Abstract
This review aims to gain insight into the major causes of hair graying (canities) and how plant-derived extracts and phytochemicals could alleviate this symptom. Research articles on human hair graying were searched and selected using the PubMed, Web of Science, and Google Scholar databases. We first examined the intrinsic and extrinsic factors associated with hair graying, such as the reduced capacity of melanin synthesis and transfer, exhaustion of melanocyte stem cells (MSCs) and melanocytes, genetics and epigenetics, race, gender, family history, aging, oxidative stress, stress hormones, systematic disorders, nutrition, smoking, alcohol consumption, lifestyle, medications, and environmental factors. We also examined various plants and phytochemicals that have shown a potential to interfere with the onset or progression of human hair graying at different levels from in vitro studies to clinical studies: the extract of Polygonum multiflorum and its major components, 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside and emodin; the extract of Eriodictyon angustifolium and its major flavonoid compounds, hydroxygenkwanin, sterubin, and luteolin; the extracts of Adzuki beans (Vigna angularis), Fuzhuan brick tea (Camellia sinensis), and Gynostemma pentaphyllum; bixin, a carotenoid compound found in Bixa orellana; and rhynchophylline, an alkaloid compound found in certain Uncaria species. Experimental evidence supports the notion that certain plant extracts and phytochemicals could alleviate hair graying by enhancing MSC maintenance or melanocyte function, reducing oxidative stress due to physiological and environmental influences, and managing the secretion and action of stress hormones to an appropriate level. It is suggested that hair graying may be reversible through the following tactical approaches: selective targeting of the p38 mitogen-activated protein kinase (MAPK)–microphthalmia-associated transcription factor (MITF) axis, nuclear factor erythroid 2-related factor 2 (NRF2), or the norepinephrine–β2 adrenergic receptor (β2AR)–protein kinase A (PKA) signaling pathway.
Keywords:
hair graying; gray hair; canities; Polygonum multiflorum; Eriodictyon angustifolium; melanocyte stem cells (MSCs); microphthalmia-associated transcription factor (MITF); p38 mitogen-activated protein kinase (MAPK); oxidative stress; nuclear factor erythroid 2-related factor 2 (NRF2); norepinephrine; β2 adrenergic receptor (β2AR) 1. Introduction
Human hair does not continue to grow throughout life, but overlapping hair cycles occur approximately 25 times, resulting in changes in the overall hair population. The hair cycle consists of the anagen (growth), catagen (regression, intermediate, or transition), and telogen (resting) phases [1]. The final stage of the telogen phase is also separately classified as the exogen (shedding) phase [2]. The hair and associated cells and matrices constitute a mini-organ called a hair follicle [3,4]. Hair follicle keratinocyte stem cells (HFKSCs) of the keratinocyte lineage reside in the hair follicle bulge area [1,5,6]. The new hair germs at the telogen phase also contain HFKSCs or progenitor cells [7]. During the anagen onset, some primed HFKSCs (in the hair germ) and quiescent HFKSCs (in the bulge) are activated and migrate to the matrix, becoming transit-amplifying cells [5]. These cells continue dividing, proliferating, differentiating, and keratinizing, leading to hair production and growth [8].
Melanoblasts, derived from neural crest cells via melanoblasts/glial bipotent progenitor cells, differentiate into melanocytes. A portion of melanoblasts dedifferentiate to melanocyte stem cells (MSCs), which can differentiate into transit-amplifying cells and then into melanocytes [9]. MSCs found alongside HFKSCs in the hair follicle bulge are located in the secondary hair germs at the telogen phase [10,11]. During the anagen onset, some MSCs differentiate into mature melanocytes via transit-amplifying cells and migrate to the matrix area where melanocytes produce and supply melanin pigments to incorporate them into the hair fibers [10]. A recent study has reported that transit-amplifying cells may dedifferentiate into MSCs, while some MSCs may become directed into other pathways and may be unable to differentiate into melanocytes [11]. Overall, coordinated differentiation of stem cell and progenitor lineages in the hair follicle is important for hair growth and pigmentation [12]. Furthermore, hair growth and pigmentation are influenced by interactions with nerves, adipocytes, and immune cells neighboring hair follicles [13,14].
Hair graying, also known as canities, is the progressive loss of pigment in hair follicles, resulting in the appearance of gray or white hair [15]. It is a representative sign of human aging, along with hair loss [16]. Hair graying begins in the 40s for most people (senile hair graying) but can occur prematurely in some individuals (premature hair graying) [17,18]. As synchronous coordination of hair development and melanin synthesis is a requirement for normal hair pigmentation, its failure can cause hair graying [17]. Although the 50/50/50 rule of thumb, stating that “at age 50 years, 50% of the population has at least 50% of gray hair”, is not necessarily valid or applicable to all races and genders, a significant number of people experience hair graying as they age [17,19,20,21]. Hair graying can occur even in young people when an insufficient amount of melanin is deposited within the growing hair shafts [22]. It causes psychological stress for some people that affects their mental health and social life [23].
Hair graying is a part of the aging process and is not generally considered a disease for most people unless closely related to a specific health condition or nutritional deficiency. In the past, hair graying was considered irreversible and had no proper countermeasure other than dyeing. However, the way we look at hair graying is changing as the understanding of the causative mechanism is deepening with new reports on cases of hair color restoration [24,25,26,27]. The repigmentation of white or gray hair is observed in patients receiving various medications [28,29]. A reversal of premature hair graying is also observed in patients treated with a topical formulation of 5% Melitane, an α-melanocyte-stimulating hormone (α-MSH) biomimetic peptide, or 2% Greyverse, an α-MSH biomimetic palmitoyl tetrapeptide-20, combined with oral supplements containing biotin and calcium pantothenate [30,31]. Therefore, we might be entering the era of medical therapy for hair graying [32,33].
This review aims to gain insight into the major causes of hair graying and how plant extracts and phytochemicals could alleviate the symptoms. Plants have unique survival strategies, distinct from animals, and synthesize secondary metabolites called phytochemicals [34]. Plant extracts have been used in traditional medicine, and some phytochemicals have been developed into active pharmaceutical ingredients or lead compounds for new drug development [35]. There is growing interest in the benefits and usability of plant-derived natural products in the cosmetics and dermatological fields [36,37,38,39]. We have recently reviewed the plant extracts and phytochemicals used to treat hair loss [40]. In line with this previous review, we explore the effects of plant extracts and phytochemicals on hair graying in the present review. In the context of existing review papers on the causes and treatments of hair graying [32,33], the present review focuses on deriving specific therapeutic molecular targets by interrelating studies on the causal mechanisms of hair graying and the biological activities of plant extracts and phytochemicals.
We first examine the latest studies on intrinsic and extrinsic factors associated with human hair graying. Then, we examine experimental studies demonstrating the therapeutic potential of plant extracts and phytochemicals in alleviating hair graying. Finally, we discuss major issues in human hair graying summarized by the following questions:
- What can be the targets for treating hair graying?
- What are the modulatory targets of plant extracts and phytochemicals?
- What should be the next research topics to advance treatments for human hair graying?
We hope this review will help identify novel therapeutic strategies and tactics for alleviating human hair graying.
2. Methods
The PubMed (https://pubmed.ncbi.nlm.nih.gov/), Web of Science (https://www.webofscience.com/), and Google Scholar (https://scholar.google.com/) databases were accessed on 4 July 2024 to search for research articles related to the topic of this narrative review. A preliminary search using several keywords, such as hair, grey, gray, graying, greying, white, canities, extract, plant, herb, phytochemical, etc., and Boolean search commands, such as ‘AND’, ‘OR’, and ‘NOT’, resulted in hundreds of articles. The search results were refined by limiting the search ranges for some keywords to title words only, followed by a brief reading of their titles and abstracts to select approximately 130 articles highly focused on human hair graying research. Most of these articles were cited and used for in-depth discussion in this review. The articles related to intrinsic and extrinsic factors associated with hair graying are mentioned mostly in Section 3. The articles related to the therapeutic potential of plant extracts and phytochemicals are mentioned mostly in Section 4.
3. Intrinsic and Extrinsic Factors Associated with Hair Graying
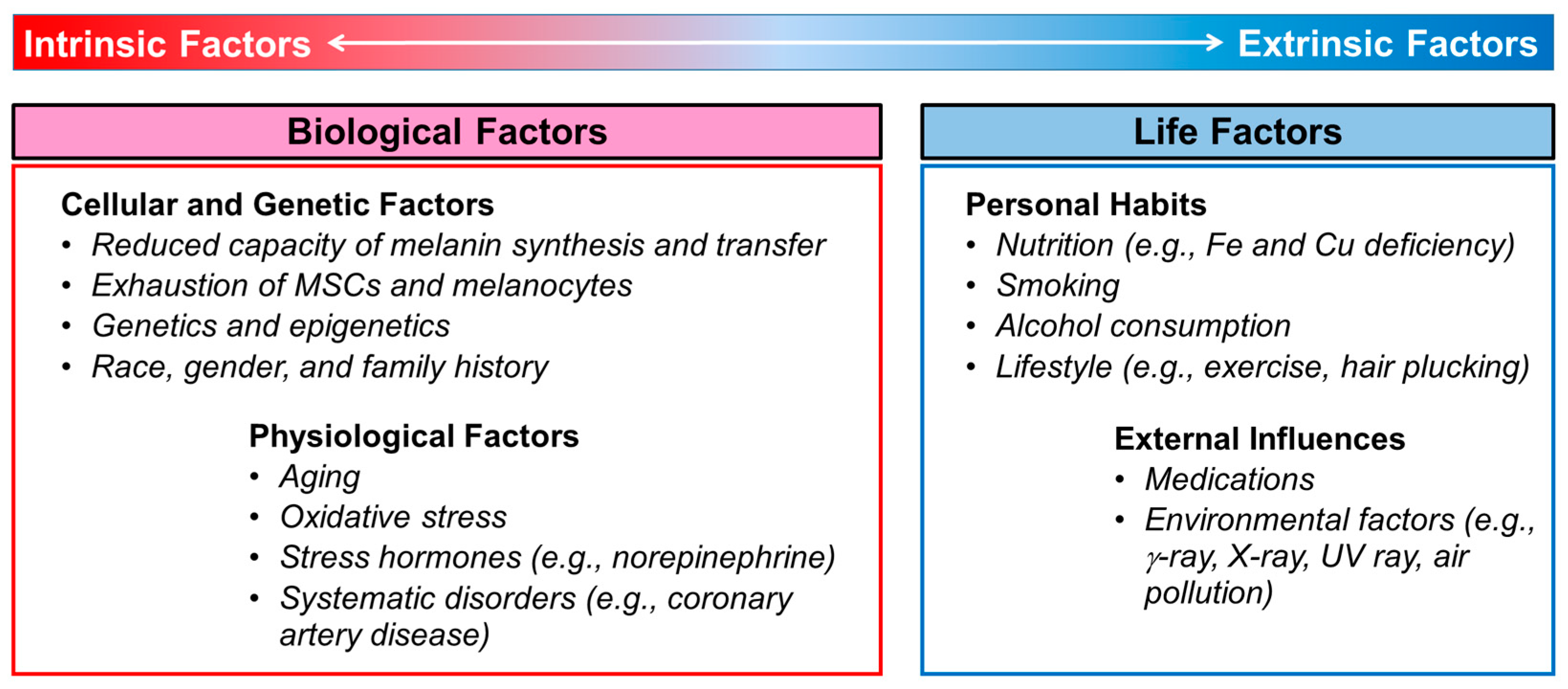
In this chapter, we examine various intrinsic and extrinsic factors directly or indirectly related to the onset and progression of hair graying. Intrinsic factors refer to biological factors, such as cellular, genetic, and physiological factors, whereas extrinsic factors refer to life factors comprising personal habits and external influences, as summarized in Figure 1. Since these factors are interconnected and influence each other, the classification provided here is arbitrary.

Figure 1.
Intrinsic and extrinsic factors associated with the incidence or progression of hair graying in humans. Abbreviations: Cu, copper; Fe, iron; MSCs, melanocyte stem cells; UV, ultraviolet.
3.1. Reduced Capacity of Melanin Synthesis and Transfer
GeneChip Array analysis of mRNA expression profiles between pigmented, gray, and white scalp hair follicles from human donors identified 194 and 192 downregulated genes and 186 and 177 upregulated genes in gray and white hair follicles, respectively, compared to the pigmented hair follicles. The downregulated genes included tyrosinase (TYR), tyrosinase-related protein 1 (TYRP1), premelanosome protein (PMEL, Pmel17, GP100), melan-A (MLANA), KIT (c-Kit, receptor for stem cell factor), and MET (c-Met, receptor for hepatocyte growth factor) [41].
Genome-wide comparison of the gene expression profiles of gray and black hair follicular cells of human patients with premature hair graying identified 127 differentially expressed genes (DEGs). Many melanogenesis-associated proteins, such as TYR, MLANA, PMEL, TYRP1, solute carrier family 45 member 2 (SLC45A2), KIT, G protein-coupled receptor 143 (GPR143), and oculocutaneous albinism 2 (OCA2) were found to be downregulated in gray hair follicles compared to black hair follicles [42].
These studies support the view that the expression of genes related to melanin synthesis, melanosome biogenesis, and other melanocyte-relevant functions is reduced in hair follicles producing white or gray hair, resulting in insufficient melanin supply and reduced melanin incorporation into hair filaments during the anagen. Most DEGs in the above-mentioned studies are target genes of the transcriptional regulation by microphthalmia-associated transcription factor (MITF); hence, reduced MITF activity in melanocytes causes hair graying.
Placental sphingolipid induced the expression of MITF and TYR via the p38 mitogen-activated protein kinase (MAPK) signaling pathway in murine melanoma B16F10 cells and its topical application increased MITF expression in follicular melanocytes and the growth of fresh dense black hair in age-onset gray-haired C57BL/6J mice [43]. Therefore, the melanin synthesis and transport capacity of melanocytes, which are controlled by MITF, are the key variables most directly related to hair pigmentation and graying.
