Abstract
People have been exposed to the 900 MHz mobile phone electromagnetic field for approximately 30 years. There is still no conclusion from immature rodent experiments regarding the potential effects of nonthermal radiofrequency (RF) 900 MHz continuous wave exposure during biological development. Here, we test the hypothesis that mother rats exposed at a whole-body specific absorption rate (wbSAR) occupational (Oc) limit of the International Commission on Non-Ionizing Radiation Protection for humans (0.4 W/kg) will show impairments in development, with less effect at the public (Pu) limit (0.08 W/kg). The wbSAR was estimated at 0.4 W/kg to mimic working mothers (OcM exposure) and 0.08 W/kg for non-working mothers, i.e., public (PuM exposure). This pre- and postnatal study is the first to compare public and occupational exposure limits on rat pup physical development. Litter endpoints and the descendants’ body weights and lengths were recorded regularly from birth concomitantly with the age of developmental landmarks. Male neonates showed earlier pinna ear detachment and earlier eye opening in both the OcM and PuM groups, but earlier incisor eruption only in the PuM group. The OcM-exposed males showed lower body weight as juveniles until adolescence. The OcM- and PuM-exposed descendant females showed earlier pinna ear detachment and eye opening with similar body weight. These data suggest variations in the development time of descendant rats when the mother rats received daily 900 MHz continuous waves at human limits for workers and non-workers (public).
1. Background
Exposure to 900 MHz radiofrequency electromagnetic fields (RF-EMFs) was first introduced in the 1990s as the second generation (2G) of wireless signals, i.e., the Global System for Mobile communication (GSM) network. Up to date, exposure to 900 MHz RF-EMF is ongoing in everyday public and professional life. Van Wel et al. [1] showed that the 2G network is still an important contributor to people’s RF-EMF exposure and the main contributor to RF-EMF penetration in the brain during phone calls with the device close to the head. To date, the limits at 900 MHz were set at 0.08 W/kg for the general public (Pu) and 0.4 W/kg for occupational (Oc) exposures to RF-EMF. At 900 MHz incident electric field (EF) reference levels were set on the whole body over 30 min, at 41.25 V/m for the Pu and 90 V/m for Oc environment exposures [2]. One may hypothesize that vulnerable populations may be physiologically impacted at lower SARs than the ICNIRP limits.
Both Pu and Oc environments include vulnerable subjects which are ubiquitously exposed to RF-EMF, i.e., pregnant women, immature (fetuses, new-born, and children), sick (ill and injured), or aging (senile). Hospitals are also the seat of the rapid growth of RF-EMF wireless medical devices and the increased use of mobile phones by health professionals [3]. The Developmental Origins of Health and Disease (DoHaD) have been supported by decades of research. From the first days of gestation until adolescence, numerous processes may be disturbed by external or internal factors and lead to long-term impacts on the fully developed organism [4]. The skulls of newborns and fetuses are less efficient in protecting their brains from RF-EMF penetration compared to the adult skull [5].
One might assume that the effects observed in rodents may be transferred to humans since the biological tissues of humans and rodents have similar dielectric properties [6]. However, this assumption requires caution, because the same penetration depth can expose the whole body of a rat but only the superficial tissues of a human [7].
Recent studies looking at the effects of 2450 MHz continuous waves during the prenatal and postnatal period on the growing rat bone development do indicate histological alteration (tibia thickness) at 10 and 15 V/m [8]. Another study exposed pregnant rats 1 hour per day from gestational day GD 13 to 21 to 900 MHz at 0.025 W/kg and indicated no effect on weight but an effect of exposure specifically on the development of vertebrae (reduced quantity of cartilage tissue) [9]. The National Toxicology Program recently indicated lower body weight for offspring at birth and during early development when exposed to 900 MHz RF-EMF at different specific absorption rate (SAR) (1.5 W/kg, 3 W/kg, or 6 W/kg) from GD 5 and up to 106 weeks with 18 hours per day of exposure [10]. No effect was shown on litter size, but a reduction in length was shown in a meta-analysis study in non-human mammals exposed to RF-EMF at a whole-body SAR of 4 W/kg [11]. A similar effect on weight at birth was observed when pregnant rats were exposed during pregnancy 2 hours per day to 900 MHz RF-EMF at 0.155 W/kg [12]. Kumlin et al. [13] exposed postnatal day (PND) 21 rats to 900 MHz SARs of 0.3 or 3.0 W/kg 2 hours per day for 5 weeks. This post-weaning exposure did not modify the rats’ body weight. With the same period of exposure, Bosquillon de Jenlis et al. [14] showed an increased weight when exposing PND 21 rats 23 hours per day for 5 weeks at 900 MHz and 0.03 W/kg. Klose et al. exposed rats from 14 until 19 months of age 2 hours per day to GSM 900 MHz at 0.7 or 2.5 W/kg. In response to this long-term exposure during adulthood, rats showed reduced body weight at the age of 17, 18, and 19 months [15]. No physical development impact was detected in two protocols with prenatal RF-EMF exposures, 1 hour per day at 2 W/kg [16] and 6 hours per day at 0.5–0.9 W/kg [17]. No physical development impact was detected in rats exposed 1 hour per day at 0.025 W/kg from GD 13 until PND 32 [18]. Physical impact was detected on weight with 8 hours per day exposures during gestation from GD 1 until 10 at 915 MHz and 0.045 µW/cm2 [19]. Physical growth impact was also detected in protocols using shorter daily exposure (30 min per day) on a longer developmental period for the entire gestation and until PND 6, 15, or 30 (up to 51 days) at 950 W/kg and 1.3–1.0 W/kg [20]. No physical impact was detected in one protocol using extended daily exposures of 22 hours per day from GD 1 for 19 days at 970 MHz and with a SAR of 0.07 and 2.4 W/kg [21].
Overall, the published data do not converge to a simple correlation between rodents’ RF-EMF exposure SAR or duration and their physical development. One may hypothesize lower physiological and developmental health protection of ICNIRP limits in vulnerable populations, i.e., day-long working, and non-working pregnant women, and young children.
Here, we aimed to assess the hypothesis that when mother rats receive a wbSAR at the occupational (Oc) limit of the ICNIRP for humans (0.4 W/kg), their descendants will show impairments on growth and development, with less effect at public (Pu) limit (0.08 W/kg). The wbSAR was estimated for mean dam rat body weight of 330 g: 0.4 W/kg to mimic working mothers (OcM exposure, n = 8) and 0.08 W/kg for non-working mothers, i.e., public (PuM exposure, n = 8). Wang et al. [22] highlight the need to assess long duration of exposure in toxicological studies to observe the impact on growth as for RF-EMF during the perinatal period.
The descendants’ body weights and lengths were examined from birth until adolescence, on pups, sex and stillbirth ratio, and on age at pinna ear detachment, incisor eruption, and eye opening.
2. Methods
2.1. Animals
Twenty-five pregnant female rats (Sprague Dawley, 10–12 weeks old) were purchased from Janvier (France). The protocols complied with the decree on vertebrate animal experiments (French State Council, 1987) and were approved by the Regional Ethical Committee N. 96 (CREMEAP) and the French Ministry of Research. The rats were received at the vivarium at GD 3. Before the start of the experiment, the rats acclimatized for 5 days in the exposure chambers.
