Use of Syzygium aromaticum L. Fermented Plant Extract to Enhance Antioxidant Potential: Fermentation Kinetics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparations of Fermented Stems Extract (FSE)
2.3. Determination of Antioxidant Activity: DPPH Assay
2.4. Determination of Total Polyphenols Content: Folin–Ciocalteu Method
2.5. Determination of Lactic Acid by GC-MS
2.6. Fermentation of Stems in the Presence of Lactic Acid Bacteria
2.7. Kinetic Modelling
2.8. Cytotoxicity Analysis
2.8.1. Cell Culture
2.8.2. Alamar Blue (AB) Assay
2.8.3. Neutral Red (NR) Uptake Assay
2.9. Reducing the Activity of Fe3+ to Fe2+
2.10. Chelating Activity of the Fe2+ Ion
2.11. GC-MS Analysis of FSE
3. Results
3.1. Studies of the Influence of Technological Parameters on Fermentation Stems and Kinetic Modelling
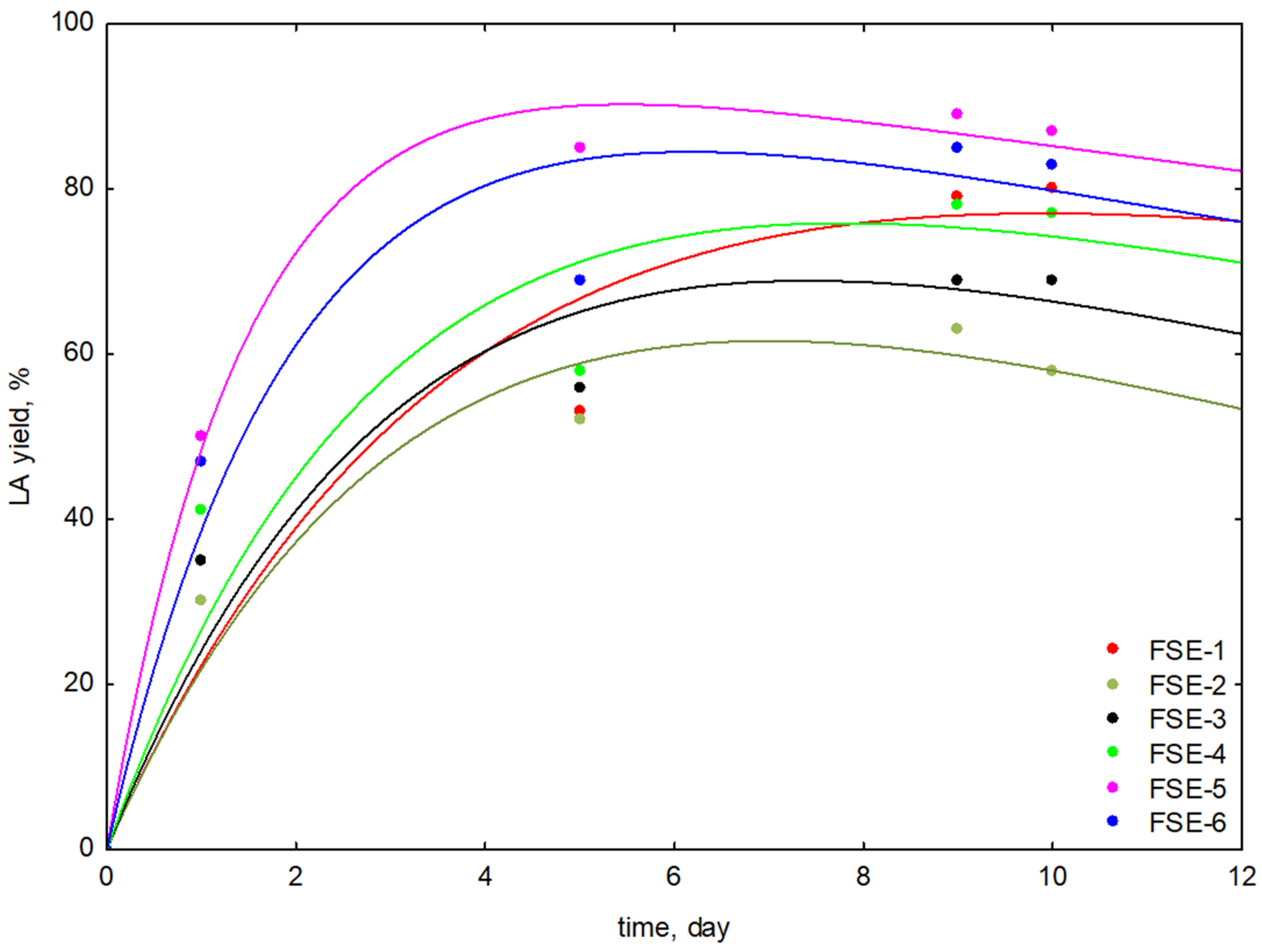
3.1.1. Studies on the Effect of Lactic Acid Bacteria Strains
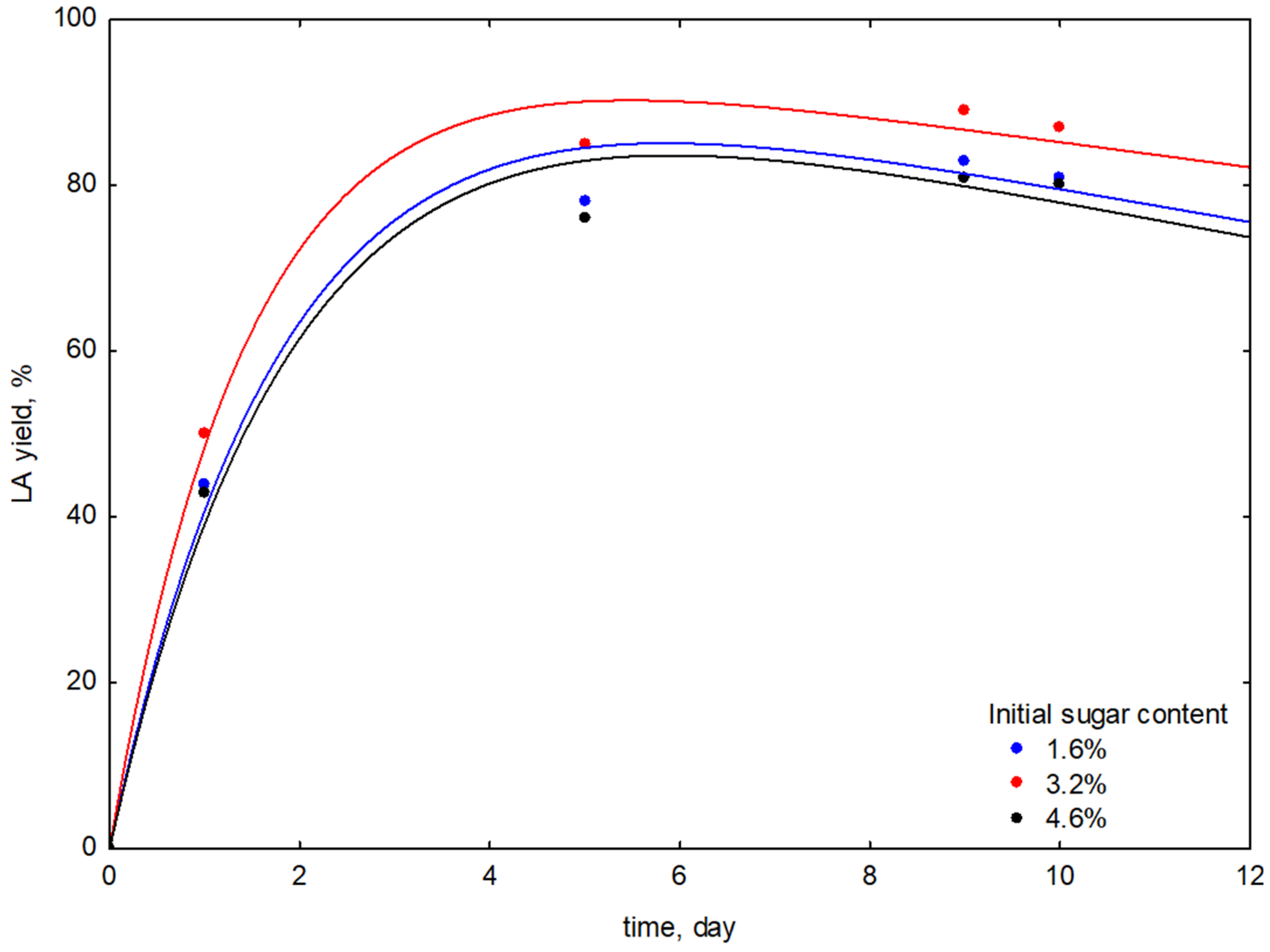
3.1.2. Studies on the Effect of the Initial Amount of Sugars
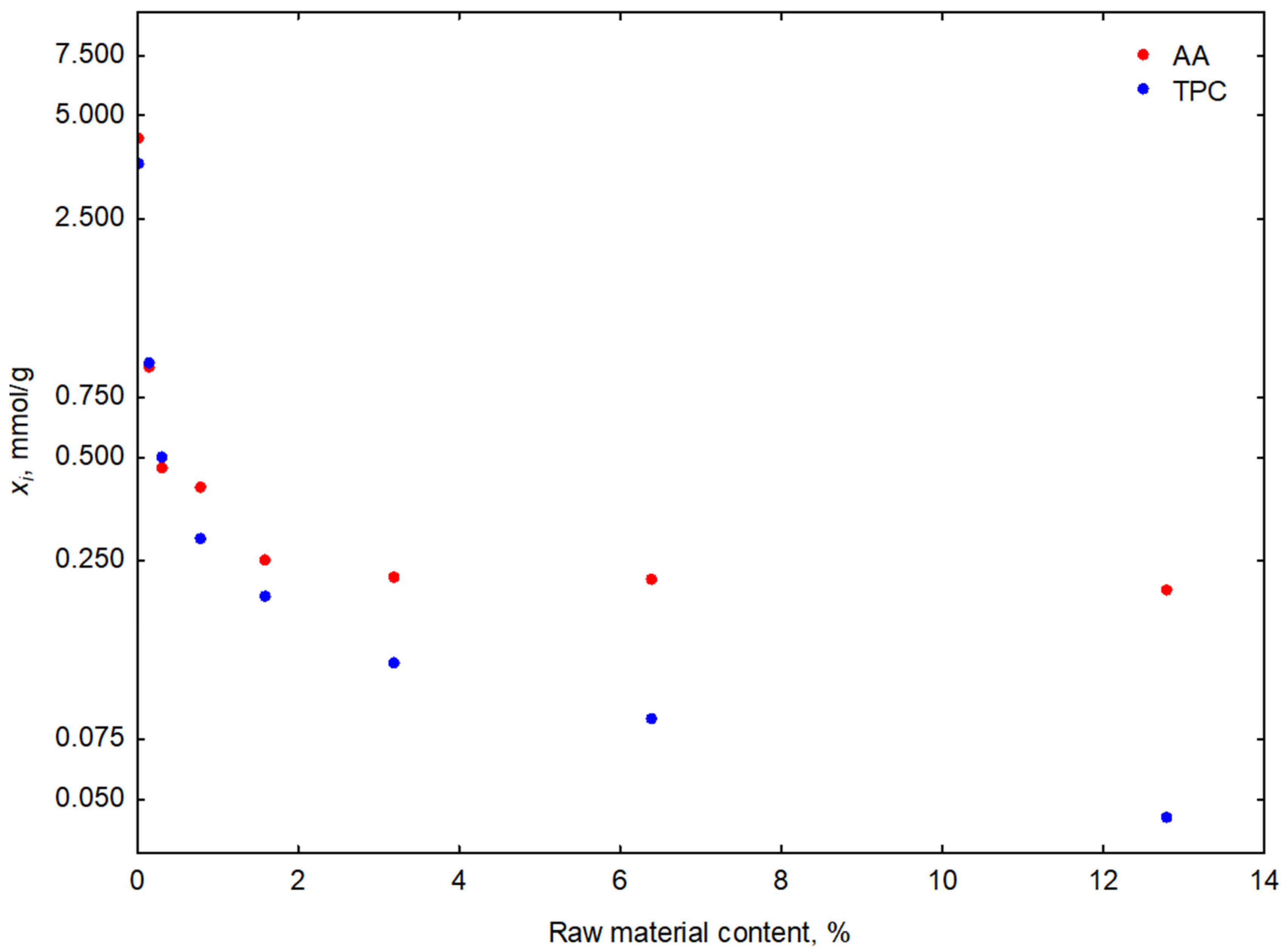
3.1.3. Studies on the Effect of the Plant Raw Material Content
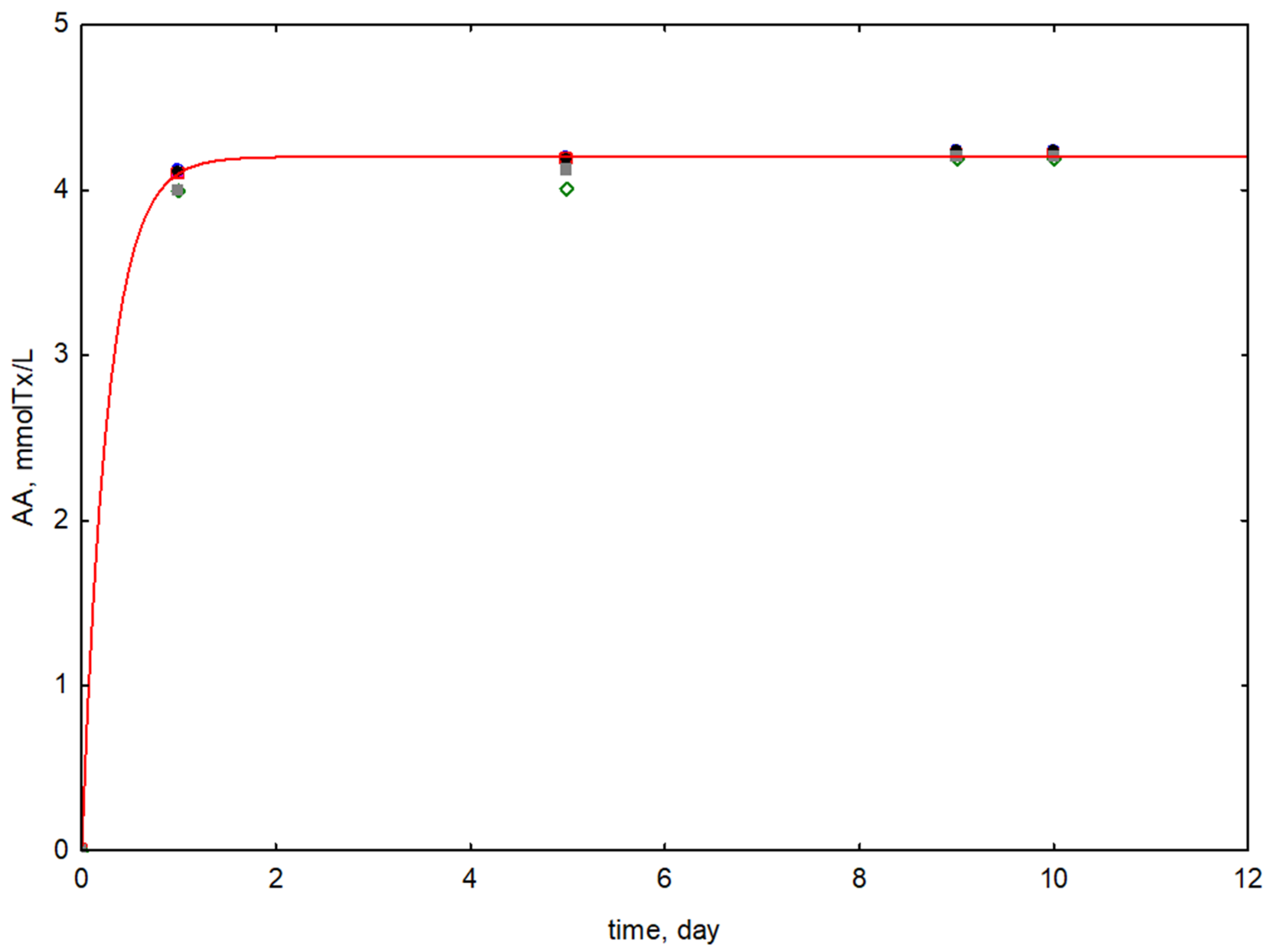
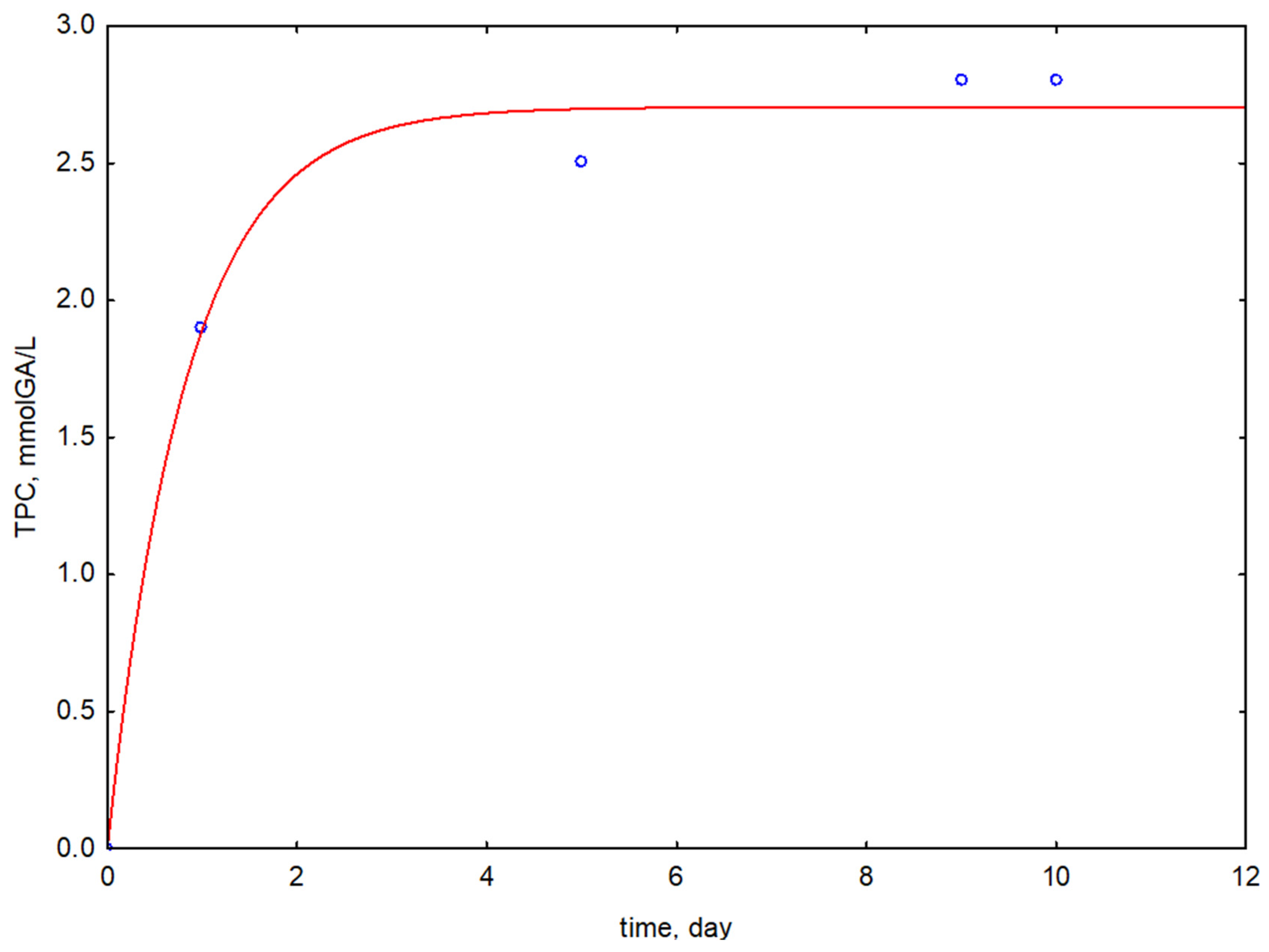
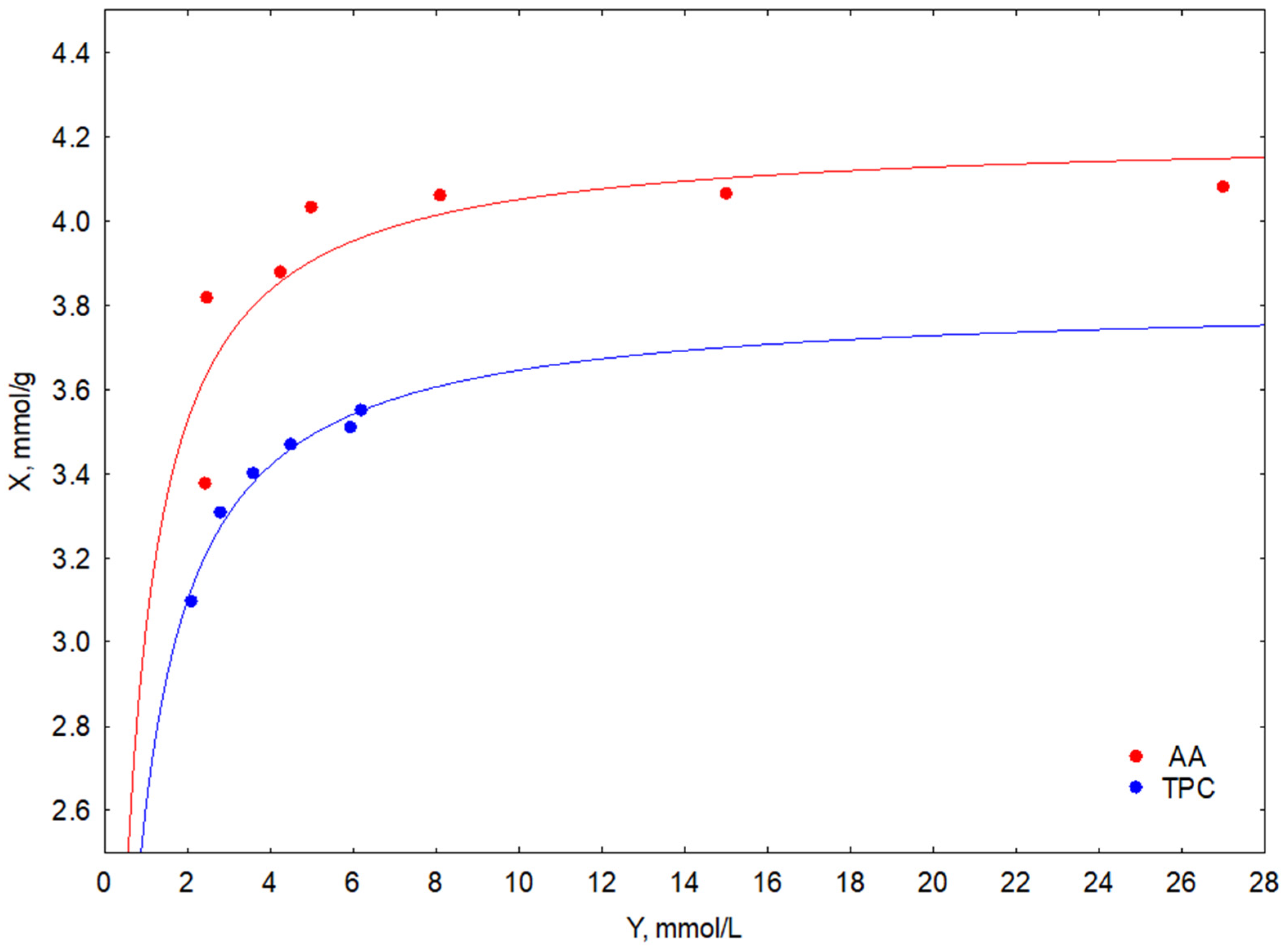
3.2. Antioxidant Activity, Total Polyphenols Content, and LA Efficiency
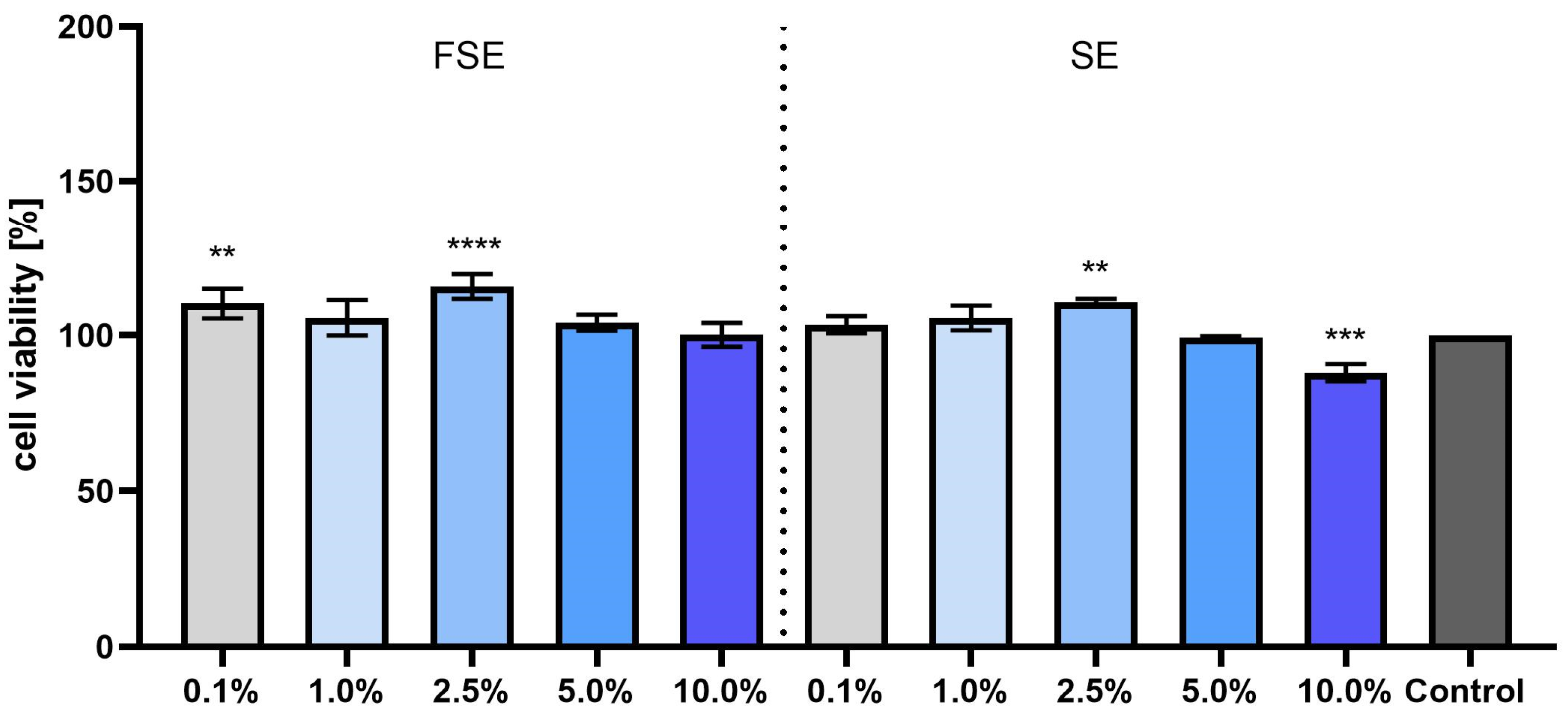
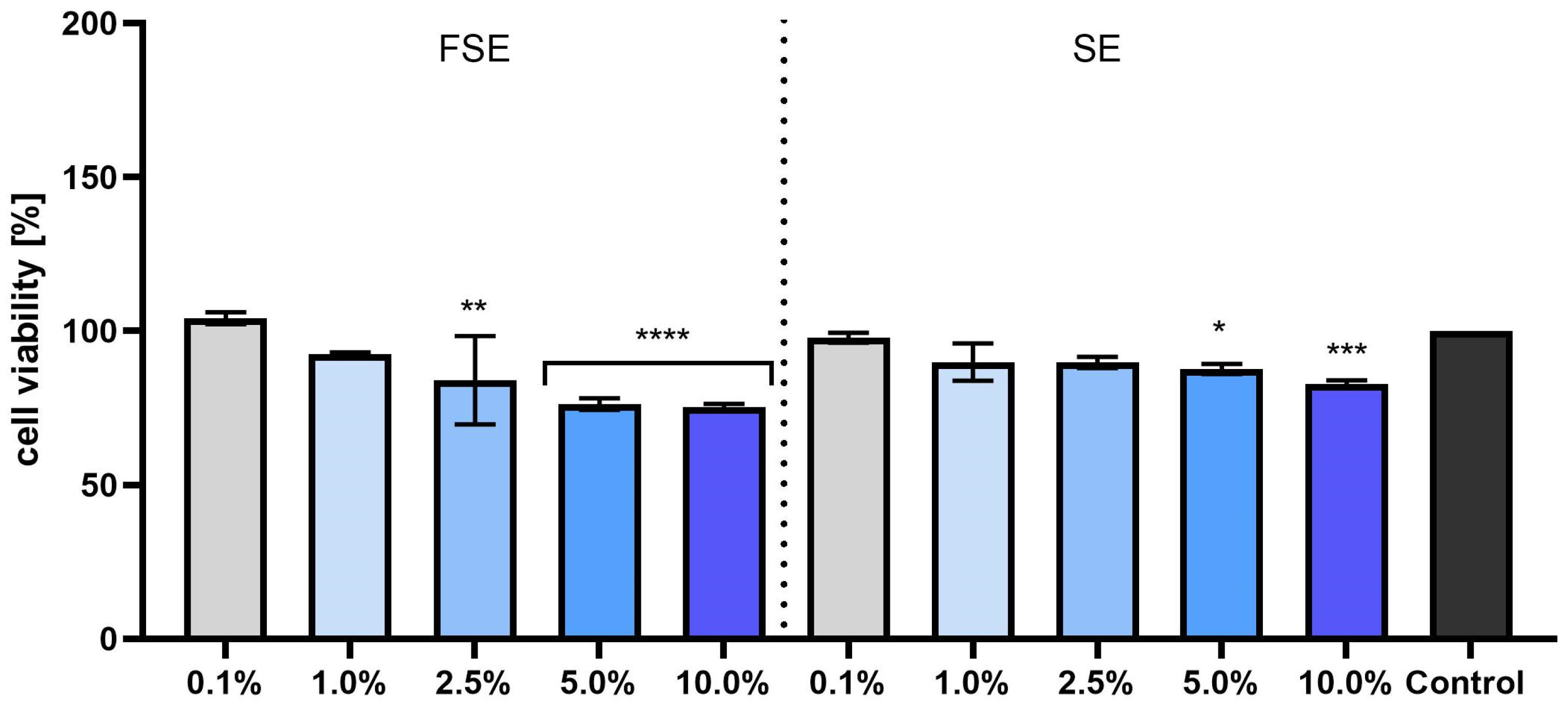
3.3. Cytotoxicity Evaluation
3.4. GC-MS Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kowalczewski, P.Ł.; Zembrzuska, J. Advances in Biological Activities and Application of Plant Extracts. Appl. Sci. 2023, 13, 9324. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Biological Activity of Fermented Plant Extracts for Potential Dermal Applications. Pharmaceutics 2023, 15, 2775. [Google Scholar] [CrossRef] [PubMed]
- Kucharska, E.; Sarpong, R.; Bobkowska, A.; Ryglewicz, J.; Nowak, A.; Kucharski, Ł.; Muzykiewicz-Szymańska, A.; Duchnik, W.; Pełech, R. Use of Silybum Marianum Extract and Bio-Ferment for Biodegradable Cosmetic Formulations to Enhance Antioxidant Potential and Effect of the Type of Vehicle on the Percutaneous Absorption and Skin Retention of Silybin and Taxifolin. Appl. Sci. 2024, 14, 169. [Google Scholar] [CrossRef]
- Pérez-Rivero, C.; López-Gómez, J.P. Unlocking the Potential of Fermentation in Cosmetics: A Review. Fermentation 2023, 9, 463. [Google Scholar] [CrossRef]
- Majchrzak, W.; Motyl, I.; Śmigielski, K. Biological and Cosmetical Importance of Fermented Raw Materials: An Overview. Molecules 2022, 27, 4845. [Google Scholar] [CrossRef] [PubMed]
- Faria, D.J.; de Carvalho, A.P.A.; Conte-Junior, C.A. Valorization of Fermented Food Wastes and Byproducts: Bioactive and Valuable Compounds, Bioproduct Synthesis, and Applications. Fermentation 2023, 9, 920. [Google Scholar] [CrossRef]
- Hussain, A.; Bose, S.; Wang, J.-H.; Yadav, M.K.; Mahajan, G.B.; Kim, H. Fermentation, a Feasible Strategy for Enhancing Bioactivity of Herbal Medicines. Food Res. Int. 2016, 81, 1–16. [Google Scholar] [CrossRef]
- Sharma, R.; Garg, P.; Kumar, P.; Bhatia, S.K.; Kulshrestha, S. Microbial Fermentation and Its Role in Quality Improvement of Fermented Foods. Fermentation 2020, 6, 106. [Google Scholar] [CrossRef]
