Magnetoelectric Nanoparticles for Wireless Peripheral Nerve Stimulation: A Computational Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Electromagnetic Simulation
2.1.1. MENPs Model
2.1.2. Nerve Model
2.1.3. Stimulation Settings
2.2. Modeling of the Neuronal Dynamics
2.3. Configurations and Data Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bavishi, S.; Rosenthal, J.; Bockbrader, M. Chapter 17—Neuroprosthetics. In Rehabilitation after Traumatic Brain Injury; Eapen, B.C., Cifu, D.X., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 241–253. [Google Scholar] [CrossRef]
- Yu, B.M.; Santhanam, G.; Sahani, M.; Shenoy, K.V. Chapter 7—Neural Decoding for Motor and Communication Prostheses. In Statistical Signal Processing for Neuroscience and Neurotechnology; Oweiss, K.G., Ed.; Academic Press: Oxford, UK, 2010; pp. 219–263. [Google Scholar] [CrossRef]
- Russell, C.; Roche, A.D.; Chakrabarty, S. Peripheral nerve bionic interface: A review of electrodes. Int. J. Intell. Robot. Appl. 2019, 3, 11–18. [Google Scholar] [CrossRef]
- Lee, S.; Lee, C. Toward advanced neural interfaces for the peripheral nervous system (PNS) and their future applications. Curr. Opin. Biomed. Eng. 2018, 6, 130–137. [Google Scholar] [CrossRef]
- Günter, C.; Delbeke, J.; Ortiz-Catalan, M. Safety of long-term electrical peripheral nerve stimulation: Review of the state of the art. J. NeuroEng. Rehabil. 2019, 16, 13. [Google Scholar] [CrossRef] [PubMed]
- Kargol, A.; Malkinski, L.; Caruntu, G.; Kargol, A.; Malkinski, L.; Caruntu, G. Biomedical Applications of Multiferroic Nanoparticles. In Advanced Magnetic Materials; IntechOpen: London, UK, 2012. [Google Scholar] [CrossRef]
- Kozielski, K.L.; Jahanshahi, A.; Gilbert, H.B.; Yu, Y.; Erin, Ö.; Francisco, D.; Sitti, M. Nonresonant powering of injectable nanoelectrodes enables wireless deep brain stimulation in freely moving mice. Sci. Adv. 2021, 7, eabc4189. [Google Scholar] [CrossRef] [PubMed]
- Apu, E.H.; Nafiujjaman, M.; Sandeep, S.; Makela, A.V.; Khaleghi, A.; Vainio, S.; Ashammakhi, N. Biomedical applications of multifunctional magnetoelectric nanoparticles. Mater. Chem. Front. 2022, 6, 1368–1390. [Google Scholar] [CrossRef]
- Khizroev, S. Technobiology’s Enabler: The Magnetoelectric Nanoparticle. Cold Spring Harb. Perspect. Med. 2019, 9, a034207. [Google Scholar] [CrossRef]