3.2. Exhaustion of MSCs and Melanocytes
Hair pigmentation can be affected by defects or depletion of MSCs, immature precursor cells, and mature melanocytes residing in the hair follicle bulge or bulb [44,45,46]. Loss of MSCs within the bulge niche preceded the loss of differentiated melanocytes in the hair matrix [47,48]. B-cell lymphoma 2 (BCL-2) deficiency caused selective apoptosis of MSCs, but not differentiated melanocytes, while MITF mutation accelerated pigmentation, differentiation, and senescence of MSCs [47]. Expressions of melanocyte markers, such as MITF-M, SRY-box transcription factor 10 (SOX10), paired box 3 (PAX3), pro-opiomelanocortin (POMC), TYR, TYRP1, and tyrosinase-related protein 2 (TYRP2), also called dopachrome tautomerase (DCT), were absent or reduced in the bulbs of white (non-pigmented) hair compared to black (pigmented) hair [49,50]. White hair shafts grew significantly faster than black hair shafts; the expression of keratins (KRTs) such as KRT6, KRT14, KRT16, KRT25, and KRT83 and keratin-associated protein (KRTAP) 4 isoforms was upregulated in white hair follicles compared to black hair follicles [51]. Therefore, the rapid growth of hair shafts may exhaust MSCs; hence, the incomplete self-maintenance of MSCs may be the causative mechanism for hair graying [47,52,53].
Various cell signaling pathways are involved in maintaining melanoblasts and MSCs in hair follicles [54]. Hair graying was observed in mice deficient in Notch1, Notch2 receptor, or RBP-J kappa transcription factor due to the disappearance of melanocytes in the hair matrix [55]. Therefore, Notch signaling might play an essential role in the melanocyte population during hair follicle cycles [56]. Phosphatase and tensin homolog (PTEN) encodes a phosphatidylinositol-3,4,5-trisphosphate 3-phosphatase which lowers phosphatidylinositol-3,4,5-trisphosphate levels in cells, thereby negatively regulating the phosphatidylinositol 3-kinase (PI3K)–protein kinase B (PKB) signaling pathway [57]. PTEN-deficient mice were resistant to MSC exhaustion in the hair follicle bulge and hair graying induced by repeated depilation in mice [58]. The increased expression of KIT ligand (KITL), also called stem cell factor (SCF), as well as of hepatocyte growth factor (HGF), and endothelin 3 (ET3), which promote melanocyte proliferation of melanoblasts and MSCs, alleviated hair graying induced by repeated hair plucking and shaving in C57BL/6 mice [59]. On the other hand, the expression of β-catenin in the anagen and telogen skin of aged mice was higher than that in young mice, and overexpression of Wingless and Int-1 (WNT) 10b promoted an excessive differentiation and depletion of MSCs, increasing hair graying [60]. Thus, the excessive consumption, ectopic differentiation, and aging of MSCs hinder the development of mature melanocytes in harmony with the hair follicle cycle, resulting in hair graying [61].
3.3. Genetics and Epigenetics
Although most people experience hair graying as they age, some people show symptoms of premature hair graying at a much earlier age [62,63]. The age criteria for classifying premature hair graying vary according to race [18,22,62]. Premature hair graying occurs mainly due to genetic factors, although it is also influenced by various other factors [22,64,65]. A familial history of premature hair graying is an important risk factor for the expression of this phenotype [66,67,68].
MITF plays a critical role at multiple stages of the melanocyte life cycle across species, with its expressional depletion resulting in a fully depigmented hair or coat [69]. The heterozygosity for MITF exacerbates hair graying in transgenic (DctSox10) mice that conditionally overexpress SOX10 by upregulating a type I interferon (IFN)-regulated gene expression signature associated with innate immunity [70].
Genome-wide association studies revealed that interferon regulatory factor 4 (IRF4) downregulation is associated with scalp hair graying [71]. IRF4 interacts with MITF and transcription factor AP-2α (TFAP2A), inducing gene expression of melanogenic enzymes, such as TYR [72]. Accordingly, hair-graying prediction models have been proposed by combining age, sex, and 13 unique single nucleotide polymorphisms (SNPs), including those in IRF4, maestro heat-like repeat family member 2A (MROH2A), kinesin family member 1A (KIF1A), and non-structural maintenance of chromosomes element 1 homolog (NSMCE1) [73].
A recent study has reported that the S519N variant of SAM and SH3 domain-containing protein 1 (SASH1) causes dysfunction in MSCs maintenance, contributing to the development of certain aging-related pigmentation disorders, namely senile lentigo of the skin and scalp hair graying in a Hispanic family [74]. SASH1 is a scaffold protein binding to tankyrase 2 (TNKS2) and regulating the division of MSCs, but its S519N variant does not function normally due to a low binding affinity to TNKS2 [74,75].
MicroRNAs, produced by the dicer (endoribonuclease type 3), are involved in lowering target gene expression by inducing mRNA degradation or suppressing translation [76]. Mimicking stress conditions, the inactivation of dicer causes the misplacement of melanocytes within the hair follicle, resulting in reduced melanin transfer to keratinocytes in the growing hair [77]. These effects were partly mediated by miR-92b, which targets integrin alpha chain 5 (ITGA5) involved in the regulation of melanocyte migration, implicating that such epigenetic mechanisms might link stress to premature hair graying.
From a mechanistic perspective, it is considered that certain genetic or epigenetic variations may cause acquired hair graying by negatively affecting MSC maintenance and melanocyte migration, whereas some genetic or epigenetic variations resulting in differences in the melanogenic function of melanocytes may affect natural hair color.
3.4. Race, Gender, and Family History
The age at which premature hair graying is defined varies by race: 20 in Caucasians, 25 in Asians, and 30 in African Americans [18,22,62]. This is because the genetic background and natural hair color of different races vary, and the time at which the results of hair graying become apparent varies [73]. Although natural hair color and brightness vary by race, there is no evidence that graying occurs more frequently or intensely in certain races [19].
In many races, men tend to develop gray hair earlier and more extensively than women of similar age [19]. Graying patterns also vary by gender [19,78]. In men, hair graying tends to occur first and is more prominent in the temporal region (the sides of the head). In women, the frontal and parietal regions (top and crown) are affected more often and early. In a survey study conducted on 620 gray-haired Korean subjects, 33.9% of men and 55.2% of women responded that they dye their hair regularly [79]. This means that women are relatively more concerned about hair graying.
Several studies have supported that hair graying is significantly affected by a hereditary component [66,67,73]. A cross-sectional survey of 100 young men less than 25 years old with premature hair graying showed that 39% of individuals had a family history, with 26% from the paternal side, 10% from the maternal side, and 3% from both parents [67]. It is noteworthy that a man’s hair graying may be more influenced by his father’s history than his mother’s.
3.5. Aging
Aging is the major factor altering the expression of hair graying-associated genes [80]. Aged transgenic mice carrying the oncogenic RET gene exhibit gray hair due to an accelerated hair cycle, accompanied by the senescence of HFKSCs expressing ET1 and a decrease in follicular MSCs expressing endothelin receptor B (ETRB) [81]. Comparison of human scalp samples from young people aged 27–40 years with less gray hair and senior people aged 61–90 years with more gray hair confirmed the age-dependent increase in senescent follicular HFKSCs, decrease in ET-1 expression, and reduction in the number of functional follicular MSCs [81]. Thus, HFKSC senescence, MSC depletion, and their altered gene expression are considered the cause of age-dependent hair graying.
Comparison of gene expression between hair follicles of young and elder volunteers, aged below 25 and above 50 years, respectively, indicated that KRT33, KRT34, KRTAP4-4, and KRTAP4-7 became downregulated with age [82]. Lipidomic analysis of follicular tissues of black and white hair obtained from female participants aged 55–65 years indicated that white hair contains reduced total lipid, sphingolipid (particularly glucosylceramide and galactosylceramide), phosphatidylserine, and phosphatidic acid levels [83]. These studies suggest that age-dependent hair graying is accompanied or influenced by structural, biochemical, and pathophysiological changes in the hair interior and surrounding microenvironment.
3.6. Oxidative Stress
As people age, there is an increase in oxidative damage and a decrease in antioxidant defenses in the body [84]. Oxidative stress also plays a major role in age-induced hair graying via multiple effects on the viability and functionality of MSCs and mature melanocytes [85,86,87]. Cross-sectional studies support that systemic redox imbalance or oxidative stress can affect the incidence and degree of premature hair graying [88,89]. Specifically, serum malondialdehyde (MDA) levels increased while serum glutathione (GSH) and superoxide dismutase (SOD) levels decreased in patients with premature canities compared to controls [89].
Various factors, including cellular melanin synthesis reaction and ultraviolet (UV) irradiation, can cause the production of reactive oxygen species (ROS) in melanocytes [90,91]. Furthermore, the age-related decrease in BCL-2 expression makes melanocytes more vulnerable to oxidative stress and apoptotic death [92].
The reduction in catalase (CAT) expression and activity in hair follicles causes hydrogen peroxide (H2O2) accumulation, impairment of methionine sulfoxide repair, melanocyte dysfunction, and cell death [93,94]. In addition to CAT downregulation, hydroxyl radical scavenging activity becomes repressed in unpigmented hair follicles compared to normally pigmented hair follicles [50]. Ataxia telangiectasia mutated (ATM) protein, a serine/threonine protein kinase involved in the protection against oxidative DNA damage, is highly expressed within the nuclei of melanocytes in anagen hair follicle bulbs, but its expression is reduced proportionally to the degree of canities [95]. Thus, the balance between oxidative stress and antioxidant defense can affect human hair follicle growth, differentiation, and pigmentation.
The role of oxidative stress as the main causal mechanism in hair graying is also supported by hair depigmentation in animal models irradiated with UV, γ, or X-rays [96,97]. Hair graying can be induced by irradiating animals or organ-cultured hair follicles with γ-rays or X-rays, providing useful experimental models for hair graying research [97,98,99]. HFKSCs are more prone to oxidative DNA damage induced by X-ray irradiation than follicular MSCs [100]. When PUVA phototherapy (a combination treatment using psoralen and UVA) was applied to the backs of black mice after their hair had been removed, gray or white hair grew instead of black hair on the affected bare skin, and this effect was attenuated by pretreatment with a gel containing SOD [96]. Dietary restriction delayed hair graying in C57BL/6 J mice induced by γ-ray irradiation, but the effect was temporary [101]. Dietary restriction prolongs the quiescence of DNA-damaged hair follicle stem cells and does not prevent their eventual death and depletion.
3.7. Stress Hormones
The autonomic nervous system (ANS) and hypothalamic–pituitary–adrenal (HPA) axis, which are connected both anatomically and functionally, mediate acute and prolonged responses to stress signals, respectively [102]. The activation of sympathetic ANS and HPA triggers the release of catecholamines (e.g., adrenaline and norepinephrine) and glucocorticoid stress hormone (e.g., cortisol in humans, corticosterone in rodents), respectively, into the bloodstream [103].
Zhang et al. demonstrated that pain induced by the injection of resiniferatoxin, a capsaicin analog, increased serum norepinephrine and corticosterone levels and number of unpigmented white hairs in C57BL/6J mice with black coat color, and this change was inhibited by buprenorphine (an opioid analgesic) [104]. MSCs in the hair follicles express the glucocorticoid receptor (GR), the melanocortin 1 receptor (MC1R), and the β2 adrenergic receptor (β2AR), and the selective depletion of β2AR (but not GR depletion, MC1R depletion, or adrenalectomy) prevented white hair development, suggesting that the hyperactivation of sympathetic nerves causes the release of norepinephrine from the skin [104,105]. Additionally, the secretion of norepinephrine led to the rapid differentiation and eventual depletion of MSCs [106].
3.8. Systematic Disorders
Premature hair graying is associated with various disorders [18], such as coronary artery disease [107,108,109], obesity [66], metabolic diseases [110], hearing impairment [111], osteopenia [112], atopy [113], and autoimmune diseases including vitiligo [70]. Premature hair graying is a strong predictive marker of coronary artery disease, especially in smokers compared to non-smokers [114,115].
3.9. Nutrition
A study analyzing serum levels of trace elements in patients with premature hair graying demonstrated lower copper levels and higher iron levels in the patient group compared to the control group while zinc levels did not differ between the two groups [64]. In another study, iron, copper, and calcium were all lower in the patient group than in the control group [116]. An 11-year-old male with slowly graying hair had low serum ferritin and hemoglobin levels, and five months of iron supplementation (oral ferrous sulfate, 40 mg daily) normalized serum ferritin and hemoglobin levels and recovered black hair color in this patient [117]. Epidemiological or cross-sectional studies also suggested that premature hair graying is related to low serum ferritin and calcium levels [118,119,120]. On the other hand, serum levels of selenium and lead did not significantly differ between the two groups with and without premature hair graying [121].
Hair loss and graying hair were observed in patients receiving intravenous fat-free fluid for a long period. These symptoms were related to a deficiency of essential fatty acids and were cured by topical safflower oil containing high linoleic acid levels [122]. Several studies investigated the link between hair graying and certain vitamins, including vitamin B12 and vitamin D, and most, but not all, results supported a possible correlation [115,118,119,120,123,124].
3.10. Smoking, Alcohol Consumption, Lifestyle, Medications, and Environmental Factors
Smoking and alcohol consumption, which cause oxidative stress, are also independent risk factors correlated with hair graying [66,113,125,126,127]. A sedentary lifestyle, which can exacerbate metabolic diseases, affects hair pigmentation, whereas adequate sun exposure and regular exercise can help promote general health and prevent hair graying [22,65,123].
Hair plucking can occur when people shampoo, comb, dye, or perm your hair, and frequent repetition can deplete melanocytes and cause hair graying. Accordingly, repeated hair plucking is a technique to induce hair graying in animal models [11,59].
