Effect of Laser Surface Treatment on the Corrosion Resistance of Zircaloy-4 at High Temperature
Abstract
:1. Introduction
2. Experimental Procedures
3. Results
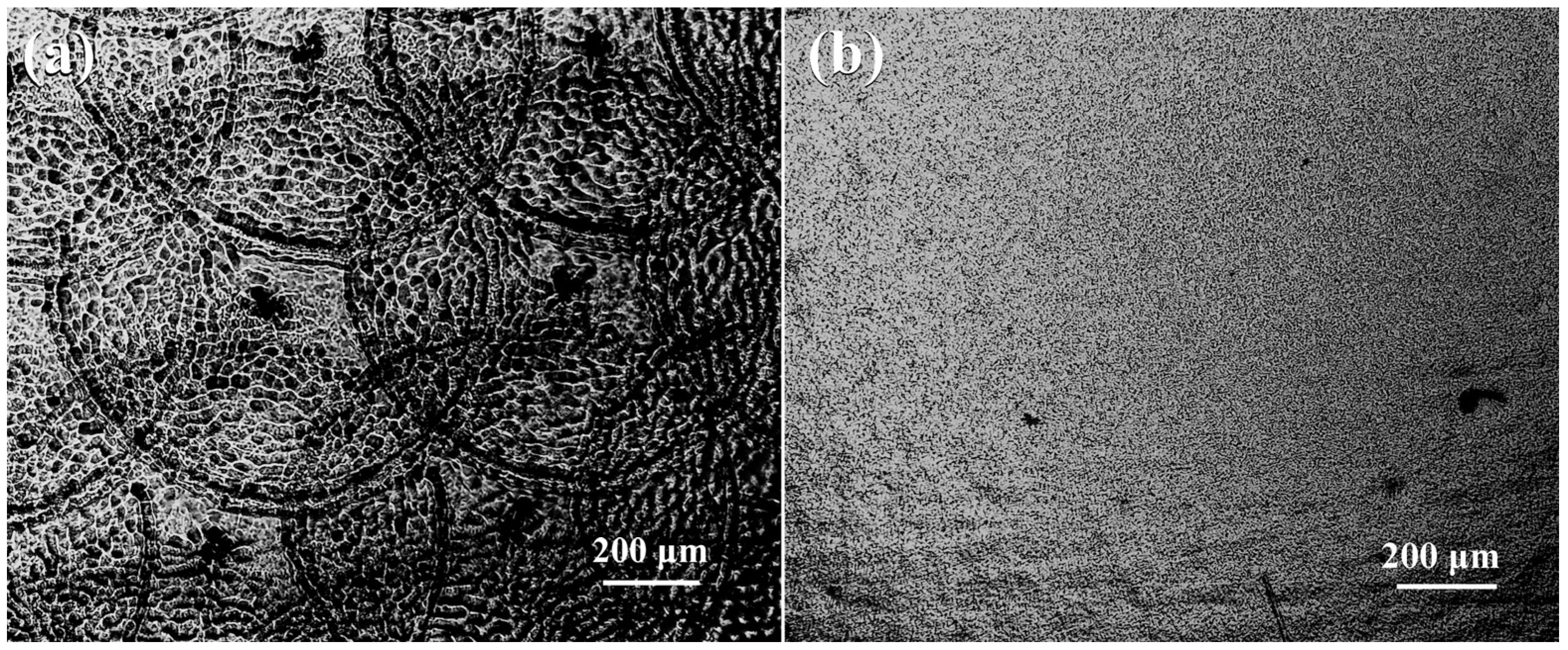
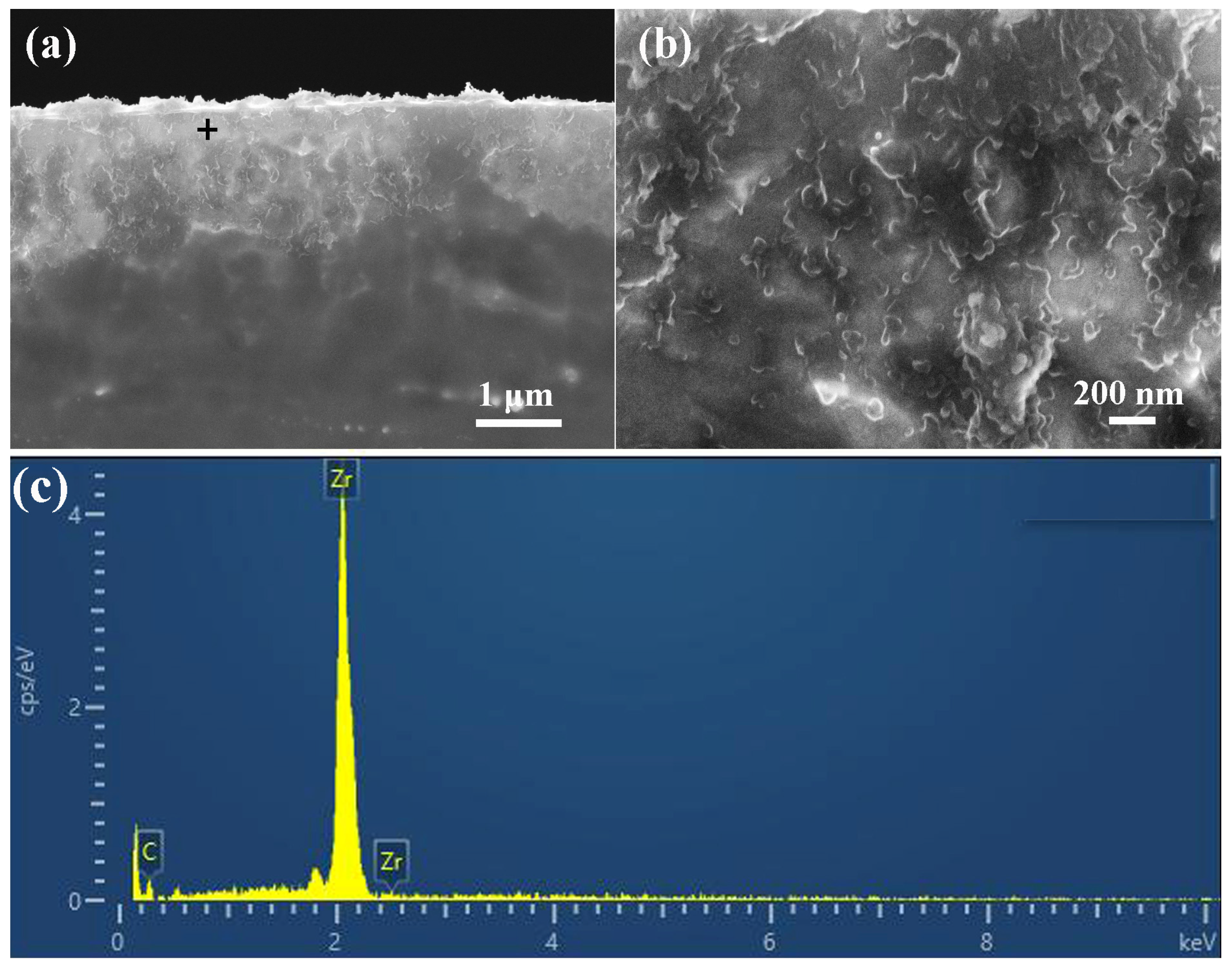
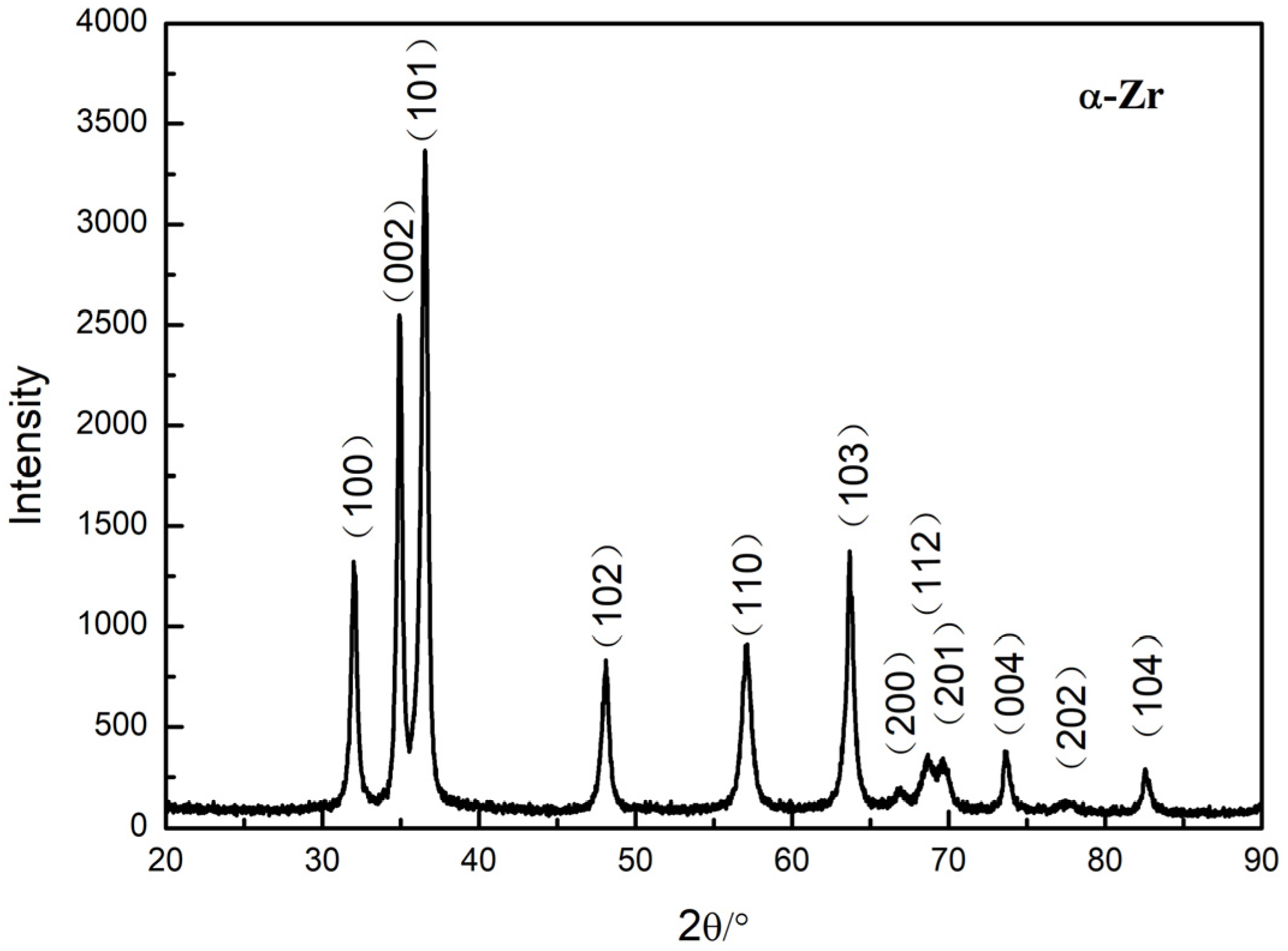
3.1. LST Layer Features
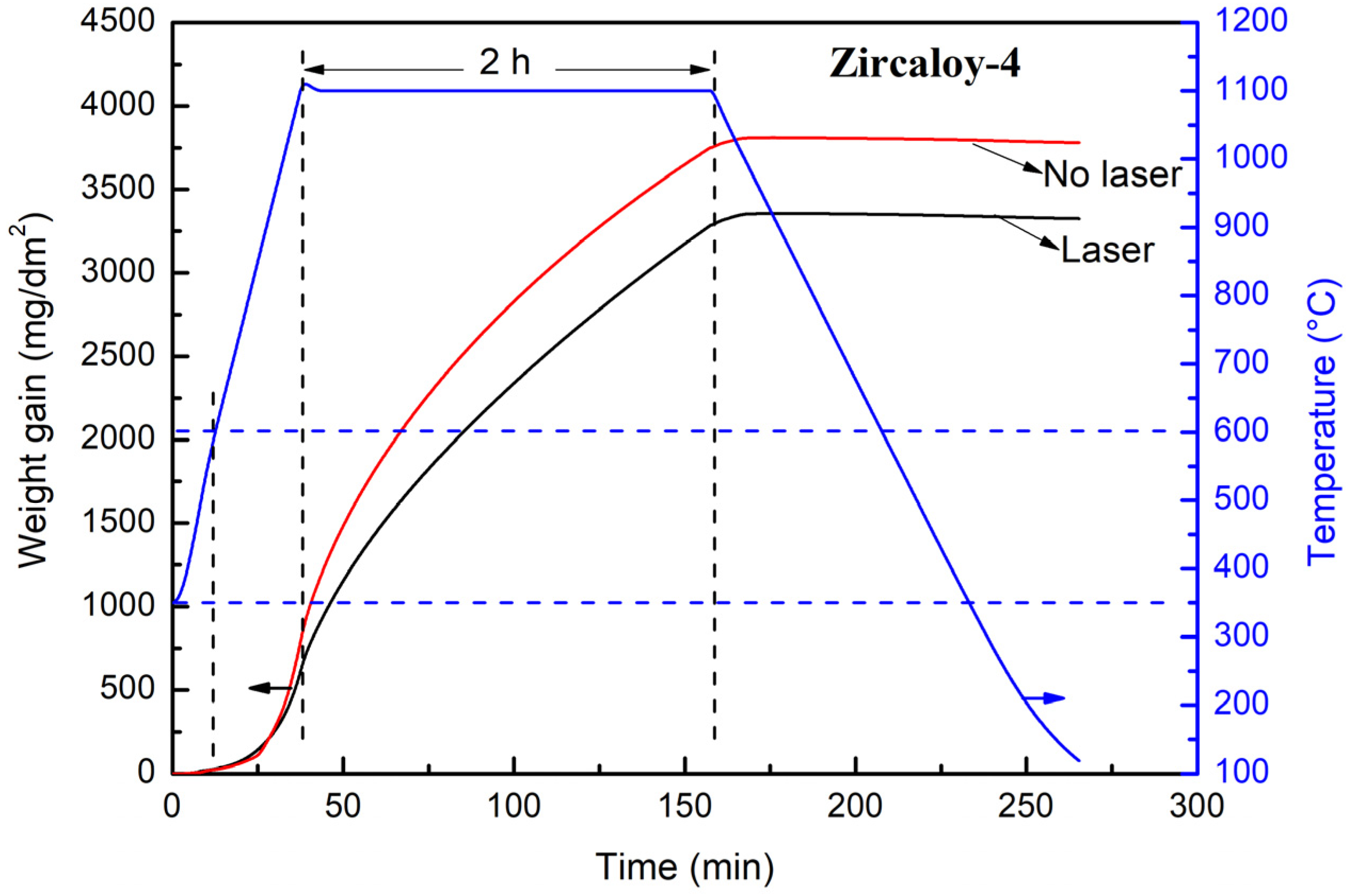
3.2. Weight Gain Curve
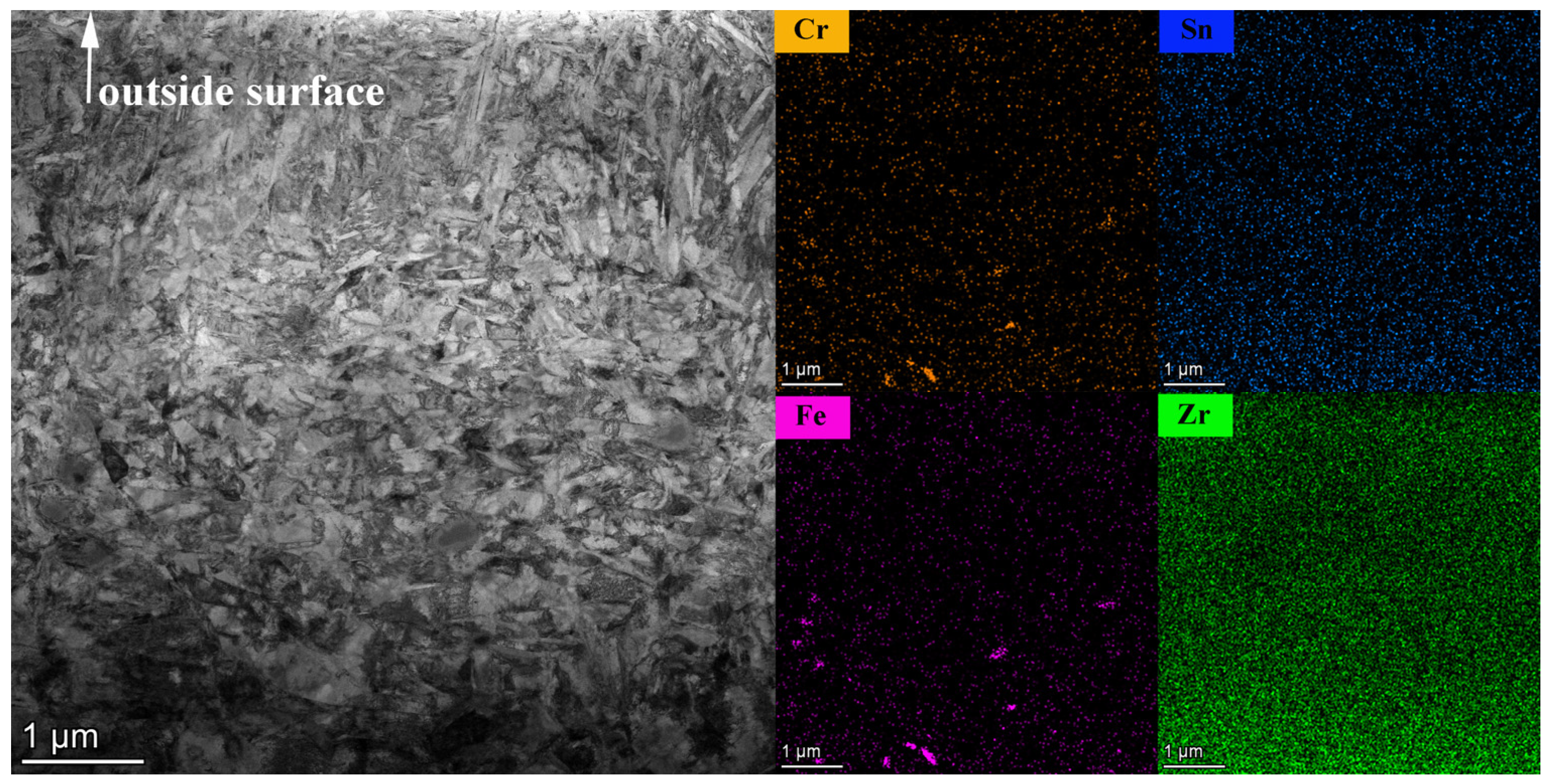
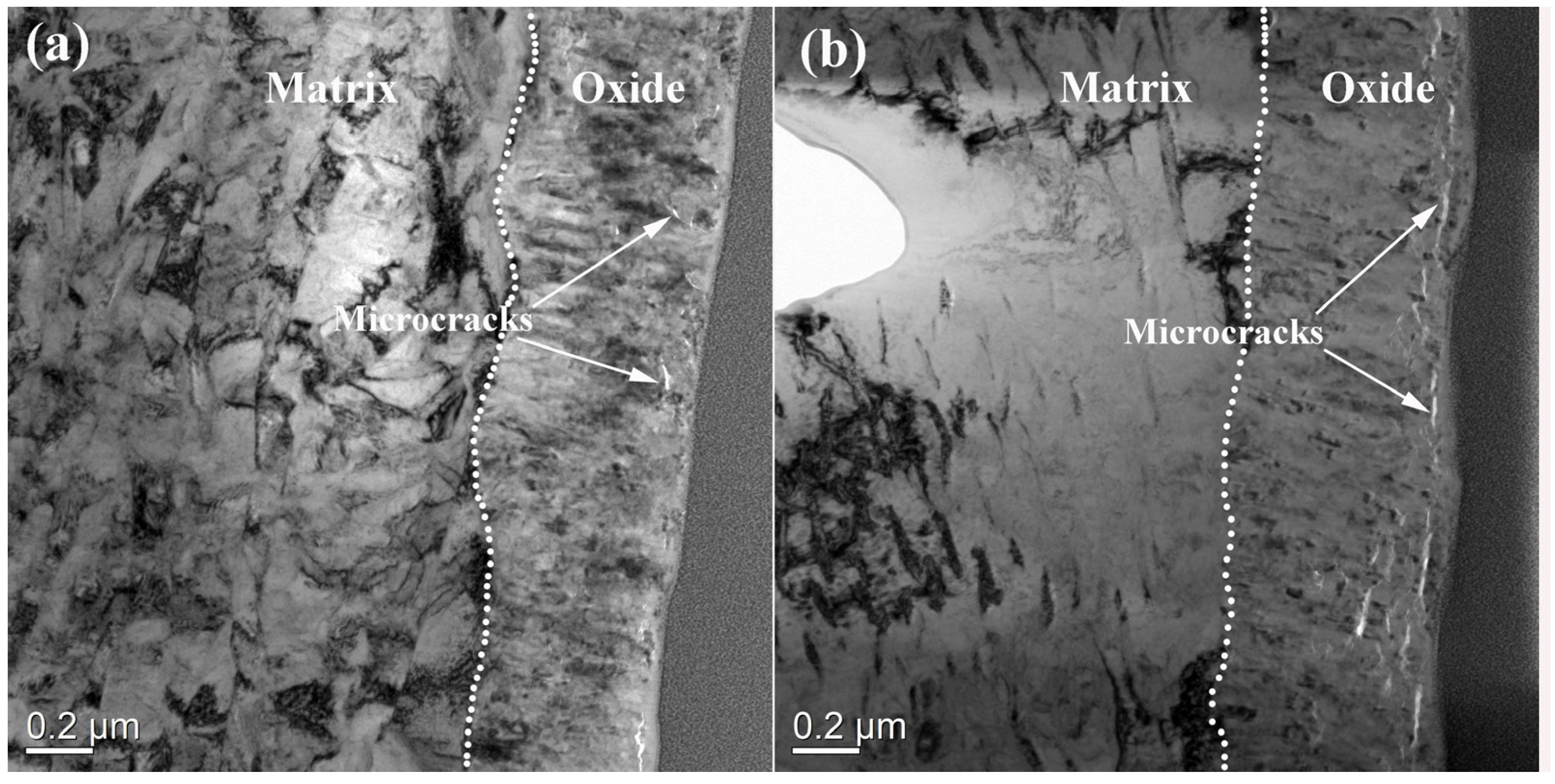
3.3. Oxide Film Morphologies
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zinkle, S.J.; Terrani, K.A.; Gehin, J.C.; Ott, L.J.; Snead, L.L. Accident tolerant fuels for LWRs: A perspective. J. Nucl. Mater. 2014, 448, 374–379. [Google Scholar] [CrossRef]
- Ott, L.J.; Robb, K.R.; Wang, D. Preliminary assessment of accident-tolerant fuels on LWR performance during normal operation and under DB and BDB accident conditions. J. Nucl. Mater. 2014, 448, 520–533. [Google Scholar] [CrossRef]
- Koo, Y.-H.; Yang, J.-H.; Park, J.-Y.; Kim, K.-S.; Kim, H.-G.; Kim, D.-J.; Jung, Y.-I.; Song, K.-W. KAERIs development of LWR accident tolerant fuel. Nucl. Technol. 2014, 186, 295–304. [Google Scholar] [CrossRef]
- Wei, K.; Cui, L.; Li, B.; Yang, Z.; Han, Y.; Kano, S.; Abe, H. Direct experimental evidence of the reduction of Cr2O3 by Zr at high temperature. Corros. Sci. 2023, 225, 111587. [Google Scholar] [CrossRef]
- Huang, J.; Zou, S.; Xiao, W.; Yang, C.; Yu, H.; Zhang, L.; Zhang, K. Microstructural evolution of Cr-coated Zircaloy-4 alloy prepared by multi-arc plating during high temperature oxidation. J. Nucl. Mater. 2022, 562, 153616. [Google Scholar] [CrossRef]
- Ševeček, M.; Gurgen, A.; Seshadri, A.; Che, Y.; Wagih, M.; Phillips, B.; Champagne, V.; Shirvan, K. Development of Cr cold spray-coated fuel cladding with enhanced accident tolerance. Nucl. Eng. Technol. 2018, 50, 229–236. [Google Scholar] [CrossRef]