- Gulsunoglu-Konuskan, Z.; Kilic-Akyilmaz, M. Microbial Bioconversion of Phenolic Compounds in Agro-Industrial Wastes: A Review of Mechanisms and Effective Factors. J. Agric. Food Chem. 2022, 70, 6901–6910. [Google Scholar] [CrossRef]
- Cvetanović, A.; Švarc-Gajić, J.; Zeković, Z.; Savić, S.; Vulić, J.; Mašković, P.; Ćetković, G. Comparative Analysis of Antioxidant, Antimicrobiological and Cytotoxic Activities of Native and Fermented Chamomile Ligulate Flower Extracts. Planta 2015, 242, 721–732. [Google Scholar] [CrossRef]
- He, X.; Zou, Y.; Yoon, W.-B.; Park, S.-J.; Park, D.-S.; Ahn, J. Effects of Probiotic Fermentation on the Enhancement of Biological and Pharmacological Activities of Codonopsis lanceolata Extracted by High Pressure Treatment. J. Biosci. Bioeng. 2011, 112, 188–193. [Google Scholar] [CrossRef] [PubMed]
- Garcia, C.; Guerin, M.; Souidi, K.; Remize, F. Lactic Fermented Fruit or Vegetable Juices: Past, Present and Future. Beverages 2020, 6, 8. [Google Scholar] [CrossRef]
- Ziemlewska, A.; Nizioł-Łukaszewska, Z.; Bujak, T.; Zagórska-Dziok, M.; Wójciak, M.; Sowa, I. Effect of Fermentation Time on the Content of Bioactive Compounds with Cosmetic and Dermatological Properties in Kombucha Yerba Mate Extracts. Sci. Rep. 2021, 11, 18792. [Google Scholar] [CrossRef]
- Vauzour, D.; Rodriguez-Mateos, A.; Corona, G.; Oruna-Concha, M.J.; Spencer, J.P.E. Polyphenols and Human Health: Prevention of Disease and Mechanisms of Action. Nutrients 2010, 2, 1106–1131. [Google Scholar] [CrossRef] [PubMed]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxidative Med. Cell. Longev. 2016, 2016, e7432797. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kim, H.-S.; Eom, S.J.; Kim, K.-T.; Paik, H.-D. Antioxidative and Anticanceric Activities of Magnolia (Magnolia denudata) Flower Petal Extract Fermented by Pediococcus Acidilactici KCCM 11614. Molecules 2015, 20, 12154–12165. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-O.; Kim, S.-J.; Kim, J.-S.; Ji, H.; Lee, E.-O.; Lee, H.-J. Comparison of the Main Components and Bioactivity of Rhus Verniciflua Stokes Extracts by Different Detoxification Processing Methods. BMC Complement. Altern. Med. 2018, 18, 242. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-H.; Jeong, G.H.; Park, S.L.; Won, S.Y.; Paek, N.S.; Lee, B.-H.; Moon, S.-K. Inhibitory Effects of Fermented Extract of Ophiopogon Japonicas on Thrombin-Induced Vascular Smooth Muscle Cells. Mol. Med. Rep. 2016, 13, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.; Jeon, S.B.; Lee, Y.; Lee, H.; Kim, J.; Kwon, B.R.; Yu, K.-Y.; Cha, J.-D.; Hwang, S.-M.; Choi, K.-M.; et al. Fermented Red Ginseng Extract Inhibits Cancer Cell Proliferation and Viability. J. Med. Food 2015, 18, 421–428. [Google Scholar] [CrossRef]
- Sunder, S.; Bauman, J.S.; Decker, S.J.; Lifton, A.R.; Kumar, A. The Yeast AMP-Activated Protein Kinase Snf1 Phosphorylates the Inositol Polyphosphate Kinase Kcs1. J. Biol. Chem. 2024, 300, 105657. [Google Scholar] [CrossRef]
- Ioannou, P.; Ziogou, A.; Giannakodimos, I.; Giannakodimos, A.; Baliou, S.; Samonis, G. Infective Endocarditis by Lactobacillus Species—A Narrative Review. Antibiotics 2024, 13, 53. [Google Scholar] [CrossRef]
- Punia Bangar, S.; Suri, S.; Trif, M.; Ozogul, F. Organic Acids Production from Lactic Acid Bacteria: A Preservation Approach. Food Biosci. 2022, 46, 101615. [Google Scholar] [CrossRef]