Various medications including certain chemotherapy drugs and antimalarial medications might cause hair graying [18]. Hair loss and graying were observed in patients during the treatment of depressive disorder with mirtazapine which fully recovered 10 weeks after medication discontinuation [128]. On the other hand, some anti-inflammatory drugs rarely promote the repigmentation of gray hair [28,29], but these cannot be applied for general treatment.
Hazardous environmental factors, such as X-rays, γ-rays, UV rays, and air pollution, can cause oxidative damage to hair follicle cells and hair filaments [129,130,131]. PUVA phototherapy or irradiation with X- or γ-rays can induce oxidative damage to cells and hair graying in animals, providing useful experimental models [96,97,101].
4. Therapeutic Potential of Plant Extracts and Phytochemicals
In this chapter, we review studies on the efficacy and mechanism of action of plant extracts and phytochemicals in various experimental hair-graying models.
4.1. Extracts and Bioactive Components of Polygonum multiflorum
Polygonum multiflorum (PM), also named Pleuropterus multiflorus, Fallopia multiflora, or Reynoutria multiflora, is the most widely used plant in the treatment of hair graying. This plant has been used in traditional medicine to treat various disorders [132]. It has two different medicinal forms, Polygonum multiflorum Radix (PMR) and Polygonum multiflorum Radix Preparata (PMRP), a processed form prepared by steaming PMR with black soybean decoction [133,134].
The main phytochemical components of PM can be classified into stilbenes, quinones, flavonoids, and others, as described in comprehensive reviews of this plant [135,136]. In high-performance liquid chromatography (HPLC) analysis, 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside (TSG), emodin, emodin-8-O-β-D-glucopyranoside, physcion, and torachrysone-8-O-β-D-glucoside appeared as the major peaks [137,138]. Of these, TSG has been most extensively studied as the main active compound responsible for the pharmacological activities of PM [139,140].
Table 1 shows the biological activities of PM extracts and their phytochemical components evaluated in various experimental models to study melanin synthesis or hair pigmentation.

Table 1.
Effects of the extracts and bioactive components of Polygonum multiflorum (PM) on melanin synthesis or hair pigmentation.
The hair-blackening effects of PMR extract, PMRP extract, and TSG in pure form were examined in 6-week-old C57BL/6J male mice (10 animals per group) with H2O2-induced hair bleaching [134]. For the model group, the 0.0375% H2O2 solution was spread on their back furs every morning for 6 weeks. Six hours after each H2O2 treatment, mice fasted for 2 h and received PMR extract (0.576 g kg−1 by gavage and 1.152 g kg−1 topically), PMRP extract (0.576 g kg−1 by gavage and 1.152 g kg −1 topically), or TSG (0.034 g kg−1 by gavage and 0.068 g kg−1 topically). The total melanin content of hairs was lowered by H2O2, which was reversed by PMR and PMRP extracts. When the expression of POMC, α-MSH, MC1R, agouti signaling protein (ASIP), MITF, TYR, TYRP1, and TYRP2 in the skin was analyzed by enzyme-linked immunosorbent assay (ELISA), only α-MSH, MC1R, and TYR levels were significantly downregulated by H2O2 and reversed by the PMR extract, PMRP extract, or TSG in the order of activity.
Sextius et al. reported the effects of PMR and PMRP extracts in protecting melanocytes from oxidative stress [141]. The water extract of PMRP reduced the intracellular ROS levels at 0.05, 0.1, 0.2, or 0.5 μg mL−1 while preserving the intracellular level of GSH at 0.05 μg mL−1 in primary human foreskin melanocytes exposed to 250 µM H2O2. PMR extract (0.01 mg mL−1) prevented cell death induced by 1100−1750 μM H2O2. PMR extract (0.1 mg mL−1) also enhanced pigmentation in human hair follicles ex vivo.
The methanolic extract of PM roots increased melanin contents and mRNA levels of MC1R, MITF, and TYR in human melanoma SKMEL-28 cells and the embryos and larvae of zebrafish at concentrations that did not cause cell death or morphological defects [142]. Considering that MC1R, MITF, and TYR mRNA levels were higher in the black hair follicles of young people than in their graying hair follicles, plant extracts that can upregulate the expression of MC1R, MITF, and TYR would help in the treatment of early hair graying and other pigmentation loss-related diseases.
The 70% ethanoic extract of PM roots (5 or 10 μg mL−1) increased the melanin content in B16F10 cells without influencing cell viability [143]. The extract (10 μg mL−1) increased the expression of cyclooxygenase-2 (COX2) and melanogenic genes, such as MITF, TYR, TYRP1, and TYRP2, at mRNA or protein levels. The extract induced p38 MAPK phosphorylation and SB203580 (inhibitor of p38 MAPK) abolished the expression of COX2, MITF, and TYR and melanin synthesis in cells stimulated by the extract of PM roots.
TSG (5 or 10 μg mL−1) increased the TYR activity and melanin content in B16 cells without influencing cell viability or proliferation [144]. TSG (10 μg mL−1) increased the mRNA and protein levels of TYR, which was associated with the phosphorylation (activation) of cyclic AMP response element-binding protein (CREB) and the increased protein levels of MITF. TSG induced p38 MAPK phosphorylation and SB203580 abolished the expression of MITF and TYR and melanin synthesis in TSG-stimulated cells.
Emodin reduced intracellular ROS levels and increased TYR activity and melanin synthesis in B16F1 cells exposed to 250 µM H2O2 [145]. It also increased TYR, TYRP1, TYRP2, MITF, NAD-dependent deacetylase sirtuin-1 (SIRT1), and forkhead box protein O1 (FOXO1) protein levels while decreasing those of Extracellular signal-regulated kinase (ERK) and phospho-ERK in H2O2-treated cells.
We could not find clinical trials evaluating the anti-hair-graying properties of PM extracts, but one clinical study investigated a hair tonic containing mixed extracts of several plants, including PM, Pueraria lobate, and Ginkgo biloba [146]. Although this tonic was not effective enough to change the gross hair grayness, it reduced the number of newly developed gray hairs per unit scalp area. Still, whether this effect is due to the phytochemicals of PM or other components remains unclear. Nonetheless, this study demonstrated the potential of herbal medicine to alleviate hair graying in humans.
4.2. Extracts and Bioactive Components of Eriodictyon angustifolium
Eriodictyon angustifolium (EA) is a plant called narrow-leaf Yerba Santa in Western America and has been used to treat respiratory and other diseases in traditional medicines and to mask the bitter flavor of pharmaceuticals [147]. It contains various flavonoid compounds (e.g., naringenin, eriodictyol, homoeriodictyol, hesperetin, sterubin, luteolin, diosmetin, chrysoeriol, hydroxygenkwanin, and isosakuranetin) and bisprenylated benzoic acid derivatives (e.g., erionic acids A, B, C, D, E, and F) [147,148,149,150].
In human gingival fibroblasts stimulated with LPS from Porphyromonas gingivalis, a crude ethanol/water extract of EA and its flavanone-rich fraction reduced the release of pro-inflammatory cytokines such as interleukin-6 (IL-6), IL-8, and macrophage chemoattractant protein-1 (MCP-1), supporting their anti-inflammatory potential [149]. Of individual flavanones, eriodictyol and naringenin had more potent anti-inflammatory effects than homoeriodictyol, sterubin, and hesperetin [149].
As summarized in Table 2, Taguchi et al. extensively studied the biological activities of EA extracts and their phytochemical components in cells, animal models, and human subjects, to examine their therapeutic potential in preventing hair graying.

Table 2.
Effects of the extracts and bioactive components of Eriodictyon angustifolium (EA) on melanin synthesis or hair pigmentation.
In one study [151], sterubin, one of the most abundant flavonoid components of EA [152], increased melanin in the human melanoma HMVII cell line, which was accompanied by an increase in β-catenin, MITF, and TYR. Sterubin also reduced the intracellular ROS level, oxidative DNA damage, mitochondrial damage, and death of normal human epidermal keratinocytes (NHEKs) irradiated with X-ray. Furthermore, the topical application of sterubin (0.1% solution) for 4 weeks restored pigmentation in the gray beard hair of a human subject.
In a subsequent study [152], the ethanol extract of EA increased melanin synthesis in the human melanoma HMVII cell line, which was accompanied by an increase in β-catenin, MITF, and TYR. However, the effects of Eriodictyon californicum (EC) extract were absent or weak. Additionally, EA extract reduced the oxidative DNA damage and death of NHEKs irradiated with X-ray more effectively than or similarly to EC extract. Furthermore, topical application of EA extract on the beard and scalp area for 1 year and 24 weeks, respectively, reduced the occurrence of gray hairs in human subjects.
As an animal model was fed water or plant extract freely for 1 month, its hair was plucked, irradiated with X-rays, and observed after 1 month, showing a reduced degree of gray hair in the group receiving the extract. When the skin was incised immediately after X-ray irradiation followed by histological analysis, the extract reduced the oxidative DNA damage in HFKSCs [150]. Thus, the extract might preserve the function of HFKSCs as a niche for MSCs, alleviating melanocyte depletion and hair graying.
When comparing the same molar concentrations of various flavonoid compounds, such as hydroxygenkwanin, sterubin, luteolin, eriodictyol, homoeriodictyol, hesperetin, and diosmetin, only hydroxygenkwanin, sterubin, and luteolin reduced the oxidative DNA damage and death of NHEKs irradiated with X-ray. Among them, hydroxygenkwanin had the strongest effect [153]. Hydroxygenkwanin further reduced the intracellular ROS level and mitochondrial damage in X-ray-irradiated NHEKs. When the animal model was topically treated with each compound for 7 days, followed by plucking its hair, irradiating it with X-rays, and observing it after 38 days, the degree of gray hair and oxidative DNA damage was reduced in the group treated with hydroxygenkwanin, sterubin, or luteolin. However, other flavonoids, such as eriodictyol, homoeriodictyol, hesperetin, and diosmetin, as well as a reference drug minoxidil, had no such effects. Furthermore, topical application of hydroxygenkwanin, sterubin, or luteolin reduced the hair graying induced by repeated hair plucking [153]. However, when hydroxygenkwanin was administered after X-ray irradiation, its inhibitory effects on hair graying were absent. Additionally, its inhibitory effects on hair graying were not seen in Kit-mutant mice, such as KitW/+ and KitV620Atg1/+ mice [154].
4.3. Other Plant Extracts and Phytochemicals
Table 3 introduces several studies on other plant extracts and phytochemicals.

Table 3.
Effects of some plant extracts and phytochemicals on melanin synthesis or hair pigmentation.
Adzuki beans (Vigna angularis), also called red beans, have a higher polyphenol content than mung beans or soybeans [160]. The partially purified fraction from the hot water extract of Adzuki beans activated MITF through the Protein kinase A (PKA) signaling pathway at the cellular level, increasing the expression of melanogenic enzymes and melanin synthesis in B16 cells [155]. This effect was also reproduced in animal experiments, as the extract increased the melanin contents of back and abdomen hair in mice [155]
Tea (Camellia sinensis) is classified into six major categories, green tea, white tea, yellow tea, oolong tea, black tea, and dark tea, in the order of increases in the fermentation process, with Fuzhuan brick tea representing a kind of dark tea [161]. The n-hexane fraction of the ethanolic extract of Fuzhuan brick tea (phytochemical components: gallic acid, theaflavin, theobromine, caffeine, epicatechin, and quercetin) stimulated both the nuclear factor erythroid 2-related factor 2 (NRF2) signaling pathways and the MAPK signaling pathways, leading to expression of heme oxygenase 1 (HO1), SOD1, CAT, glutathione peroxidase 1 (GPX1), MITF, TYR, TYRP1, and TYRP2, as well as melanin synthesis in Melan-A cells [156]. It also stimulated hair pigmentation in seven-week-old male C57BL/6 mice treated with hydroquinone.
The extract of Gynostemma pentaphyllum (GP) may help prevent both hair loss and graying. It stimulated the proliferation of human dermal papilla cells by upregulating the WNT signaling pathway while downregulating the transforming growth factor-β (TGF-β) signaling pathway. Furthermore, its topical application enhanced hair growth in mice [157]. The extract stimulated the proliferation of murine melanoma B16 cells and their expression of MITF, MITF, TYR, TYRP1, and TYRP2 [157]. It further increased melanin content and TYR activity in B16 cells in the presence of epinephrine [157].
Bixin is an apocarotenoid compound found in Bixa orellana that can stimulate the NRF2 pathway by modifying cysteine residue in kelch-like ECH-associated protein 1 (KEAP1) [162]. Its systemic administration reduced the oxidative DNA damage and inflammatory reaction in wild-type SKH-1 mice, but such effects were not seen in Nrf2(−/−) mice [162]. Topical bixin treatment reduced UV-induced oxidative DNA damage induced by UV in wild-type SKH-1 mice, but not in Nrf2(−/−) mice [158]. Additionally, topical bixin retarded hair graying induced by PUVA phototherapy in wild-type C57BL/6J mice, but not in Nrf2(−/−) mice [158]. Thus, bixin can alleviate photo-oxidative skin damage or hair graying through NRF2 activation.
Rhynchophylline is an alkaloid compound found in certain Uncaria species (Rubiaceae) [163]. Rhynchophylline was identified as a blocker of β2AR, inhibiting the norepinephrine–β2AR–PKA signaling pathway [159]. Rhynchophylline restored the melanogenic pathway and intracellular calcium balance and attenuated the apoptosis of the A2058 and murine melanoma B16F10 cell lines influenced by norepinephrine. Its topical application reduced hair graying in seven-week-old male C57BL/6 mice exposed to resiniferatoxin [159].
5. Discussion
5.1. What Can Be the Targets for Treating Hair Graying?
Premature or senile hair graying occurs when hair growth is relatively normal, but pigmentation is insufficient. Therefore, the most direct cause of hair graying is the decline in the viability and function of melanocytes that synthesize and supply melanin [164].