- Yang, J.; Steinbrück, M.; Tang, C.C.; Grobe, M.; Liu, J.; Zhang, J.; Yin, D.; Wang, S.Z. Review on chromium coated zirconium alloy accident tolerant fuel cladding. J. Alloys Compd. 2022, 895, 162450. [Google Scholar] [CrossRef]
- Xiang, Y.; Yang, H.; Chen, Q.; Peng, X.; Zhang, R.; Wei, T.; Liu, C.; Chen, H. Long-term high-temperature steam oxidation behavior of Cr-coated Zircaloy-4 alloy for accident tolerant fuel. Mater. Corros. 2022, 73, 2009–2018. [Google Scholar] [CrossRef]
- Han, X.; Xue, J.; Peng, S.; Zhang, H. An interesting oxidation phenomenon of Cr coatings on Zry-4 substrates in high temperature steam environment. Corros. Sci. 2019, 156, 117–124. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Zhou, Q.; Xue, W.; Jin, X.; Du, J.; Li, Y.; Li, S. TEM characterizations of a ZrO2/Cr composite coating on Zr-1Nb alloy after 1200 °C steam oxidation. Mater. Charact. 2023, 197, 112701. [Google Scholar] [CrossRef]
- Liao, H.; Huang, W.; Ruan, H.; Zhang, T.; Ning, Y.; Zhu, P.; Xu, X.; Xu, M.; Yin, S.; Su, Y.; et al. Oxidation behavior and microstructural evolution of Cr coatings prepared by multi-arc ion plating on Zry-4 in steam environments up to 1400 °C. J. Nucl. Mater. 2023, 583, 154530. [Google Scholar] [CrossRef]
- Meng, C.; Ma, J.; Wang, H.; Liu, W.; Hu, Y.; Zhang, B.; Tu, M.; Yuan, C.; He, X. Enhancing the oxidation behaviors of Zr alloys for nuclear fuel cladding using nanolamerllar Cr/CrN coating. Corros. Sci. 2024, 227, 111725. [Google Scholar] [CrossRef]
- Li, Q.; Song, P.; Zhang, R.; Li, Z.; Li, Z.; Wang, Y.; Du, P.; Lu, J. Oxidation behavior and Cr-Zr diffusion of Cr coating prepared by atmospheric plasma spraying on Zircaloy-4 cladding in steam at 1300 °C. Corros. Sci. 2022, 203, 110378. [Google Scholar] [CrossRef]