2.2. Procedures and Experimental Groups
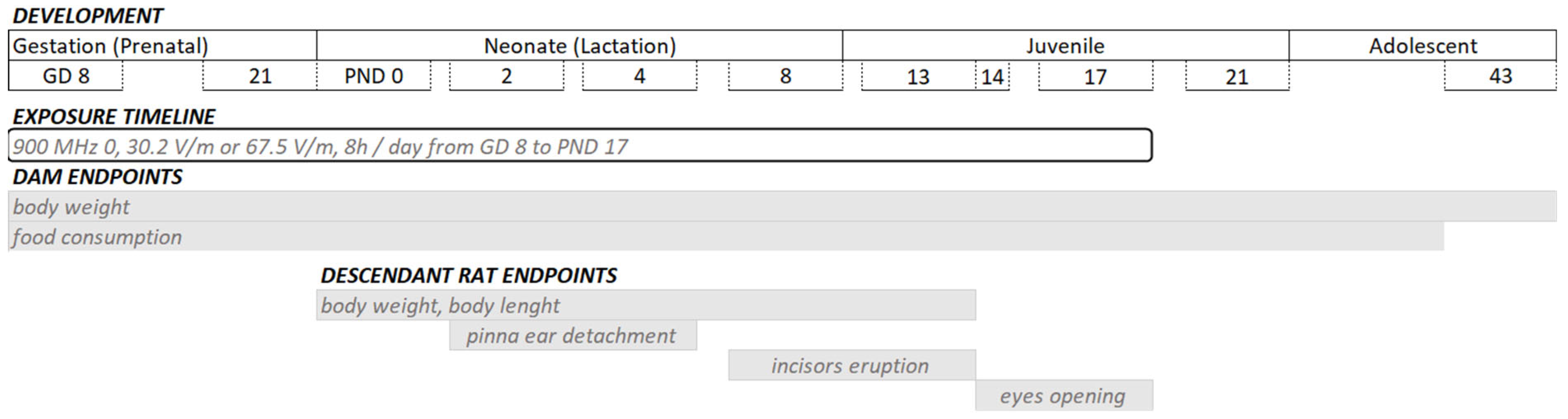
The animals were kept in a 12 h light/dark cycle (light: 300 lux) at 22 °C room temperature with food and tap water ad libitum. The 25 pregnant rats were randomly assigned to the sham-exposed group (control, n = 9), the general public wbSAR for mothers group (PuM; n = 8), or the occupational wbSAR for mothers group (OcM; n = 8). Three exposure chambers were used (dimensions: 1.42 m × 1.795 m × 2.35 m). One chamber was allocated for one exposure level. The pregnant rats were exposed to continuous waves at a frequency of 900 MHz from GD 8 until the weaning of pups at PND 17 (Figure 1). All the rats were exposed from 11:00 a.m. to 7:00 p.m. The pregnant rats were housed individually.

Figure 1.
Experimental design. From gestational day (GD) 8 until postnatal day (PND) 17, the rats were daily exposed to 900 MHz radiofrequency (RF) from 11:00 a.m. to 7:00 p.m. of 0 (sham-exposed group), 30.2 V/m electric field and 0.08 W/kg (PuM: whole-body limits of the general public applied to mother rats), or 67.5 V/m electric field and 0.4 W/kg (OcM: whole-body occupational limits applied to mother rats). The dam’s body weight was assessed from GD 8 until PND 22, and food consumption was assessed from GD 9 until PND 20.
The experimental design was planned for each litter and each sex before birth. The semi-random numbering of pups was performed as previously described [23]. The assigned age of sacrifice for the 1st, 2nd, and 3rd rats of each sex per litter, were, respectively, PND 8, 17, and 43. At birth, the litters were reduced to 3 males and 3 females. Each litter was housed with the mother until PND 20. After weaning, rats from the same litter were housed 4 or 3 per cage per gender and experimental group.
2.3. Exposure and Field Measurements
Details of reverberating chambers are described by Maalouf et al. [24]. Briefly, RF-EMF exposures were performed in 3 reverberation chambers commercialized by IT’IS Foundation (Zurich, Switzerland) [25]. A signal generator (SMIQ02B generator, Rohde and Schwarz Munich, Germany) produced a 900 MHz band RF-EMF (continuous waves). It was coupled with amplifiers (700 MHz–2200 MHz TE UMS 015 AA, Zurich, Switzerland) and with 3 log-periodic antennas manufactured by SPEAG. The antennas were directed to one of the two stirrers (vertical and horizontal) for good RF-EMF signal homogeneity. The exposure to the electromagnetic field (EMF) was monitored with two probes (ER3DV5, SPEAG, Zurich, Switzerland). The rats were free to move in their cages during the 900 MHz RF-EMF exposure from the generator. IT’IS Foundation performed the dosimetry using the finite difference time domain (FDTD) method as previously described [26]. Exposures were off anytime the investigators had to open the exposure apparatus doors to manipulate, weigh, check the rats, and check the system (before 11 a.m. or after 7 p.m.). To determine the SAR in rats at mobile phone frequencies, a simplified 12-plane wave model for the average exposure in a reverberation chamber was used in conjunction with a variety of anatomical rat model within the FDTD solver in SEMCAD X (SPEAG, Switzerland). The measurement indicates a variation of 0.2 dB standard deviation for the E-Field strength.
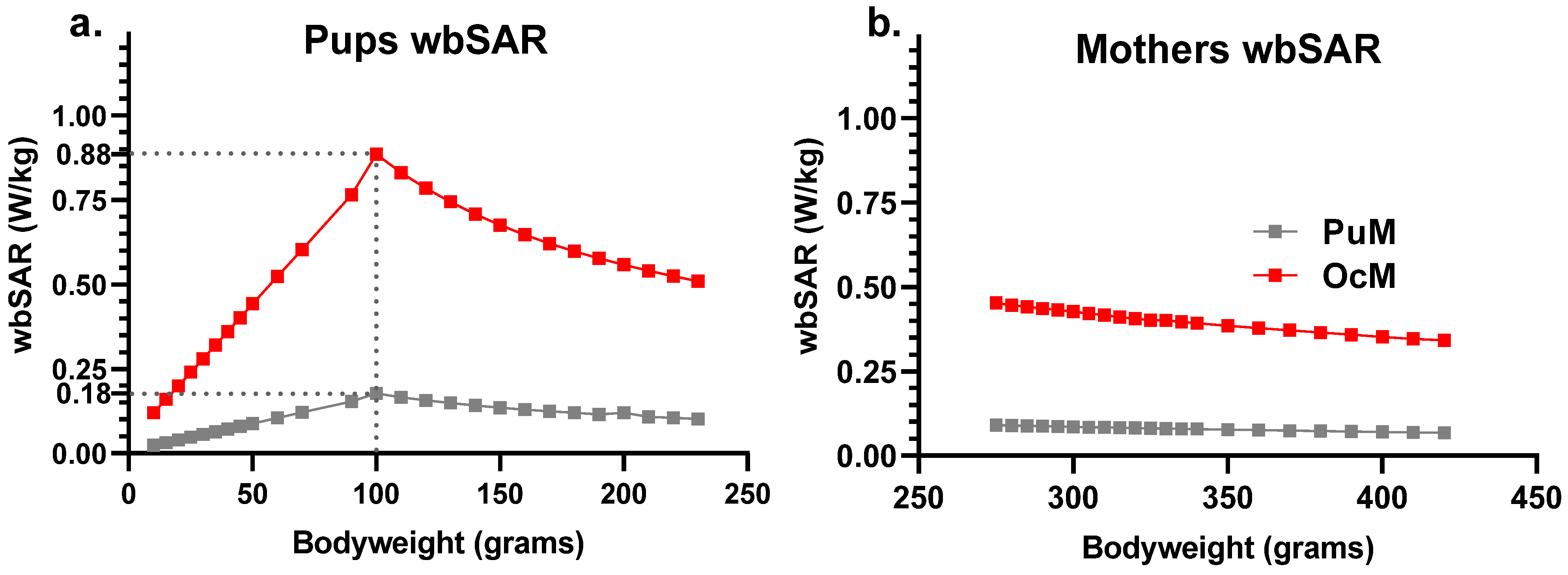
Exposure was set at 30.2 V/m and 67.5 V/m EF to reach 0.08 W/kg (PuM group) and 0.4 W/kg (OcM group) for the wbSAR of a 330 g adult rat. The corresponding postnatal pups wbSAR were deduced from extraction from the exposure software with theoretical weights (Figure 2) or from the approximation formula from Gong et al. [27] with measured weight of pups or mothers (Figure 2 and Figure 3). The wbSAR of a 10 g pup was approximately 0.12 W/kg and 0.024 W/kg when its 330 g mother was exposed at 0.4 W/kg (EF: 67.5 V/m) or 0.08 W/kg (EF: 30.2 V/m), respectively. The wbSAR then grew with the body weight and reached, respectively, 0.4 or 0.08 W/kg when the pups weighed 45 g around the age of 14 days. The wbSAR peak for the pups was reached at PND 27 (weight around 100 g) with approximately 0.90 W/kg and 0.20 W/kg, respectively, for the OcM and PuM groups.
