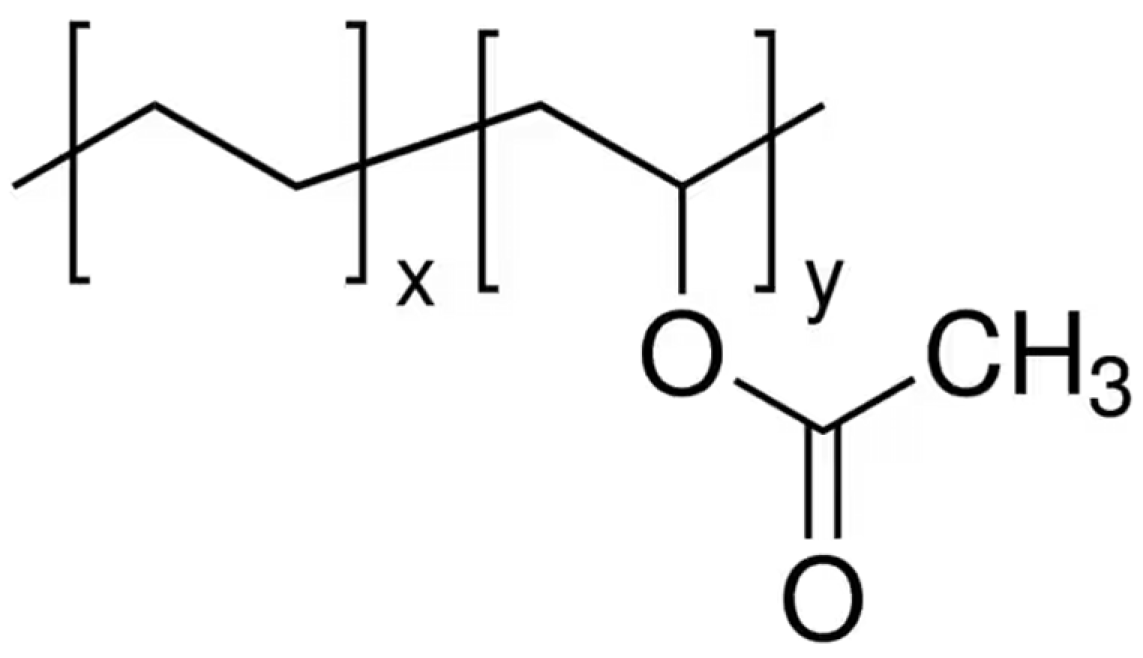
5. Composites of Poly(lactic acid) with Poly(ethylene-co-vinyl acetate)
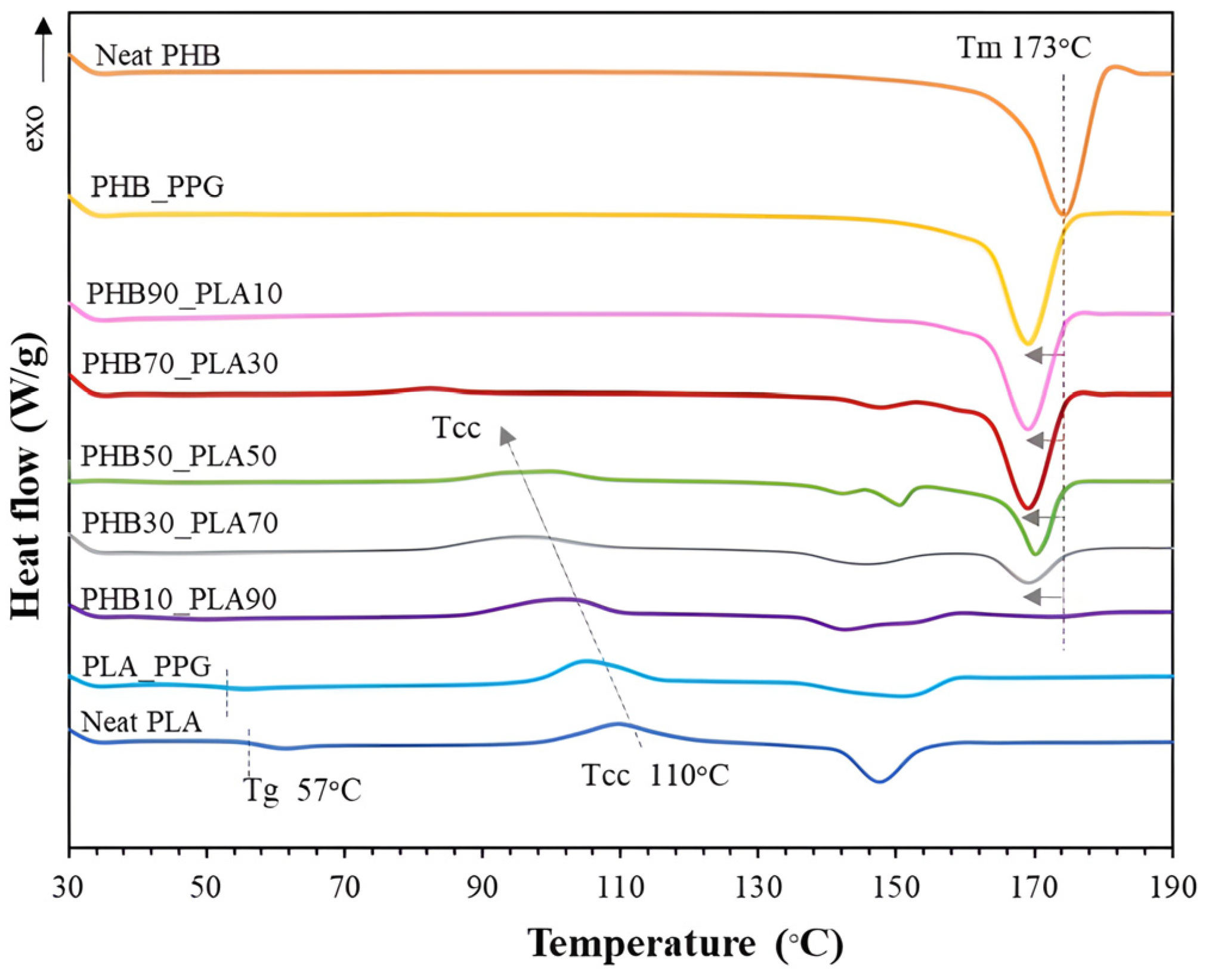
6. Composites of Poly(lactic acid) with Poly(3-hydroxybutyrate)
7. Discussion
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nagarajan, V.; Mohanty, A.K.; Misra, M. Perspective on Polylactic Acid (PLA) Based Sustainable Materials for Durable Applications: Focus on Toughness and Heat Resistance. ACS Sustain. Chem. Eng. 2016, 4, 2899–2916. [Google Scholar] [CrossRef]
- Rajeshkumar, L. Biodegradable Polymer Blends and Composites Renewable Resources. In Biodegradable Polymers, Blends and Composites; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar] [CrossRef]
- Jalabert, M.; Fraschini, C.; Prud’Homme, R.E. Synthesis and Characterization of Poly(L-Lactide)s and Poly(D-Lactide)s of Controlled Molecular Weight. J. Polym. Sci. A Polym. Chem. 2007, 45, 1944–1955. [Google Scholar] [CrossRef]
- Mastalygina, E.E.; Olkhov, A.A.; Vorontsov, N.V.; Kiselev, N.V.; Khaidarov, T.B.; Khaydarov, B.B.; Kolesnikov, E.A.; Burmistrov, I.N. Influence of Copper-Based Fillers on Structural and Mechanical Properties of Polylactic Acid Composites. J. Compos. Sci. 2022, 6, 386. [Google Scholar] [CrossRef]
- Feng, L.; Bian, X.; Li, G.; Chen, X. Thermal Properties and Structural Evolution of Poly(l-Lactide)/Poly(d-Lactide) Blends. Macromolecules 2021, 54, 10163–10176. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Zinjarde, S.S.; Gokhale, D.V. Polylactic Acid: Synthesis and Biomedical Applications. J. Appl. Microbiol. 2019, 127, 1612–1626. [Google Scholar] [CrossRef]
- Murariu, M.; Dubois, P. PLA Composites: From Production to Properties. Adv. Drug. Deliv. Rev. 2016, 107, 17–46. [Google Scholar] [CrossRef]
- Södergård, A.; Stolt, M. Properties of Lactic Acid Based Polymers and Their Correlation with Composition. Prog. Polym. Sci. 2002, 27, 1123–1163. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and Biological Application of Polylactic Acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef]
- Suryanegara, L.; Nakagaito, A.N.; Yano, H. The Effect of Crystallization of PLA on the Thermal and Mechanical Properties of Microfibrillated Cellulose-Reinforced PLA Composites. Compos. Sci. Technol. 2009, 69, 1187–1192. [Google Scholar] [CrossRef]
- Carrasco, F.; Pagès, P.; Gámez-Pérez, J.; Santana, O.O.; Maspoch, M.L. Processing of Poly(Lactic Acid): Characterization of Chemical Structure, Thermal Stability and Mechanical Properties. Polym. Degrad. Stab. 2010, 95, 116–125. [Google Scholar] [CrossRef]
- Fortunati, E.; Armentano, I.; Zhou, Q.; Iannoni, A.; Saino, E.; Visai, L.; Berglund, L.A.; Kenny, J.M. Multifunctional Bionanocomposite Films of Poly(Lactic Acid), Cellulose Nanocrystals and Silver Nanoparticles. Carbohydr. Polym. 2012, 87, 1596–1605. [Google Scholar] [CrossRef]
- Inkinen, S.; Hakkarainen, M.; Albertsson, A.C.; Södergård, A. From Lactic Acid to Poly(Lactic Acid) (PLA): Characterization and Analysis of PLA and Its Precursors. Biomacromolecules 2011, 12, 523–532. [Google Scholar] [CrossRef]
- Vasir, J.K.; Labhasetwar, V. Biodegradable Nanoparticles for Cytosolic Delivery of Therapeutics. Adv. Drug. Deliv. Rev. 2007, 59, 718–728. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and Mechanical Properties of PLA, and Their Functions in Widespread Applications—A Comprehensive Review. Adv. Drug. Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Sawyer, D.J. Bioprocessing—No Longer a Field of Dreams. In Macromolecular Symposia; WILEY-VCH Verlag: Weinheim, Germany, 2003; Volume 201. [Google Scholar] [CrossRef]