- Kim, H.; Kim, I.; Jung, Y.; Park, D.; Park, J.; Koo, Y. Adhesion property and high temperature oxidation behavior of Cr-coated Zircaloy-4 cladding tube prepared by 3D laser coating. J. Nucl. Mater. 2015, 465, 531–539. [Google Scholar] [CrossRef]
- Han, X.; Chen, C.; Tan, Y.; Feng, W.; Peng, S.; Zhang, H. A systematic study of the oxidation behavior of Cr coatings on Zry4 substrates in high temperature steam environment. Corros. Sci. 2020, 174, 108826. [Google Scholar] [CrossRef]
- Brachet, J.-C.; Rouesne, E.; Ribis, J.; Guilbert, T.; Urvoy, S.; Nony, G.; Toffolon-Masclet, C.; Le Saux, M.; Chaabane, N.; Palancher, H.; et al. High temperature steam oxidation of chromium-coated zirconium based alloys: Kinetics and process. Corros. Sci. 2020, 167, 108537. [Google Scholar] [CrossRef]
- Liu, H.; Feng, Y.; Yao, Y.; Li, B.; Wang, R.; Shi, X.; Li, P.; Shu, J.; Huang, F.; Huang, Q.; et al. Effect of the 345 °C and 16.5 MPa autoclave corrosion on the oxidation behavior of Cr-coated zirconium claddings in the high-temperature steam. Corros. Sci. 2021, 189, 109608. [Google Scholar] [CrossRef]
- Wang, D.; Zhong, R.; Zhang, Y.; Chen, P.; Lan, Y.; Yu, J.; Su, G.H.; Qiu, S.; Tian, W. Isothermal experiments on steam oxidation of magnetron-sputtered chromiumcoated zirconium alloy cladding at 1200 °C. Corros. Sci. 2022, 206, 110544. [Google Scholar] [CrossRef]
- Liu, J.; Cui, Z.; Hao, Z.; Ma, D.; Lu, J.; Cui, Y.; Li, C.; Liu, W.; Xie, S.; Hu, P.; et al. Steam oxidation of Cr-coated Sn-containing Zircaloy solid rod at 1000 °C. Corros. Sci. 2021, 190, 109682. [Google Scholar] [CrossRef]
- Liu, J.; Steinbrück, M.; Große, M.; Stegmaier, U.; Tang, C.; Yun, D.; Yang, J.; Cui, Y.; Seifert, H.J. Systematic investigations on the coating degradation mechanism during the steam oxidation of Cr-coated Zry-4 at 1200 °C. Corros. Sci. 2022, 202, 110310. [Google Scholar] [CrossRef]