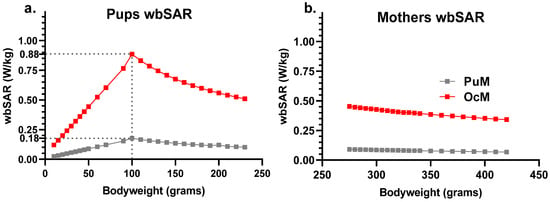
Figure 2.
Estimate of the wbSAR in the pups and mothers showed linear growth before a peak at 100 g body weight (around PND 27) followed by a linear decrease with 0.4 W/kg and 0.08 W/kg attained for 330 g body weight. The wbSAR was calculated for the pups (a) and mothers (b) on a “coupling factor” dependent on the animal’s body weight.
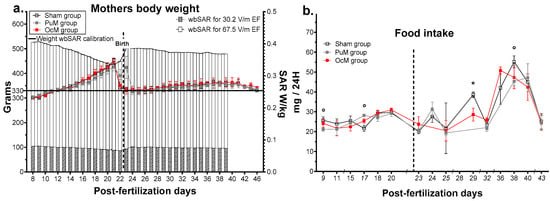
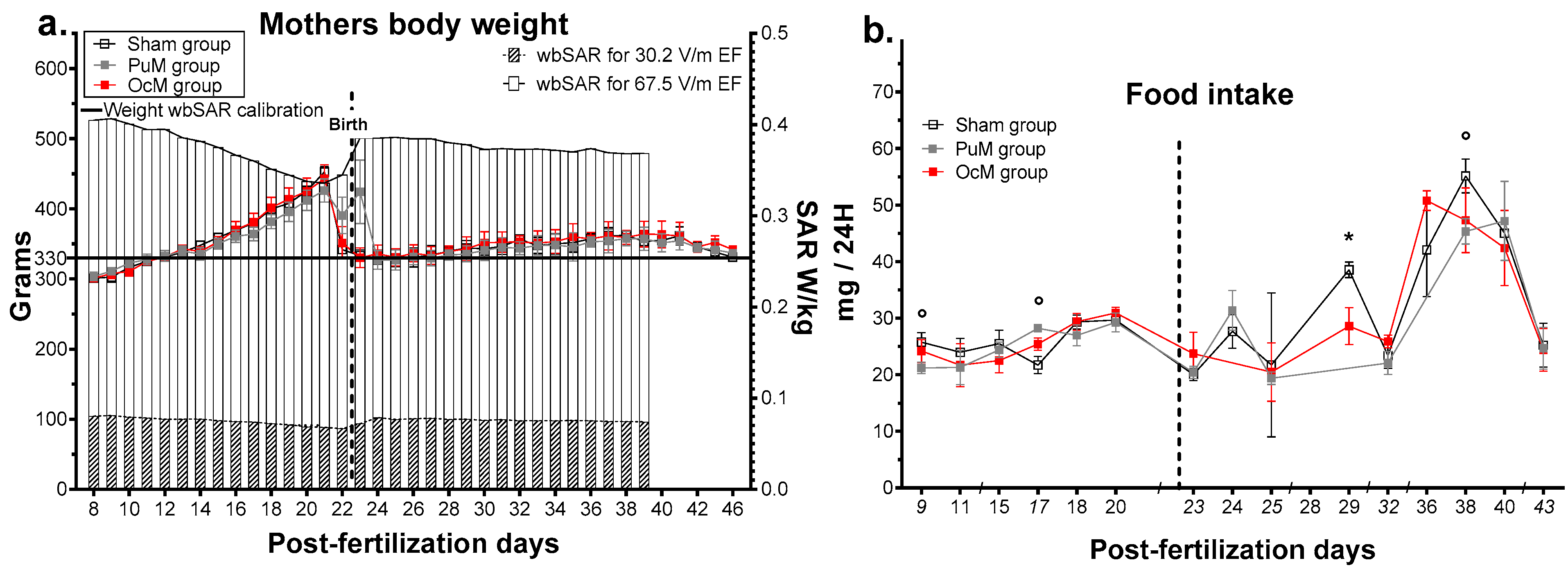
Figure 3.
Effects of pre- and postnatal 900 MHz exposure on the mothers’ body weight (a) and on the litter food intake (b). * p < 0.05 between the OcM and sham group, ° p < 0.05 between the PuM and sham group. n = 8–9/group. wbSAR was calculated numerically with an approximation formula. Left Y axis indicates the weight in grams for the sham, PuM, and OcM groups. Right Y axis indicates the wbSAR evolution in W/kg.
2.4. Physical Development
Every four days from birth, body weight, and length were measured for the pups. The mothers’ weight was measured every day from GD 8 to delivery and then every four days. Each pup was observed every day to determine the age of eye opening, incisor eruption, and pinna ear detachment. The timeline for these markers occurs around PND 2–4 for pinna ear detachment, PND 8–13 for incisor eruption, and PND 14–17 for eye opening.
2.5. Statistical Analysis
Means ± standard error were analyzed using GraphPad Prism (Version 8.0.2, GraphPad Software, Boston, MA, USA) and R Studio (Version 3.6.2, Vienna, Austria). In complete data sets, two-way ANOVA was performed. Time, RF-EMF exposure, and sex were considered between-subject factors. A mixed effects analysis was performed for incomplete data sets (missing mother’s body weight data). We performed Sidak’s or Tukey’s t test post hoc. Permutation tests were performed with the R package ‘statmod’ (version 1.5.0, [28]) with the function “compareGrowthCurves” for the weight and size of the pups (set with 1 × 106 random permutations) with complementary multiple t test analyses. Significant effects were found when p-value < 0.05.
3. Results
3.1. Mothers and Litter Endpoints
The effects of daily exposure at 900 MHz on the mothers and their litters are shown in Figure 3. The weights of the mothers with the corresponding wbSAR are presented in Figure 3a. The mixed-effects analysis indicated no effect of 900 MHz exposure on the mothers’ weight (Table 1), but an effect of interaction between age and exposure was observed (F72,599 = 2.514, p-value < 0.0001, Table 1). Multiple comparisons confirmed no effect of exposures for all the ages but showed an effect of age due to the increase in weight during gestation. The mixed-effects analysis indicated a significant difference in estimated SAR over time (F36,397 = 20.35, p-value < 0.0001). The variation in the mean SAR was calculated between 0.067 W/kg and 0.081 W/kg (max standard deviation: 0.005) for the PuM group and between 0.336 W/kg and 0.407 W/kg (max standard deviation: 0.028) for the OcM group.

Table 1.
Results of the analysis of variance for physical development and SAR exposure.
The mother’s food intake per cage is presented in Figure 3b. The multiple t test indicated that maternal food intake was impacted punctually by 900 MHz exposure. For the OcM group, the mothers ate less at 29 days postfertilization (t-ratio = 2.559, p-value = 0.037). For the PuM group, the mothers ate less at 9 days (t-ratio = 2.408, p-value = 0.047), more at 17 days (t-ratio = 3.596, p-value = 0.0088), and less at 38 days postfertilization (t-ratio = 2.623, p-value = 0.039).
