Unlocking the Detoxification of Phenanthrene from Water Using Alkali-Activated Slag Mortar
Abstract
:1. Introduction
2. Materials and Methods
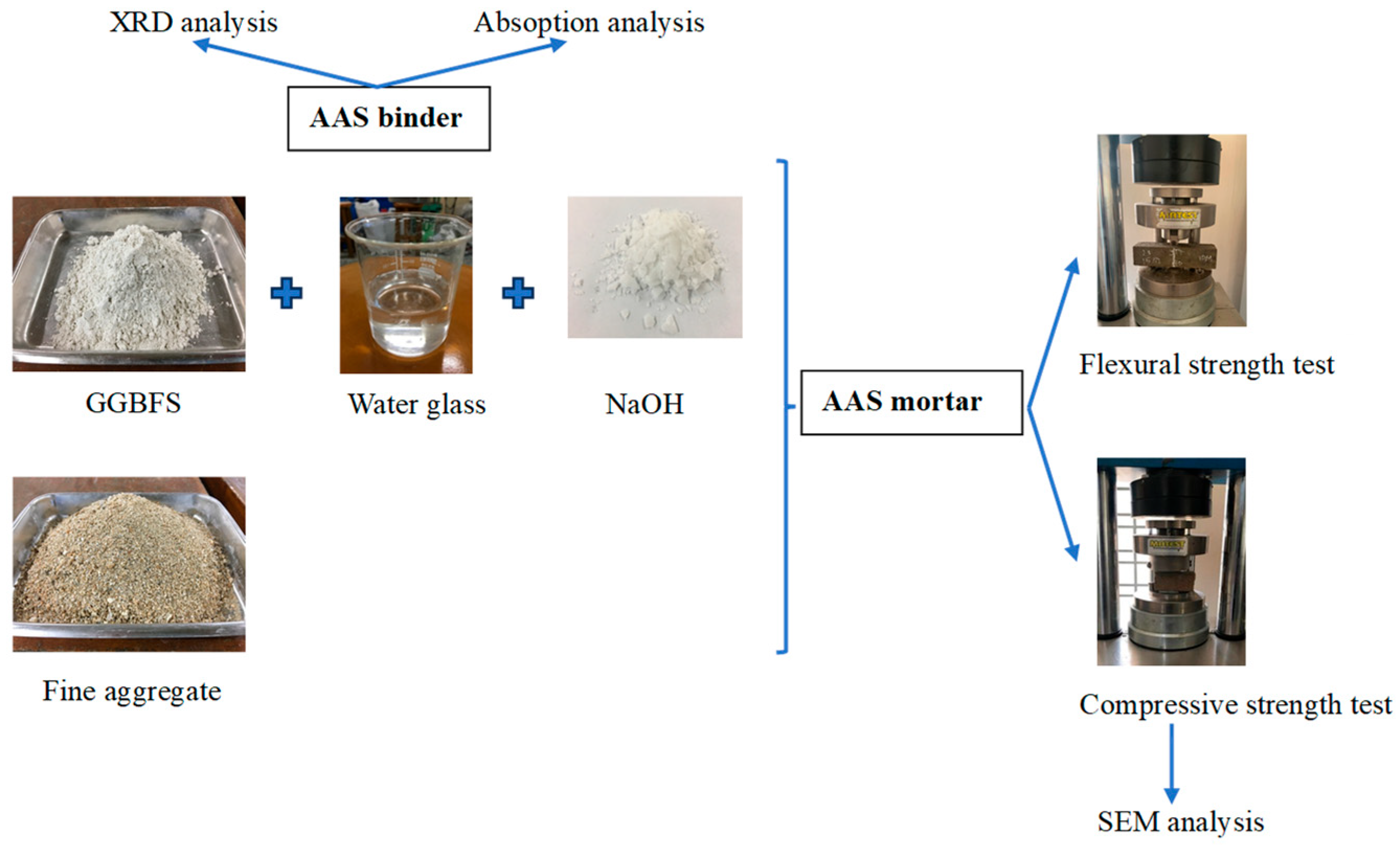
2.1. Materials
2.2. Sample Preparation
2.3. Sorption Experiment
2.4. Sample Testing and Characterization
3. Results and Discussions
3.1. Characterization of Ground Granulated Blast Furnace Slag (GGBFS) and Alkali-Activated Slag (AAS)
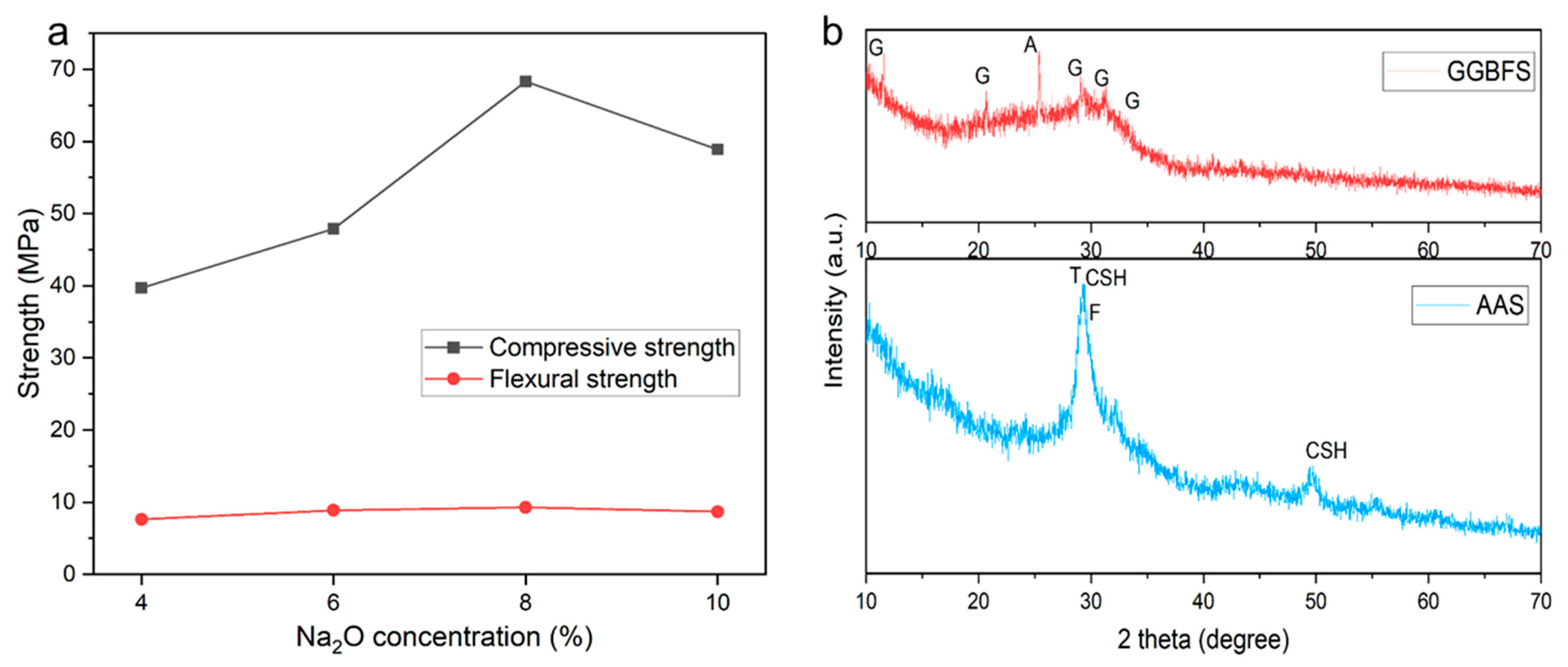
3.1.1. Mechanical Strength
3.1.2. X-ray Diffraction Analysis
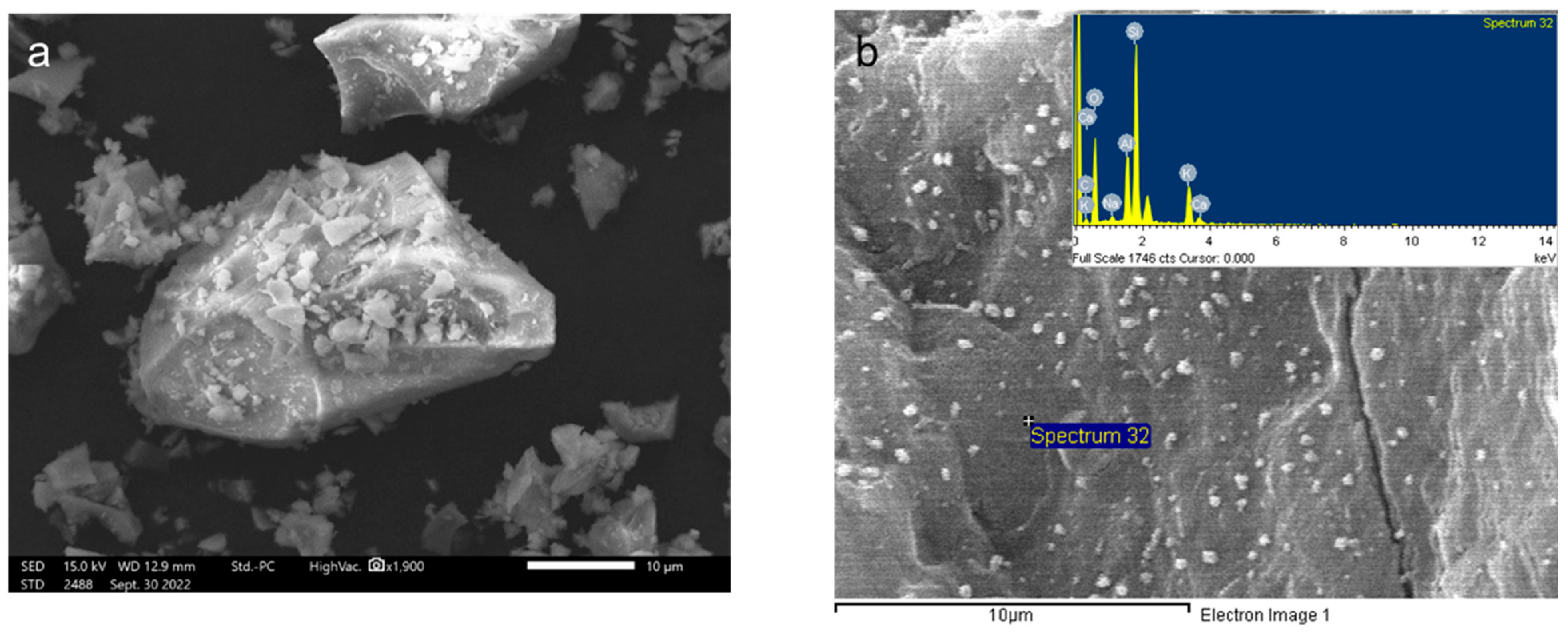
3.1.3. Scanning Electron Microscope Analysis
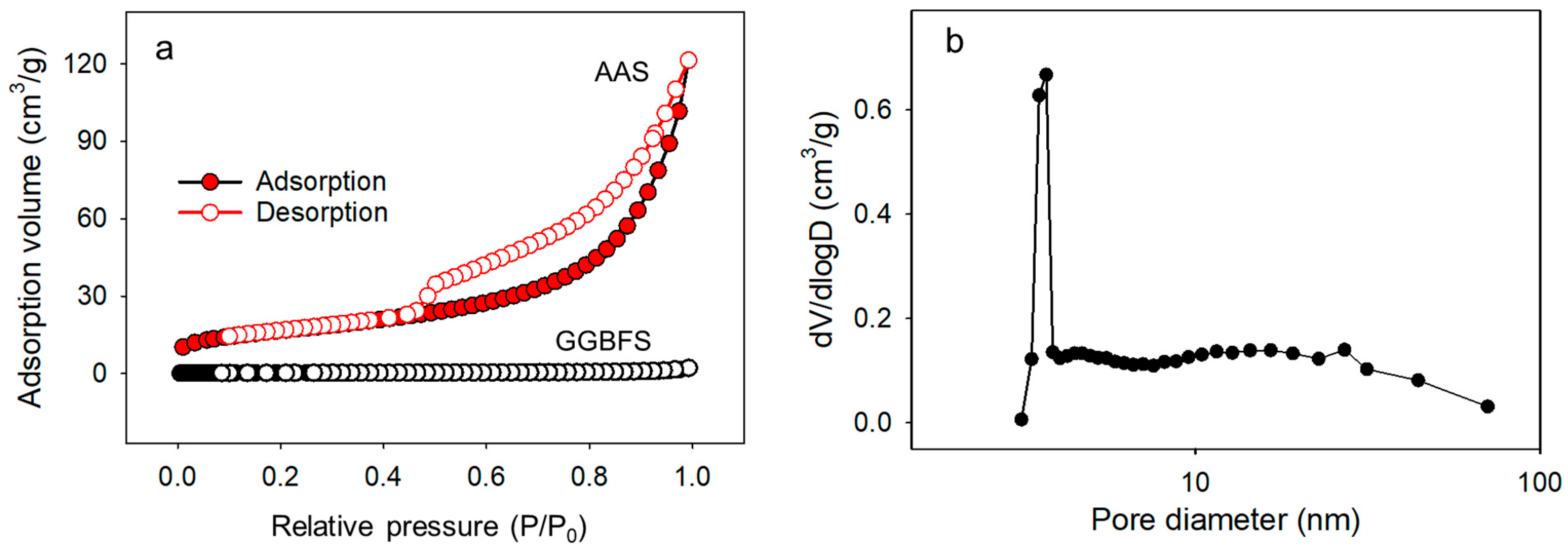
3.1.4. Nitrogen Sorption–Desorption Isotherm
3.2. Phenanthrene Sorption Performance
3.2.1. Sorption Kinetics
3.2.2. Sorption Equilibrium of PHE Using GGBFS and AAS
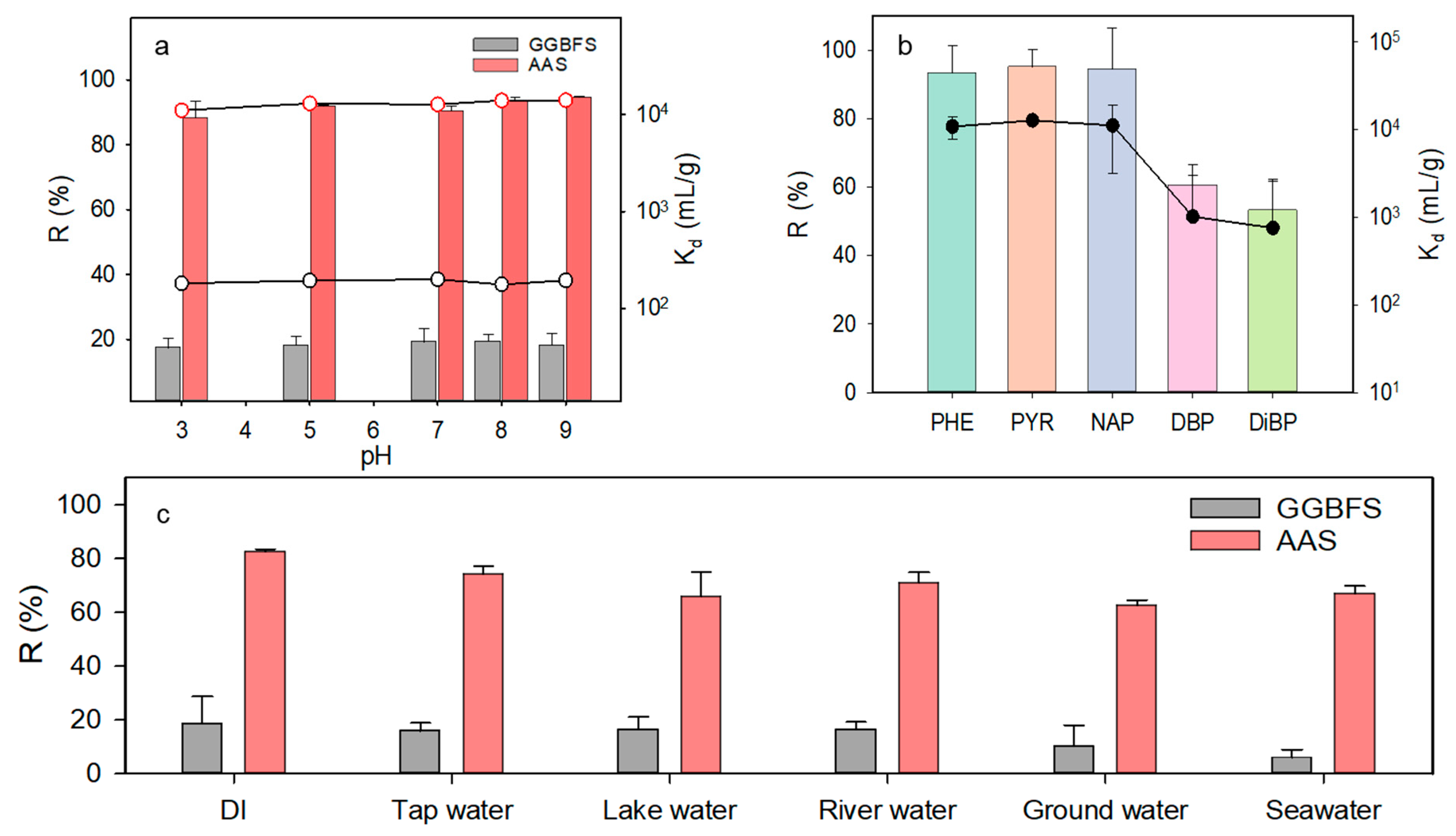
3.2.3. Effect of pH
3.2.4. Effect of Competitive Hydrophobic Organic Carbons (HOCs)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Huseien, G.F.; Sam, A.R.M.; Mirza, J.; Tahir, M.M.; Asaad, M.A.; Ismail, M.; Shah, K.W. Waste ceramic powder incorporated alkali activated mortars exposed to elevated Temperatures: Performance evaluation. Constr. Build. Mater. 2018, 187, 307–317. [Google Scholar] [CrossRef]