- Fatani, S.A. Substance Use Disorders Management Educational Intervention: Development and Evaluating a Tailored Substance Use Disorder Intervention for Community Pharmacists in Saskatoon. Ph.D. Thesis, The University of Saskatchewan, Saskatoon, SK, Canada, 2021. [Google Scholar]
- Tariq, S.; Wani, S.; Rasool, W.; Shafi, K.; Bhat, M.A.; Prabhakar, A.; Shalla, A.H.; Rather, M.A. A Comprehensive Review of the Antibacterial, Antifungal and Antiviral Potential of Essential Oils and Their Chemical Constituents against Drug-Resistant Microbial Pathogens. Microb. Pathog. 2019, 134, 103580. [Google Scholar] [CrossRef]
- Caputo, L.; Capozzolo, F.; Amato, G.; De Feo, V.; Fratianni, F.; Vivenzio, G.; Nazzaro, F. Chemical Composition, Antibiofilm, Cytotoxic, and Anti-Acetylcholinesterase Activities of Myrtus communis L. Leaves Essential Oil. BMC Complement. Med. Ther. 2022, 22, 142. [Google Scholar] [CrossRef]
- Djipa, C.D.; Delmée, M.; Quetin-Leclercq, J. Antimicrobial Activity of Bark Extracts of Syzygium jambos (L.) Alston (Myrtaceae). J. Ethnopharmacol. 2000, 71, 307–313. [Google Scholar] [CrossRef]
- Da Silva, V.P.; Alves, C.C.F.; Miranda, M.L.D.; Bretanha, L.C.; Balleste, M.P.; Micke, G.A.; Silveira, E.V.; Martins, C.H.G.; Ambrosio, M.A.L.V.; De Souza Silva, T.; et al. Chemical Composition and In Vitro Leishmanicidal, Antibacterial and Cytotoxic Activities of Essential Oils of the Myrtaceae Family Occurring in the Cerrado Biome. Ind. Crops Prod. 2018, 123, 638–645. [Google Scholar] [CrossRef]
- Ferreira Macedo, J.G.; Linhares Rangel, J.M.; De Oliveira Santos, M.; Camilo, C.J.; Martins Da Costa, J.G.; Maria De Almeida Souza, M. Therapeutic Indications, Chemical Composition and Biological Activity of Native Brazilian Species from Psidium Genus (Myrtaceae): A Review. J. Ethnopharmacol. 2021, 278, 114248. [Google Scholar] [CrossRef]
- De Oliveira Raphaelli, C.; Camargo, T.M.; Radünz, M.; Dos Santos Pereira, E.; Da Rocha Concenço, F.I.G.; Vizzotto, M.; Da Rosa Zavareze, E.; Nora, L. Eugenia Pyriformis Cambess-Myrtaceae: An Unexplored Brazilian Specieswith Antimicrobial and Antioxidant Potential. CNF 2024, 20, 687–702. [Google Scholar] [CrossRef]
- Ojewole, J.A.O. Anti-Inflammatory and Analgesic Effects of Psidium Guajava Linn. (Myrtaceae) Leaf Aqueous Extracts in Rats and Mice. Methods Find. Exp. Clin. Pharmacol. 2006, 28, 441. [Google Scholar] [CrossRef] [PubMed]
- García, Y.M.; Ramos, A.L.C.C.; De Oliveira Júnior, A.H.; De Paula, A.C.C.F.F.; De Melo, A.C.; Andrino, M.A.; Silva, M.R.; Augusti, R.; De Araújo, R.L.B.; De Lemos, E.E.P.; et al. Physicochemical Characterization and Paper Spray Mass Spectrometry Analysis of Myrciaria Floribunda (H. West Ex Willd.) O. Berg Accessions. Molecules 2021, 26, 7206. [Google Scholar] [CrossRef] [PubMed]
- Olajide, O.A.; Awe, S.O.; Makinde, J.M. Pharmacological Studies on the Leaf of Psidium Guajava. Fitoterapia 1999, 70, 25–31. [Google Scholar] [CrossRef]
- Dexheimer, G.M.; De Oliveira Becker Delving, L.K.; De Oliveira, H.S.; Biolchi, V.; Goettert, M.I.; Pozzobon, A. Calyptranthes Grandifolia O.Berg (Myrtaceae) Ethanolic Extract Inhibits TNF-α Gene Expression and Cytokine Release In Vitro. Mol. Med. Rep. 2017, 15, 2873–2880. [Google Scholar] [CrossRef] [PubMed]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove Essential Oil (Syzygium aromaticum L. Myrtaceae): Extraction, Chemical Composition, Food Applications, and Essential Bioactivity for Human Health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef] [PubMed]