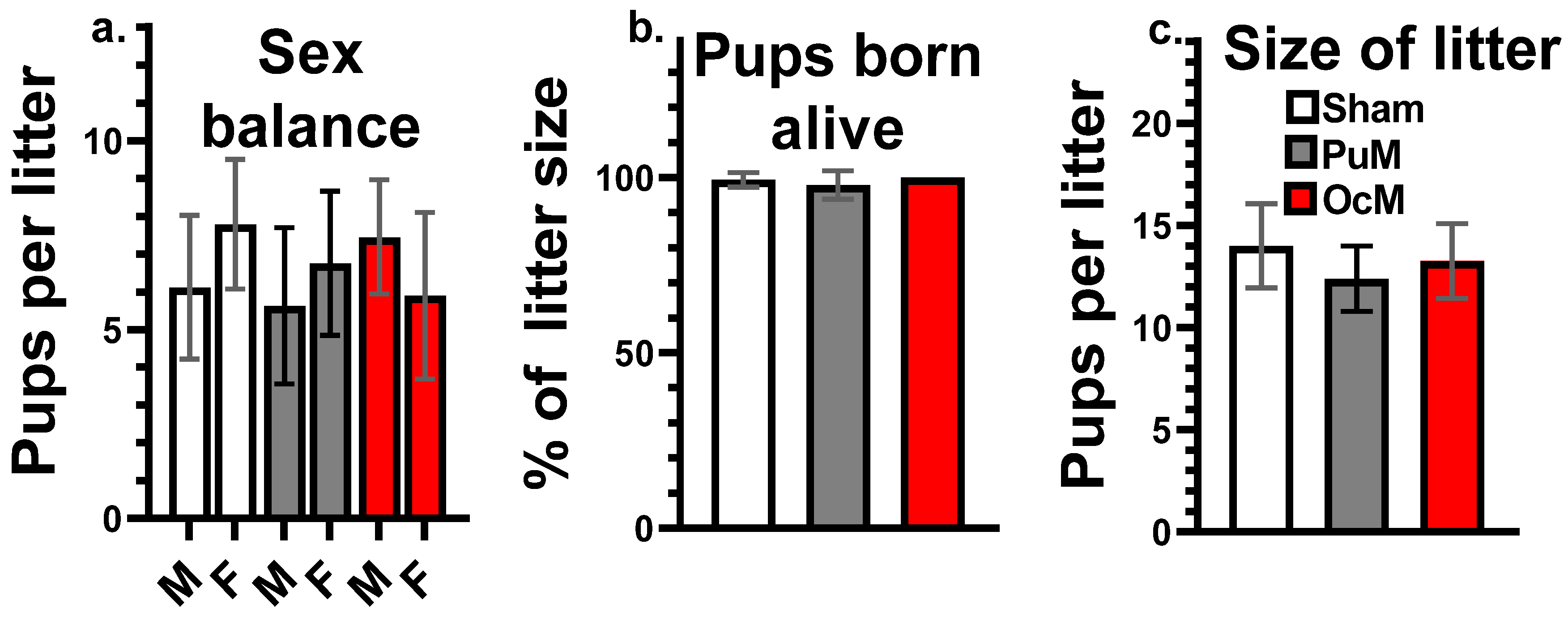
No effect of exposures was shown at PND 0 on the sex balance of the litters presented in Figure 4a, the percent of the pups born alive presented in Figure 4b, or the size of the litters presented in Figure 4c, (Table 1).
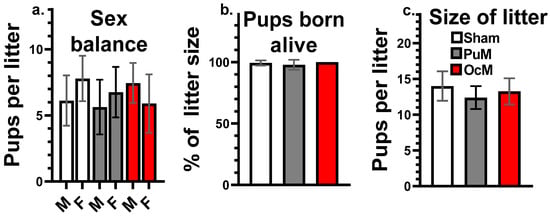
Figure 4.
No effects of exposures on litter parameters at birth. Proportion of males and females per litters (a), death pups at birth (percentage of pups born alive) (b), and total number of pups per litter (litter size) (c). n = 8–9/group.
3.2. Pups’ Physical Development
The effects of 900 MHz exposure on the physical development of pups (weight and size from PND 2 to PND 43) are presented in Figure 5.
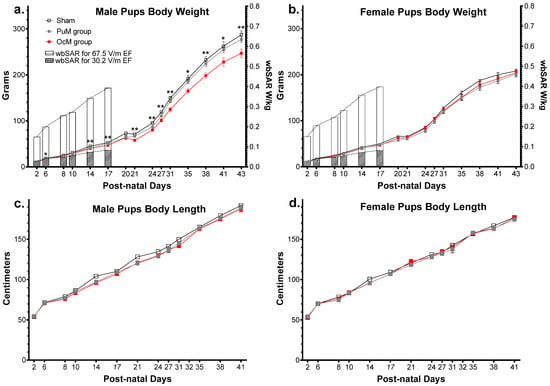
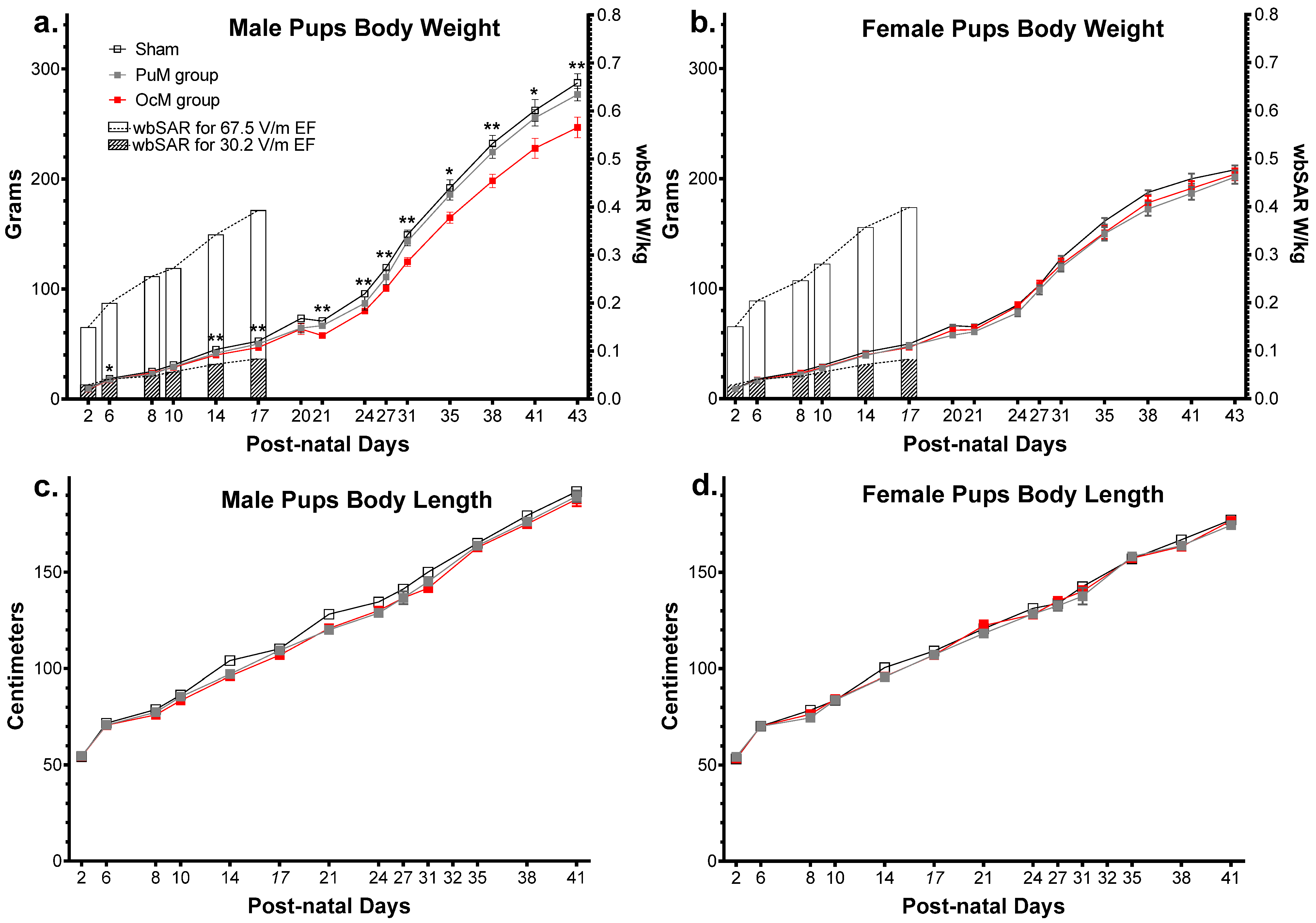
Figure 5.