- Asaad, M.A.; Sarbini, N.N.; Sulaiman, A.; Ismail, M.; Huseien, G.F.; Majid, Z.A.; Raja, P.B. Improved corrosion resistance of mild steel against acid activation: Impact of novel Elaeis guineensis and silver nanoparticles. J. Ind. Eng. Chem. 2018, 63, 139–148. [Google Scholar] [CrossRef]
- Yang, L.Y.; Jia, Z.J.; Zhang, Y.M.; Dai, J.G. Effects of nano-TiO2 on strength, shrinkage and microstructure of alkali activated slag pastes. Cem. Concr. Compos. 2015, 57, 1–7. [Google Scholar] [CrossRef]
- Rashad, A.M.; Bai, Y.; Basheer, P.A.M.; Milestone, N.B.; Collier, N.C. Hydration and properties of sodium sulfate activated slag. Cem. Concr. Compos. 2013, 37, 20–29. [Google Scholar] [CrossRef]
- Lee, N.K.; Koh, K.T.; An, G.H.; Ryu, G.S. Influence of binder composition on the gel structure in alkali activated fly ash/slag pastes exposed to elevated temperatures. Ceram. Int. 2017, 43, 2471–2480. [Google Scholar] [CrossRef]
- Kong, D.L.Y.; Sanjayan, J.G. Effect of elevated temperatures on geopolymer paste, mortar and concrete. Cem. Concr. Res. 2010, 40, 334–339. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. Calorim. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Kong, D.L.Y.; Sanjayan, J.G.; Sagoe-Crentsil, K. Comparative performance of geopolymers made with metakaolin and fly ash after exposure to elevated temperatures. Cem. Concr. Res. 2007, 37, 1583–1589. [Google Scholar] [CrossRef]
- Aydın, S.; Baradan, B. Effect of activator type and content on properties of alkali-activated slag mortars. Compos. Part B Eng. 2014, 57, 166–172. [Google Scholar] [CrossRef]
- Fernández-Jiménez, A.; Palomo, J.G.; Puertas, F. Alkali-activated slag mortars: Mechanical strength behaviour. Cem. Concr. Res. 1999, 29, 1313–1321. [Google Scholar] [CrossRef]
- Bakharev, T.; Sanjayan, J.G.; Cheng, Y.B. Effect of elevated temperature curing on properties of alkali-activated slag concrete. Cem. Concr. Res. 1999, 29, 1619–1625. [Google Scholar] [CrossRef]
- Wang, S.-D.; Scrivener, K.L. Hydration products of alkali activated slag cement. Cem. Concr. Res. 1995, 25, 561–571. [Google Scholar] [CrossRef]