MITF performs a central regulatory function in the differentiation and proliferation of melanocytes and melanin synthesis [69,165]. Therefore, various factors can influence hair graying if they are upstream regulators of MITF, downstream targets of MITF, or interacting partners of MITF. The SCF/c-KIT, ET1/ETRB, WNT/Frizzled, and α-MSH/MC1R signaling pathways are involved in MITF regulation [80]. Mutations and expression levels of MITF and several proteins that interact with it, such as SOX10 and IRF4, can affect the differentiation of MSCs and the death and function of melanocytes, leading to hair graying [70,71]. Topical α-MSH mimetic peptides have been proven effective in alleviating hair graying in patients [30,31]. Topical placental sphingolipid increased MITF level in follicular melanocytes and black hair growth in age-onset gray-haired C57BL/6J mice [43].
Oxidative stress is caused by the overproduction of ROS or other pro-oxidants and a lack of antioxidants or antioxidant enzymes, resulting in oxidative damage to the key cellular molecules, such as nucleic acids, lipids, and proteins [89,95]. Oxidative stress is both a cause and a consequence of aging and various diseases. Poor nutrition, lack of exercise, smoking, alcohol consumption, radiation, and air pollution can all increase oxidative stress, accelerating hair graying [85,86,87,88,89]. Oxidative stress due to X-ray irradiation, PUVA phototherapy, H2O2, or hydroquinone can induce hair graying, which symptoms can be alleviated by various antioxidant strategies: inhibition of ROS generation, scavenging ROS using antioxidants or antioxidant enzymes, and increasing the antioxidant capacity of cells [96,101,156,158].
Stress hormones are the main triggers of hair graying [104,105], while male hormones are one of the main triggers of alopecia [166]. An injection of resiniferatoxin causes pain stress and hair graying in animal models by stimulating norepinephrine secretion from the affected area [104,105]. The norepinephrine–β2AR–PKA signaling pathway mediates the pain stress responses in the body, including rapid differentiation and depletion of MSCs in the hair follicles, leading to hair graying [104,105]. A natural alkaloid compound, rhynchophylline, was identified as a novel β2AR blocker and its biological activity antagonizing the action of norepinephrine was verified in cells [159]. In animal models, rhynchophylline alleviated resiniferatoxin-induced hair graying [159]. Repeated hair plucking also caused hair graying in animal models probably via pain induction, and several EA-derived flavonoid compounds reduced the occurrence of white or gray hair [104,153].
Taken together, pharmacological approaches enhancing the expression and activity of MITF in melanocytes or effectively mitigating the negative effects of oxidative stress and stress hormones are expected to provide useful strategies for treating hair graying.
5.2. What Are the Modulatory Targets of Plant Extracts and Phytochemicals?
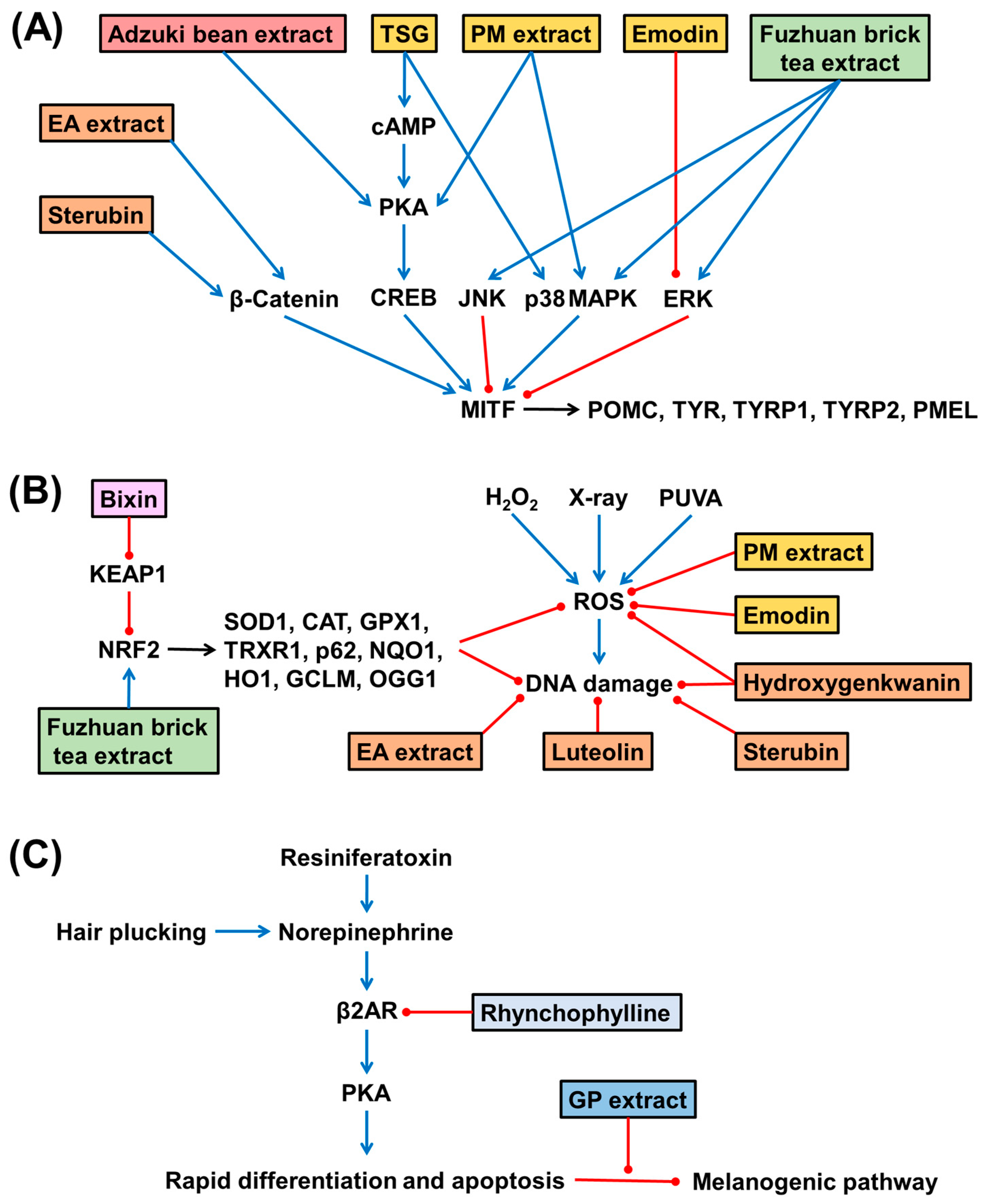
As summarized in Figure 2, various plant extracts and phytochemicals activate MITF, alleviate oxidative stress, or block stress hormone-induced response in melanocytes.

Figure 2.
Effects of plant extracts and phytochemicals on the MITF-melanogenic pathways (A), ROS-mediated oxidative stress (B), and the stress hormone-mediated response (C) in melanocytic cells and animal models. Extracts and phytochemicals derived from the same plant are indicated with the same color boxes. The stimulatory and suppressive effects are indicated by blue sharp arrows and red blunt arrows, respectively. Abbreviations; CAT, catalase; CREB, cyclic AMP response element-binding protein; EA, Eriodictyon angustifolium; ERK, extracellular signal-regulated kinase; GCLM, glutamate-cysteine ligase’s modifier subunit; GP, Gynostemma pentaphyllum; GPX1, glutathione peroxidase 1; HO1, heme oxygenase 1; JNK, c-jun N-terminal kinase; KEAP1, kelch-like ECH-associated protein 1; MAPK, mitogen-activated protein kinase; MITF, microphthalmia-associated transcription factor; NQO1, NAD(P)H quinone oxidoreductase 1; NRF2, nuclear factor erythroid 2-related factor 2; OGG1, 8-oxoguanine DNA glycosylase; PKA, protein kinase A; PM, Polygonum multiflorum; PMEL, premelanosome protein; POMC, pro-opiomelanocortin; PUVA, psoralen plus UVA; ROS, reactive oxygen species; SOD1, superoxide dismutase 1; TRXR1, thioredoxin reductase 1; TSG, 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside; TYR, tyrosinase; TYRP1, tyrosinase-related protein 1; TYRP2, tyrosinase-related protein 1; β2AR, β2 adrenergic receptor.
The extract of PM and its phytochemical components, such as TSG and emodin, the extract of EA and its components, such as sterubin, and the extracts of Adzuki beans and Fuzhuan brick tea activated MITF in melanocytes. PM extract, TSG, and emodin stimulated p38 MAPK or PKA activation or suppressed ERK activation, increasing the expression of MITF and target genes impacted by H2O2 [143,144,145]. EA extract and sterubin activated the β-catenin-dependent WNT signaling pathway and increased the expression of MITF and target genes impacted by X-ray irradiation [151,152]. Adzuki bean extract activated PKA and increased the expression of MITF and target genes [155]. The extract of Fuzhuan brick tea stimulated the phosphorylation of p38 MAPK, JNK, and ERK, which was associated with an increased expression of MITF and target genes [156].
The extracts of PM and its component (emodin), the extract of EA and its components (hydroxygenkwaninm, sterubin, and luteolin), the extract of Fuzhuan brick tea, and a carotenoid compound, bixin, all could alleviate oxidative stress in cells. The PM extract and emodin reduced the H2O2-induced cellular ROS levels [141,145]. The EA extract and its components, such as hydroxygenkwanin, sterubin, and luteolin, reduced the intracellular ROS level, oxidative DNA damage, and mitochondrial damage induced by X-ray irradiation [150,151,153]. The extract of Fuzhuan brick tea and bixin activated NRF2 and alleviated oxidative stress triggered by hydroquinone treatment or PUVA phototherapy [156,158].
Moreover, the extract of GP leaves and an alkaloid compound, rhynchophylline, blocked the stress hormone-induced response in cells. They inhibited the action of norepinephrine or resiniferatoxin and restored the melanogenesis pathway [157,159].
5.3. What Should Be the Next Research Topics to Advance Treatments for Human Hair Graying?
PKA and MAPKs play important roles in directly or indirectly regulating MITF expression and activity [167,168]. PKA activates CREB, which increases MITF expression in melanocytes stimulated by α-MSH [169]. Of the MAPKs, p38 MAPK directly phosphorylates MITF at Ser307 to increase its transcriptional activity [170] or activates PKA or other protein kinases, such as MAP kinase-activated protein kinase 2 (MAPKAPK2), to contribute to increased MITF expression through CREB [43,171]. ERK phosphorylates MITF at Ser73, enhancing both the transcriptional activity and proteasomal degradation of MITF, up- or downregulating MITF activity and expression depending on the cellular context [167]. JNK phosphorylates CREB-regulated transcription coactivator 3 (CRTC3), thereby inhibiting nuclear translocation and transcriptional activity, and suppressing MITF expression [172]. Thus, p38 MAPK has a consistent and greater influence than JNK and ERK in regulating MITF expression and activity.
The PM extract and TSG increased p38 MAPK activation and MITF expression, and this effect was neutralized by a selective inhibitor of p38 MAPK (SB203580). Additionally, placental sphingolipid and recombinant human IL-33 increased MITF expression through a similar mechanism [32,155]. These observations highlight the importance of p38 MAPK in regulating MITF expression and activity. Therefore, future studies should focus on the selective targeting of p38 MAPK as a strategy for treating hair graying due to melanocyte dysfunction.
NRF2 is a transcription factor regulating the gene expression of Phase II metabolism/antioxidant enzymes by binding the antioxidant-responsive elements (AREs) in the promoter of target genes, enhancing the cellular antioxidant defense capacity [173]. In basal state cells, cytosolic NRF2 is captured by dimeric KEAP1 or other proteins, which promote ubiquitylation and proteasomal degradation of NRF2. Under stimulated conditions, NRF2 escapes from KEAP1 or proteasomal degradation and translocates to the nucleus where it induces the transcription of target genes [174].
Topical administration of a fraction of Fuzhuan brick tea extract or an apocarotenoid compound bixin to mice increased the expression of NRF2 and its target genes in the skin and attenuated hair graying induced by hydroquinone treatment or PUVA phototherapy, respectively [156,158]. Therefore, an effective NRF2 activator could help attenuate hair graying due to oxidative stress. Consequently, future studies need to systemically examine the effects on hair graying of many known and unknown NRF2 modulators [174].
The rapid growth or turnover of hair might cause hair graying due to accelerated differentiation and depletion of MSCs [47,52,53]. Supporting this notion, less pigmented hair grew faster than pigmented black hair; in other words, hair that grew faster was less pigmented [51]. Although the cause of accelerated differentiation and depletion of MSCs during rapid hair growth is unclear, it might be due to excessive activation of the β-catenin-dependent WNT signaling pathway [60]. If so, the β-catenin-dependent WNT signaling pathway cannot be an ideal therapeutic target for alleviating hair graying, although it could be a promising therapeutic target for promoting hair growth [175,176].
Resiniferatoxin injection and repeated hair plucking commonly induce hair graying, probably by stimulating the release of norepinephrine, a stress hormone [104,153]. Interestingly, norepinephrine promoted hair follicle growth in an organotypic skin culture model [177], despite accelerating the differentiation and depletion of MSCs [106]. Therefore, proper regulation of the norepinephrine–β2AR–PKA signaling pathway is important for maintaining a balance between hair growth and pigmentation. Understanding how to control the secretion and action of norepinephrine to treat hair graying and hair loss will be an intriguing topic of future research.
Only a few studies have evaluated the anti-hair-graying efficacy of plant extracts, mixtures, or bioactive compounds through clinical trials [146,151,152], and most studies were conducted using human cells or mice. There are limitations to predicting their therapeutic efficacy for human hair graying based on mouse model studies because of the higher complexity of controlling hair pigmentation in humans. There may be other similar studies not covered in this review. For example, glycosylated polyphenols derived from Larix europea wood extract and Camellia sinensis leaf extract reduced oxidative stress and protected MSCs in human hair follicles ex vivo, and in a clinical study carried out for 4 months on 44 Caucasian male volunteers with 30% to 95% gray hair, a lotion containing the active ingredient reduced the proportion of gray hairs to the total hairs and the number of gray hairs per unit scalp area relative to day 0 values to a greater extent compared to the placebo control [178]. More expanded clinical trials are needed to evaluate the efficacy of various plant-derived natural products on human hair graying.