- Rasal, R.M.; Janorkar, A.V.; Hirt, D.E. Poly(Lactic Acid) Modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Ren, J. Modification of PLA. In Biodegradable Poly(Lactic Acid): Synthesis, Modification, Processing and Applications; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Huda, M.S.; Drzal, L.T.; Mohanty, A.K.; Misra, M. Effect of Fiber Surface-Treatments on the Properties of Laminated Biocomposites from Poly(Lactic Acid) (PLA) and Kenaf Fibers. Compos. Sci. Technol. 2008, 68, 424–432. [Google Scholar] [CrossRef]
- Tsuji, H. Hydrolytic Degradation. In Poly(Lactic Acid); John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010; pp. 343–381. [Google Scholar] [CrossRef]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the Structure of Solution Grown Crystals of Lactide Copolymers by Means of Chemical Reactions. Kolloid-Z. Z. Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- Ho, K.L.G.; Pometto, A.L.; Gadea-Rivas, A.; Briceño, J.A.; Rojas, A. Degradation of Polylactic Acid (PLA) Plastic in Costa Rican Soil and Iowa State University Compost Rows. J. Environ. Polym. Degrad. 1999, 7, 173–177. [Google Scholar] [CrossRef]
- Podzorova, M.V.; Tertyshnaya, Y.V.; Popov, A.A. The Effect of Environmental Factors on Biodegradable Polylactide-Based Materials. Polym. Sci. Ser. D 2017, 10, 289–292. [Google Scholar] [CrossRef]
- Rogovina, S.Z.; Aleksanyan, K.V.; Vladimirov, L.V.; Berlin, A.A. Biodegradable Polymer Materials Based on Polylactide. Russ. J. Phys. Chem. B 2019, 13, 812–818. [Google Scholar] [CrossRef]
- Mohamed, R.M.; Yusoh, K. A Review on the Recent Research of Polycaprolactone (PCL). Adv. Mater. Res. 2015, 1134, 249–255. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as Biomaterial for Bone Scaffolds: Review of Literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Xiao, A.; Yu, B.; Bhat, G.; Zhu, F. Effect of PCL and Compatibilizer on the Tensile and Barrier Properties of PLA/PCL Films. Polymer 2017, 41, 181. [Google Scholar] [CrossRef]
- Jeong, H.; Rho, J.; Shin, J.Y.; Lee, D.Y.; Hwang, T.; Kim, K.J. Mechanical Properties and Cytotoxicity of PLA/PCL Films. Biomed. Eng. Lett. 2018, 8, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Bulatović, V.O.; Grgić, D.K.; Slouf, M.; Ostafinska, A.; Dybal, J.; Jozinović, A. Biodegradability of Blends Based on Aliphatic Polyester and Thermoplastic Starch. Chem. Pap. 2019, 73, 1121–1134. [Google Scholar] [CrossRef]
- Noroozi, N.; Schafer, L.L.; Hatzikiriakos, S.G. Thermorheological Properties of Poly (ε-Caprolactone)/Polylactide Blends. Polym. Eng. Sci. 2012, 52, 2348–2359. [Google Scholar] [CrossRef]
- Dell’Erba, R.; Groeninckx, G.; Maglio, G.; Malinconico, M.; Migliozzi, A. Immiscible Polymer Blends of Semicrystalline Biocompatible Components: Thermal Properties and Phase Morphology Analysis of PLLA/PCL Blends. Polymer 2001, 42, 7831–7840. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, Y.; Zhang, M.; Zhou, W. Phase Behavior and Its Viscoelastic Response of Polylactide/Poly(ε-Caprolactone) Blend. Eur. Polym. J. 2008, 44, 2171–2183. [Google Scholar] [CrossRef]
- Botlhoko, O.J.; Ramontja, J.; Ray, S.S. A New Insight into Morphological, Thermal, and Mechanical Properties of Melt-Processed Polylactide/Poly(ε-Caprolactone) Blends. Polym. Degrad. Stab. 2018, 154, 84–95. [Google Scholar] [CrossRef]
- Navarro-Baena, I.; Sessini, V.; Dominici, F.; Torre, L.; Kenny, J.M.; Peponi, L. Design of Biodegradable Blends Based on PLA and PCL: From Morphological, Thermal and Mechanical Studies to Shape Memory Behavior. Polym. Degrad. Stab. 2016, 132, 97–108. [Google Scholar] [CrossRef]
- Broz, M.E.; VanderHart, D.L.; Washburn, N.R. Structure and Mechanical Properties of Poly(D,L-Lactic Acid)/Poly(ε-Caprolactone) Blends. Biomaterials 2003, 24, 4181–4190. [Google Scholar] [CrossRef]