- Nuñez, L.; D’ Aquino, M. Microbicide Activity of Clove Essential Oil (Eugenia caryophyllata). Braz. J. Microbiol. 2012, 43, 1255–1260. [Google Scholar] [CrossRef] [PubMed]
- El-Saber Batiha, G.; Alkazmi, L.M.; Wasef, L.G.; Beshbishy, A.M.; Nadwa, E.H.; Rashwan, E.K. Syzygium aromaticum L. (Myrtaceae): Traditional Uses, Bioactive Chemical Constituents, Pharmacological and Toxicological Activities. Biomolecules 2020, 10, 202. [Google Scholar] [CrossRef] [PubMed]
- Makuch, E.; Nowak, A.; Günther, A.; Pełech, R.; Kucharski, Ł.; Duchnik, W.; Klimowicz, A. Enhancement of the Antioxidant and Skin Permeation Properties of Eugenol by the Esterification of Eugenol to New Derivatives. AMB Express 2020, 10, 187. [Google Scholar] [CrossRef] [PubMed]
- Makuch, E.; Nowak, A.; Günther, A.; Pełech, R.; Kucharski, Ł.; Duchnik, W.; Klimowicz, A. The Effect of Cream and Gel Vehicles on the Percutaneous Absorption and Skin Retention of a New Eugenol Derivative With Antioxidant Activity. Front. Pharmacol. 2021, 12, 658381. [Google Scholar] [CrossRef] [PubMed]
- Danthu, P.; Simanjuntak, R.; Fawbush, F.; Leong Pock Tsy, J.-M.; Razafimamonjison, G.; Abdillahi, M.M.; Jahiel, M.; Penot, E. The Clove Tree and Its Products (Clove Bud, Clove Oil, Eugenol): Prosperous Today but What of Tomorrow’s Restrictions? Fruits 2020, 75, 224–242. [Google Scholar] [CrossRef]
- Ratri, P.J.; Ayurini, M.; Khumaini, K.; Rohbiya, A. Clove Oil Extraction by Steam Distillation and Utilization of Clove Buds Waste as Potential Candidate for Eco-Friendly Packaging. J. Bahan Alam Terbarukan 2020, 9, 47–54. [Google Scholar] [CrossRef]
- Maddheshiya, S.; Ahmad, A.; Ahmad, W.; Zakir, F.; Aggarwal, G. Essential Oils for the Treatment of Skin Anomalies: Scope and Potential. South. Afr. J. Bot. 2022, 151, 187–197. [Google Scholar] [CrossRef]
- Esposito, E.; Nastruzzi, C.; Sguizzato, M.; Cortesi, R. Nanomedicines to Treat Skin Pathologies with Natural Molecules. Curr. Pharm. Des. 2019, 25, 2323–2337. [Google Scholar] [CrossRef] [PubMed]
- Parsaeimehr, A.; Martinez-Chapa, S.O.; Parra-Saldívar, R. Chapter 13—Medicinal Plants Versus Skin Disorders: A Survey From Ancient to Modern Herbalism. In The Microbiology of Skin, Soft Tissue, Bone and Joint Infections; Kon, K., Rai, M., Eds.; Clinical Microbiology: Diagnosis, Treatments and Prophylaxis of Infections; Academic Press: Cambridge, MA, USA, 2017; Volume 2, pp. 205–221. [Google Scholar]
- Ahmad, S.; Ullah, F.; Sadiq, A.; Ayaz, M.; Imran, M.; Ali, I.; Zeb, A.; Ullah, F.; Shah, M.R. Chemical Composition, Antioxidant and Anticholinesterase Potentials of Essential Oil of Rumex Hastatus D. Don Collected from the North West of Pakistan. BMC Complement. Altern. Med. 2016, 16, 29. [Google Scholar] [CrossRef] [PubMed]
- Adbhai, A.R.; Dewanjee, S.; Patel, K.G.; Karmakar, N. Sugar Beet Molasses Production and Utilization. In Sugar Beet Cultivation, Management and Processing; Misra, V., Srivastava, S., Mall, A.K., Eds.; Springer Nature: Singapore, 2022; pp. 885–904. ISBN 978-981-19273-0-0. [Google Scholar]