- Eerenstein, W.; Mathur, N.D.; Scott, J.F. Multiferroic and magnetoelectric materials. Nature 2006, 442, 759–765. [Google Scholar] [CrossRef]
- Kopyl, S.; Surmenev, R.; Surmeneva, M.; Fetisov, Y.; Kholkin, A. Magnetoelectric effect: Principles and applications in biology and medicine—A review. Mater. Today Bio 2021, 12, 100149. [Google Scholar] [CrossRef]
- Kaushik, A.; Jayant, R.D.; Nikkhah-Moshaie, R.; Bhardwaj, V.; Roy, U.; Huang, Z.; Ruiz, A.; Yndart, A.; Atluri, V.; El-Hage, N.; et al. Magnetically guided central nervous system delivery and toxicity evaluation of magneto-electric nanocarriers. Sci. Rep. 2016, 6, 25309. [Google Scholar] [CrossRef]
- Rodzinski, A.; Guduru, R.; Liang, P.; Hadjikhani, A.; Stewart, T.; Stimphil, E.; Runowicz, C.; Cote, R.; Altman, N.; Datar, R.; et al. Targeted and controlled anticancer drug delivery and release with magnetoelectric nanoparticles. Sci. Rep. 2016, 6, 20867. [Google Scholar] [CrossRef]
- Guduru, R.; Liang, P.; Hong, J.; Rodzinski, A.; Hadjikhani, A.; Horstmyer, J.; Khizroev, S. Magnetoelectric “spin” on stimulating the brain. Nanomedicine 2015, 10, 2051–2061. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.; Gao, J.; Wang, P.; Nagesetti, A.; Andrews, P.; Masood, S.; Jin, X. In Vivo Wireless Brain Stimulation via Non-invasive and Targeted Delivery of Magnetoelectric Nanoparticles. Neurother. J. Am. Soc. Exp. Neurother. 2021, 18, 2091–2106. [Google Scholar] [CrossRef]
- Pardo, M.; Roberts, E.R.; Pimentel, K.; Yildirim, Y.A.; Navarrete, B.; Wang, P.; Khizroev, S. Size-dependent intranasal administration of magnetoelectric nanoparticles for targeted brain localization. Nanomed. Nanotechnol. Biol. Med. 2021, 32, 102337. [Google Scholar] [CrossRef] [PubMed]
- Hadjikhani, A.; Rodzinski, A.; Wang, P.; Nagesetti, A.; Guduru, R.; Liang, P.; Khizroev, S. Biodistribution and clearance of magnetoelectric nanoparticles for nanomedical applications using energy dispersive spectroscopy. Nanomedicine 2017, 12, 1801–1822. [Google Scholar] [CrossRef] [PubMed]
- Fiocchi, S.; Chiaramello, E.; Marrella, A.; Suarato, G.; Bonato, M.; Parazzini, M.; Ravazzani, P. Modeling of core-shell magneto-electric nanoparticles for biomedical applications: Effect of composition, dimension, and magnetic field features on magnetoelectric response. PLoS ONE 2022, 17, e0274676. [Google Scholar] [CrossRef] [PubMed]