- Todo, M.; Takayam, T. Fracture Mechanisms of Biodegradable PLA and PLA/PCL Blends. In Biomaterials—Physics and Chemistry; InTech: Rijeka, Croatia, 2011; pp. 375–394. [Google Scholar] [CrossRef]
- Ostafinska, A.; Fortelny, I.; Nevoralova, M.; Hodan, J.; Kredatusova, J.; Slouf, M. Synergistic Effects in Mechanical Properties of PLA/PCL Blends with Optimized Composition, Processing, and Morphology. RSC Adv. 2015, 5, 98971–98982. [Google Scholar] [CrossRef]
- Ostafinska, A.; Fortelný, I.; Hodan, J.; Krejčíková, S.; Nevoralová, M.; Kredatusová, J.; Kruliš, Z.; Kotek, J.; Šlouf, M. Strong Synergistic Effects in PLA/PCL Blends: Impact of PLA Matrix Viscosity. J. Mech. Behav. Biomed. Mater. 2017, 69, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Bai, H.; Huang, C.; Xiu, H.; Gao, Y.; Zhang, Q.; Fu, Q. Toughening of Poly(l-Lactide) with Poly(ε-Caprolactone): Combined Effects of Matrix Crystallization and Impact Modifier Particle Size. Polymer 2013, 54, 5257–5266. [Google Scholar] [CrossRef]
- Urquijo, J.; Guerrica-Echevarría, G.; Eguiazábal, J.I. Melt Processed PLA/PCL Blends: Effect of Processing Method on Phase Structure, Morphology, and Mechanical Properties. J. Appl. Polym. Sci. 2015, 132, 42641. [Google Scholar] [CrossRef]
- Semba, T.; Kitagawa, K.; Ishiaku, U.S.; Hamada, H. The Effect of Crosslinking on the Mechanical Properties of Polylactic Acid/Polycaprolactone Blends. J. Appl. Polym. Sci. 2006, 101, 1816–1825. [Google Scholar] [CrossRef]
- Przybysz-Romatowska, M.; Haponiuk, J.; Formela, K. Poly(ε-Caprolactone)/Poly(Lactic Acid) Blends Compatibilized by Peroxide Initiators: Comparison of Two Strategies. Polymers 2020, 12, 228. [Google Scholar] [CrossRef]
- Chee, W.K.; Ibrahim, N.A.; Zainuddin, N.; Abd Rahman, M.F.; Chieng, B.W. Impact Toughness and Ductility Enhancement of Biodegradable Poly(Lactic Acid)/Poly(ε-Caprolactone) Blends via Addition of Glycidyl Methacrylate. Adv. Mater. Sci. Eng. 2013, 2013, 976373. [Google Scholar] [CrossRef]
- Tsuji, H.; Yamada, T. Blends of Aliphatic Polyesters. VIII. Effects of Poly(L-Lactide-Co-ε-Caprolactone) on Enzymatic Hydrolysis of Poly(L-Lactide), Poly(ε-Caprolactone), and Their Blend Films. J. Appl. Polym. Sci. 2003, 87, 412–419. [Google Scholar] [CrossRef]
- Jem, K.J.; Tan, B. The Development and Challenges of Poly (Lactic Acid) and Poly (Glycolic Acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Middleton, J.C.; Tipton, A.J. Synthetic Biodegradable Polymers as Medical Devices. Med. Device Diagn. Ind. News Prod. Suppliers 1998, 21, 1–8. [Google Scholar]
- Lee, S.; Hongo, C.; Nishino, T. Crystal Modulus of Poly(Glycolic Acid) and Its Temperature Dependence. Macromolecules 2017, 50, 5074–5079. [Google Scholar] [CrossRef]
- Samantaray, P.K.; Little, A.; Haddleton, D.M.; McNally, T.; Tan, B.; Sun, Z.; Huang, W.; Ji, Y.; Wan, C. Poly(Glycolic Acid) (PGA): A Versatile Building Block Expanding High Performance and Sustainable Bioplastic Applications. Green Chem. 2020, 22, 4055–4081. [Google Scholar] [CrossRef]
- Miller, R.A.; Brady, J.M.; Cutright, D.E. Degradation Rates of Oral Resorbable Implants (Polylactates and Polyglycolates): Rate Modification with Changes in PLA/PGA Copolymer Ratios. J. Biomed. Mater. Res. 1977, 11, 711–719. [Google Scholar] [CrossRef]
- You, Y.; Youk, J.H.; Lee, S.W.; Min, B.-M.; Lee, S.J.; Park, W.H. Preparation of Porous Ultrafine PGA Fibers via Selective Dissolution of Electrospun PGA/PLA Blend Fibers. Mater. Lett. 2006, 60, 757–760. [Google Scholar] [CrossRef]
- Mazitova, A.K.; Aminova, G.K.; Zaripov, I.I.; Vikhareva, I.N. Biodegradable Polymer Materials and Modifying Additives: State of the Art. Part II. Nanotechnol. Constr. 2021, 13, 32–38. [Google Scholar] [CrossRef]