- Qu, J.; Wang, Y.; Tian, X.; Jiang, Z.; Deng, F.; Tao, Y.; Jiang, Q.; Wang, L.; Zhang, Y. KOH-activated porous biochar with high specific surface area for adsorptive removal of chromium (VI) and naphthalene from water: Affecting factors, mechanisms and reusability exploration. J. Hazard. Mater. 2021, 401, 123292. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Shi, J.; Sun, S.; Wang, J.; Biney, B.W.; Al-shiaani, N.H.; Wang, S.; Guo, A.; Chen, K.; Wang, Z. In-situ decontamination of heavy metal containing wastewater from oil refineries into catalyst for polycyclic aromatic hydrocarbons hydrogenation coupled with water-gas shift reaction. Sep. Purif. Technol. 2023, 307, 122802. [Google Scholar] [CrossRef]
- Carlson, L.M.; Dean, J.; Stanek, J.; Zhao, J.; Kaiser, J.P. Provisional Peer-Reviewed Toxicity Values for 3, 5-Dinitroaniline (CASRN 618-87-1); U.S. Environmental Protection Agency: Cincinnati, OH, USA, 2021.
- Hanafi, M.F.; Sapawe, N. A review on the current techniques and technologies of organic pollutants removal from water/wastewater. Mater. Today Proc. 2020, 31, A158–A165. [Google Scholar] [CrossRef]