- Wei, T.; Zhang, R.; Yang, H.; Liu, H.; Qiu, S.; Wang, Y.; Du, P.; He, K.; Hu, X.; Dong, C. Microstructure, corrosion resistance and oxidation behavior of Cr-coatings on Zircaloy-4 prepared by vacuum arc plasma deposition. Corros. Sci. 2019, 158, 108077. [Google Scholar] [CrossRef]
- Xie, S.J.; Liu, K.Q.; Meng, R.Z.; Cui, Y.G.; Wang, C.; Lu, J.Q.; Song, Z.X.; Wang, D.W.; Liu, W.B.; Qiu, J.; et al. Formation of intragranular nanocavities in the Cr2O3 layer during the corrosion of Cr3C2+Cr coated ZirloTM. Corror. Sci. 2024, 233, 112117. [Google Scholar] [CrossRef]
- Yeom, H.; Maier, B.; Johnson, G.; Dabney, T.; Lenling, M.; Sridharan, K. High temperature oxidation and microstructural evolution of cold spray chromium coatings on Zircaloy-4 in steam environments. J. Nucl. Mater. 2019, 526, 151737. [Google Scholar] [CrossRef]
- Yang, J.; Stegmaier, U.; Tang, C.; Steinbrück, M.; Große, M.; Wang, S.; Seifert, H.J. High temperature Cr-Zr interaction of two types of Cr-coated Zr alloys in inert gas environment. J. Nucl. Mater. 2021, 547, 152806. [Google Scholar] [CrossRef]
- Pysarenko, V.; Kuznetsova, T.; Samelyuk, A. Structure and mechanical properties of ZrCr2 intermetallic compound within the temperature range 20–1300 °C. Mater. Sci. 2004, 40, 83–88. [Google Scholar] [CrossRef]
- Zhang, L.; Lai, P.; Liu, Q.; Zeng, Q.; Lu, J.; Guo, X. Fretting wear behavior of zirconium alloy in B-Li water at 300 °C. J. Nucl. Mater. 2018, 499, 401–409. [Google Scholar] [CrossRef]
- Jiang, J.; Ma, X.; Wang, B. Positive or negative role of preoxidation in the crack arresting of Cr coating for accident tolerant fuel cladding. Corros. Sci. 2021, 193, 109870. [Google Scholar] [CrossRef]
- Kim, D.; Steinbrück, M.; Grosse, M.; Tang, C.; Lee, Y. Eutectic reaction and oxidation behavior of Cr-coated Zircaloy-4 accident-tolerant fuel cladding under various heating rates. J. Nucl. Mater. 2023, 583, 154538. [Google Scholar] [CrossRef]