- Palmonari, A.; Cavallini, D.; Sniffen, C.J.; Fernandes, L.; Holder, P.; Fagioli, L.; Fusaro, I.; Biagi, G.; Formigoni, A.; Mammi, L. Short Communication: Characterization of Molasses Chemical Composition. J. Dairy Sci. 2020, 103, 6244–6249. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhumri, S.A.; Al Mosallam, M.S.; Zhang, W.; Alharbi, S.; Abou-Elwafa, S.F. Application of Molasses as an Eco-Innovative Approach Substitutes Mineral Nitrogen Fertilization and Enhances Sugar Beet Productivity. Waste Biomass Valor. 2023, 14, 287–296. [Google Scholar] [CrossRef]
- Mordenti, A.L.; Giaretta, E.; Campidonico, L.; Parazza, P.; Formigoni, A. A Review Regarding the Use of Molasses in Animal Nutrition. Animals 2021, 11, 115. [Google Scholar] [CrossRef] [PubMed]
- Leonard, W.; Zhang, P.; Ying, D.; Adhikari, B.; Fang, Z. Fermentation Transforms the Phenolic Profiles and Bioactivities of Plant-Based Foods. Biotechnol. Adv. 2021, 49, 107763. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, M.; Amakura, Y.; Yoshida, T. Polyphenolic Compounds in Clove and Pimento and Their Antioxidative Activities. Biosci. Biotechnol. Biochem. 2011, 75, 2207–2212. [Google Scholar] [CrossRef] [PubMed]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and Mechanisms of Antioxidant Activity Using the DPPH.Free Radical Method. LWT Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Anissi, J.; El Hassouni, M.; Ouardaoui, A.; Sendide, K. A Comparative Study of the Antioxidant Scavenging Activity of Green Tea, Black Tea and Coffee Extracts: A Kinetic Approach. Food Chem. 2014, 150, 438–447. [Google Scholar] [CrossRef]
- Page, B.; Page, M.; Noel, C. A New Fluorometric Assay for Cytotoxicity Measurements In-Vitro. Int. J. Oncol. 1993, 3, 473–476. [Google Scholar] [CrossRef]
- Zagórska-Dziok, M.; Ziemlewska, A.; Mokrzyńska, A.; Nizioł-Łukaszewska, Z.; Wójciak, M.; Sowa, I. Evaluation of the Biological Activity of Hydrogel with Cornus mas L. Extract and Its Potential Use in Dermatology and Cosmetology. Molecules 2023, 28, 7384. [Google Scholar] [CrossRef] [PubMed]
- Karamać, M.; Pegg, R.B. Limitations of the Tetramethylmurexide Assay for Investigating the Fe(II) Chelation Activity of Phenolic Compounds. J. Agric. Food Chem. 2009, 57, 6425–6431. [Google Scholar] [CrossRef]
- Hossain, M.A.; Piyatida, P.; da Silva, J.A.T.; Fujita, M. Molecular Mechanism of Heavy Metal Toxicity and Tolerance in Plants: Central Role of Glutathione in Detoxification of Reactive Oxygen Species and Methylglyoxal and in Heavy Metal Chelation. J. Bot. 2012, 2012, e872875. [Google Scholar] [CrossRef]
- Lima, É.C.; Adebayo, M.A.; Machado, F.M. Kinetic and Equilibrium Models of Adsorption. In Carbon Nanomaterials as Adsorbents for Environmental and Biological Applications; Bergmann, C.P., Machado, F.M., Eds.; Carbon Nanostructures; Springer International Publishing: Cham, Switzerland, 2015; pp. 33–69. ISBN 978-3-319-18874-4. [Google Scholar]
- Moriasi, G.A.; Ireri, A.M.; Ngugi, M.P. In Vivo Cognitive-Enhancing, Ex Vivo Malondialdehyde-Lowering Activities and Phytochemical Profiles of Aqueous and Methanolic Stem Bark Extracts of Piliostigma thonningii (Schum.). Int. J. Alzheimer’s Dis. 2020, 2020, 1367075. [Google Scholar] [CrossRef] [PubMed]
- Yuenyong, J.; Pokkanta, P.; Phuangsaijai, N.; Kittiwachana, S.; Mahatheeranont, S.; Sookwong, P. GC-MS and HPLC-DAD Analysis of Fatty Acid Profile and Functional Phytochemicals in Fifty Cold-Pressed Plant Oils in Thailand. Heliyon 2021, 7, e06304. [Google Scholar] [CrossRef]