- Marrella, A.; Suarato, G.; Fiocchi, S.; Chiaramello, E.; Bonato, M.; Parazzini, M.; Ravazzani, P. Magnetoelectric nanoparticles shape modulates their electrical output. Front. Bioeng. Biotechnol. 2023, 11, 1219777. [Google Scholar] [CrossRef] [PubMed]
- Stefano, M.; Cordella, F.; Loppini, A.; Filippi, S.; Zollo, L. A Multiscale Approach to Axon and Nerve Stimulation Modeling: A Review. IEEE Trans. Neural Syst. Rehabil. Eng. 2021, 29, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Stefano, M.; Cordella, F.; Gioi, S.M.L.; Zollo, L. Electrical stimulation of the human median nerve: A comparison between anatomical and simplified simulation models. In Proceedings of the 2021 10th International IEEE/EMBS Conference on Neural Engineering (NER), Virtual, 4–6 May 2021; pp. 769–772. [Google Scholar] [CrossRef]
- Gonçalves, R.; Larrea, A.; Sebastian, M.S.; Sebastian, V.; Martins, P.; Lanceros-Mendez, S. Synthesis and size dependent magnetostrictive response of ferrite nanoparticles and their application in magnetoelectric polymer-based multiferroic sensors. J. Mater. Chem. C 2016, 4, 10701–10706. [Google Scholar] [CrossRef]
- Wang, P.; Zhang, E.; Toledo, D.; Smith, I.T.; Navarrete, B.; Furman, N.; Hernandez, A.F.; Telusma, M.; McDaniel, D.; Liang, P.; et al. Colossal Magnetoelectric Effect in Core-Shell Magnetoelectric Nanoparticles. Nano Lett. 2020, 20, 5765–5772. [Google Scholar] [CrossRef]
- Chiaramello, E.; Fiocchi, S.; Bonato, M.; Marrella, A.; Suarato, G.; Parazzini, M.; Ravazzani, P. Magnetoelectric Nanoparticles: Evaluating Stimulation Feasibility of the Possible Next Generation Approach for Deep Brain Stimulation. IEEE Access 2022, 10, 124884–124893. [Google Scholar] [CrossRef]
- Fiocchi, S.; Chiaramello, E.; Marrella, A.; Bonato, M.; Parazzini, M.; Ravazzani, P. Modelling of magnetoelectric nanoparticles for non-invasive brain stimulation: A computational study. J. Neural Eng. 2022, 19, 056020. [Google Scholar] [CrossRef]
- Romeni, S.; Valle, G.; Mazzoni, A.; Micera, S. Tutorial: A computational framework for the design and optimization of peripheral neural interfaces. Nat. Protoc. 2020, 15, 3129–3153. [Google Scholar] [CrossRef] [PubMed]