Early diagnosis and patient-tailored treatment are essential. As the possible causative factors for hair graying are known, specifying the exact cause through questionnaires and biochemical analysis is important for specific patients. In reported cases, hair graying occurring due to iron or essential fatty acid deficiency was treated by supplying each nutrient [117,122]. Mirtazapine-induced hair graying could be treated by discontinuing this medication [128]. Analysis of the patient’s oxidative stress status and stress hormone levels will also provide an important guide to patient-tailored treatment.
Since plant-derived natural products have both pros and cons as medicines, they must be used for appropriate purposes in appropriate amounts to find a balance between efficacy and side effects [37,39,40]. For example, PM extract may be used to alleviate both hair loss and graying [133,134,179], but caution should be taken because its high doses can cause liver toxicity [180,181]. Molecular targets for treating hair graying are being specified, and information on the efficacy and mechanism of action of individual phytochemicals is expanding. We hope that by using such information, an effective treatment that will help maintain the health and beauty of hair will be developed in the near future.
6. Conclusions
This review addressed various intrinsic and extrinsic factors influencing the onset or progression of premature and senile hair graying. It further addressed the efficacy and mechanism of action of numerous plant extracts and phytochemicals that can help alleviate these symptoms. Certain types of hair graying can be prevented or treated by enhancing MSC maintenance or melanocyte function, reducing oxidative stress, and managing the secretion and action of stress hormones. Tactical approaches to pursue this goal may include a selective activation of the p38 MAPK–MITF axis, enhancing cellular antioxidant capacity through activating NRF2, and modulating the norepinephrine–β2AR–PKA signaling pathway. Furthermore, accurately diagnosing the cause of hair graying for specific patients and applying customized treatment as early as possible are important.
Funding
This research received no external funding.
Conflicts of Interest
The author declares no conflicts of interest.
References
- Morgan, B.A. The Dermal Papilla: An Instructive Niche for Epithelial Stem and Progenitor Cells in Development and Regeneration of the Hair Follicle. CSH Perspect. Med. 2014, 4, a015180. [Google Scholar] [CrossRef]
- Houschyar, K.S.; Borrelli, M.R.; Tapking, C.; Popp, D.; Puladi, B.; Ooms, M.; Chelliah, M.P.; Rein, S.; Pförringer, D.; Thor, D.; et al. Molecular Mechanisms of Hair Growth and Regeneration: Current Understanding and Novel Paradigms. Dermatology 2020, 236, 271–280. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.Y.; Zhu, L.; He, J. Morphogenesis, Growth Cycle and Molecular Regulation of Hair Follicles. Front. Cell Dev. Biol. 2022, 10, 899095. [Google Scholar] [CrossRef] [PubMed]
- Schneider, M.R.; Schmidt-Ullrich, R.; Paus, R. The Hair Follicle as a Dynamic Miniorgan. Curr. Biol. 2009, 19, R132–R142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Chen, T. Local and systemic mechanisms that control the hair follicle stem cell niche. Nat. Rev. Mol. Cell Biol. 2024, 25, 87–100. [Google Scholar] [CrossRef]
- Morita, R.; Sanzen, N.; Sasaki, H.; Hayashi, T.; Umeda, M.; Yoshimura, M.; Yamamoto, T.; Shibata, T.; Abe, T.; Kiyonari, H.; et al. Tracing the origin of hair follicle stem cells. Nature 2021, 594, 547. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, S. Deciphering the molecular mechanisms of stem cell dynamics in hair follicle regeneration. Exp. Mol. Med. 2024, 56, 110–117. [Google Scholar] [CrossRef]
- Natarelli, N.; Gahoonia, N.; Sivamani, R.K. Integrative and Mechanistic Approach to the Hair Growth Cycle and Hair Loss. J. Clin. Med. 2023, 12, 893. [Google Scholar] [CrossRef]
- Mort, R.L.; Jackson, I.J.; Patton, E.E. The melanocyte lineage in development and disease. Development 2015, 142, 620–632. [Google Scholar] [CrossRef]
- Huang, L.L.; Zuo, Y.Z.; Li, S.L.; Li, C.Y. Melanocyte stem cells in the skin: Origin, biological characteristics, homeostatic maintenance and therapeutic potential. Clin. Transl. Med. 2024, 14, e1720. [Google Scholar] [CrossRef]
- Sun, Q.; Lee, W.; Hu, H.; Ogawa, T.; De Leon, S.; Katehis, I.; Lim, C.H.; Takeo, M.; Cammer, M.; Taketo, M.M.; et al. Dedifferentiation maintains melanocyte stem cells in a dynamic niche. Nature 2023, 616, 774. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.J.; Yu, Y.; Liu, C.Y.; Zhang, X.; Zhu, P.Y.; Peng, Y.; Yan, X.Y.; Li, Y.; Hua, P.; Li, Q.F.; et al. Single-cell transcriptomics reveals lineage trajectory of human scalp hair follicle and informs mechanisms of hair graying. Cell Discov. 2022, 8, 49. [Google Scholar] [CrossRef]
- Chen, J.; Zheng, Y.X.; Hu, C.; Jin, X.X.; Chen, X.P.; Xiao, Y.; Wang, C.C. Hair Graying Regulators Beyond Hair Follicle. Front. Physiol. 2022, 13, 839859. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Fan, Z.X.; Zhu, D.C.; Guo, Y.L.; Ye, K.; Dai, D.; Guo, Z.; Hu, Z.Q.; Miao, Y.; Qu, Q. Emerging Role of Dermal White Adipose Tissue in Modulating Hair Follicle Development During Aging. Front. Cell Dev. Biol. 2021, 9, 728188. [Google Scholar] [CrossRef]
- Singal, A.; Daulatabad, D.; Grover, C. Graying severity score: A useful tool for evaluation of premature canities. Indian Dermatol. Online J. 2016, 7, 164–167. [Google Scholar] [CrossRef]
- Mirmirani, P. Age-related hair changes in men: Mechanisms and management of alopecia and graying. Maturitas 2015, 80, 58–62. [Google Scholar] [CrossRef]
- O’Sullivan, J.D.B.; Nicu, C.; Picard, M.; Cheret, J.; Bedogni, B.; Tobin, D.J.; Paus, R. The biology of human hair greying. Biol. Rev. Camb. Philos. Soc. 2021, 96, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.B.; Shamim, H.; Nagaraju, U. Premature Graying of Hair: Review with Updates. Int. J. Trichology 2018, 10, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Panhard, S.; Lozano, I.; Loussouarn, G. Greying of the human hair: A worldwide survey, revisiting the ‘50’ rule of thumb. Br. J. Dermatol. 2012, 167, 865–873. [Google Scholar] [CrossRef]
- Acer, E.; Arslantas, D.; Emiral, G.Ö.; Ünsal, A.; Atalay, B.I.; Göktas, S. Clinical and epidemiological characteristics and associated factors of hair graying: A population-based, cross-sectional study in Turkey. An. Bras. Dermatol. 2020, 95, 439–446. [Google Scholar] [CrossRef]
- Tobin, D.J.; Paus, R. Graying: Gerontobiology of the hair follicle pigmentary unit. Exp. Gerontol. 2001, 36, 29–54. [Google Scholar] [CrossRef] [PubMed]
- Kaur, K.; Kaur, R.; Bala, I. Therapeutics of premature hair graying: A long journey ahead. J. Cosmet. Dermatol. 2019, 18, 1206–1214. [Google Scholar] [CrossRef] [PubMed]
- Saad, M.; Babar, N.F.; Majeed, R.; Rehman, A.U.; Khan, O.A.; Chatha, D.E.; Aamir, U.; Nadeem, A.; Abbas, S. Impact of Premature Greying of Hair on Socio-cultural Adjustment and Self-esteem among Medical Undergraduates in Foundation University, Islamabad. Cureus 2019, 11, e5083. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, A.M.; Rausser, S.; Ren, J.; Mosharov, E.V.; Sturm, G.; Ogden, R.T.; Patel, P.; Kumar Soni, R.; Lacefield, C.; Tobin, D.J.; et al. Quantitative mapping of human hair greying and reversal in relation to life stress. Elife 2021, 10, e67437. [Google Scholar] [CrossRef]
- Pandhi, D.; Khanna, D. Premature graying of hair. Indian J. Dermatol. Venereol. Leprol. 2013, 79, 641–653. [Google Scholar] [CrossRef]
- Chow, E.Y.; Salopek, T.G. Acitretin-Induced Repigmentation of Gray Hair: A Case Report. Cureus 2024, 16, e58261. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, Q.; Wang, Y.; Yang, D. Two case studies of persistent white hair regrowth after alopecia areata turning pigmented following treatment. J. Cosmet. Dermatol. 2024, 23, 2490–2495. [Google Scholar] [CrossRef]
- Dasanu, C.A.; Mitsis, D.; Alexandrescu, D.T. Hair repigmentation associated with the use of lenalidomide: Graying may not be an irreversible process! J. Oncol. Pharm. Pract. 2013, 19, 165–169. [Google Scholar] [CrossRef]
- Yale, K.; Juhasz, M.; Atanaskova Mesinkovska, N. Medication-Induced Repigmentation of Gray Hair: A Systematic Review. Skin. Appendage Disord. 2020, 6, 1–10. [Google Scholar] [CrossRef]
- Sakhiya, J.J.; Sakhiya, D.J.; Patel, M.R.; Daruwala, F.R. Case report on premature hair graying treated with Melitane 5% and oral hair supplements. Indian J. Pharmacol. 2019, 51, 346–349. [Google Scholar] [CrossRef]
- Chavan, D. Reversal of Premature Hair Graying Treated with a Topical Formulation Containing α-Melanocyte-Stimulating Hormone Agonist (Greyverse Solution 2%). Int. J. Trichology 2022, 14, 207–209. [Google Scholar] [CrossRef]
- Feng, Z.R.; Qin, Y.; Jiang, G. Reversing Gray Hair: Inspiring the Development of New Therapies Through Research on Hair Pigmentation and Repigmentation Progress. Int. J. Biol. Sci. 2023, 19, 4588–4607. [Google Scholar] [CrossRef] [PubMed]
- Paus, R.; Sevilla, A.; Grichnik, J.M. Human Hair Graying Revisited: Principles, Misconceptions, and Key Research Frontiers. J. Investig. Dermatol. 2024, 144, 474–491. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Samota, M.K.; Choudhary, M.; Choudhary, M.; Pandey, A.K.; Sharma, A.; Thakur, J. How do plants defend themselves against pathogens—Biochemical mechanisms and genetic interventions. Physiol. Mol. Biol. Plants 2022, 28, 485–504. [Google Scholar] [CrossRef]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-derived natural products for drug discovery: Current approaches and prospects. Nucleus 2022, 65, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Emerging Strategies to Protect the Skin from Ultraviolet Rays Using Plant-Derived Materials. Antioxidants 2020, 9, 637. [Google Scholar] [CrossRef]
- Boo, Y.C. Can Plant Phenolic Compounds Protect the Skin from Airborne Particulate Matter? Antioxidants 2019, 8, 379. [Google Scholar] [CrossRef]
- Sitarek, P.; Kowalczyk, T.; Wieezfinska, J.; Merecz-Sadowska, A.; Górski, K.; Sliwinski, T.; Skala, E. Plant Extracts as a Natural Source of Bioactive Compounds and Potential Remedy for the Treatment of Certain Skin Diseases. Curr. Pharm. Des. 2020, 26, 2859–2875. [Google Scholar] [CrossRef]
- Boo, Y.C. Insights into How Plant-Derived Extracts and Compounds Can Help in the Prevention and Treatment of Keloid Disease: Established and Emerging Therapeutic Targets. Int. J. Mol. Sci. 2024, 25, 1235. [Google Scholar] [CrossRef]
- Choi, J.Y.; Boo, M.Y.; Boo, Y.C. Can Plant Extracts Help Prevent Hair Loss or Promote Hair Growth? A Review Comparing Their Therapeutic Efficacies, Phytochemical Components, and Modulatory Targets. Molecules 2024, 29, 29102288. [Google Scholar] [CrossRef]
- Peters, E.M.; Liezmann, C.; Spatz, K.; Ungethum, U.; Kuban, R.J.; Daniltchenko, M.; Kruse, J.; Imfeld, D.; Klapp, B.F.; Campiche, R. Profiling mRNA of the graying human hair follicle constitutes a promising state-of-the-art tool to assess its aging: An exemplary report. J. Investig. Dermatol. 2013, 133, 1150–1160. [Google Scholar] [CrossRef]