- Poletto, P.; Pereira, G.N.; Monteiro, C.R.M.; Pereira, M.A.F.; Bordignon, S.E.; De Oliveira, D. Xylooligosaccharides: Transforming the Lignocellulosic Biomasses into Valuable 5-Carbon Sugar Prebiotics. Process Biochem. 2020, 91, 352–363. [Google Scholar] [CrossRef]
- Othman, M.; Ariff, A.B.; Rios-Solis, L.; Halim, M. Extractive Fermentation of Lactic Acid in Lactic Acid Bacteria Cultivation: A Review. Front. Microbiol. 2017, 8, 2285. [Google Scholar] [CrossRef] [PubMed]
- Filipčev, B.; Lević, L.; Bodroža-Solarov, M.; Mišljenović, N.; Koprivica, G. Quality Characteristics and Antioxidant Properties of Breads Supplemented with Sugar Beet Molasses-Based Ingredients. Int. J. Food Prop. 2010, 13, 1035–1053. [Google Scholar] [CrossRef]
- Juan, M.-Y.; Chou, C.-C. Enhancement of Antioxidant Activity, Total Phenolic and Flavonoid Content of Black Soybeans by Solid State Fermentation with Bacillus subtilis BCRC 14715. Food Microbiol. 2010, 27, 586–591. [Google Scholar] [CrossRef]
- Zhou, Z.; Fu, C. A New Flavanone and Other Constituents from the Rhizomes of Cyperus Rotundus and Their Antioxidant Activities. Chem. Nat. Compd. 2013, 48, 963–965. [Google Scholar] [CrossRef]
- Cho, K.M.; Lee, J.H.; Yun, H.D.; Ahn, B.Y.; Kim, H.; Seo, W.T. Changes of Phytochemical Constituents (Isoflavones, Flavanols, and Phenolic Acids) during Cheonggukjang Soybeans Fermentation Using Potential Probiotics Bacillus Subtilis CS90. J. Food Compos. Anal. 2011, 24, 402–410. [Google Scholar] [CrossRef]
- Devi, A.; Konerira Aiyappaa, A.; Waterhouse, A.L. Adsorption and Biotransformation of Anthocyanin Glucosides and Quercetin Glycosides by Oenococcus oeni and Lactobacillus plantarum in Model Wine Solution. J. Sci. Food Agric. 2020, 100, 2110–2120. [Google Scholar] [CrossRef] [PubMed]
- YiRmiBeşoğlu, S.S.S.; Tefon Öztürk, B.E. Comparing Microbiological Profiles, Bioactivities, and Physicochemical and Sensory Properties of Donkey Milk Kefir and Cow Milk Kefir. Turk. J. Vet. Anim. Sci. 2020, 44, 774–781. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Tao, Y.; Li, D.; Han, Y.; Show, P.L.; Wen, G.; Zhou, J. Fermentation of Blueberry and Blackberry Juices Using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacterium bifidum: Growth of Probiotics, Metabolism of Phenolics, Antioxidant Capacity In Vitro and Sensory Evaluation. Food Chem. 2021, 348, 129083. [Google Scholar] [CrossRef] [PubMed]
- Sinz, S.; Kunz, C.; Liesegang, A.; Braun, U.; Marquardt, S.; Soliva, C.R.; Kreuzer, M. In Vitro Bioactivity of Various Pure Flavonoids in Ruminal Fermentation, with Special Reference to Methane Formation. Czech J. Anim. Sci. 2018, 63, 293–304. [Google Scholar] [CrossRef]
- Sordon, S.; Popłoński, J.; Tronina, T.; Huszcza, E. Microbial Glycosylation of Daidzein, Genistein and Biochanin A: Two New Glucosides of Biochanin A. Molecules 2017, 22, 81. [Google Scholar] [CrossRef]
- Prashar, A.; Locke, I.C.; Evans, C.S. Cytotoxicity of Clove (Syzygium aromaticum) Oil and Its Major Components to Human Skin Cells. Cell Prolif. 2006, 39, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Hwang, Y.; Chang, B.; Kim, D.; Cho, H.; Kim, S. Effects of the Syzygium aromaticum L. Extract on Antioxidation and Inhibition of Matrix Metalloproteinase in Human Dermal Fibroblast. Asian Pac. J. Trop. Biomed. 2019, 9, 53. [Google Scholar] [CrossRef]