- Ramdhanie, L.I.; Aubuchon, S.R.; Boland, E.D.; Knapp, D.C.; Barnes, C.P.; Simpson, D.G.; Wnek, G.E.; Bowlin, G.L. Thermal and Mechanical Characterization of Electrospun Blends of Poly(Lactic Acid) and Poly(Glycolic Acid). Polym. J. 2006, 38, 1137–1145. [Google Scholar] [CrossRef]
- Choi, R.S.; Riegler, M.; Pothoulakis, C.; Kim, B.S.; Mooney, D.; Vacanti, M.; Vacanti, J.P. Studies of Brush Border Enzymes, Basement Membrane Components, and Electrophysiology of Tissue-Engineered Neointestine. J. Pediatr. Surg. 1998, 33, 991–997. [Google Scholar] [CrossRef]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic Acid (PLA) Controlled Delivery Carriers for Biomedical Applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef]
- Pawar, R.P.; Tekale, S.U.; Shisodia, S.U.; Totre, J.T.; Domb, A.J. Biomedical Applications of Poly(Lactic Acid). Recent Pat. Regen. Med. 2014, 4, 40–51. [Google Scholar] [CrossRef]
- Cantón, I.; Mckean, R.; Charnley, M.; Blackwood, K.A.; Fiorica, C.; Ryan, A.J.; MacNeil, S. Development of an Ibuprofen-Releasing Biodegradable PLA/PGA Electrospun Scaffold for Tissue Regeneration. Biotechnol. Bioeng. 2010, 105, 396–408. [Google Scholar] [CrossRef]
- Jian, J.; Xiangbin, Z.; Xianbo, H. An Overview on Synthesis, Properties and Applications of Poly(Butylene-Adipate-Co-Terephthalate)–PBAT. Adv. Ind. Eng. Polym. Res. 2020, 3, 19–26. [Google Scholar] [CrossRef]
- Herrera, R.; Franco, L.; Rodríguez-Galán, A.; Puiggalí, J. Characterization and Degradation Behavior of Poly(Butylene Adipate-Co-Terephthalate)s. J. Polym. Sci. A Polym. Chem. 2002, 40, 4141–4157. [Google Scholar] [CrossRef]
- Lee, S.H.; Lim, S.W.; Lee, K.H. Properties of Potentially Biodegradable Copolyesters of (Succinic Acid–1,4-butanediol)/(Dimethyl Terephthalate–1,4-butanediol). Polym. Int. 1999, 48, 861–867. [Google Scholar] [CrossRef]
- Chandra, R.; Rustgi, R. Biodegradable Polymers. Prog. Polym. Sci. 1998, 23, 1273–1335. [Google Scholar] [CrossRef]
- Müller, R.J.; Witt, U.; Rantze, E.; Deckwer, W.D. Architecture of Biodegradable Copolyesters Containing Aromatic Constituents. Polym. Degrad. Stab. 1998, 59, 203–208. [Google Scholar] [CrossRef]
- Shahlari, M.; Lee, S. Biodegradable Polymer/Clay Nanocomposites Based on Poly(Butylene Adipate-Co-Terephthalate) and Poly(Lactic Acid). In Proceedings of the AIChE Annual Meeting, Conference Proceedings, Philadelphia, PA, USA, 16–21 November 2008. [Google Scholar]
- Su, S.; Duhme, M.; Kopitzky, R. Uncompatibilized Pbat/Pla Blends: Manufacturability, Miscibility and Properties. Materials 2020, 13, 4897. [Google Scholar] [CrossRef]
- Liu, T.Y.; Lin, W.C.; Yang, M.C.; Chen, S.Y. Miscibility, Thermal Characterization and Crystallization of Poly(l-Lactide) and Poly(Tetramethylene Adipate-Co-Terephthalate) Blend Membranes. Polymer 2005, 46, 12586–12594. [Google Scholar] [CrossRef]
- Yeh, J.T.; Tsou, C.H.; Huang, C.Y.; Chen, K.N.; Wu, C.S.; Chai, W.L. Compatible and Crystallization Properties of Poly(Lactic Acid)/Poly(Butylene Adipate-Co-Terephthalate) Blends. J. Appl. Polym. Sci. 2010, 116, 680–687. [Google Scholar] [CrossRef]
- Chiu, H.-T.; Huang, S.-Y.; Chen, Y.-F.; Kuo, M.-T.; Chiang, T.-Y.; Chang, C.-Y.; Wang, Y.-H. Heat Treatment Effects on the Mechanical Properties and Morphologies of Poly (Lactic Acid)/Poly (Butylene Adipate-Co-Terephthalate) Blends. Int. J. Polym. Sci. 2013, 2013, 951696. [Google Scholar] [CrossRef]
- Meaurio, E.; Zuza, E.; Sarasua, J.R. Direct Measurement of the Enthalpy of Mixing in Miscible Blends of Poly(DL-Lactide) with Poly(Vinylphenol). Macromolecules 2005, 38, 9221–9228. [Google Scholar] [CrossRef]
- Kumara, P.H.S.; Nagasawa, N.; Yagi, T.; Tamada, M. Radiation-Induced Crosslinking and Mechanical Properties of Blends of Poly(Lactic Acid) and Poly(Butylene Terephthalate-Co-Adipate). J. Appl. Polym. Sci. 2008, 109, 3321–3328. [Google Scholar] [CrossRef]