- Bian, Y.M.; Wei, G.; Song, X.; Yuan, L.; Chen, H.Y.; Ni, T.; Lu, D.R. Global downregulation of pigmentation-associated genes in human premature hair graying. Exp. Ther. Med. 2019, 18, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Singh, S.K.; Mallick, S.; Bera, R.; Datta, P.K.; Mandal, M.; Roy, S.; Bhadra, R. Sphingolipid-mediated restoration of Mitf expression and repigmentation in vivo in a mouse model of hair graying. Pigment. Cell Melanoma Res. 2009, 22, 205–218. [Google Scholar] [CrossRef] [PubMed]
- Commo, S.; Gaillard, O.; Bernard, B.A. Human hair greying is linked to a specific depletion of hair follicle melanocytes affecting both the bulb and the outer root sheath. Br. J. Dermatol. 2004, 150, 435–443. [Google Scholar] [CrossRef] [PubMed]
- Peters, E.M.; Imfeld, D.; Graub, R. Graying of the human hair follicle. J. Cosmet. Sci. 2011, 62, 121–125. [Google Scholar]
- Pss, R.; Madhunapantula, S.V.; Betkerur, J.B.; Bovilla, V.R.; Shastry, V. Melanogenesis Markers Expression in Premature Graying of Hair: A Cross-Sectional Study. Skin Pharmacol. Physiol. 2022, 35, 180–186. [Google Scholar] [CrossRef]
- Nishimura, E.K.; Granter, S.R.; Fisher, D.E. Mechanisms of hair graying: Incomplete melanocyte stem cell maintenance in the niche. Science 2005, 307, 720–724. [Google Scholar] [CrossRef]
- Ji, J.; Ho, B.S.; Qian, G.; Xie, X.M.; Bigliardi, P.L.; Bigliardi-Qi, M. Aging in hair follicle stem cells and niche microenvironment. J. Dermatol. 2017, 44, 1097–1104. [Google Scholar] [CrossRef]
- Choi, Y.J.; Yoon, T.J.; Lee, Y.H. Changing expression of the genes related to human hair graying. Eur. J. Dermatol. 2008, 18, 397–399. [Google Scholar] [CrossRef]
- Shi, Y.; Luo, L.F.; Liu, X.M.; Zhou, Q.; Xu, S.Z.; Lei, T.C. Premature Graying as a Consequence of Compromised Antioxidant Activity in Hair Bulb Melanocytes and Their Precursors. PLoS ONE 2014, 9, e93589. [Google Scholar] [CrossRef]
- Choi, H.I.; Choi, G.I.; Kim, E.K.; Choi, Y.J.; Sohn, K.C.; Lee, Y.; Kim, C.D.; Yoon, T.J.; Sohn, H.J.; Han, S.H.; et al. Hair greying is associated with active hair growth. Br. J. Dermatol. 2011, 165, 1183–1189. [Google Scholar] [CrossRef] [PubMed]
- Steingrímsson, E.; Copeland, N.G.; Jenkins, N.A. Melanocyte stem cell maintenance and hair graying. Cell 2005, 121, 9–12. [Google Scholar] [CrossRef]
- Jo, S.K.; Lee, J.Y.; Lee, Y.; Kim, C.D.; Lee, J.H.; Lee, Y.H. Three Streams for the Mechanism of Hair Graying. Ann. Dermatol. 2018, 30, 397–401. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.X.; Liu, Y.H.; He, J.; Wang, J.R.; Chen, X.D.; Yang, R.H. Regulation of signaling pathways in hair follicle stem cells. Burns Trauma 2022, 10, tkac022. [Google Scholar] [CrossRef] [PubMed]
- Schouwey, K.; Delmas, V.; Larue, L.; Zimber-Strobl, U.; Strobl, L.J.; Radtke, F.; Beermann, F. Notch1 and Notch2 receptors influence progressive hair graying in a dose-dependent manner. Dev. Dyn. 2007, 236, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Schouwey, K.; Beermann, F. The Notch pathway: Hair graying and pigment cell homeostasis. Histol. Histopathol. 2008, 23, 609–619. [Google Scholar] [CrossRef]
- Lee, Y.R.; Pandolfi, P.P. PTEN Mouse Models of Cancer Initiation and Progression. Cold Spring Harb. Perspect. Med. 2020, 10, a037283. [Google Scholar] [CrossRef]
- Inoue-Narita, T.; Hamada, K.; Sasaki, T.; Hatakeyama, S.; Fujita, S.; Kawahara, K.; Sasaki, M.; Kishimoto, H.; Eguchi, S.; Kojima, I.; et al. Pten deficiency in melanocytes results in resistance to hair graying and susceptibility to carcinogen-induced melanomagenesis. Cancer Res. 2008, 68, 5760–5768. [Google Scholar] [CrossRef]
- Endou, M.; Aoki, H.; Kobayashi, T.; Kunisada, T. Prevention of hair graying by factors that promote the growth and differentiation of melanocytes. J. Dermatol. 2014, 41, 716–723. [Google Scholar] [CrossRef]
- Zhang, Z.; Lei, M.; Xin, H.; Hu, C.; Yang, T.; Xing, Y.; Li, Y.; Guo, H.; Lian, X.; Deng, F. Wnt/β-catenin signaling promotes aging-associated hair graying in mice. Oncotarget 2017, 8, 69316–69327. [Google Scholar] [CrossRef]
- Zhang, X.; Zhu, J.; Zhang, J.; Zhao, H. Melanocyte stem cells and hair graying. J. Cosmet. Dermatol. 2023, 22, 1720–1723. [Google Scholar] [CrossRef]
- Phan, B.; Ali, A.M.; Black, T.A.; Kashyap, A.; Niazi, M.; Rashid, R.M. Fractal Pattern in the Premature Graying of Hair: A Case Report. Cureus 2024, 16, e59994. [Google Scholar] [CrossRef] [PubMed]
- Rangu, S.A.; Oza, V.S. Poliosis, hair pigment dilution, and premature graying of the hair: A diagnostic approach in pediatric patients and review of the literature. Pediatr. Dermatol. 2024, 41, 197–203. [Google Scholar] [CrossRef]
- Fatemi Naieni, F.; Ebrahimi, B.; Vakilian, H.R.; Shahmoradi, Z. Serum iron, zinc, and copper concentration in premature graying of hair. Biol. Trace Elem. Res. 2012, 146, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Nath, B.; Gupta, V.; Kumari, R. A Community Based Study to Estimate Prevalence and Determine Correlates of Premature Graying of Hair among Young Adults in Srinagar, Uttarakhand, India. Int. J. Trichology 2020, 12, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Ryu, H.H.; Yoon, J.; Jo, S.; Jang, S.; Choi, M.; Kwon, O.; Jo, S.J. Association of premature hair graying with family history, smoking, and obesity: A cross-sectional study. J. Am. Acad. Dermatol. 2015, 72, 321–327. [Google Scholar] [CrossRef] [PubMed]
- Anggraini, D.R.; Feriyawati, L.; Hidayat, H.; Wahyuni, A.S. Risk Factors Associated with Premature Hair Greying of Young Adult. Open Access Maced. J. Med. Sci. 2019, 7, 3762–3764. [Google Scholar] [CrossRef]
- Thompson, K.G.; Marchitto, M.C.; Ly, B.C.K.; Chien, A.L. Evaluation of Physiological, Psychological, and Lifestyle Factors Associated with Premature Hair Graying. Int. J. Trichology 2019, 11, 153–158. [Google Scholar] [CrossRef]
- Steingrímsson, E.; Arnheiter, H.; Hallsson, J.H.; Lamoreux, M.L.; Copeland, N.G.; Jenkins, N.A. Interallelic complementation at the mouse Mitf locus. Genetics 2003, 163, 267–276. [Google Scholar] [CrossRef]
- Harris, M.L.; Fufa, T.D.; Palmer, J.W.; Joshi, S.S.; Larson, D.M.; Incao, A.; Gildea, D.E.; Trivedi, N.S.; Lee, A.N.; Day, C.P.; et al. A direct link between MITF, innate immunity, and hair graying. PLoS Biol. 2018, 16, e2003648. [Google Scholar] [CrossRef]
- Adhikari, K.; Fontanil, T.; Cal, S.; Mendoza-Revilla, J.; Fuentes-Guajardo, M.; Chacón-Duque, J.C.; Al-Saadi, F.; Johansson, J.A.; Quinto-Sanchez, M.; Acuña-Alonzo, V.; et al. A genome-wide association scan in admixed Latin Americans identifies loci influencing facial and scalp hair features. Nat. Commun. 2016, 7, 10815. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, C.; Grill, C.; Stacey, S.N.; Metcalf, A.M.; Gorkin, D.U.; Robinson, K.C.; Van Otterloo, E.; Kim, R.S.Q.; Bergsteinsdottir, K.; Ogmundsdottir, M.H.; et al. A Polymorphism in IRF4 Affects Human Pigmentation through a Tyrosinase-Dependent MITF/TFAP2A Pathway. Cell 2013, 155, 1022–1033. [Google Scholar] [CrossRef] [PubMed]
- Pospiech, E.; Kukla-Bartoszek, M.; Karlowska-Pik, J.; Zielinski, P.; Wozniak, A.; Boron, M.; Dabrowski, M.; Zubanska, M.; Jarosz, A.; Grzybowski, T.; et al. Exploring the possibility of predicting human head hair greying from DNA using whole-exome and targeted NGS data. BMC Genom. 2020, 21, 538. [Google Scholar] [CrossRef] [PubMed]
- Lambert, K.A.; Clements, C.M.; Mukherjee, N.; Pacheco, T.R.; Shellman, S.X.; Henen, M.A.; Vogeli, B.; Goldstein, N.B.; Birlea, S.; Hintzsche, J.; et al. SASH1 S519N Variant Links Skin Hyperpigmentation and Premature Hair Graying to Dysfunction of Melanocyte Lineage. J. Investig. Dermatol. 2024, S0022-0202X(0024)00393-00392. [Google Scholar] [CrossRef]
- Lambert, K.A.; Clements, C.M.; Mukherjee, N.; Pacheco, T.R.; Shellman, S.X.; Henen, M.A.; Vogeli, B.; Goldstein, N.B.; Birlea, S.; Hintzsche, J.; et al. SASH1 interacts with TNKS2 and promotes human melanocyte stem cell maintenance. bioRxiv 2023. [Google Scholar] [CrossRef]
- Yao, Q.; Chen, Y.; Zhou, X. The roles of microRNAs in epigenetic regulation. Curr. Opin. Chem. Biol. 2019, 51, 11–17. [Google Scholar] [CrossRef]
- Bertrand, J.U.; Petit, V.; Aktary, Z.; de la Grange, P.; Elkoshi, N.; Sohier, P.; Delmas, V.; Levy, C.; Larue, L. Loss of Dicer in Newborn Melanocytes Leads to Premature Hair Graying and Changes in Integrin Expression. J. Investig. Dermatol. 2024, 144, 601–611. [Google Scholar] [CrossRef]
- Jo, S.J.; Paik, S.H.; Choi, J.W.; Lee, J.H.; Cho, S.; Kim, K.H.; Eun, H.C.; Kwon, O.S. Hair Graying Pattern Depends on Gender, Onset Age and Smoking Habits. Acta Derm.-Venereol. 2012, 92, 160–161. [Google Scholar] [CrossRef]
- Jo, S.J.; Shin, H.; Paik, S.H.; Choi, J.W.; Lee, J.H.; Cho, S.; Kwon, O. The Pattern of Hair Dyeing in Koreans with Gray Hair. Ann. Dermatol. 2013, 25, 401–404. [Google Scholar] [CrossRef]
- Wang, S.; Kang, Y.; Qi, F.; Jin, H. Genetics of hair graying with age. Ageing Res. Rev. 2023, 89, 101977. [Google Scholar] [CrossRef]
- Iida, M.; Tazaki, A.; Yajima, I.; Ohgami, N.; Taguchi, N.; Goto, Y.; Kumasaka, M.Y.; Prevost-Blondel, A.; Kono, M.; Akiyama, M.; et al. Hair graying with aging in mice carrying oncogenic RET. Aging Cell 2020, 19, e13273. [Google Scholar] [CrossRef] [PubMed]
- Giesen, M.; Gruedl, S.; Holtkoetter, O.; Fuhrmann, G.; Koerner, A.; Petersohn, D. Ageing processes influence keratin and KAP expression in human hair follicles. Exp. Dermatol. 2011, 20, 759–761. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; He, C. Exploration of potential lipid biomarkers for age-induced hair graying by lipidomic analyses of hair shaft roots with follicular tissue attached. J. Cosmet. Dermatol. 2022, 21, 6118–6123. [Google Scholar] [CrossRef]
- Tan, B.L.; Norhaizan, M.E.; Liew, W.P.; Sulaiman Rahman, H. Antioxidant and Oxidative Stress: A Mutual Interplay in Age-Related Diseases. Front. Pharmacol. 2018, 9, 1162. [Google Scholar] [CrossRef]
- Seiberg, M. Age-induced hair greying—The multiple effects of oxidative stress. Int. J. Cosmet. Sci. 2013, 35, 532–538. [Google Scholar] [CrossRef]
- Trueb, R.M. Oxidative stress in ageing of hair. Int. J. Trichology 2009, 1, 6–14. [Google Scholar] [CrossRef]
- Arck, P.C.; Overall, R.; Spatz, K.; Liezman, C.; Handjiski, B.; Klapp, B.F.; Birch-Machin, M.A.; Peters, E.M.J. Towards a “free radical theory of graying”: Melanocyte apoptosis in the aging human hair follicle is an indicator of oxidative stress induced tissue damage. FASEB J. 2006, 20, 1567–1569. [Google Scholar] [CrossRef]
- Acer, E.; Kaya Erdogan, H.; Kocaturk, E.; Saracoglu, Z.N.; Alatas, O.; Bilgin, M. Evaluation of oxidative stress and psychoemotional status in premature hair graying. J. Cosmet. Dermatol. 2020, 19, 3403–3407. [Google Scholar] [CrossRef] [PubMed]
- Saxena, S.; Gautam, R.K.; Gupta, A.; Chitkara, A. Evaluation of Systemic Oxidative Stress in Patients with Premature Canities and Correlation of Severity of Hair Graying with the Degree of Redox Imbalance. Int. J. Trichology 2020, 12, 16–23. [Google Scholar] [CrossRef]
- Chen, J.J.; Liu, Y.; Zhao, Z.; Qiu, J. Oxidative stress in the skin: Impact and related protection. Int. J. Cosmet. Sci. 2021, 43, 495–509. [Google Scholar] [CrossRef]