- Zhao, Y.L.; Liu, J.Z. The effect of laser surface treatment on the nodular corrosion of Zircaloy-4. Rare Metal. Mat. Eng. 1996, 25, 44–46. [Google Scholar]
- Yang, J.X.; Wang, X.; Wen, Q.; Wang, X.B.; Wang, R.S.; Zhang, Y.W.; Xue, W.B. The effect of microarc oxidation and excimer laser processing on the microstructure and corrosion resistance of Zr-1Nb alloy. J. Nucl. Mater. 2015, 467, 186–193. [Google Scholar] [CrossRef]
- Andersson, T.; Thorvaldsson, T.; Wilson, A.; Wardle, A.M. Improvement in Water Reactor Fuel Technology and Utilization; IAEA: Vienna, Austria, 1987; pp. 435–449. [Google Scholar]
- Lee, S.J.; Park, C.J.; Lim, Y.S.; Kwon, H.S. Influences of laser surface alloying with niobium on the corrosion resistance of Zircaloy-4. J. Nucl. Mater. 2003, 321, 177–183. [Google Scholar] [CrossRef]
- Chai, L.J.; Wu, H.; Wang, S.Y.; Chen, K.; Wang, T.T.; Xia, J.Y. Characterization of microstructure and hardness of a Zr-2.5 Nb alloy surface-treated by pulsed laser. Mater. Chem. Phys. 2017, 198, 303–309. [Google Scholar] [CrossRef]
- Chai, L.J.; Chen, K.; Zhi, Y.; Murty, K.L.; Chen, L.Y.; Yang, Z.N. Nanotwins induced by pulsed laser and their hardening effect in a Zr alloy. J. Alloys Compd. 2018, 748, 163–170. [Google Scholar] [CrossRef]
- Tang, C.; Zhu, W.-H.; Ma, J.-G.; Ma, Y.; Zhu, H.-M.; Wang, X.-L.; Chen, Z.-Y. Microstructure and properties of composite ceramic coating on Zr-4 alloy surface by laser cladding, Zircaloy. Equip. Manuf. Technol. 2016, 1, 29–31. [Google Scholar]
- Wu, H.; Fan, H.-Y.; Ying, S.-H.; Huang, X.-Q. Investigation of laser surface alloying with niobium on the depth and the structure of Zircaloy-4. Eng. Mater. 2005, 8, 50–52. [Google Scholar]
- Liu, Y.Y.; Yang, Y.L.; Dong, D.Y.; Wang, J.; Zhou, L.L. Improving wear resistance of Zr-2.5 Nb alloy by formation of microtextured nitride layer produced via laser surface texturing/plasma nitriding technology. Surf. Interfaces 2020, 20, 100638. [Google Scholar] [CrossRef]