- El Ghallab, Y.; Al Jahid, A.; Jamal Eddine, J.; Ait Haj Said, A.; Zarayby, L.; Derfoufi, S. Syzygium aromaticum L.: Phytochemical Investigation and Comparison of the Scavenging Activity of Essential Oil, Extracts and Eugenol. Adv. Tradit. Med. 2020, 20, 153–158. [Google Scholar] [CrossRef]
- Triandriani, W.; Sogandi; Saputri, D.D.; Suhendar, U. Antioxidant Activity of Endophytic Bacterial Extract Isolated from Clove Leaf (Syzygium aromaticum L.). JAAB 2020, 1, 9–17. [Google Scholar] [CrossRef]
- Sharma, A.; Bhardwaj, G.; Sohal, H.S.; Gohain, A. Eugenol. In Nutraceuticals and Health Care; Elsevier: Amsterdam, The Netherlands, 2022; pp. 177–198. ISBN 978-0-323-89779-2. [Google Scholar]
- Ulanowska, M.; Olas, B. Biological Properties and Prospects for the Application of Eugenol—A Review. IJMS 2021, 22, 3671. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I. Antioxidant Activity of Eugenol: A Structure–Activity Relationship Study. J. Med. Food 2011, 14, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Alexa, V.T.; Galuscan, A.; Soica, C.M.; Cozma, A.; Coricovac, D.; Borcan, F.; Popescu, I.; Mioc, A.; Szuhanek, C.; Dehelean, C.A.; et al. In Vitro Assessment of the Cytotoxic and Antiproliferative Profile of Natural Preparations Containing Bergamot, Orange and Clove Essential Oils. Molecules 2022, 27, 990. [Google Scholar] [CrossRef] [PubMed]


| Parameters of the Fermentation Process | ||
|---|---|---|
| Unit | Ranges of Change | |
| Type of microorganism | - | L. reuteri MI_0168 L. salivarius LY_0652 L. brevis LY_1120 L. acidophilus MI-0078 L. rhamnosus MI-0272 L. plantarum MI-0102 |
| Initial sugar content | % | 1.60–4.60 |
| Plant raw material content | % | 0.032–12.80 |
| Fermentation time | days | 1–10 |
| Fermented Stems Extract/Type of Microorganism | Fermentation Time | AA | TPC | LAe |
|---|---|---|---|---|
| Days | mmol Tx/L | mmol GA/L | % | |
| FSE-1 L. reuteri MI_0168 | 1 | 4.12 ± 0.11 | 1.91 ± 0.09 | 40 ± 1 |
| 5 | 4.20 ± 0.17 | 2.53 ± 0.16 | 53 ± 1 | |
| 9 | 4.23 ± 0.19 | 2.83 ± 0.20 | 79 ± 1 | |
| 10 | 4.23 ± 0.18 | 2.83 ± 0.13 | 80 ± 1 | |
| FSE-2 L. salivarius LY_0652 | 1 | 4.10 ± 0.19 | 1.90 ± 0.12 | 42 ± 1 |
| 5 | 4.18 ± 0.11 | 2.52 ± 0.19 | 52 ± 1 | |
| 9 | 4.21 ± 0.09 | 2.81 ± 0.13 | 63 ± 1 | |
| 10 | 4.21 ± 0.21 | 2.81 ± 0.11 | 58 ± 1 | |
| FSE-3 L. brevis LY_1120 | 1 | 3.99 ± 0.09 | 1.89 ± 0.09 | 41 ± 1 |
| 5 | 4.00 ± 0.07 | 2.44 ± 0.03 | 56 ± 1 | |
| 9 | 4.19 ± 0.11 | 2.65 ± 0.01 | 69 ± 1 | |
| 10 | 4.19 ± 0.19 | 2.65 ± 0.06 | 69 ± 1 | |
| FSE-4 L. acidophilus MI-0078 | 1 | 4.11 ± 0.19 | 1.89 ± 0.11 | 41 ± 1 |
| 5 | 4.13 ± 0.14 | 2.44 ± 0.12 | 58 ± 1 | |
| 9 | 4.22 ± 0.19 | 2.65 ± 0.11 | 70 ± 1 | |
| 10 | 4.22 ± 0.16 | 2.65 ± 0.11 | 77 ± 1 | |
| FSE-5 L. rhamnosus MI-0272 | 1 | 4.11 ± 0.10 | 1.92 ± 0.09 | 50 ± 1 |
| 5 | 4.19 ± 0.11 | 2.52 ± 0.21 | 85 ± 1 | |
| 9 | 4.23 ± 0.11 | 2.82 ± 0.13 | 89 ± 1 | |
| 10 | 4.23 ± 0.13 | 2.82 ± 0.15 | 87 ± 1 | |
| FSE-6 L. plantarum MI-0102 | 1 | 3.99 ± 0.22 | 1.91 ± 0.13 | 47 ± 1 |
| 5 | 4.12 ± 0.19 | 2.51 ± 0.13 | 69 ± 1 | |
| 9 | 4.20 ± 0.17 | 2.81 ± 0.20 | 85 ± 1 | |
| 10 | 4.20 ± 0.17 | 2.81 ± 0.21 | 85 ± 1 |
| Fermented Stems Extract/Initial Sugars Content | Fermentation Time | AA | TPC | LAe |
|---|---|---|---|---|
| Days | mmol Tx/L | mmol GA/L | % | |
| FSE-7 1.60% | 1 | 4.09 ± 0.21 | 1.90 ± 0.11 | 44 ± 1 |