Effect of perinatal exposure to 900 MHz on pup weight. There was a significant difference between the exposed and sham groups in male pup body weight from PND 6 to PND 43 (a). There was no difference between the groups regarding the body weight in females (b) and the body length in males (c) and females (d). n = 8–9/group. * p < 0.05; ** p < 0.01. SAR was calculated numerically with an approximation formula.
The weights of the male pups are shown in Figure 5a. The results of the 1 × 106 random permutation tests suggested that there was a significant decrease in weight between the OcM group and sham group (adjusted p-value = 0.0025, Table 1). This difference was refined using a complementary multiple t test analysis showing a significant decrease at PND 6 (p-value = 0.007) and until PND 43 (p-value = 0.006). There was no difference between the PuM group and sham group (Table 1) but a decrease between the PuM and OcM groups (p-value = 0.0125). Weight increased with age in all the groups (p-value < 0.0001).
The weights of the female pups are presented in Figure 5b. No difference was found between the sham and exposed groups for the female pups’ weight (Table 1). Weight increased with age in all the groups (p-value < 0.0001). The body length of the male pups is presented in Figure 5c. No difference was found between the groups for male pup length (Table 1). The body length of the female pups is presented in Figure 5d. No difference was found between the groups for female pup length (Table 1). Length for both male and female pups increased with age in all the groups (p-value < 0.0001).
The result of the three-way ANOVA on mean wbSAR variation showed no significant effect for sex, age/sex interaction, sex/treatment interaction, or age/sex/treatment interaction. The statistical test showed a significant effect of age (F2.020,46.16 = 449.4, p-value < 0.001, Table 1) between the treatments (F1,28 = 1176, p-value < 0.0001, Table 1) and interaction between age and treatment (F7,160 = 190.2, p-value < 0.0001, Table 1). The variation in the mean SAR was calculated to be between 0.03 W/kg and 0.103 W/kg (max standard deviation: 0.004) for the PuM group (EF: 30.2 V/m) and between 0.150 W/kg and 0.506 W/kg (max standard deviation: 0.018) for the OcM group (EF: 67.5 V/m).
The effects of exposure to 900 MHz radiation on the clinical markers of development, for ear detachment, incisor eruption, and eye opening, are presented in Figure 6.
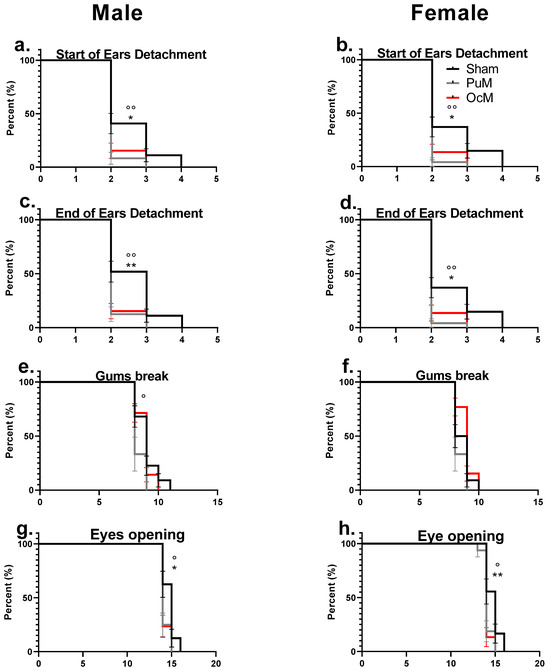
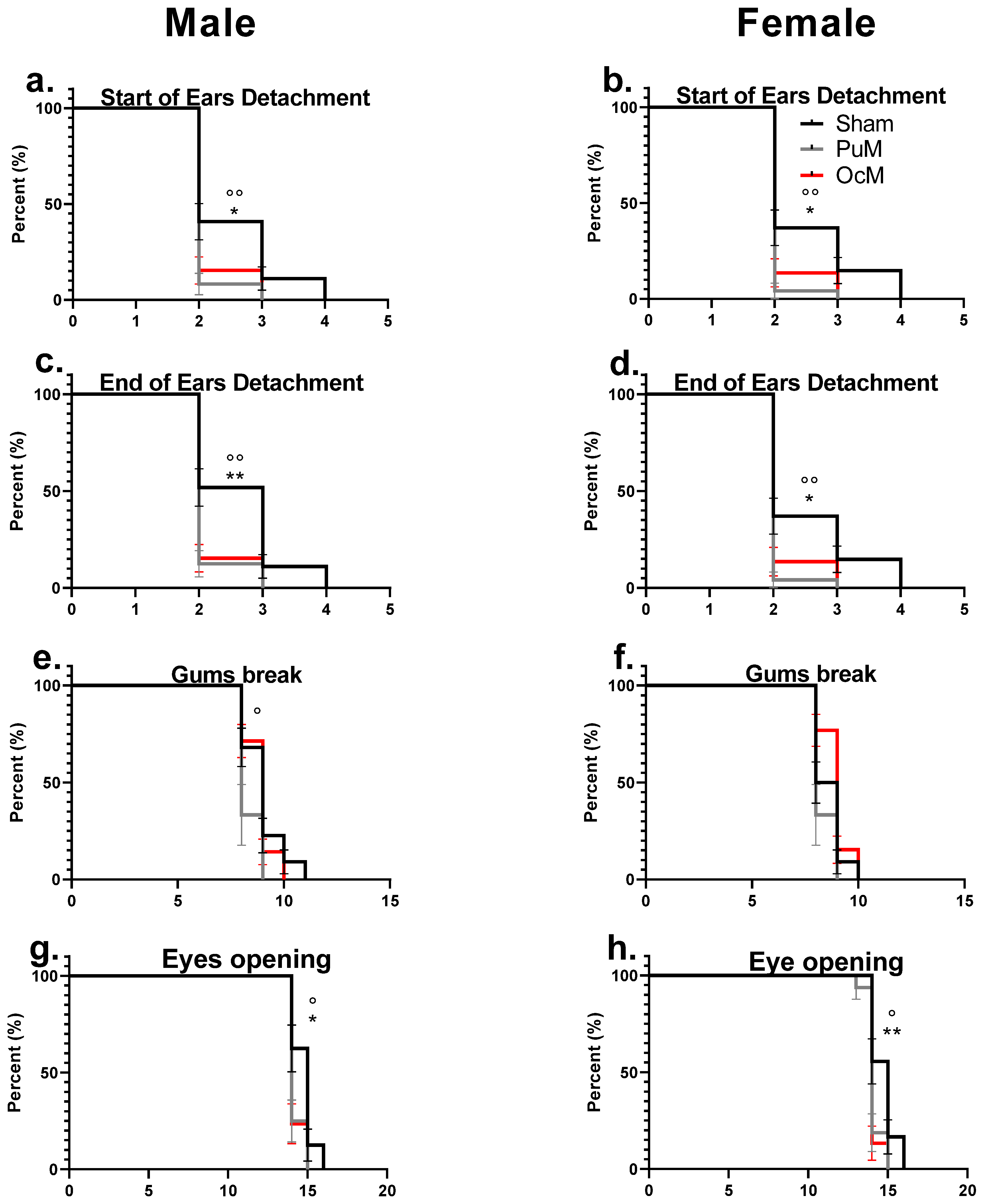
Figure 6.
Precocious phenotype exhibited by developmental landmarks. There was a significant difference between the exposed groups and the sham-exposed group regarding the start of ear detachment for males (a) and females (b) (p < 0.05). The end of pinna ear detachment was significantly early for the exposed groups in males (c) and in females (d) compared to the sham group. Incisor eruption (gum break) was significantly early in the PuM group compared to sham in males (e), but equivalent between sham and exposed in females (f). Eye opening was significantly early in both exposed groups in males (g) and in both SAR groups for females (h). n = 8–9/group. *: sham vs 0.4 W/kg p < 0.05, ** p< 0.01. °: sham vs. 0.08 W/kg p < 0.05, °° p < 0.01.