- Zhang, H.X.; Fruchart, D.; Hlil, E.K.; Ortega, L.; Li, Z.K.; Zhang, J.J.; Sun, J.; Zhou, L. Crystal structure, corrosion kinetics of new zirconium alloys and residual stress analysis of oxide films. J. Nucl. Mater. 2010, 396, 65–70. [Google Scholar] [CrossRef]
- Grandjean, A.; Serruys, Y. Metal and oxygen mobilities during Zircaloy-4 oxidation at high temperature. J. Nucl. Mater. 1999, 273, 111–115. [Google Scholar] [CrossRef]
- Müller, S.; Lanzani, L. Corrosion of Zr-1Nb and Zr-2.5Nb in 0.1 M LiOH at 343 °C. Procedia Mater. Sci. 2015, 8, 46–55. [Google Scholar] [CrossRef]
- Cox, B. Some thoughts on the mechanisms of in-reactor corrosion of zirconium alloys. J. Nucl. Mater. 2005, 336, 331–368. [Google Scholar] [CrossRef]
- Dawson, J.K.; Long, G.; Seddon, W.E.; White, J.F. The kinetics and mechanism of the oxidation of zricaloy-2 at 350–500 °C. J. Nucl. Mater. 1968, 25, 179–200. [Google Scholar] [CrossRef]
- Cox, B. Pore structure in oxide films on irradiated and unirradiated zirconium alloys. J. Nucl. Mater. 1987, 148, 332–343. [Google Scholar] [CrossRef]
- Liao, J.J.; Yang, Z.; Qiu, S.; Peng, Q.; Li, Z.; Zhang, J. The correlation between tetragonal phase and the undulated metal/oxide interface in the oxide films of zirconium alloys. J. Nucl. Mater. 2019, 524, 101–110. [Google Scholar] [CrossRef]
- Zhong, X.Y.; Yang, B.; Li, M.C.; Yao, M.Y.; Zhou, B.X. EIS characterization of the oxide film on Zr-4 alloy surface in super-heated steam. Rare Metal Mater. Eng. 2010, 39, 2155–2168. (In Chinese) [Google Scholar]
- Weidinger, H.G.; Ruhmann, H.; Cheliotis, G.; Maguire, M.; Yau, T.L. Corrosion-electrochemical properties of zirconium intermetallics. In 9th International Symposium on Zirconium in the Nuclear Industry; ASTM STP 1132; ASTM International: West Conshohocken, PA, USA, 1991; pp. 499–535. [Google Scholar]