- Kaminski, K.; Kazimierczak, U.; Kolenda, T. Oxidative stress in melanogenesis and melanoma development. Wspolczesna Onkol./Contemp. Oncol. 2022, 26, 112447. [Google Scholar] [CrossRef]
- Geueke, A.; Mantellato, G.; Kuester, F.; Schettina, P.; Nelles, M.; Seeger, J.M.; Kashkar, H.; Niemann, C. The anti-apoptotic Bcl-2 protein regulates hair follicle stem cell function. EMBO Rep. 2021, 22, e52301. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.M.; Decker, H.; Hartmann, H.; Chavan, B.; Rokos, H.; Spencer, J.D.; Hasse, S.; Thornton, M.J.; Shalbaf, M.; Paus, R.; et al. Senile hair graying: H2O2-mediated oxidative stress affects human hair color by blunting methionine sulfoxide repair. FASEB J. 2009, 23, 2065–2075. [Google Scholar] [CrossRef] [PubMed]
- Kauser, S.; Westgate, G.E.; Green, M.R.; Tobin, D.J. Human Hair Follicle and Epidermal Melanocytes Exhibit Striking Differences in Their Aging Profile which Involves Catalase. J. Investig. Dermatol. 2011, 131, 979–982. [Google Scholar] [CrossRef]
- Sikkink, S.K.; Mine, S.; Freis, O.; Danoux, L.; Tobin, D.J. Stress-sensing in the human greying hair follicle: Ataxia Telangiectasia Mutated (ATM) depletion in hair bulb melanocytes in canities-prone scalp. Sci. Rep. 2020, 10, 18711. [Google Scholar] [CrossRef]
- Emerit, I.; Filipe, P.; Freitas, J.; Vassy, J. Protective effect of superoxide dismutase against hair graying in a mouse model. Photochem. Photobiol. 2004, 80, 579–582. [Google Scholar] [CrossRef] [PubMed]
- Ungvari, A.; Kiss, T.; Gulej, R.; Tarantini, S.; Csik, B.; Yabluchanskiy, A.; Mukli, P.; Csiszar, A.; Harris, M.L.; Ungvari, Z. Irradiation-induced hair graying in mice: An experimental model to evaluate the effectiveness of interventions targeting oxidative stress, DNA damage prevention, and cellular senescence. Geroscience 2024, 46, 3105–3122. [Google Scholar] [CrossRef]
- Dai, D.M.; He, Y.; Guan, Q.; Fan, Z.X.; Zhu, Y.M.; Wang, J.; Wu, S.L.; Chen, J.; Le, D.J.; Hu, Z.Q.; et al. Modeling human gray hair by irradiation as a valuable tool to study aspects of tissue aging. Geroscience 2023, 45, 1215–1230. [Google Scholar] [CrossRef]
- Anderson, Z.T.; Palmer, J.W.; Idris, M.I.; Villavicencio, K.M.; Le, G.; Cowart, J.; Weinstein, D.E.; Harris, M.L. Topical RT1640 treatment effectively reverses gray hair and stem cell loss in a mouse model of radiation-induced canities. Pigment Cell Melanoma Res. 2021, 34, 89–100. [Google Scholar] [CrossRef]
- Aoki, H.; Hara, A.; Motohashi, T.; Kunisada, T. Keratinocyte stem cells but not melanocyte stem cells are the primary target for radiation-induced hair graying. J. Investig. Dermatol. 2013, 133, 2143–2151. [Google Scholar] [CrossRef]
- Qiu, R.; Qiu, X.; Su, M.; Sun, M.; Wang, Y.; Wu, J.; Wang, H.; Tang, D.; Tao, S. Dietary Restriction Delays But Cannot Heal Irradiation-Induced Hair Graying by Preserving Hair Follicle Stem Cells in Quiescence. Rejuvenation Res. 2023, 26, 242–252. [Google Scholar] [CrossRef]
- Mueller, B.; Figueroa, A.; Robinson-Papp, J. Structural and functional connections between the autonomic nervous system, hypothalamic-pituitary-adrenal axis, and the immune system: A context and time dependent stress response network. Neurol. Sci. 2022, 43, 951–960. [Google Scholar] [CrossRef] [PubMed]
- Ulrich-Lai, Y.M.; Herman, J.P. Neural regulation of endocrine and autonomic stress responses. Nat. Rev. Neurosci. 2009, 10, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Ma, S.; Rachmin, I.; He, M.; Baral, P.; Choi, S.; Goncalves, W.A.; Shwartz, Y.; Fast, E.M.; Su, Y.; et al. Hyperactivation of sympathetic nerves drives depletion of melanocyte stem cells. Nature 2020, 577, 676–681. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; He, M.G.; Rachmin, I.; Yu, X.L.; Kim, S.; Fisher, D.E.; Hsu, Y.C. Melanocortin 1 receptor is dispensable for acute stress induced hair graying in mice. Exp. Dermatol. 2021, 30, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Mendelsohn, A.R.; Larrick, J.W. The Danger of Being Too Sympathetic: Norepinephrine in Alzheimer’s Disease and Graying of Hair. Rejuvenation Res. 2020, 23, 68–72. [Google Scholar] [CrossRef]
- Kocaman, S.A.; Cetin, M.; Durakoglugil, M.E.; Erdogan, T.; Canga, A.; Cicek, Y.; Dogan, S.; Sahin, I.; Satiroglu, O.; Bostan, M. The degree of premature hair graying as an independent risk marker for coronary artery disease: A predictor of biological age rather than chronological age. Anadolu Kardiyol. Derg. 2012, 12, 457–463. [Google Scholar] [CrossRef]
- Sharma, K.; Humane, D.; Shah, K.; Patil, S.; Charaniya, R.; Meniya, J. Androgenic alopecia, premature graying, and hair thinning as independent predictors of coronary artery disease in young Asian males. Cardiovasc. Endocrinol. 2017, 6, 152–158. [Google Scholar] [CrossRef]
- ElFaramawy, A.A.A.; Hanna, I.S.; Darweesh, R.M.; Ismail, A.S.; Kandil, H.I. The degree of hair graying as an independent risk marker for coronary artery disease, a CT coronary angiography study. Egypt. Heart J. 2018, 70, 15–19. [Google Scholar] [CrossRef]
- Paik, S.H.; Jang, S.; Joh, H.K.; Lim, C.S.; Cho, B.; Kwon, O.; Jo, S.J. Association Between Premature Hair Greying and Metabolic Risk Factors: A Cross-sectional Study. Acta Derm.-Venereol. 2018, 98, 748–752. [Google Scholar] [CrossRef]
- Ozbay, I.; Kahraman, C.; Kucur, C.; Namdar, N.D.; Oghan, F. Is there a relationship between premature hair greying and hearing impairment? J. Laryngol. Otol. 2015, 129, 1097–1100. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Choudhary, A.; Kumar, A.; Zaidi, A.; Mohanty, S.; Yadav, S. A Study of Association of Premature Graying of Hair and Osteopenia in North Indian Population. Int. J. Trichology 2020, 12, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Acer, E.; Erdogan, H.K.; Igrek, A.; Parlak, H.; Saraçoglu, Z.N.; Bilgin, M. Relationship between diet, atopy, family history, and premature hair graying. J. Cosmet. Dermatol. 2019, 18, 665–670. [Google Scholar] [CrossRef]
- Aggarwal, A.; Srivastava, S.; Agarwal, M.P.; Dwivedi, S. Premature Graying of Hair: An Independent Risk Marker for Coronary Artery Disease in Smokers—A Retrospective Case Control Study. Ethiop. J. Health Sci. 2015, 25, 123–128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mahendiratta, S.; Sarma, P.; Kaur, H.; Kaur, S.; Kaur, H.; Bansal, S.; Prasad, D.; Prajapat, M.; Upadhay, S.; Kumar, S.; et al. Premature graying of hair: Risk factors, co-morbid conditions, pharmacotherapy and reversal—A systematic review and meta-analysis. Dermatol. Ther. 2020, 33, e13990. [Google Scholar] [CrossRef]
- El-Sheikh, A.M.; Elfar, N.N.; Mourad, H.A.; Hewedy, E.S. Relationship between Trace Elements and Premature Hair Graying. Int. J. Trichology 2018, 10, 278–283. [Google Scholar] [CrossRef]
- Choi, J.W.; Lew, B.L.; Sim, W.Y. A Case of Premature Hair Graying Treated with Ferrous Sulfate. Ann. Dermatol. 2016, 28, 775–776. [Google Scholar] [CrossRef]
- Bhat, R.M.; Sharma, R.; Pinto, A.C.; Dandekeri, S.; Martis, J. Epidemiological and investigative study of premature graying of hair in higher secondary and pre-university school children. Int. J. Trichology 2013, 5, 17–21. [Google Scholar] [CrossRef]
- Chakrabarty, S.; Krishnappa, P.G.; Gowda, D.G.; Hiremath, J. Factors Associated with Premature Hair Graying in a Young Indian Population. Int. J. Trichology 2016, 8, 11–14. [Google Scholar] [CrossRef]
- El-Husseiny, R.; Alrgig, N.T.; Abdel Fattah, N.S.A. Epidemiological and biochemical factors (serum ferritin and vitamin D) associated with premature hair graying in Egyptian population. J. Cosmet. Dermatol. 2021, 20, 1860–1866. [Google Scholar] [CrossRef]
- Kiafar, B.; Taheri, A.; Isaac Hashemy, S.; Saki, A.; Mahdeianfar, B.; Taghavi, F.; Vahabi-Amlashi, S. Serum levels of lead and selenium in patients with premature graying of the hair. J. Cosmet. Dermatol. 2020, 19, 1513–1516. [Google Scholar] [CrossRef] [PubMed]
- Skolnik, P.; Eaglstein, W.H.; Ziboh, V.A. Human essential fatty acid deficiency: Treatment by topical application of linoleic acid. Arch. Dermatol. 1977, 113, 939–941. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Dogra, D. Association of Epidemiological and Biochemical Factors with Premature Graying of Hair: A Case-Control Study. Int. J. Trichology 2018, 10, 211–217. [Google Scholar] [CrossRef]
- Sonthalia, S.; Priya, A.; Tobin, D.J. Demographic Characteristics and Association of Serum Vitamin B12, Ferritin and Thyroid Function with Premature Canities in Indian Patients from an Urban Skin Clinic of North India: A Retrospective Analysis of 71 Cases. Indian J. Dermatol. 2017, 62, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Zayed, A.A.; Shahait, A.D.; Ayoub, M.N.; Yousef, A.M. Smokers’ hair: Does smoking cause premature hair graying? Indian Dermatol. Online J. 2013, 4, 90–92. [Google Scholar] [CrossRef] [PubMed]
- Sabharwal, R.; Gupta, A.; Moon, N.; Mahendra, A.; Sargaiyan, V.; Gupta, A.; Subudhi, S.K.; Gupta, S. Association between use of tobacco and age on graying of hair. Niger. J. Surg. 2014, 20, 83–86. [Google Scholar] [CrossRef]
- Akin Belli, A.; Etgu, F.; Ozbas Gok, S.; Kara, B.; Dogan, G. Risk Factors for Premature Hair Graying in Young Turkish Adults. Pediatr. Dermatol. 2016, 33, 438–442. [Google Scholar] [CrossRef]
- Osman, M.; McCauley, M.D. Psychiatric shades of grey: Mirtazapine-induced hair discoloration and hair loss. Ir. J. Psychol. Med. 2016, 33, 175–178. [Google Scholar] [CrossRef]
- Richena, M.; Silveira, M.; Rezende, C.A.; Joekes, I. Yellowing and bleaching of grey hair caused by photo and thermal degradation. J. Photochem. Photobiol. B-Biol. 2014, 138, 172–181. [Google Scholar] [CrossRef]
- Zhai, X.; Gong, M.H.; Peng, Y.X.; Yang, D.P. Effects of UV Induced-Photoaging on the Hair Follicle Cycle of C57BL6/J Mice. Clin. Cosmet. Investig. Dermatol. 2021, 14, 527–539. [Google Scholar] [CrossRef]
- Naudin, G.; Bastien, P.; Mezzache, S.; Trehu, E.; Bourokba, N.; Appenzeller, B.M.R.; Soeur, J.; Bornschlögl, T. Human pollution exposure correlates with accelerated ultrastructural degradation of hair fibers. Proc. Natl. Acad. Sci. USA 2019, 116, 18410–18415. [Google Scholar] [CrossRef]
- Bounda, G.A.; Feng, Y.U. Review of clinical studies of Polygonum multiflorum Thunb. and its isolated bioactive compounds. Pharmacogn. Res. 2015, 7, 225–236. [Google Scholar] [CrossRef]
- Li, Y.; Han, M.; Lin, P.; He, Y.; Yu, J.; Zhao, R. Hair Growth Promotion Activity and Its Mechanism of Polygonum multiflorum. Evid.-Based Complement. Altern. Med. 2015, 2015, 517901. [Google Scholar] [CrossRef] [PubMed]
- Han, M.N.; Lu, J.M.; Zhang, G.Y.; Yu, J.; Zhao, R.H. Mechanistic Studies on the Use of Polygonum multiflorum for the Treatment of Hair Graying. Biomed. Res. Int. 2015, 2015, 651048. [Google Scholar] [CrossRef]
- Lin, L.; Ni, B.; Lin, H.; Zhang, M.; Li, X.; Yin, X.; Qu, C.; Ni, J. Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: A review. J. Ethnopharmacol. 2015, 159, 158–183. [Google Scholar] [CrossRef] [PubMed]
- Ho, T.T.; Murthy, H.N.; Dalawai, D.; Bhat, M.A.; Paek, K.Y.; Park, S.Y. Attributes of Polygonum multiflorum to transfigure red biotechnology. Appl. Microbiol. Biotechnol. 2019, 103, 3317–3326. [Google Scholar] [CrossRef]