- Yang, L.; Qian, X.; Wang, Z.; Li, Y.; Bai, H.; Li, H. Steel slag as low-cost adsorbent for the removal of phenanthrene and naphthalene. Adsorpt. Sci. Technol. 2018, 36, 1160–1177. [Google Scholar] [CrossRef]
- Miroshnichenko, D.; Shmeltser, K.; Kormer, M.; Sahalai, D.; Pyshyev, S.; Kukhar, O.; Korchak, B.; Chervinskyy, T. Influence of Raw Materials and Technological Factors on the Sorption Properties of Blast-Fuel Coke. ChemEngineering 2024, 8, 30. [Google Scholar] [CrossRef]
- Adams, R.G.; Lohmann, R.; Fernandez, L.A.; MacFarlane, J.K.; Gschwend, P.M. Polyethylene devices: Passive samplers for measuring dissolved hydrophobic organic compounds in aquatic environments. Environ. Sci. Technol. 2007, 41, 1317–1323. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, H.; Duan, S.; Liu, X.; Sun, J.; Hayat, T.; Alsaedi, A.; Li, J. A New Application of a Mesoporous Hybrid of Tungsten Oxide and Carbon as an Adsorbent for Elimination of Sr2+ and Co2+ from an Aquatic Environment. ACS Sustain. Chem. Eng. 2018, 6, 2462–2473. [Google Scholar] [CrossRef]