- Signori, F.; Coltelli, M.-B.; Bronco, S. Thermal Degradation of Poly(Lactic Acid) (PLA) and Poly(Butylene Adipate-Co-Terephthalate) (PBAT) and Their Blends upon Melt Processing. Polym. Degrad. Stab. 2009, 94, 74–82. [Google Scholar] [CrossRef]
- Jiang, L.; Wolcott, M.P.; Zhang, J. Study of Biodegradable Polylactide/Poly(Butylene Adipate-Co.-Terephthalate) Blends. Biomacromolecules 2006, 7, 199–207. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, H.; Wang, X.; Yu, X.; Zhou, W.; Peng, S. Super Tough Poly(Lactic Acid) Blends: A Comprehensive Review. RSC Adv. 2020, 10, 13316–13368. [Google Scholar] [CrossRef]
- He, H.Z.; Liu, B.D.; Xue, B.; Zhu, Z.W.; Xue, F.; Liu, S.M.; Chen, M.; Wang, G.Z.; Zhan, Z.M.; Chen, Q.H.; et al. Preparation, Mechanical and Thermal Properties of PLA/PBAT/EGMA Blends. Key Eng. Mater. 2018, 783, 18–22. [Google Scholar] [CrossRef]
- Deng, Y.; Yu, C.; Wongwiwattana, P.; Thomas, N.L. Optimising Ductility of Poly(Lactic Acid)/Poly(Butylene Adipate-Co-Terephthalate) Blends Through Co-Continuous Phase Morphology. J. Polym. Environ. 2018, 26, 3802–3816. [Google Scholar] [CrossRef]
- Sritham, E.; Phunsombat, P.; Chaishome, J. Tensile Properties of PLA/PBAT Blends and PLA Fibre-Reinforced PBAT Composite. MATEC Web Conf. 2018, 192, 03014. [Google Scholar] [CrossRef]
- Palsikowski, P.A.; Kuchnier, C.N.; Pinheiro, I.F.; Morales, A.R. Biodegradation in Soil of PLA/PBAT Blends Compatibilized with Chain Extender. J. Polym. Environ. 2018, 26, 330–341. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, G.; Bian, X.; Zeng, J.; Weng, Y. Biodegradation Behavior of Poly(Butylene Adipate-Co-Terephthalate) (PBAT), Poly(Lactic Acid) (PLA), and Their Blend in Freshwater with Sediment. Molecules 2020, 25, 3946. [Google Scholar] [CrossRef]
- Zumstein, M.T.; Rechsteiner, D.; Roduner, N.; Perz, V.; Ribitsch, D.; Guebitz, G.M.; Kohler, H.P.E.; McNeill, K.; Sander, M. Enzymatic Hydrolysis of Polyester Thin Films at the Nanoscale: Effects of Polyester Structure and Enzyme Active-Site Accessibility. Environ. Sci. Technol. 2017, 51, 7476–7485. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.M. Ethylene-Vinyl Acetate (EVA) Copolymers: A General Review. IEEE Electr. Insul. Mag. 1993, 9, 30–38. [Google Scholar] [CrossRef]
- Singla, R.K.; Zafar, M.T.; Maiti, S.N.; Ghosh, A.K. Physical Blends of PLA with High Vinyl Acetate Containing EVA and Their Rheological, Thermo-Mechanical and Morphological Responses. Polym. Test. 2017, 63, 398–406. [Google Scholar] [CrossRef]
- Yeh, J.T.; Wu, C.J.; Tsou, C.H.; Chai, W.L.; Chow, J.D.; Huang, C.Y.; Chen, K.N.; Wu, C.S. Study on the Crystallization, Miscibility, Morphology, Properties of Poly(Lactic Acid)/Poly(ε-Caprolactone) Blends. Polym.—Plast. Technol. Eng. 2009, 48, 571–578. [Google Scholar] [CrossRef]
- Ma, P.; Hristova-Bogaerds, D.G.; Goossens, J.G.P.; Spoelstra, A.B.; Zhang, Y.; Lemstra, P.J. Toughening of Poly(Lactic Acid) by Ethylene-Co-Vinyl Acetate Copolymer with Different Vinyl Acetate Contents. Eur. Polym. J. 2012, 48, 146–154. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, Y.; Yin, J.; Zhao, G.; Jiang, W. High Impact Poly(Lactic Acid)/Poly(Ethylene Octene) Blends Prepared by Reactive Blending. Polym. Eng. Sci. 2013, 53, 389–396. [Google Scholar] [CrossRef]
- Li, K.; Peng, J.; Turng, L.S.; Huang, H.X. Dynamic Rheological Behavior and Morphology of Polylactide/Poly(Butylenes Adipate-Co-Terephthalate) Blends with Various Composition Ratios. Adv. Polym. Technol. 2011, 30, 67–162. [Google Scholar] [CrossRef]