- Proff, C.; Abolhassani, S.; Lemaignan, C. Oxidation behavior of binary zirconium alloys containing intermetallic precipitates. J. Nucl. Mater. 2011, 416, 125–134. [Google Scholar] [CrossRef]
- Proff, S.; Abolhassani, S.; Lemaignan, C. Oxidation behaviour of zirconium alloys and their precipitates—A mechanistic study. J. Nucl. Mater. 2013, 432, 222–238. [Google Scholar] [CrossRef]
- Baek, J.H.; Jeong, Y.H.N.; Kim, I.S. Effects of the accumulated annealing parameter on the corrosion characteristics of a Zr-0.5Nb-1.0Sn-0.5Fe-0.25Cr alloy. J. Nucl. Mater. 2000, 280, 235–245. [Google Scholar] [CrossRef]
- Baek, J.H.; Jeong, Y.H. Depletion of Fe and Cr within precipitates during Zircaloy-4 oxidation. J. Nucl. Mater. 2002, 304, 107–116. [Google Scholar] [CrossRef]
- Huang, J.; Yao, M.-Y.; Gao, C.-Y.; Liang, X.; Peng, J.C.; Zhang, J.-L.; Zhou, B.-X. The influence of second phase particles on the crack formation in oxide films formed on zirconium alloys. Corros. Sci. 2015, 99, 172–177. [Google Scholar] [CrossRef]
- Preuss, M.; Frankel, P.; Polatidis, E.; Wei, J.; Smith, J.; English, C.; Wang, F.; Cottis, B.; Lyon, S.; Lozano-Perez, S.; et al. Studies regarding corrosion mechanisms in zirconium alloys. In Proceedings of the International Symposium on Zirconium in the Nuclear Industry, Chengdu, China, 9–13 May 2010; p. 649. [Google Scholar]
- Bouvier, P.; Godlewski, J.; Lucazeau, G. A Raman study of the nanocrystallite size effect on the pressure-temperature phase diagram of zirconia grown by zirconium-based alloys oxidation. J. Nucl. Mater. 2002, 300, 118–126. [Google Scholar] [CrossRef]
- Yasuda, K.; Arai, S.; Itoh, M.; Wada, K. Influence of Y2O3 distribution on the rate of tetragonal to monoclinic phase transformation of yttria-stabilized zirconia during hydrothermal aging. J. Mater. Sci. 1999, 34, 3597–3604. [Google Scholar] [CrossRef]