- Do, D.D. Adsorption Analysis: Equilibria and Kinetics; Imperial College Press: London, UK, 1998. [Google Scholar]
- Choi, Y.; Cho, Y.-M.; Gala, W.R.; Luthy, R.G. Measurement and modeling of activated carbon performance for the sequestration of parent-and alkylated-polycyclic aromatic hydrocarbons in petroleum-impacted sediments. Environ. Sci. Technol. 2013, 47, 1024–1032. [Google Scholar] [CrossRef]
- Le, Q.T.N.; Lee, H.H.; Hwang, I. Evaluation of the use of biochar to stabilize polycyclic aromatic hydrocarbons and phthalates in sediment. Environ. Pollut. 2023, 317, 120644. [Google Scholar] [CrossRef] [PubMed]
- ASTM C109/C109M-2013; Standard Test Method for Compressive Strength of Hydraulic Cement Mortars (Using 2-in. or [50-mm] Cube Specimens). ASTM International: West Conshohocken, PA, USA, 2013.
- ASTM C348:2021; Standard Test Method for Flexural Strength of Hydraulic-Cement Mortars. ASTM International: West Conshohocken, PA, USA, 2021.
- U.S. Environmental Protection Agency. Definition and Procedure for the Determination of the Method Detection Limit, Revision 2; Environmental Protection Agency EPA: Washington, DC, USA, 2016.