- Tao, Y.; Zhou, X.; Li, W.; Cai, B. Simultaneous Quantitation of Five Bioactive Ingredients in Raw and Processed Fallopia multiflora by Employing UHPLC-Q-TOF-MS. J. Chromatogr. Sci. 2019, 57, 618–624. [Google Scholar] [CrossRef]
- Xia, X.H.; Yuan, Y.Y.; Liu, M. The assessment of the chronic hepatotoxicity induced by Polygoni Multiflori Radix in rats: A pilot study by using untargeted metabolomics method. J. Ethnopharmacol. 2017, 203, 182–190. [Google Scholar] [CrossRef]
- Ling, S.; Xu, J.W. Biological Activities of 2,3,5,4′-Tetrahydroxystilbene-2-O-beta-D-Glucoside in Antiaging and Antiaging-Related Disease Treatments. Oxid. Med. Cell Longev. 2016, 2016, 4973239. [Google Scholar] [CrossRef]
- Lee, S.Y.; Ahn, S.M.; Wang, Z.; Choi, Y.W.; Shin, H.K.; Choi, B.T. Neuroprotective effects of 2,3,5,4′-tetrahydoxystilbene-2-O-beta-D-glucoside from Polygonum multiflorum against glutamate-induced oxidative toxicity in HT22 cells. J. Ethnopharmacol. 2017, 195, 64–70. [Google Scholar] [CrossRef]
- Sextius, P.; Betts, R.; Benkhalifa, I.; Commo, S.; Eilstein, J.; Massironi, M.; Wang, P.; Michelet, J.F.; Qiu, J.; Tan, X.; et al. Polygonum multiflorum Radix extract protects human foreskin melanocytes from oxidative stress in vitro and potentiates hair follicle pigmentation ex vivo. Int. J. Cosmet. Sci. 2017, 39, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Thang, N.D.; Diep, P.N.; Lien, P.T.H.; Lien, L.T. Polygonum multiflorum root extract as a potential candidate for treatment of early graying hair. J. Adv. Pharm. Technol. Res. 2017, 8, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Kim, H.J.; Jun, H.S. Polygonum multiflorum Thunb. Extract Stimulates Melanogenesis by Induction of COX2 Expression through the Activation of p38 MAPK in B16F10 Mouse Melanoma Cells. Evid.-Based Complement. Altern. Med. 2020, 2020, 7642019. [Google Scholar] [CrossRef]
- Jiang, Z.; Xu, J.; Long, M.; Tu, Z.; Yang, G.; He, G. 2,3,5,4′-tetrahydroxystilbene-2-O-beta-D-glucoside (THSG) induces melanogenesis in B16 cells by MAP kinase activation and tyrosinase upregulation. Life Sci. 2009, 85, 345–350. [Google Scholar] [CrossRef]
- Kim, J.; Kim, M.M. The effect of emodin on melanogenesis through the modulation of ERK and MITF signaling pathway. Nat. Product. Res. 2022, 36, 1084–1088. [Google Scholar] [CrossRef]
- Jo, S.J.; Shin, H.; Paik, S.H.; Na, S.J.; Jin, Y.; Park, W.S.; Kim, S.N.; Kwon, O.S. Efficacy and Safety of Pueraria lobata Extract in Gray Hair Prevention: A Randomized, Double-Blind, Placebo-Controlled Study. Ann. Dermatol. 2013, 25, 218–222. [Google Scholar] [CrossRef]
- Reichelt, K.V.; Hartmann, B.; Weber, B.; Ley, J.P.; Krammer, G.E.; Engel, K.H. Identification of bisprenylated benzoic acid derivatives from yerba santa (Eriodictyon ssp.) using sensory-guided fractionation. J. Agric. Food Chem. 2010, 58, 1850–1859. [Google Scholar] [CrossRef]
- Yang, J.; Liang, Q.; Wang, M.; Jeffries, C.; Smithson, D.; Tu, Y.; Boulos, N.; Jacob, M.R.; Shelat, A.A.; Wu, Y.S.; et al. UPLC-MS-ELSD-PDA as a Powerful Dereplication Tool to Facilitate Compound Identification from Small-Molecule Natural Product Libraries. J. Nat. Prod. 2014, 77, 902–909. [Google Scholar] [CrossRef]
- Walker, J.; Reichelt, K.V.; Obst, K.; Widder, S.; Hans, J.; Krammer, G.E.; Ley, J.P.; Somoza, V. Identification of an anti-inflammatory potential of Eriodictyon angustifolium compounds in human gingival fibroblasts. Food Funct. 2016, 7, 3046–3055. [Google Scholar] [CrossRef]
- Taguchi, N.; Homma, T.; Aoki, H.; Kunisada, T. Dietary Eriodictyon angustifolium Tea Supports Prevention of Hair Graying by Reducing DNA Damage in CD34+ Hair Follicular Keratinocyte Stem Cells. Biol. Pharm. Bull. 2020, 43, 1451–1454. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, N.; Hata, T.; Kamiya, E.; Kobayashi, A.; Aoki, H.; Kunisada, T. Reduction in human hair graying by sterubin, an active flavonoid of Eriodictyon angustifolium. J. Dermatol. Sci. 2018, 92, 286–289. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, N.; Hata, T.; Kamiya, E.; Homma, T.; Kobayashi, A.; Aoki, H.; Kunisada, T. Eriodictyon angustifolium extract, but not Eriodictyon californicum extract, reduces human hair greying. Int. J. Cosmet. Sci. 2020, 42, 336–345. [Google Scholar] [CrossRef] [PubMed]
- Taguchi, N.; Kitai, R.; Ando, T.; Nishimura, T.; Aoki, H.; Kunisada, T. Protective Effect of Hydroxygenkwanin against Hair Graying Induced by X-Ray Irradiation and Repetitive Plucking. JID Innov. 2022, 2, 100121. [Google Scholar] [CrossRef]
- Aoki, H.; Yamada, Y.; Hara, A.; Kunisada, T. Two distinct types of mouse melanocyte: Differential signaling requirement for the maintenance of non-cutaneous and dermal versus epidermal melanocytes. Development 2009, 136, 2511–2521. [Google Scholar] [CrossRef] [PubMed]
- Itoh, T.; Furuichi, Y. Hot-water extracts from adzuki beans (Vigna angularis) stimulate not only melanogenesis in cultured mouse B16 melanoma cells but also pigmentation of hair color in C3H mice. Biosci. Biotechnol. Biochem. 2005, 69, 873–882. [Google Scholar] [CrossRef]
- Zhao, P.J.; Park, N.H.; Alam, M.B.; Lee, S.H. Fuzhuan Brick Tea Boosts Melanogenesis and Prevents Hair Graying through Reduction of Oxidative Stress via NRF2-HO-1 Signaling. Antioxidants 2022, 11, 599. [Google Scholar] [CrossRef]
- Liu, X.; Lv, X.; Ji, T.; Hu, H.; Chang, L. Gynostemma pentaphyllum Makino extract induces hair growth and exhibits an anti-graying effect via multiple mechanisms. J. Cosmet. Dermatol. 2024, 23, 648–657. [Google Scholar] [CrossRef] [PubMed]
- de la Vega, M.R.; Zhang, D.D.; Wondrak, G.T. Topical Bixin Confers NRF2-Dependent Protection Against Photodamage and Hair Graying in Mouse Skin. Front. Pharmacol. 2018, 9, 287. [Google Scholar] [CrossRef] [PubMed]
- Li, X.X.; Shi, R.L.; Yan, L.C.; Chu, W.W.; Sun, R.S.; Zheng, B.K.; Wang, S.; Tan, H.; Wang, X.S.; Gao, Y. Natural product rhynchophylline prevents stress-induced hair graying by preserving melanocyte stem cells via the β2 adrenergic pathway suppression. Nat. Prod. Bioprospecting 2023, 13, 54. [Google Scholar] [CrossRef]
- Wang, Y.; Yao, X.; Shen, H.; Zhao, R.; Li, Z.; Shen, X.; Wang, F.; Chen, K.; Zhou, Y.; Li, B.; et al. Nutritional Composition, Efficacy, and Processing of Vigna angularis (Adzuki Bean) for the Human Diet: An Overview. Molecules 2022, 27, 6079. [Google Scholar] [CrossRef]
- Chen, G.; Peng, Y.; Xie, M.; Xu, W.; Chen, C.; Zeng, X.; Liu, Z. A critical review of Fuzhuan brick tea: Processing, chemical constituents, health benefits and potential risk. Crit. Rev. Food Sci. Nutr. 2023, 63, 5447–5464. [Google Scholar] [CrossRef] [PubMed]
- Tao, S.; Park, S.L.; Rojo de la Vega, M.; Zhang, D.D.; Wondrak, G.T. Systemic administration of the apocarotenoid bixin protects skin against solar UV-induced damage through activation of NRF2. Free Radic. Biol. Med. 2015, 89, 690–700. [Google Scholar] [CrossRef] [PubMed]
- Qin, N.; Lu, X.; Liu, Y.; Qiao, Y.; Qu, W.; Feng, F.; Sun, H. Recent research progress of Uncaria spp. based on alkaloids: Phytochemistry, pharmacology and structural chemistry. Eur. J. Med. Chem. 2021, 210, 112960. [Google Scholar] [CrossRef] [PubMed]
- Tobin, D.J. Aging of the hair follicle pigmentation system. Int. J. Trichology 2009, 1, 83–93. [Google Scholar] [CrossRef] [PubMed]
- Gelmi, M.C.; Houtzagers, L.E.; Strub, T.; Krossa, I.; Jager, M.J. MITF in Normal Melanocytes, Cutaneous and Uveal Melanoma: A Delicate Balance. Int. J. Mol. Sci. 2022, 23, 6001. [Google Scholar] [CrossRef]
- Heilmann-Heimbach, S.; Hochfeld, L.M.; Henne, S.K.; Nothen, M.M. Hormonal regulation in male androgenetic alopecia-Sex hormones and beyond: Evidence from recent genetic studies. Exp. Dermatol. 2020, 29, 814–827. [Google Scholar] [CrossRef]
- Wellbrock, C.; Arozarena, I. Microphthalmia-associated transcription factor in melanoma development and MAP-kinase pathway targeted therapy. Pigment Cell Melanoma Res. 2015, 28, 390–406. [Google Scholar] [CrossRef]
- Lee, A.R.; Lim, J.; Lim, J.S. Emerging roles of MITF as a crucial regulator of immunity. Exp. Mol. Med. 2024, 56, 311–318. [Google Scholar] [CrossRef]
- Kim, J.H.; Seok, J.K.; Kim, Y.M.; Boo, Y.C. Identification of small peptides and glycinamide that inhibit melanin synthesis using a positional scanning synthetic peptide combinatorial library. Br. J. Dermatol. 2019, 181, 128–137. [Google Scholar] [CrossRef]
- Mansky, K.C.; Sankar, U.; Han, J.H.; Ostrowski, M.C. Microphthalmia transcription factor is a target of the p38 MAPK pathway in response to receptor activator of NF-κB ligand signaling. J. Biol. Chem. 2002, 277, 11077–11083. [Google Scholar] [CrossRef]
- Zhou, J.; Song, J.; Ping, F.F.; Shang, J. Enhancement of the p38 MAPK and PKA signaling pathways is associated with the pro-melanogenic activity of Interleukin 33 in primary melanocytes. J. Dermatol. Sci. 2014, 73, 110–116. [Google Scholar] [CrossRef]
- Kim, J.H.; Hong, A.R.; Kim, Y.H.; Yoo, H.; Kang, S.W.; Chang, S.E.; Song, Y. JNK suppresses melanogenesis by interfering with CREB-regulated transcription coactivator 3-dependent MITF expression. Theranostics 2020, 10, 4017–4029. [Google Scholar] [CrossRef] [PubMed]
- Tonelli, C.; Chio, I.I.C.; Tuveson, D.A. Transcriptional Regulation by Nrf2. Antioxid. Redox Signal 2018, 29, 1727–1745. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Natural Nrf2 Modulators for Skin Protection. Antioxidants 2020, 9, 812. [Google Scholar] [CrossRef]
- Kwack, M.H.; Sung, Y.K.; Chung, E.J.; Im, S.U.; Ahn, J.S.; Kim, M.K.; Kim, J.C. Dihydrotestosterone-inducible dickkopf 1 from balding dermal papilla cells causes apoptosis in follicular keratinocytes. J. Investig. Dermatol. 2008, 128, 262–269. [Google Scholar] [CrossRef]
- Papukashvili, D.; Rcheulishvili, N.; Liu, C.; Xie, F.F.; Tyagi, D.; He, Y.J.; Wang, P.G. Perspectives on miRNAs Targeting DKK1 for Developing Hair Regeneration Therapy. Cells 2021, 10, 2957. [Google Scholar] [CrossRef] [PubMed]
- Kong, Y.N.; Liu, Y.S.; Pan, L.L.; Cheng, B.A.; Liu, H.W. Norepinephrine Regulates Keratinocyte Proliferation to Promote the Growth of Hair Follicles. Cells Tissues Organs 2015, 201, 423–435. [Google Scholar] [CrossRef]
- De Tollenaere, M.; Chapuis, E.; Auriol, P.; Auriol, D.; Scandolera, A.; Reynaud, R. Global Repigmentation Strategy of Grey Hair Follicles by Targeting Oxidative Stress and Stem Cells Protection. Appl. Sci. 2021, 11, 1533. [Google Scholar] [CrossRef]
- Park, H.J.; Zhang, N.; Park, D.K. Topical application of Polygonum multiflorum extract induces hair growth of resting hair follicles through upregulating Shh and β-catenin expression in C57BL/6 mice. J. Ethnopharmacol. 2011, 135, 369–375. [Google Scholar] [CrossRef]
- Yu, H.S.; Wang, L.L.; He, Y.; Han, L.F.; Ding, H.; Song, X.B.; Gao, X.M.; Yun, N.R.; Li, Z. Advances in the Study of the Potential Hepatotoxic Components and Mechanism of Polygonum multiflorum. Evid.-Based Complement. Altern. Med. 2020, 2020, 6489648. [Google Scholar] [CrossRef]
- Teka, T.; Wang, L.M.; Gao, J.; Mou, J.J.; Pan, G.X.; Yu, H.Y.; Gao, X.M.; Han, L.F. Polygonum multiflorum: Recent updates on newly isolated compounds, potential hepatotoxic compounds and their mechanisms. J. Ethnopharmacol. 2021, 271, 113864. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).