- Hasgall, P.A.; Neufeld, E.; Gosselin, M.C.; Klingenböck, A.; Kuster, N.; Kuster, N.; Hasgall, P.; Gosselin, M. IT’IS Database for Thermal and Electromagnetic Parameters of Biological Tissues; Version 4.1; IT’IS Foundation: Zürich, Switzerland, 2022. [Google Scholar] [CrossRef]
- McIntyre, C.C.; Richardson, A.G.; Grill, W.M. Modeling the Excitability of Mammalian Nerve Fibers: Influence of Afterpotentials on the Recovery Cycle. J. Neurophysiol. 2002, 87, 995–1006. [Google Scholar] [CrossRef] [PubMed]
- Pardo, M.; Khizroev, S. Where do we stand now regarding treatment of psychiatric and neurodegenerative disorders? Considerations in using magnetoelectric nanoparticles as an innovative approach. WIREs Nanomed. Nanobiotechnol. 2022, 14, e1781. [Google Scholar] [CrossRef]
- Warman, E.; Grill, W.; Durand, D. Modeling the effects of electric fields on nerve fibers: Determination of excitation thresholds. IEEE Trans. Biomed. Eng. 1993, 39, 1244–1254. [Google Scholar] [CrossRef]
- Richardson, A.G.; McIntyre, C.C.; Grill, W.M. Modelling the effects of electric fields on nerve fibres: Influence of the myelin sheath. Med. Biol. Eng. Comput. 2000, 38, 438–446. [Google Scholar] [CrossRef]
- Grinberg, Y.; Schiefer, M.A.; Tyler, D.J.; Gustafson, K.J.; Thickness, F.P. Size, and Position Affect Model Predictions of Neural Excitation. IEEE Trans. Neural Syst. Rehabil. Eng. Publ. IEEE Eng. Med. Biol. Soc. 2008, 16, 572–581. [Google Scholar] [CrossRef] [PubMed]
- Adewole, D.O.; Serruya, M.D.; Harris, J.P.; Burrell, J.P.; Petrov, D.; Chen, H.I.; Wolf, J.A.; Cullen, D.K. The Evolution of Neuroprosthetic Interfaces. Crit. Rev. Biomed. Eng. 2016, 44, 123–152. [Google Scholar] [CrossRef]
- Winkler, T.; Stålberg, E. Surface anodal stimulation of human peripheral nerves. Exp. Brain Res. 1988, 73, 481–488. [Google Scholar] [CrossRef]
- Paffi, A.; Apollonio, F.; Puxeddu, M.G.; Parazzini, M.; D’inzeo, G.; Ravazzani, P.; Liberti, M. A numerical study to compare stimulations by intraoperative microelectrodes and chronic macroelectrodes in the DBS technique. BioMed Res. Int. 2013, 2013, 262739. [Google Scholar] [CrossRef]
- McIntyre, C.C.; Mori, S.; Sherman, D.L.; Thakor, N.V.; Vitek, J.L. Electric field and stimulating influence generated by deep brain stimulation of the subthalamic nucleus. Clin. Neurophysiol. Off. J. Int. Fed. Clin. Neurophysiol. 2004, 115, 589–595. [Google Scholar] [CrossRef] [PubMed]