- Yuan, D.; Xu, C.; Chen, Z.; Chen, Y. Crosslinked Bicontinuous Biobased Polylactide/Natural Rubber Materials: Super Toughness, “Net-like”-Structure of NR Phase and Excellent Interfacial Adhesion. Polym. Test. 2014, 38, 73–80. [Google Scholar] [CrossRef]
- Pracella, M.; Haque, M.M.U.; Paci, M.; Alvarez, V. Property Tuning of Poly(Lactic Acid)/Cellulose Bio-Composites through Blending with Modified Ethylene-Vinyl Acetate Copolymer. Carbohydr. Polym. 2016, 137, 515–524. [Google Scholar] [CrossRef]
- Wang, Y.; Wei, Z.; Li, Y. Highly Toughened Polylactide/Epoxidized Poly(Styrene-b-Butadiene-b-Styrene) Blends with Excellent Tensile Performance. Eur. Polym. J. 2016, 85, 92–104. [Google Scholar] [CrossRef]
- Sangeetha, V.H.; Valapa, R.B.; Nayak, S.K.; Varghese, T.O. Investigation on the Influence of EVA Content on the Mechanical and Thermal Characteristics of Poly(Lactic Acid) Blends. J. Polym. Environ. 2018, 26, 1–14. [Google Scholar] [CrossRef]
- Sinha, P.; Mathur, S.; Sharma, P.; Kumar, V. Potential of Pine Needles for PLA-Based Composites. Polym. Compos. 2018, 39, 1339–1349. [Google Scholar] [CrossRef]
- Makhetha, T.; Mpitso, K.; Luyt, A. Preparation and Characterization of EVA/PLA/Sugarcane Bagasse Composites for Water Purification. J. Compos. Mater. 2017, 51, 1169–1186. [Google Scholar] [CrossRef]
- Van Cong, D.; Hoang, T.; Giang, N.V.; Ha, N.T.; Lam, T.D.; Sumita, M. A Novel Enzymatic Biodegradable Route for PLA/EVA Blends under Agricultural Soil of Vietnam. Mater. Sci. Eng. C 2012, 32, 558–563. [Google Scholar] [CrossRef]
- Lohrasbi, P.; Yeganeh, J.K. Synergistic Toughening of Poly(Lactic Acid)/Poly(Ethylene Vinyl Acetate) (PLA/EVA) by Dynamic Vulcanization and Presence of Hydrophobic Nanoparticles. Polym. Adv. Technol. 2021, 32, 4326–4339. [Google Scholar] [CrossRef]
- Han, D.-H.; Choi, M.-C.; Nagappan, S.; Kim, Y.-M.; Kim, H.-S. Ethylene Vinyl Acetate (EVA)/Poly(Lactic Acid) (PLA) Blends and Their Foams. Mol. Cryst. Liq. Cryst. 2020, 707, 38–45. [Google Scholar] [CrossRef]
- Zhang, T.; Chen, X.; Guo, Z.; Zhao, Y.; Xiu, H.; Bai, H.; Zhang, Q.; Fu, Q. Controlling the Selective Distribution of Hydrophilic Silica Nanoparticles in Polylactide/Ethylene-Co-Vinyl-Acetate Blends via Tailoring the OH Surface Concentration of Silica. Compos. Commun. 2021, 25, 100737. [Google Scholar] [CrossRef]
- McAdam, B.; Brennan Fournet, M.; McDonald, P.; Mojicevic, M. Production of Polyhydroxybutyrate (PHB) and Factors Impacting Its Chemical and Mechanical Characteristics. Polymers 2020, 12, 2908. [Google Scholar] [CrossRef]
- Tertyshnaya, Y.V.; Podzorova, M.V. Composite Materials Based on Polylactide and Poly-3-Hydroxybutyrate “Green” Polymers. Russ. J. Appl. Chem. 2018, 91, 417–423. [Google Scholar] [CrossRef]
- Olkhov, A.; Gur’ev, V.; Akatov, V.; Mastalygina, E.; Iordanskii, A.; Sevastyanov, V.I. Composite Tendon Implant Based on Nanofibrillar Polyhydroxybutyrate and Polyamide Filaments. J. Biomed. Mater. Res. A 2018, 106, 2708–2713. [Google Scholar] [CrossRef]
- Patel, M.K.; Hansson, F.; Pitkänen, O.; Geng, S.; Oksman, K. Biopolymer Blends of Poly(Lactic Acid) and Poly(Hydroxybutyrate) and Their Functionalization with Glycerol Triacetate and Chitin Nanocrystals for Food Packaging Applications. ACS Appl. Polym. Mater. 2022, 4, 6592–6601. [Google Scholar] [CrossRef]
- Abdelwahab, M.A.; Flynn, A.; Chiou, B.-S.; Imam, S.; Orts, W.; Chiellini, E. Thermal, Mechanical and Morphological Characterization of Plasticized PLA–PHB Blends. Polym. Degrad. Stab. 2012, 97, 1822–1828. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Castro-López, M.D.M.; Rayón, E.; Barral-Losada, L.F.; López-Vilariño, J.M.; López, J.; González-Rodríguez, M.V. Plasticized Poly(Lactic Acid)-Poly(Hydroxybutyrate) (PLA-PHB) Blends Incorporated with Catechin Intended for Active Food-Packaging Applications. J. Agric. Food Chem. 2014, 62, 10170–10180. [Google Scholar] [CrossRef]