Figure 6a,b represent the age of the start of ear detachment for males and females, respectively. The log-rank test indicated a significant effect of 900 MHz exposure in the PuM groups with earlier ear detachment for the female pups (Chi square = 8.504, p-value = 0.0035, Table 2) and for the male pups (Chi square = 7.524, p-value = 0.0061, Table 2) compared to the sham group. For the OcM group compared to the sham group, the start of ear detachment occurred earlier for both the male pups (Chi square = 5.334, p-value = 0.0209, Table 2) and female pups (Chi square = 4.801, p-value = 0.0284, Table 2).

Table 2.
Results of the analysis of variance for clinical markers in the pups.
Figure 6c,d represent the age of the end of ear detachment for males and females, respectively. The log-rank test indicated a significant effect of 900 MHz exposure for the PuM group for the female pups (Chi square = 8.504, p-value = 0.0035, Table 2) and for the male pups compared to the sham group (Chi square = 9.374, p-value = 0.0022, Table 2). For the OcM group compared to the sham group, the end of ear detachment occurred earlier for the female pups (Chi square = 4.801, p-value = 0.0284, Table 2) and for the male pups (Chi square = 8.669, p-value = 0.0032, Table 2).
Figure 6e,f represent the age of incisor eruption for males and females, respectively. No effect of exposures with the log-rank test (Table 2) on the age of incisor eruption for the female pups in both groups (PuM and OcM) and for the male pups in the OcM group were observed. A significant effect of exposure compared to the sham group was shown only for the male pups in the PuM group (Chi square = 4.377, p-value = 0.0364, Table 2).
Figure 6g,h represent the age of eye opening occurrence for males and females, respectively. The log-rank test indicated an effect of 900 MHz exposure in the PuM group in both the female (Chi square = 6.303, p-value = 0.0121, Table 2) pups and males (Chi square = 5.295, p-value = 0.0214, Table 2) compared to the sham group. An effect of exposure was also observed for the female pups (Chi square = 6.819, p-value = 0.009, Table 2) and for the male pups in the OcM group (Chi square = 5.836, p-value = 0.0157, Table 2) compared to the sham group.
No sex effect was observed for ear detachment, incisor eruption, and eye opening in all the groups.
4. Discussion
The present study examined clinical endpoints after the perinatal exposure of rats to a 900 MHz signal at the general public and occupational reglementary thresholds (ICNIRP, respectively, 0.08 and 0.4 W/kg). Here, the male rats showed lower body weight from PND 6 to PND 43 (−15%) in the OcM group. The female pups showed an earlier start of ear detachment and eye opening in both the exposed groups. Only males showed earlier ear detachment in the OcM group and earlier eye opening in the PuM group.
According to our exposure system setup, and to some models in the literature, we presented an estimate of the exposure of the litters from conception until withdrawal. In our estimate based on previous publication [23], offspring absorbed less energy than their mothers from the 2nd PND (−55 to −60%) until PND 14, while they absorbed more energy than their mother from PND 14 until PND 17 (+30 to +40%).
The current results showed no deleterious effect of perinatal exposure to 900 MHz signal radiation on gestation delivery outcomes (size of litters, sex ratio, or number of stillbirths) and on the weight and length of the pups at birth. These findings agree with the results we found in a previous study at a similar level of wbSAR (0.07 W/kg) but at the 5G frequency of 3.5 GHz [23].
Interestingly, in the OcM-exposed group, a significant decrease in body mass was observed in males from PND 6 until PND 43. Our results are in accordance with those of Sangun et al. [29], who reported that prenatal 2450 MHz exposure at 0.1 W/kg wbSAR in mothers resulted in a reduction (−30%) of rat growth after birth. GSM exposed fetus rats at low SAR (0.048 W/kg) starting from GD 0 showed impacts on pre- and postnatal development including a decrease in body mass at GD 20 ([30]. Reduction in weight effect of RF-EMF exposure on male pups has also been shown by previous studies. Male pups, when paternal mice were exposed for 10 weeks to a 2000 MHz signal prior to mating and mothers during the gestation period, exhibited significantly lower weight during the adolescence period (4–12 weeks). This study also found a disturbance in glucose metabolism [31]. On the opposite, higher weight gain (+10%) was shown in juvenile male rats exposed for 5 weeks (PND 8 to PND 43), 23 hours per day, at 900 MHz at 0.03 W/kg SAR [13]. Similar results in other studies indicate that prenatal exposure at equivalent wbSAR (0.1 to 0.4 W/kg) but at different frequencies (900 MHz, 2000 MHz, or 2450 MHz) induces decreased weight in male pups. However, exposure at the later stages of development showed higher weight gain in juvenile male rats.
Recently, Maalouf et al. [24] showed that the exposure of adult male mice to 900 MHz at 0.4 W/kg or 0.1 W/kg induced changes in the transcription of genes involved in metabolism and thermoregulation in males. Differences in glucose metabolism between male and female rats have been observed previously [32]. Also, female rats showed a delay in weight gain and less metabolic complications than males when exposed to a high-fat diet [33].
The body length of male pups was lower but not significantly with the permutation test for the OcM (p-value = 0.0557) and PuM (p-value = 0.0557) groups. Also, the daily food intake of the litters was sporadically decreased at GD 9, increased at GD 17, and decreased at PND 15 for the PuM group and decreased at PND 6 for the OcM group. Food intake follow-up (grams per hour) showed an increase when juvenile rats were housed in a room at an ambient temperature of 31 °C and exposed for 5 weeks to 900 MHz RF-EMF at 1 V/m [34]. This increased food intake could be the result of compensatory behavior stimulated by higher energy expenditure after RF-EMF exposure.
Our study is the first to show an effect of 900 MHz on precocious development. Data indicated earlier eye opening and pinna ear separation in males, respectively, at OcM or PuM and female pups at both wbSAR after pre- and postnatal exposure. The incisor eruption occurred earlier only in the PuM male group. The ear and eye endpoints were not affected in response to a WIFI signal (2400 MHz) and 1800 MHz at 1 mW/cm2 exposure for 21 days of pregnancy [35] but incisor eruption was delayed in response to 3500 MHz exposure at a wbSAR of 0.07 W/kg [24]. However, premature eye opening was shown in rat new-borns by Cai et al. [36] in both males and females when exposed to tributylin, a biocide used in the industry, with an increase in serotonin and dopamine levels at PND 57 in males. Neurotransmitter alterations were also observed in males in the F1 to F3 generation when only ancestors were exposed to tributylin (F0). After pharmacological treatment with methamphetamine in pregnant rats (inducing increased dopamine released in the synaptic cleft), a delay in eye opening, ear detachment, as well as incisor was observed in the offspring [37].
The physical development effects found in our study were also shown when toxicants impacted serotonin. Abu-Taweel [38] reported a delay in eye opening in mice treated with mercuric chloride from GD 1 to PND 15 with decreased levels of both serotonin and dopamine observed in the forebrain (from PND 7 to PND 36). The neurotoxic effect is shown to be induced by increased oxidative stress and the downregulation of Na+/K(+)-ATPase [39]. Interestingly, the same animals showed decreased body weight. Premature eye opening was also reported in pups injected with fluvoxamine (a selective serotonin reuptake inhibitor) from PND 1 to PND 14 with reduced weight from PND 1 to PND 16 [40]. The association between RF-EMF exposure and increased oxidative stress is one of the most-studied effects following exposure to further elucidate the mechanisms of action [41]. In rats, increased oxidative stress has been shown following 850 MHz or 900 MHz exposure in the testis [42,43], and in the brain [44]. The increased oxidative stress in the brain has also been associated with changes in serotonin levels [45], which may be of interest to further investigate this pathway after RF-EMF exposure.
Our exposure for the male pups in the OcM group resulted in earlier ear detachment and eye opening with reduced body weight. These data may suggest a metabolic disturbance with higher energy expenditure. One possible mechanism for lower weight gain may be the activation of brown adipose tissue, increased energy consumption, and non-shivering thermogenesis [46]. It would be worth following the rats over a longer life period to assess adult body weight and possible food intake with behavior or learning alterations. General public exposure is much weaker than the ICNIRP exposure limits evaluated in the present study [47]. Here, our goal was not to mimic environmental exposure but to question the reglementary threshold set to prevent the impact of RF-EMF on health.