- Park, S.M.; Jang, J.G.; Lee, N.K.; Lee, H.-K. Physicochemical properties of binder gel in alkali-activated fly ash/slag exposed to high temperatures. Cem. Concr. Res. 2016, 89, 72–79. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Alkali-activated binders: A review: Part; terminology, reaction mechanisms and hydration products. Constr. Build. Mater. 2008, 22, 1305–1314. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Le, Q.T.; Vivas, E.L.; Cho, K. Calcium oxalate/calcium silicate hydrate (Ca-Ox/CSH) from blast furnace slag for the highly efficient removal of Pb2+ and Cd2+ from water. J. Environ. Chem. Eng. 2021, 9, 106287. [Google Scholar] [CrossRef]
- Yuan, G.; Tian, Y.; Liu, J.; Tu, H.; Liao, J.; Yang, J.; Yang, Y.; Wang, D.; Liu, N. Schiff base anchored on metal-organic framework for Co(II) removal from aqueous solution. Chem. Eng. J. 2017, 326, 691–699. [Google Scholar] [CrossRef]
- Shao, N.; Tang, S.; Liu, Z.; Li, L.; Yan, F.; Liu, F.; Li, S.; Zhang, Z. Hierarchically structured calcium silicate hydrate-based nanocomposites derived from steel slag for highly efficient heavy metal removal from wastewater. ACS Sustain. Chem. Eng. 2018, 6, 14926–14935. [Google Scholar] [CrossRef]
- Hundal, L.S.; Thompson, M.L.; Laird, D.A.; Carmo, A.M. Sorption of phenanthrene by reference smectites. Environ. Sci. Technol. 2001, 35, 3456–3461. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Ju, F.; Song, Q.; Pan, H.; Ling, H. A simple empirical model for phenanthrene adsorption on soil clay minerals. J. Hazard. Mater. 2022, 429, 127849. [Google Scholar] [CrossRef]
- Tian, H.; Zhang, Q.; Tian, X.; Xie, Z.-F.; Pu, F.; Wang, Q.-J. Analysis of influencing factors of phenanthrene adsorption by different soils in Guanzhong basin based on response surface method. Sci. Rep. 2022, 12, 20906. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, H.; Qiao, M.; Liu, J.; Zhang, Z.; Li, H. Removal of PAHs from aqueous solutions by adsorption using different types of waste bricks. Int. J. Environ. Sci. Technol. 2023, 20, 8773–8784. [Google Scholar] [CrossRef]
- Huang, D.; Xu, B.; Wu, J.; Brookes, P.C.; Xu, J. Adsorption and desorption of phenanthrene by magnetic graphene nanomaterials from water: Roles of pH, heavy metal ions and natural organic matter. Chem. Eng. J. 2019, 368, 390–399. [Google Scholar] [CrossRef]
- Le, Q.T.N.; Hwang, I. Stabilization of hydrophobic organic compounds in sediment using alkali-activated rice husk biochar: Enhancement of sorption by dissolved organic matter. Chem. Eng. J. 2023, 476, 146513. [Google Scholar] [CrossRef]
- Patnaik, P.; Yang, M.; Powers, E.J.W.R. Kinetics of phthalate reactions with ammonium hydroxide in aqueous matrix. Water Res. 2001, 35, 1587–1591. [Google Scholar] [CrossRef]
- Chen, Q.; Zheng, J.; Yang, Q.; Dang, Z.; Zhang, L. Effect of carbon chain structure on the phthalic acid esters (PAEs) adsorption mechanism by mesoporous cellulose biochar. Chem. Eng. J. 2019, 362, 383–391. [Google Scholar] [CrossRef]