- Arrieta, M.; Samper, M.; Aldas, M.; López, J. On the Use of PLA-PHB Blends for Sustainable Food Packaging Applications. Materials 2017, 10, 1008. [Google Scholar] [CrossRef] [PubMed]
- Aydemir, D.; Gardner, D.J. Biopolymer Blends of Polyhydroxybutyrate and Polylactic Acid Reinforced with Cellulose Nanofibrils. Carbohydr. Polym. 2020, 250, 116867. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Campo, M.J.; Quiles-Carrillo, L.; Sanchez-Nacher, L.; Balart, R.; Montanes, N. High Toughness Poly(Lactic Acid) (PLA) Formulations Obtained by Ternary Blends with Poly(3-Hydroxybutyrate) (PHB) and Flexible Polyesters from Succinic Acid. Polym. Bull. 2019, 76, 1839–1859. [Google Scholar] [CrossRef]
- Vanovčanová, Z.; Alexy, P.; Feranc, J.; Plavec, R.; Bočkaj, J.; Kaliňáková, L.; Tomanová, K.; Perďochová, D.; Šariský, D.; Gálisová, I. Effect of PHB on the Properties of Biodegradable PLA Blends. Chem. Pap. 2016, 70, 1408–1415. [Google Scholar] [CrossRef]
- Zhang, M. Development of Polyhydroxybutyrate Based Blends for Compostable Packaging. Ph.D. Thesis, Loughborough University, London, UK, 2010. [Google Scholar]
- Armentano, I.; Fortunati, E.; Burgos, N.; Dominici, F.; Luzi, F.; Fiori, S.; Jimenez, A.; Yoon, K.; Ahn, J.; Kang, S.; et al. Processing and Characterization of Plasticized PLA/PHB Blends for Biodegradable Multiphase Systems. Express Polym. Lett. 2015, 9, 583–596. [Google Scholar] [CrossRef]
- Cárdenas-Triviño, G.; Linares-Bermúdez, N.; Núñez-Decap, M. Synthesis and Properties of Bionanocomposites of Polyhydroxybutyrate-Polylactic Acid Doped with Copper and Silver Nanoparticles. Int. J. Polym. Sci. 2019, 2019, 4520927. [Google Scholar] [CrossRef]
- Kervran, M.; Vagner, C.; Cochez, M.; Ponçot, M.; Saeb, M.R.; Vahabi, H. Thermal Degradation of Polylactic Acid (PLA)/Polyhydroxybutyrate (PHB) Blends: A Systematic Review. Polym. Degrad. Stab. 2022, 201, 109995. [Google Scholar] [CrossRef]
- Lai, S.-M.; Liu, Y.-H.; Huang, C.-T.; Don, T.-M. Miscibility and Toughness Improvement of Poly(Lactic Acid)/Poly(3-Hydroxybutyrate) Blends Using a Melt-Induced Degradation Approach. J. Polym. Res. 2017, 24, 102. [Google Scholar] [CrossRef]
- Kong, D.; Eng, B. Development of Polylactic Acid-Polyhydroxybutyrate Blends for Packaging Applications. Ph.D. Thesis, RMIT University, Melbourne, Australia, 2017. [Google Scholar]
- Surisaeng, J.; Kanabenja, W.; Passornraprasit, N.; Aumnate, C.; Potiyaraj’, P. Polyhydroxybutyrate/Polylactic Acid Blends: An Alternative Feedstock for 3D Printed Bone Scaffold Model. J. Phys. Conf. Ser. 2022, 2175, 012021. [Google Scholar] [CrossRef]
- Culenova, M.; Birova, I.; Alexy, P.; Galfiova, P.; Nicodemou, A.; Moncmanova, B.; Plavec, R.; Tomanova, K.; Mencik, P.; Ziaran, S.; et al. In Vitro Characterization of Poly(Lactic Acid)/Poly(Hydroxybutyrate)/Thermoplastic Starch Blends for Tissue Engineering Application. Cell Transpl. 2021, 30, 096368972110210. [Google Scholar] [CrossRef]
- Nofar, M.; Park, C.B. Poly (Lactic Acid) Foaming. Prog. Polym. Sci. 2014, 39, 1721–1741. [Google Scholar] [CrossRef]
- Gupta, B.; Revagade, N.; Hilborn, J. Poly(Lactic Acid) Fiber: An Overview. Prog. Polym. Sci. 2007, 32, 455–482. [Google Scholar] [CrossRef]
- Saeidlou, S.; Huneault, M.A.; Li, H.; Park, C.B. Poly(Lactic Acid) Crystallization. Prog. Polym. Sci. 2012, 37, 1657–1677. [Google Scholar] [CrossRef]
- Arrighi, V.; Cowie, J.M.G.; Fuhrmann, S.; Youssef, A. Miscibility Criterion in Polymer Blends and Its Determination. In Encyclopedia of Polymer Blends; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 153–198. [Google Scholar] [CrossRef]