- Devan, R.S.; Chougule, B.K. Effect of composition on coupled electric, magnetic, and dielectric properties of two phase particulate magnetoelectric composite. J. Appl. Phys. 2007, 101, 014109. [Google Scholar] [CrossRef]
- Zhang, E.; Abdel-Mottaleb, M.; Liang, P.; Navarrete, B.; Yildirim, Y.A.; Campos, M.A.; Smith, I.; Wang, P.; Yildirim, B.; Yang, L.; et al. Magnetic-field-synchronized wireless modulation of neural activity by magnetoelectric nanoparticles. Brain Stimulat. 2022, 15, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
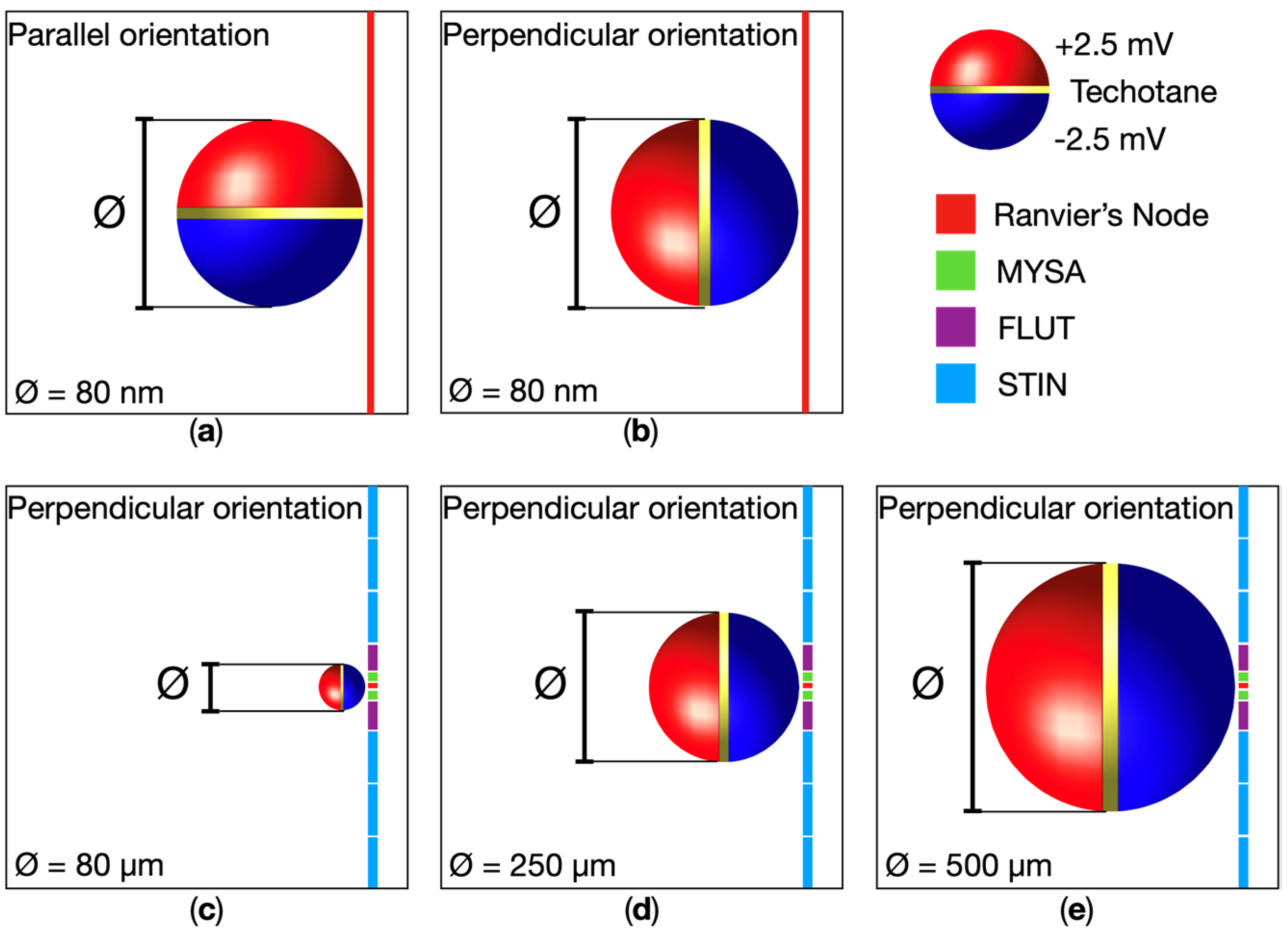




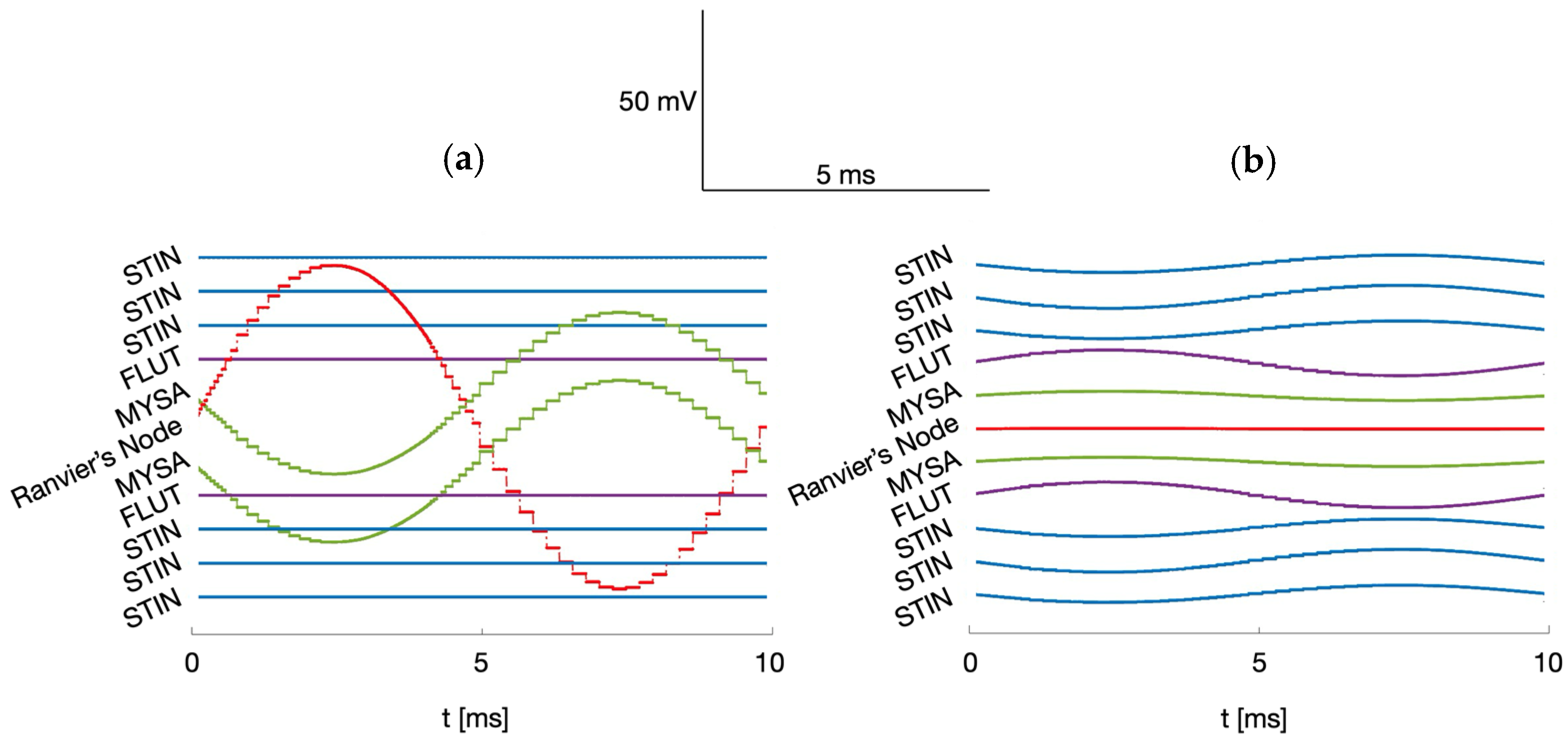
| Dipole Diameter | Parallel Orientation | Perpendicular Orientation |
|---|---|---|
| MENP–Fiber Distance | MENP–Fiber Distance | |
| 80 nm | 1R | 1R, 2R, 3R |
| 80 μm | 1R, 2R, 3R | 1R, 2R, 3R |
| 250 μm | 1R, 2R, 3R | 1R, 2R, 3R |
| 500 μm | 1R, 2R, 3R | 1R, 2R, 3R |
| MENPs–Fiber Distance | Perpendicular Orientation | Parallel Orientation | |
|---|---|---|---|
| MENP 80 nm | 1R | 4.8 | 4.7 |
| 2R | 13.7 | ||
| 3R | 31.1 | ||
| Cluster 80 μm | 1R | 5.8 | 7.9 |
| 2R | 16.7 | 67.9 | |
| 3R | 34.2 | 85.8 | |
| Cluster 250 μm | 1R | 5.7 | 6.3 |
| 2R | 13.2 | 22 | |
| 3R | 21.9 | 45 | |
| Cluster 500 μm | 1R | 2.9 | 3.9 |
| 2R | 7.4 | 23.5 | |
| 3R | 27.8 | 17.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galletta, V.; Chiaramello, E.; Fiocchi, S.; Parazzini, M.; Ravazzani, P. Magnetoelectric Nanoparticles for Wireless Peripheral Nerve Stimulation: A Computational Study. Appl. Sci. 2024, 14, 5372. https://doi.org/10.3390/app14135372
Galletta V, Chiaramello E, Fiocchi S, Parazzini M, Ravazzani P. Magnetoelectric Nanoparticles for Wireless Peripheral Nerve Stimulation: A Computational Study. Applied Sciences. 2024; 14(13):5372. https://doi.org/10.3390/app14135372
Chicago/Turabian StyleGalletta, Valentina, Emma Chiaramello, Serena Fiocchi, Marta Parazzini, and Paolo Ravazzani. 2024. "Magnetoelectric Nanoparticles for Wireless Peripheral Nerve Stimulation: A Computational Study" Applied Sciences 14, no. 13: 5372. https://doi.org/10.3390/app14135372
APA StyleGalletta, V., Chiaramello, E., Fiocchi, S., Parazzini, M., & Ravazzani, P. (2024). Magnetoelectric Nanoparticles for Wireless Peripheral Nerve Stimulation: A Computational Study. Applied Sciences, 14(13), 5372. https://doi.org/10.3390/app14135372