- Chen, J.; Xia, X.; Chu, S.; Wang, H.; Zhang, Z.; Xi, N.; Gan, J. Cation−π interactions with coexisting heavy metals enhanced the uptake and accumulation of polycyclic aromatic hydrocarbons in spinach. Environ. Sci. Technol. 2020, 54, 7261–7270. [Google Scholar] [CrossRef]
- Ying, F.; Jichao, Z.; Yanzheng, W.; Yanzhen, Y. Resource preparation of poly-Al–Zn–Fe (PAZF) coagulant from galvanized aluminum slag: Characteristics, simultaneous removal efficiency and mechanism of nitrogen and organic matters. Chem. Eng. J. 2012, 203, 301–308. [Google Scholar] [CrossRef]

| Component | wt% |
|---|---|
| CaO | 42.4 |
| SiO2 | 33.5 |
| Al2O3 | 11.3 |
| MgO | 8.5 |
| SO3 | 1.9 |
| TiO2 | 0.7 |
| Na2O | 0.4 |
| K2O | 0.4 |
| Fe2O3 | 0.4 |
| MnO | 0.2 |
| ZrO2 | 0.1 |
| SrO | 0.1 |
| Cl | 0.1 |
| Sample | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Pore Size (Å) |
|---|---|---|---|
| GGBFS | 0.53 | 0.0065 | 14.128 |
| AAS | 61.59 | 0.15 | 97.4 |
| Material | Qe,exp (mg/g) | Pseudo-First-Order Model | Pseudo-Second-Order Model | ||||
|---|---|---|---|---|---|---|---|
| K1 (1/min) | Qe,cal (mg/g) | r2 | K2 (g/mg/min) | Qe,cal (mg/g) | r2 | ||
| BFS | 2.36 ± 0.58 | 0.007 | 1.833 | 0.771 | 0.064 | 2.450 | 0.974 |
| AAS | 15.2 ± 0.11 | 0.123 | 0.531 | 0.530 | 0.247 | 15.2 | 0.957 |
| Materials | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qm (mg/g) | KL (L/mg) | r2 | KF ((mg/g) · (L/mg)1/n) | nF | r2 | |
| BFS | 3.79 | 4.691 | 0.990 | 3.436 | 0.435 | 0.922 |
| AAS | 44.00 | 7.823 | 0.959 | 51.61 | 0.456 | 0.983 |
| Adsorbent | Qm (mg/g) | Ref. |
|---|---|---|
| Alkali-activated slag | 44.0 | This study |
| Smectite | 55.0 | [33] |
| Magnesite–talc mixture | ~60 | [34] |
| Loess | 0.81 | [35] |
| Waste brick | 0.03 | [36] |
| Steel slag | 41 | [17] |
| Magnetic graphene | 43.00 | [37] |
| Alkali-activated biochar | 38.82 | [38] |
| Tap Water | Lake Water | River Water | Wastewater | Ground Water | Seawater | |
|---|---|---|---|---|---|---|
| pH | 7.15 | 6.54 | 7.03 | 7.62 | 6.91 | 8.02 |
| TOC (mg/L) | - | 13.5 | 18.1 | 10.2 | 15.6 | 3.1 |
| Na | 55.41 | 26.49 | 0.26 | 605 | 9.83 | 468 |
| K | 4.26 | 12.87 | 0.38 | 25.3 | 1.78 | 10.2 |
| Mg | 16.96 | 7.28 | 0.17 | 194.6 | 4.3 | 53.1 |
| Ca | 40.70 | 53.36 | 0.07 | 252 | 4.01 | 10.3 |
| Cl | 0.1 | 21.98 | 1.48 | 15.3 | 19.5 | 605.14 |
| SO4 | 13.0 | 15.78 | 0.22 | 1.3 | 5.88 | 28.4 |
| HCO3 | - | 62.24 | 0.11 | 2.6 | 3.86 | 5.39 |
| Br | - | - | 0.96 | - | - | 0.84 |
| F- | 0.05 | - | - | 1.8 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, T.T.; Le, Q.T.N. Unlocking the Detoxification of Phenanthrene from Water Using Alkali-Activated Slag Mortar. Appl. Sci. 2024, 14, 6991. https://doi.org/10.3390/app14166991
Tran TT, Le QTN. Unlocking the Detoxification of Phenanthrene from Water Using Alkali-Activated Slag Mortar. Applied Sciences. 2024; 14(16):6991. https://doi.org/10.3390/app14166991
Chicago/Turabian StyleTran, Thanh Tai, and Quynh Thi Ngoc Le. 2024. "Unlocking the Detoxification of Phenanthrene from Water Using Alkali-Activated Slag Mortar" Applied Sciences 14, no. 16: 6991. https://doi.org/10.3390/app14166991