5. Conclusions
Our pre- and postnatal study was the first to compare public and occupational exposure limits on rat pup physical development. The data suggested more physical development disturbances after the occupational limit exposure than after the public limit exposure. The public and occupational limit-exposed groups showed precocious development in male and female pups. In addition, the occupational limit-exposed male pups had a decreased body weight until adolescence. Our data support the hypothesis of the possible impact of RF-EMF exposure at occupational limits on developmental biology in rats.
Author Contributions
R.B. and A.-S.V. contributed to the study conception and design. Material preparation, data collection, and analysis were performed by R.B., F.R., A.L., S.R. and A.-S.V. The first draft of the manuscript was written by R.B.; A.-S.V. commented on the previous versions of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was funded by the French Ministry of Ecology Program 190.
Institutional Review Board Statement
The protocols complied with the decree on vertebrate animal experiments (French State Council, 1987) and were approved by the Regional Ethical Committee N. 96 (CREMEAP) and the French Ministry of Research (APAFIS#19003, 26/08/2019).
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflicts of interest.
List of Abbreviations
| 2G | 2nd generation |
| CREMEAP | Regional Ethical Committee |
| DoHaD | Developmental Origins of Health and Disease |
| EF | Electric field |
| EMF | Electromagnetic field |
| FDTD | Finite difference time domain |
| GD | Gestational day |
| GSM | Global System for Mobile |
| ICNIRP | International Commission on Non-Ionizing Radiation Protection |
| Oc | Occupational |
| OcM | Occupational whole-body specific absorption rate for mothers |
| Pu | Public |
| PuM | General public whole-body specific absorption rate for mothers |
| PND | Postnatal day |
| RF | Radiofrequency |
| RF-EMF | Radiofrequency electromagnetic field |
| SAR | Specific absorption rate |
| wbSAR | whole-body specific absorption rate |
References
- van Wel, L.; Liorni, I.; Huss, A.; Thielens, A.; Wiart, J.; Joseph, W.; Röösli, M.; Foerster, M.; Massardier-Pilonchery, A.; Capstick, M.; et al. Radio-frequency electromagnetic field exposure and contribution of sources in the general population: An organ-specific integrative exposure assessment. J. Expo. Sci. Environ. Epidemiol. 2021, 31, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Guidelines for Limiting Exposure to Electromagnetic Fields (100 kHz to 300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar] [CrossRef] [PubMed]
- Nerminathan, A.; Harrison, A.; Phelps, M.; Alexander, S.; Scott, K.M. Doctors’ use of mobile devices in the clinical setting: A mixed methods study. Intern. Med. J. 2017, 47, 291–298, Erratum in Intern. Med. J. 2017, 47, 978. [Google Scholar] [CrossRef] [PubMed]
- Fleming, T.P.; Velazquez, M.A.; Eckert, J.J. Embryos, DOHaD and David Barker. J. Dev. Orig. Health Dis. 2015, 6, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Fernández, C.; de Salles, A.A.; Sears, M.E.; Morris, R.D.; Davis, D.L. Absorption of wireless radiation in the child versus adult brain and eye from cell phone conversation or virtual reality. Environ. Res. 2018, 167, 694–699. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C. The Dielectric Properties of Biological Materials. In Radiofrequency Radiation Standards; Klauenberg, B.J., Grandolfo, M., Erwin, D.N., Eds.; NATO ASI Series; Springer: Boston, MA, USA, 1995; Volume 274. [Google Scholar] [CrossRef]
- Kodera, S.; Hirata, A. Comparison of Thermal Response for RF Exposure in Human and Rat Models. Int. J. Environ. Res. Public. Health 2018, 15, 2320. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Karadayi, A.; Sarsmaz, H.; Çigel, A.; Engiz, B.; Ünal, N.; Ürkmez, S.; Gürgen, S. Does Microwave Exposure at Different Doses in the Pre/Postnatal Period Affect Growing Rat Bone Development? Physiol. Res. 2024, 73, 157–172. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Keleş, A.İ. Morphological changes in the vertebrae and central canal of rat pups born after exposure to the electromagnetic field of pregnant rats. Acta Histochem. 2020, 122, 151652. [Google Scholar] [CrossRef] [PubMed]
- Wyde, M.; Cesta, M.; Blystone, C.; Elmore, S.; Foster, P.; Hooth, M.; Kissling, G.; Malarkey, D.; Sills, R.; Stout, M.; et al. Report of Partial Findings from the National Toxicology Program Carcinogenesis Studies of Cell Phone Radiofrequency Radiation in Hsd: Sprague Dawley® SD Rats (Whole Body Exposures). bioRxiv 2016, 055699. [Google Scholar] [CrossRef]
- Cordelli, E.; Ardoino, L.; Benassi, B.; Consales, C.; Eleuteri, P.; Marino, C.; Sciortino, M.; Villani, P.; Brinkworth, M.H.; Chen, G.; et al. Effects of Radiofrequency Electromagnetic Field (RF-EMF) exposure on pregnancy and birth outcomes: A systematic review of experimental studies on non-human mammals. Environ. Int. 2023, 180, 108178. [Google Scholar] [CrossRef] [PubMed]
- Daşdağ, S.; Akdağ, M.Z.; Ayyıldız, O.; Demirtaş, C.; Yayla, M.; Sert, C. Do Cellular Phones Alter Blood Parameters and Birth Weight of Rats? Electro Magnetobiol. 2000, 19, 107–113. [Google Scholar] [CrossRef]
- Kumlin, T.; Iivonen, H.; Miettinen, P.; Juvonen, A.; van Groen, T.; Puranen, L.; Pitkäaho, R.; Juutilainen, J.; Tanila, H. Mobile phone radiation and the developing brain: Behavioral and morphological effects in juvenile rats. Radiat. Res. 2007, 168, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Bosquillon de Jenlis, A.; Del Vecchio, F.; Delanaud, S.; Bach, V.; Pelletier, A. Effects of coexposure to 900 MHz radiofrequency electromagnetic fields and high-level noise on sleep, weight, and food intake parameters in juvenile rats. Environ. Pollut. 2020, 256, 113461. [Google Scholar] [CrossRef] [PubMed]
- Klose, M.; Grote, K.; Spathmann, O.; Streckert, J.; Clemens, M.; Hansen, V.W.; Lerchl, A. Effects of early-onset radiofrequency electromagnetic field exposure (GSM 900 MHz) on behavior and memory in rats. Radiat. Res. 2014, 182, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Odaci, E.; Bas, O.; Kaplan, S. Effects of prenatal exposure to a 900 MHz electromagnetic field on the dentate gyrus of rats: A stereological and histopathological study. Brain Res. 2008, 1238, 224–229. [Google Scholar] [CrossRef] [PubMed]
- Haghani, M.; Shabani, M.; Moazzami, K. Maternal mobile phone exposure adversely affects the electrophysiological properties of Purkinje neurons in rat offspring. Neuroscience 2013, 250, 588–598. [Google Scholar] [CrossRef] [PubMed]
- Odacı, E.; Özyılmaz, C. Exposure to a 900 MHz electromagnetic field for 1 hour a day over 30 days does change the histopathology and biochemistry of the rat testis. Int. J. Radiat. Biol. 2015, 91, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Gur, F.; Keles, A.I.; Erol, H.; Guven, C.; Taskin, E.; Kaya, H.; Gur, H.; Odaci, E.; Halici, M.; Timurkaan, S. The effect of 900-MHz radiofrequency electromagnetic fields during the adolescence on the histological structure of rat testis and its androgen and estrogen receptors localization. Int. J. Radiat. Res. 2021, 19, 135–144. [Google Scholar] [CrossRef]