| 5 | 4.17 ± 0.20 | 2.50 ± 0.17 | 78 ± 1 | |
| 9 | 4.21 ± 0.18 | 2.80 ± 0.18 | 83 ± 1 | |
| FSE-8 3.20% | 1 | 4.11 ± 0.20 | 1.92 ± 0.21 | 50 ± 1 |
| 5 | 4.19 ± 0.17 | 2.52 ± 0.21 | 85 ± 1 | |
| 9 | 4.23 ± 0.13 | 2.82 ± 0.18 | 89 ± 1 | |
| FSE-9 4.60% | 1 | 4.24 ± 0.17 | 1.93 ± 0.19 | 43 ± 1 |
| 5 | 4.25 ± 0.21 | 2.53 ± 0.19 | 76 ± 1 | |
| 9 | 4.29 ± 0.22 | 2.84 ± 0.09 | 81 ± 1 |
| Fermented Stems Extract/Plant Material Content | Fermentation Time | AA | TPC | LAe |
|---|---|---|---|---|
| Days | mmol Tx/L | mmol GA/L | % | |
| FSE-10 0.032% | 1 | 1.36 ± 0.11 | 1.02 ± 0.11 | 42 ± 1 |
| 5 | 2.13 ± 0.11 | 1.25 ± 0.11 | 49 ± 1 | |
| 9 | 2.33 ± 0.14 | 1.65 ± 0.19 | 79 ± 1 | |
| 10 | 2.37 ± 0.14 | 2.43 ± 0.19 | 87 ± 1 | |
| FSE-11 0.16% | 1 | 1.77 ± 0.17 | 1.18 ± 0.13 | 43 ± 1 |
| 5 | 2.21 ± 0.18 | 1.25 ± 0.13 | 54 ± 1 | |
| 9 | 2.41 ± 0.19 | 1.88 ± 0.17 | 57 ± 1 | |
| 10 | 2.45 ± 0.13 | 2.04 ± 0.17 | 86 ± 1 | |
| FSE-12 0.32% | 1 | 1.77 ± 0.11 | 1.25 ± 0.19 | 40 ± 1 |
| 5 | 2.37 ± 0.12 | 1.49 ± 0.13 | 53 ± 1 | |
| 9 | 2.45 ± 0.21 | 2.19 ± 0.13 | 87 ± 1 | |
| 10 | 2.49 ± 0.19 | 2.35 ± 0.19 | 86 ± 1 | |
| FSE-13 0.80% | 1 | 2.81 ± 0.19 | 1.33 ± 0.12 | 36 ± 1 |
| 5 | 4.01 ± 0.21 | 1.49 ± 0.15 | 55 ± 1 | |
| 9 | 4.25 ± 0.19 | 2.19 ± 0.19 | 71 ± 1 | |
| 10 | 4.29 ± 0.23 | 2.35 ± 0.17 | 88 ± 1 | |
| FSE-14 1.60% | 1 | 4.17 ± 0.19 | 1.46 ± 0.09 | 50 ± 1 |
| 5 | 4.19 ± 0.22 | 1.57 ± 0.21 | 85 ± 1 | |
| 9 | 4.29 ± 0.16 | 2.66 ± 0.21 | 89 ± 1 | |
| 10 | 4.29 ± 0.11 | 2.82 ± 0.23 | 87 ± 1 | |
| FSE-15 3.20% | 1 | 5.70 ± 0.19 | 2.09 ± 0.18 | 50 ± 1 |
| 5 | 6.22 ± 0.12 | 2.19 ± 0.18 | 85 ± 1 | |
| 9 | 8.10 ± 0.12 | 3.53 ± 0.21 | 88 ± 1 | |
| 10 | 8.14 ± 0.10 | 3.76 ± 0.21 | 87 ± 1 | |
| FSE-16 6.40% | 1 | 9.15 ± 0.13 | 5.09 ± 0.19 | 50 ± 1 |
| 5 | 11.11 ± 0.13 | 5.33 ± 0.20 | 85 ± 1 | |
| 9 | 26.88 ± 0.21 | 5.96 ± 0.19 | 88 ± 1 | |
| 10 | 26.88 ± 0.21 | 5.96 ± 0.19 | 85 ± 1 | |
| FSE-17 12.80% | 1 | 9.71 ± 0.11 | 5.17 ± 0.11 | 49 ± 1 |
| 5 | 11.15 ± 0.22 | 5.41 ± 0.18 | 83 ± 1 | |
| 9 | 26.92 ± 0.22 | 6.03 ± 0.16 | 83 ± 1 | |
| 10 | 27.00 ± 0.26 | 6.19 ± 0.13 | 81 ± 1 |
| Cosmetic Raw Material | Chelating Activity ChA | Reduction Activity RA | Antioxidant Activity AA | Total Polyphenol Content TPC | LA Efficiency LAe |
|---|---|---|---|---|---|
| mmol Fe2+/L FSE | mmol Fe3+/L FSE | mmol Tx/L | mmol GA/L | % | |
| FSE * | 0.30 ± 0.01 | 46.22 ± 0.29 | 26.88 ± 0.28 | 5.96 ± 0.19 | 88 ± 1 |
| SE ** | 0.12 ± 0.01 | 29.31 ± 0.21 | 13.24 ± 0.19 | 2.64 ± 0.11 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kucharska, E.; Zagórska-Dziok, M.; Bilewicz, P.; Kowalczyk, S.; Pełech, R. Use of Syzygium aromaticum L. Fermented Plant Extract to Enhance Antioxidant Potential: Fermentation Kinetics. Appl. Sci. 2024, 14, 4900. https://doi.org/10.3390/app14114900
Kucharska E, Zagórska-Dziok M, Bilewicz P, Kowalczyk S, Pełech R. Use of Syzygium aromaticum L. Fermented Plant Extract to Enhance Antioxidant Potential: Fermentation Kinetics. Applied Sciences. 2024; 14(11):4900. https://doi.org/10.3390/app14114900
Chicago/Turabian StyleKucharska, Edyta, Martyna Zagórska-Dziok, Paweł Bilewicz, Sebastian Kowalczyk, and Robert Pełech. 2024. "Use of Syzygium aromaticum L. Fermented Plant Extract to Enhance Antioxidant Potential: Fermentation Kinetics" Applied Sciences 14, no. 11: 4900. https://doi.org/10.3390/app14114900