- Sankar, U.; Satgunam, M.; Amiriyan, M.; Singh, R.; Teng, W.D. Sintering and densification behavior of Zno-doped Y-TZP ceramics. Appl. Mech. Mater. 2011, 83, 197–203. [Google Scholar] [CrossRef]
- Ramesh, S.; Meenaloshini, S.; Tan, C.Y.; Chew, W.J.K.; Teng, W.D. Effect of manganese oxide on the sintered properties and low temperature degradation of Y-TZP ceramics. Ceram. Int. 2008, 34, 1603–1608. [Google Scholar] [CrossRef]
- Hulme, H.; Baxter, F.; Babu, R.P.; Denecke, M.A.; Gass, M.; Steuwer, A.; Norén, K.; Carlson, S.; Preuss, M. An X-ray absorption near-edge structure (XANES) study of the Sn L3 edge in zirconium alloy oxide films formed during autoclave corrosion. Corros. Sci. 2016, 105, 202–208. [Google Scholar] [CrossRef]
- Froideval, A.; Degueldre, C.; Segre, C.U.; Pouchon, M.A.; Grolimund, D. Niobium speciation at the metal/oxide interface of corroded niobium-doped Zircaloys: A X-ray absorption near-edge structure study. Corros. Sci. 2008, 50, 1313–1320. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, S.; Meng, R.; Shi, T.; Yu, Y.; Liu, J.; Guo, Y.; Qiu, J.; Liu, W.; Yun, D. Effect of Laser Surface Treatment on the Corrosion Resistance of Zircaloy-4 at High Temperature. Appl. Sci. 2024, 14, 4977. https://doi.org/10.3390/app14124977
Xie S, Meng R, Shi T, Yu Y, Liu J, Guo Y, Qiu J, Liu W, Yun D. Effect of Laser Surface Treatment on the Corrosion Resistance of Zircaloy-4 at High Temperature. Applied Sciences. 2024; 14(12):4977. https://doi.org/10.3390/app14124977
Chicago/Turabian StyleXie, Shijing, Ruizhi Meng, Tong Shi, Yihang Yu, Jianhang Liu, Yiwen Guo, Jie Qiu, Wenbo Liu, and Di Yun. 2024. "Effect of Laser Surface Treatment on the Corrosion Resistance of Zircaloy-4 at High Temperature" Applied Sciences 14, no. 12: 4977. https://doi.org/10.3390/app14124977