- Alimohammadi, I.; Ashtarinezhad, A.; Asl, B.M.; Masruri, B.; Moghadasi, N. The effects of radiofrequency radiation on mice fetus weight, length and tissues. Data Brief. 2018, 19, 2189–2194. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Furtado-Filho, O.V.; Borba, J.B.; Dallegrave, A.; Pizzolato, T.M.; Henriques, J.A.P.; Moreira, J.C.F.; Saffi, J. Effect of 950 MHz UHF electromagnetic radiation on biomarkers of oxidative damage, metabolism of UFA and antioxidants in the livers of young rats of different ages. Int. J. Radiat. Biol. 2014, 90, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Berman, E.; Weil, C.; Philips, P.A.; Carter, H.B.; House, D.E. Fetal and Maternal Effects of Continual Exposure of Rats to 970-Mhz Circularly-Polarized Microwaves. Electro Magnetobiology 1992, 11, 43–54. [Google Scholar] [CrossRef]
- Wang, M.; Guckland, A.; Murfitt, R.; Ebeling, M.; Sprenger, D.; Foudoulakis, M.; Koutsaftis, A. Relationship between magnitude of body weight effects and exposure duration in mammalian toxicology studies and implications for ecotoxicological risk assessment. Environ. Sci. Eur. 2019, 31, 38. [Google Scholar] [CrossRef]
- Bodin, R.; Seewooruttun, C.; Corona, A.; Delanaud, S.; Pelletier, A.; Villégier, A.S. Sex-dependent impact of perinatal 5G electromagnetic field exposure in the adolescent rat behavior. Environ. Sci. Pollut. Res. Int. 2023, 30, 113704–113717. [Google Scholar] [CrossRef] [PubMed]
- Maalouf, J.; Pelletier, A.; Corona, A.; Gay-Quéheillard, J.; Bach, V.; de Seze, R.; Selmaoui, B. Dose- and Time-Dependent Effects of Radiofrequency Electromagnetic Field on Adipose Tissue: Implications of Thermoregulation and Mitochondrial Signaling. Int. J. Mol. Sci. 2023, 24, 10628. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Capstick, M.; Kuster, N.; Kuehn, S.; Berdinas-Torres, V.; Gong, Y.; Wilson, P.; Ladbury, J.; Koepke, G.; McCormick, D.L.; Gauger, J.; et al. A Radio Frequency Radiation Exposure System for Rodents based on Reverberation Chambers. IEEE Trans. Electromagn. Compat. 2017, 59, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Capstick, M.; Kuehn, S.; Wilson, P.; Ladbury, J.; Koepke, G.; McCormick, D.L.; Melnick, R.L.; Kuster, N. Life-Time Dosimetric Assessment for Mice and Rats Exposed in Reverberation Chambers of the 2-Year NTP Cancer Bioassay Study on Cell Phone Radiation. IEEE Trans. Electromagn. Compat. 2017, 59, 1798–1808. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Phipson, B.; Smyth, G.K. Permutation p-values should never be zero: Calculating exact p-values when permutations are randomly drawn. Stat. Appl. Genet. Mol. Biol. 2010, 9, 39. [Google Scholar] [CrossRef] [PubMed]
- Sangun, O.; Dundar, B.; Darici, H.; Comlekci, S.; Doguc, D.K.; Celik, S. The effects of long-term exposure to a 2450 MHz electromagnetic field on growth and pubertal development in female Wistar rats. Electromagn Biol Med. 2015, 34, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Alchalabi, A.S.; Aklilu, E.; Aziz, A.R.; Malek, F.; Ronald, S.; Khan, M.A. Different periods of intrauterine exposure to electromagnetic field: Influence on female rats’ fertility, prenatal and postnatal development. Asian Pac. J. Reprod. 2016, 5, 14–23. [Google Scholar] [CrossRef]
- Yan, S.; Ju, Y.; Dong, J.; Lei, H.; Wang, J.; Xu, Q.; Ma, Y.; Wang, J.; Wang, X. Paternal Radiofrequency Electromagnetic Radiation Exposure Causes Sex-Specific Differences in Body Weight Trajectory and Glucose Metabolism in Offspring Mice. Front. Public Health 2022, 10, 872198. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bagheripuor, F.; Ghanbari, M.; Zahediasl, S.; Ghasemi, A. Comparison of the effects of fetal hypothyroidism on glucose tolerance in male and female rat offspring. J. Physiol. Sci. 2015, 65, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Maric, I.; Krieger, J.P.; van der Velden, P.; Börchers, S.; Asker, M.; Vujicic, M.; Wernstedt Asterholm, I.; Skibicka, K.P. Sex and Species Differences in the Development of Diet-Induced Obesity and Metabolic Disturbances in Rodents. Front. Nutr. 2022, 9, 828522. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pelletier, A.; Delanaud, S.; Décima, P.; Thuroczy, G.; de Seze, R.; Cerri, M.; Bach, V.; Libert, J.P.; Loos, N. Effects of chronic exposure to radiofrequency electromagnetic fields on energy balance in developing rats. Environ. Sci. Pollut. Res. Int. 2013, 20, 2735–2746. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.Q.; Zhang, Y.; Wan, Y.M.; Zhou, Q.; Liu, C.; Wu, H.X.; Mu, Y.Z.; He, Y.F.; Rauniyar, R.; Wu, X.N. Testing of behavioral and cognitive development in rats after prenatal exposure to 1800 and 2400 MHz radiofrequency fields. J. Radiat. Res. 2020, 61, 197–206. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cai, H.; Chen, M.; Gao, Y.; Ruan, J.; He, C.; Zuo, Z. Transgenerational Effects and Mechanisms of Tributyltin Exposure on Neurodevelopment in the Male Offspring of Rats. Environ. Sci. Technol. 2023, 57, 10201–10210. [Google Scholar] [CrossRef] [PubMed]
- Rüedi-Bettschen, D.; Platt, D.M. Detrimental effects of self-administered methamphetamine during pregnancy on offspring development in the rat. Drug Alcohol Depend. 2017, 177, 171. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Abu-Taweel, G.M. Neurobehavioral protective properties of curcumin against the mercury chloride treated mice offspring. Saudi J. Biol. Sci. 2019, 26, 736–743. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, C.F.; Hsu, C.J.; Liu, S.H.; Lin-Shiau, S.Y. Neurotoxicological mechanism of methylmercury induced by low-dose and long-term exposure in mice: Oxidative stress and down-regulated Na+/K(+)-ATPase involved. Toxicol. Lett. 2008, 176, 188–197. [Google Scholar] [CrossRef] [PubMed]
- Glazova, N.Y.; Merchieva, S.A.; Volodina, M.A.; Sebentsova, E.A.; Manchenko, D.M.; Kudrun, V.S.; Levitskaya, N.G. Effects of neonatal fluvoxamine administration on the physical development and activity of the serotoninergic system in white rats. Acta Naturae 2014, 6, 98–105. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mumtaz, S.; Rana, J.N.; Choi, E.H.; Han, I. Microwave Radiation and the Brain: Mechanisms, Current Status, and Future Prospects. Int. J. Mol. Sci. 2022, 23, 9288. [Google Scholar] [CrossRef]
- Gorpinchenko, I.; Nikitin, O.; Banyra, O.; Shulyak, A. The influence of direct mobile phone radiation on sperm quality. Cent. Eur. J. Urol. 2014, 67, 65–71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zalata, A.; El-Samanoudy, A.Z.; Shaalan, D.; El-Baiomy, Y.; Mostafa, T. In vitro effect of cell phone radiation on motility, DNA fragmentation and clusterin gene expression in human sperm. Int. J. Fertil. Steril. 2015, 9, 129–136. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alkis, M.E.; Bilgin, H.M.; Akpolat, V.; Dasdag, S.; Yegin, K.; Yavas, M.C.; Akdag, M.Z. Effect of 900-, 1800-, and 2100-MHz radiofrequency radiation on DNA and oxidative stress in brain. Electromagn. Biol. Med. 2019, 38, 32–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, Z.; Gao, Y.; Zhang, C. Effects of fetal microwave radiation exposure on offspring behavior in mice. J. Radiat. Res. 2015, 56, 261–268. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Correia, A.S.; Cardoso, A.; Vale, N. Oxidative Stress in Depression: The Link with the Stress Response, Neuroinflammation, Serotonin, Neurogenesis and Synaptic Plasticity. Antioxidants 2023, 12, 470. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Iakovidis, S.; Apostolidis, C.; Manassas, A.; Samaras, T. Electromagnetic Fields Exposure Assessment in Europe Utilizing Publicly Available Data. Sensors 2022, 22, 8481. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).