- Fredrickson, G.H.; Liu, A.J.; Bates, F.S. Entropic Corrections to the Flory-Huggins Theory of Polymer Blends: Architectural and Conformational Effects. Macromolecules 1994, 27, 2503–2511. [Google Scholar] [CrossRef]
- Mei, H.; Laws, T.S.; Mahalik, J.P.; Li, J.; Mah, A.H.; Terlier, T.; Bonnesen, P.; Uhrig, D.; Kumar, R.; Stein, G.E.; et al. Entropy and Enthalpy Mediated Segregation of Bottlebrush Copolymers to Interfaces. Macromolecules 2019, 52, 8910–8922. [Google Scholar] [CrossRef]
- Mah, A.H.; Laws, T.; Li, W.; Mei, H.; Brown, C.C.; Ievlev, A.; Kumar, R.; Verduzco, R.; Stein, G.E. Entropic and Enthalpic Effects in Thin Film Blends of Homopolymers and Bottlebrush Polymers. Macromolecules 2019, 52, 1526–1535. [Google Scholar] [CrossRef]
- Magazzini, L.; Grilli, S.; Fenni, S.E.; Donetti, A.; Cavallo, D.; Monticelli, O. The Blending of Poly(Glycolic Acid) with Polycaprolactone and Poly(l-Lactide): Promising Combinations. Polymers 2021, 13, 2780. [Google Scholar] [CrossRef] [PubMed]
- Ko, H.-S.; Lee, S.; Lee, D.; Jho, J.Y. Mechanical Properties and Bioactivity of Poly(Lactic Acid) Composites Containing Poly(Glycolic Acid) Fiber and Hydroxyapatite Particles. Nanomaterials 2021, 11, 249. [Google Scholar] [CrossRef]
- Wang, X.; Peng, S.; Chen, H.; Yu, X.; Zhao, X. Mechanical Properties, Rheological Behaviors, and Phase Morphologies of High-Toughness PLA/PBAT Blends by in-Situ Reactive Compatibilization. Compos. B. Eng. 2019, 173, 107028. [Google Scholar] [CrossRef]
- Al-Itry, R.; Lamnawar, K.; Maazouz, A.; Billon, N.; Combeaud, C. Effect of the Simultaneous Biaxial Stretching on the Structural and Mechanical Properties of PLA, PBAT and Their Blends at Rubbery State. Eur. Polym. J. 2015, 68, 288–301. [Google Scholar] [CrossRef]
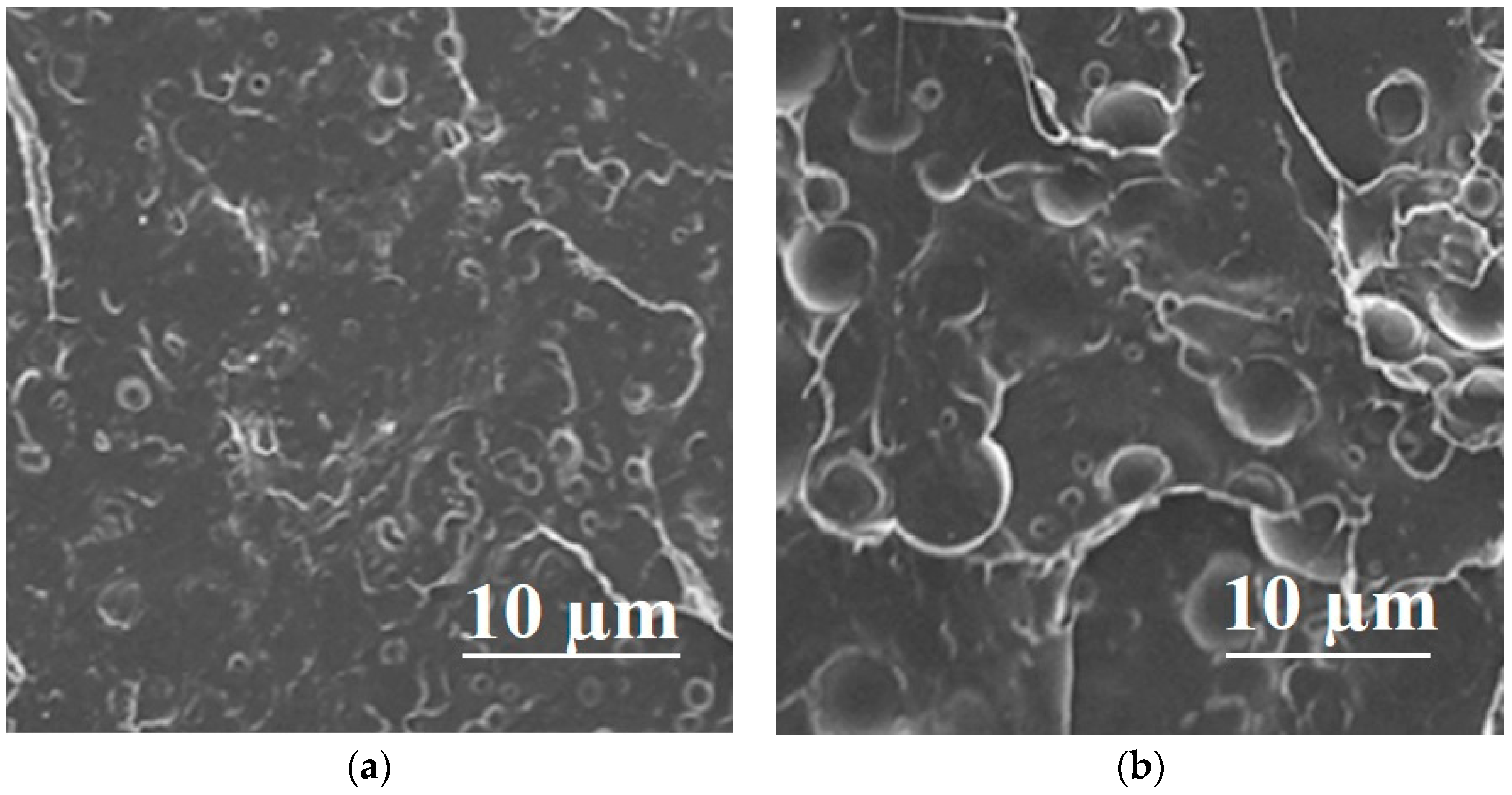
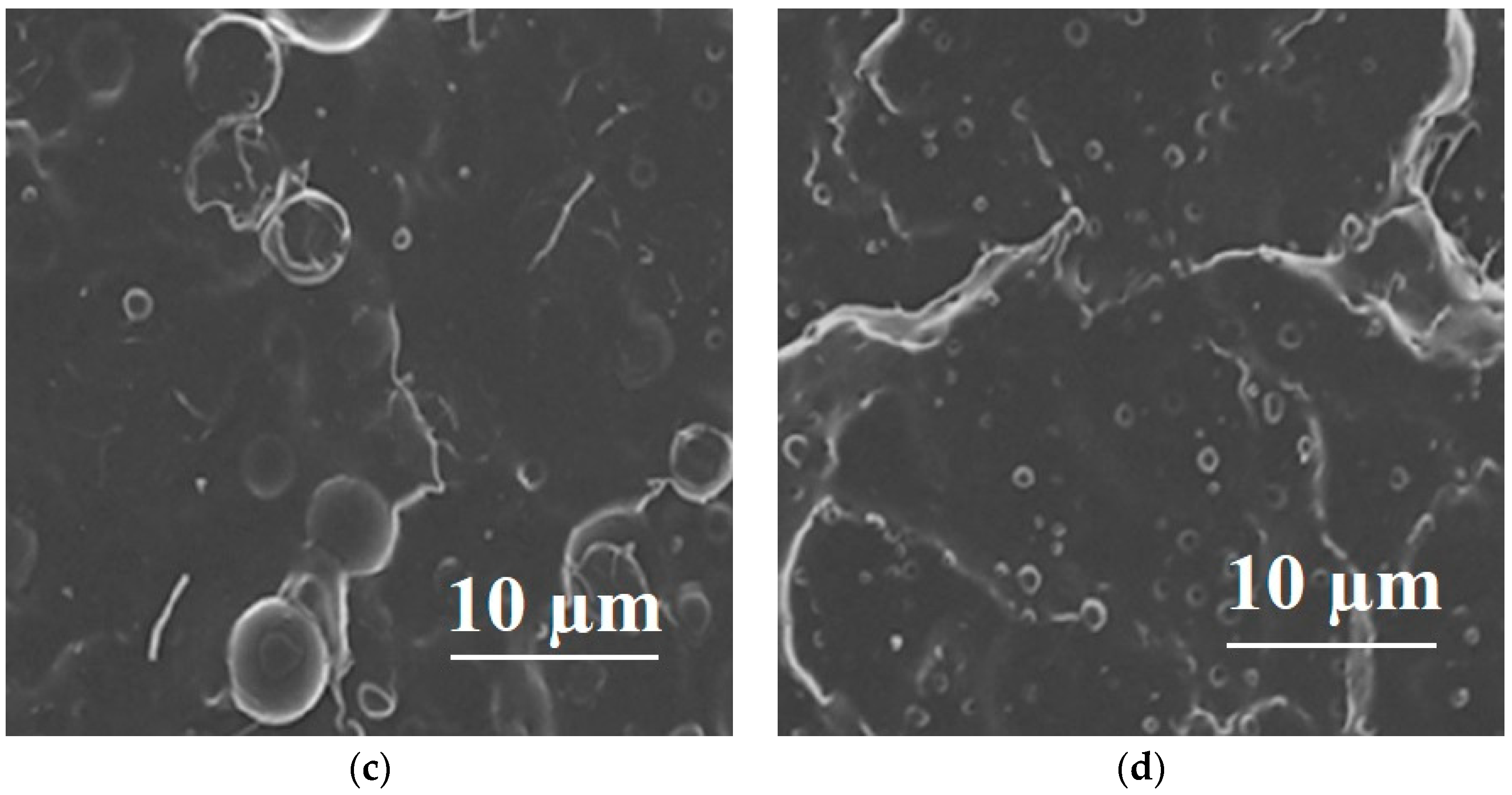

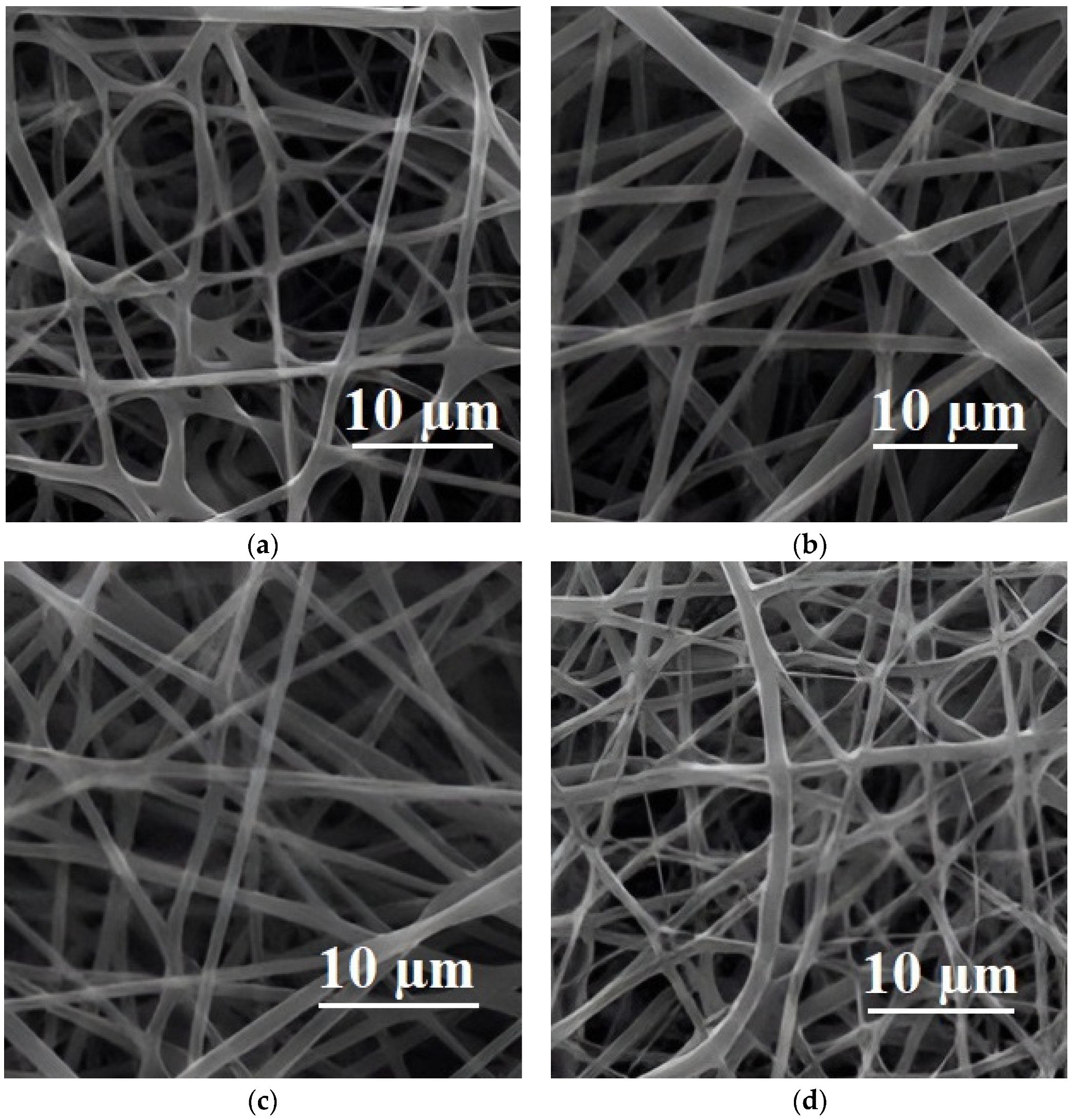
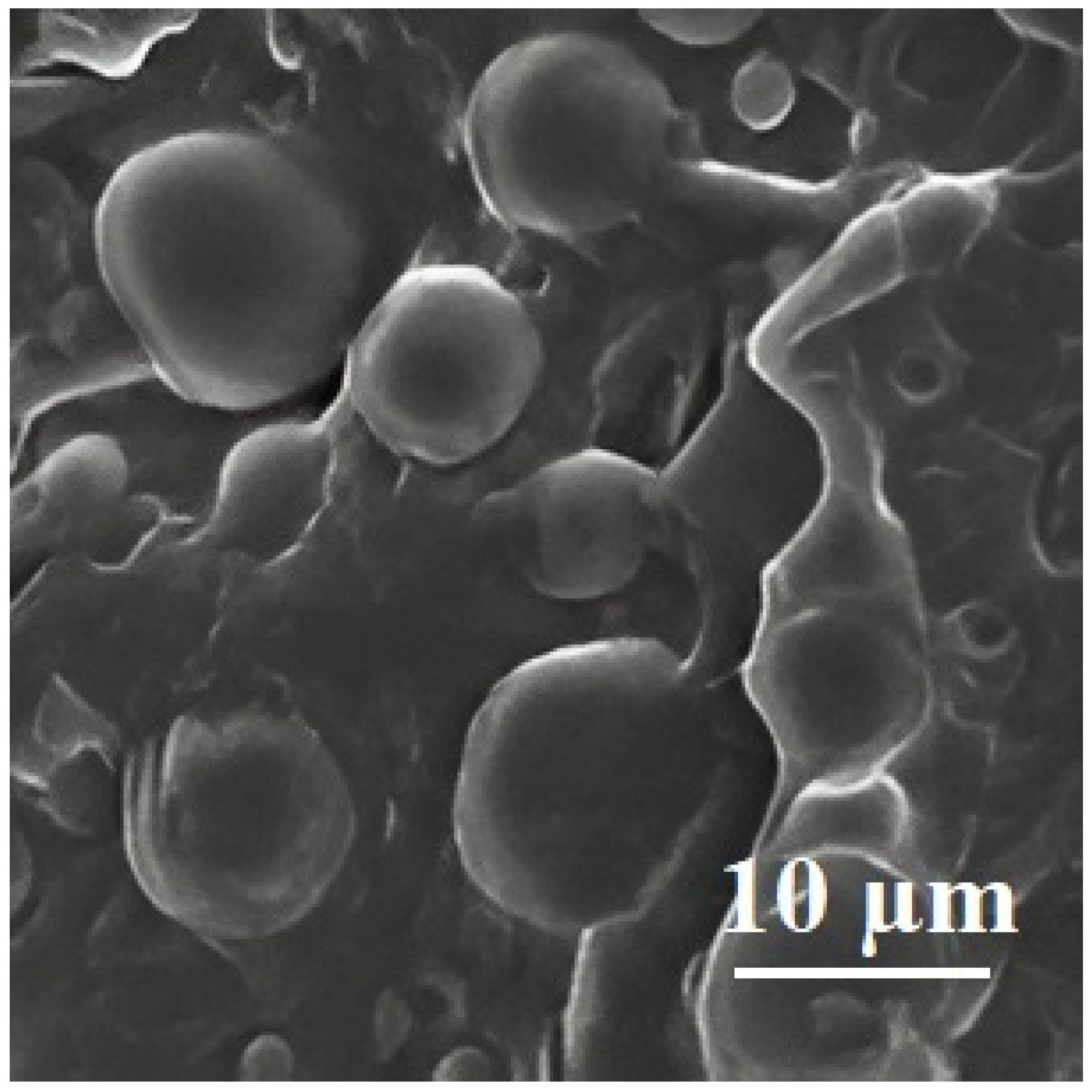

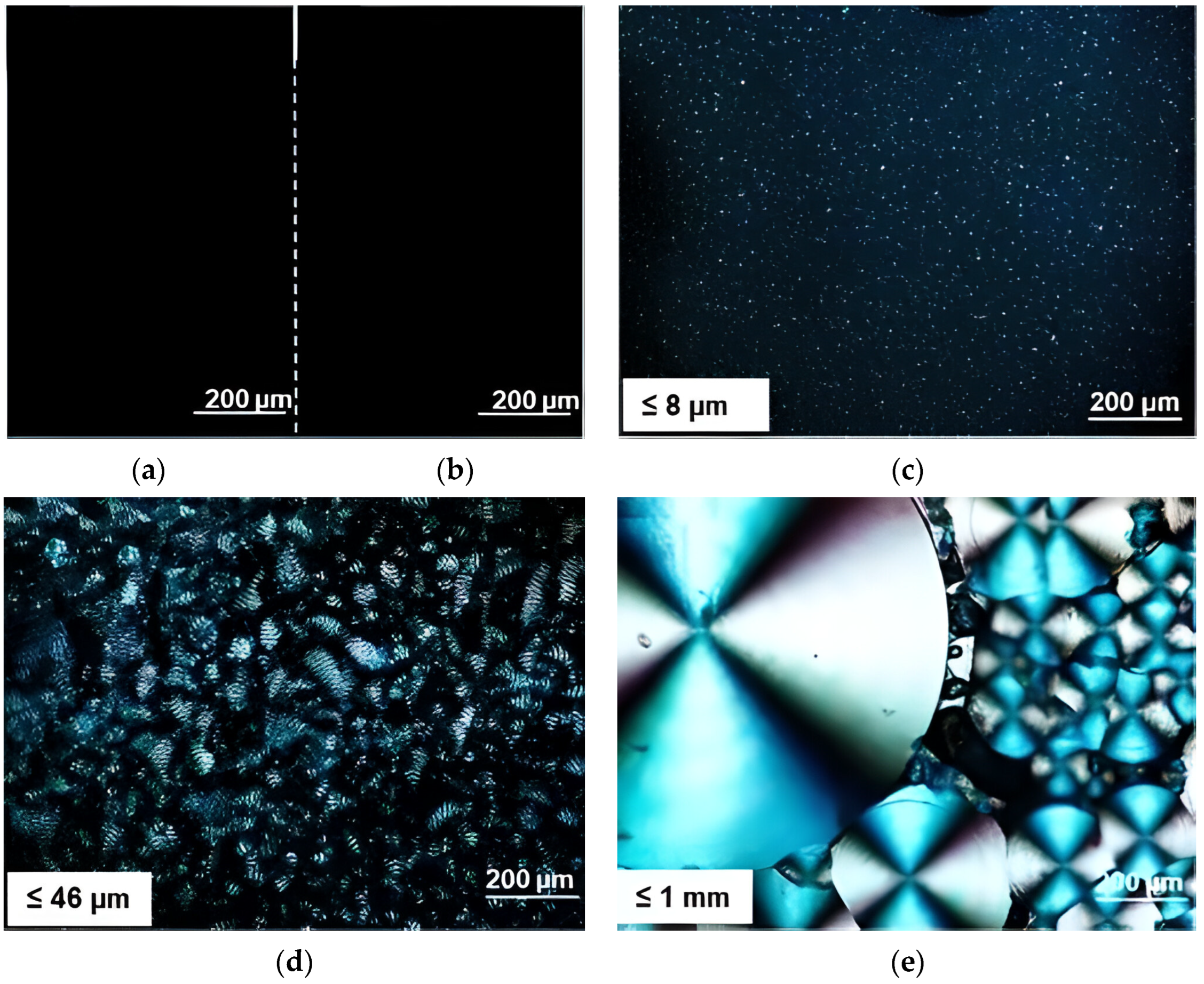
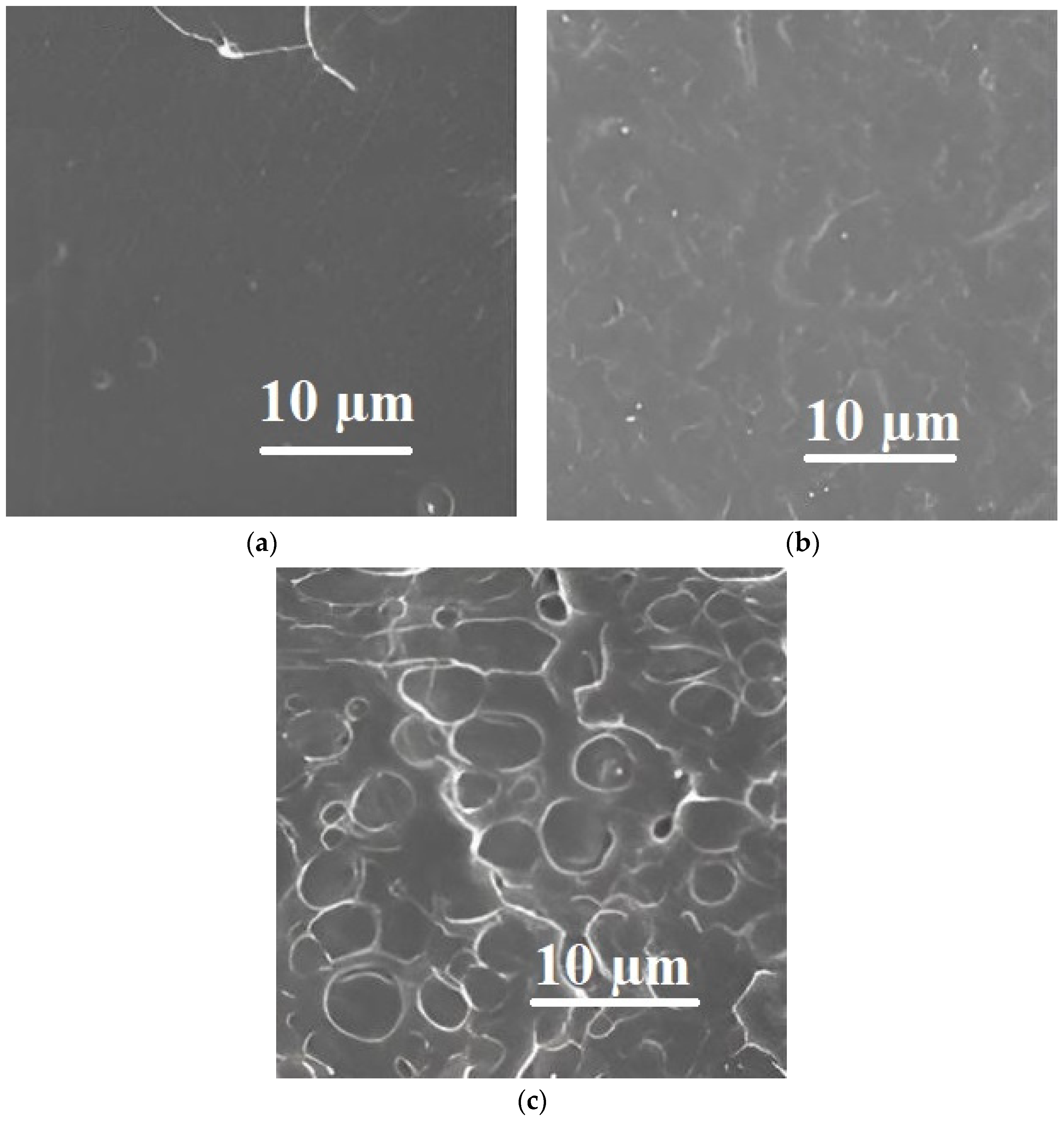
| Polymer Blend Systems | Morphology (SEM) | Tensile Strength, MPa | Elongation at Break, % | Elastic Modulus, MPa | Impact Strength, kJ/m2 | Degree of Crystallinity, % | Degradation Temperature, °C | Researchers |
|---|---|---|---|---|---|---|---|---|
| 80PLA/ 20PCL | 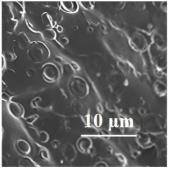 | 68 ± 2 | 20 ± 2 | 2600 ± 100 | 37 ± 1 | 62.39/28.63 | 361.93 ± 2.65 | [33] |
| 80PLA/ 20PGA | 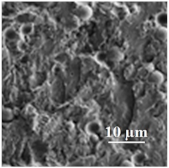 | 130 | 22.6 | 3.9 | - | 39/35 | 354 | [50,119,120] |
| 80PLA/ 20PBAT | 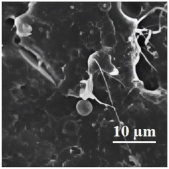 | 38.95 ± 1.21 | 387.85 ± 26.88 | 2250 | 4.85 ± 0.27 | 39 | 422.9/378.7 | [121,122] |
| 80PLA/ 20EVA | 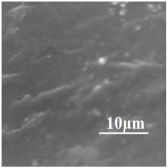 | 45 | 340 | 64 | 8.28 ± 0.11 | 3.8 | 331 | [87,90] |
| 75PLA/ 25PHB | 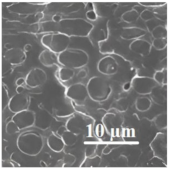 | 16 ± 3 | 7.1 ± 1 | 1270 ± 110 | 2.5 ± 0.5 | 36 | 356/283 | [98,104] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vayshbeyn, L.I.; Mastalygina, E.E.; Olkhov, A.A.; Podzorova, M.V. Poly(lactic acid)-Based Blends: A Comprehensive Review. Appl. Sci. 2023, 13, 5148. https://doi.org/10.3390/app13085148
Vayshbeyn LI, Mastalygina EE, Olkhov AA, Podzorova MV. Poly(lactic acid)-Based Blends: A Comprehensive Review. Applied Sciences. 2023; 13(8):5148. https://doi.org/10.3390/app13085148
Chicago/Turabian StyleVayshbeyn, Leonid Ilyich, Elena Evgenyevna Mastalygina, Anatoly Aleksandrovich Olkhov, and Maria Victorovna Podzorova. 2023. "Poly(lactic acid)-Based Blends: A Comprehensive Review" Applied Sciences 13, no. 8: 5148. https://doi.org/10.3390/app13085148
APA StyleVayshbeyn, L. I., Mastalygina, E. E., Olkhov, A. A., & Podzorova, M. V. (2023). Poly(lactic acid)-Based Blends: A Comprehensive Review. Applied Sciences, 13(8), 5148. https://doi.org/10.3390/app13085148








