Applied Research on Atopic Dermatitis with Special Emphasis on the Role of Emollients in This Disorder: A Review
Abstract
1. Introduction
2. Characteristics of Atopic Dermatitis: Types and Clinical Symptoms
3. Phenotypic and Endotypic Differences among Children and Adults with AD
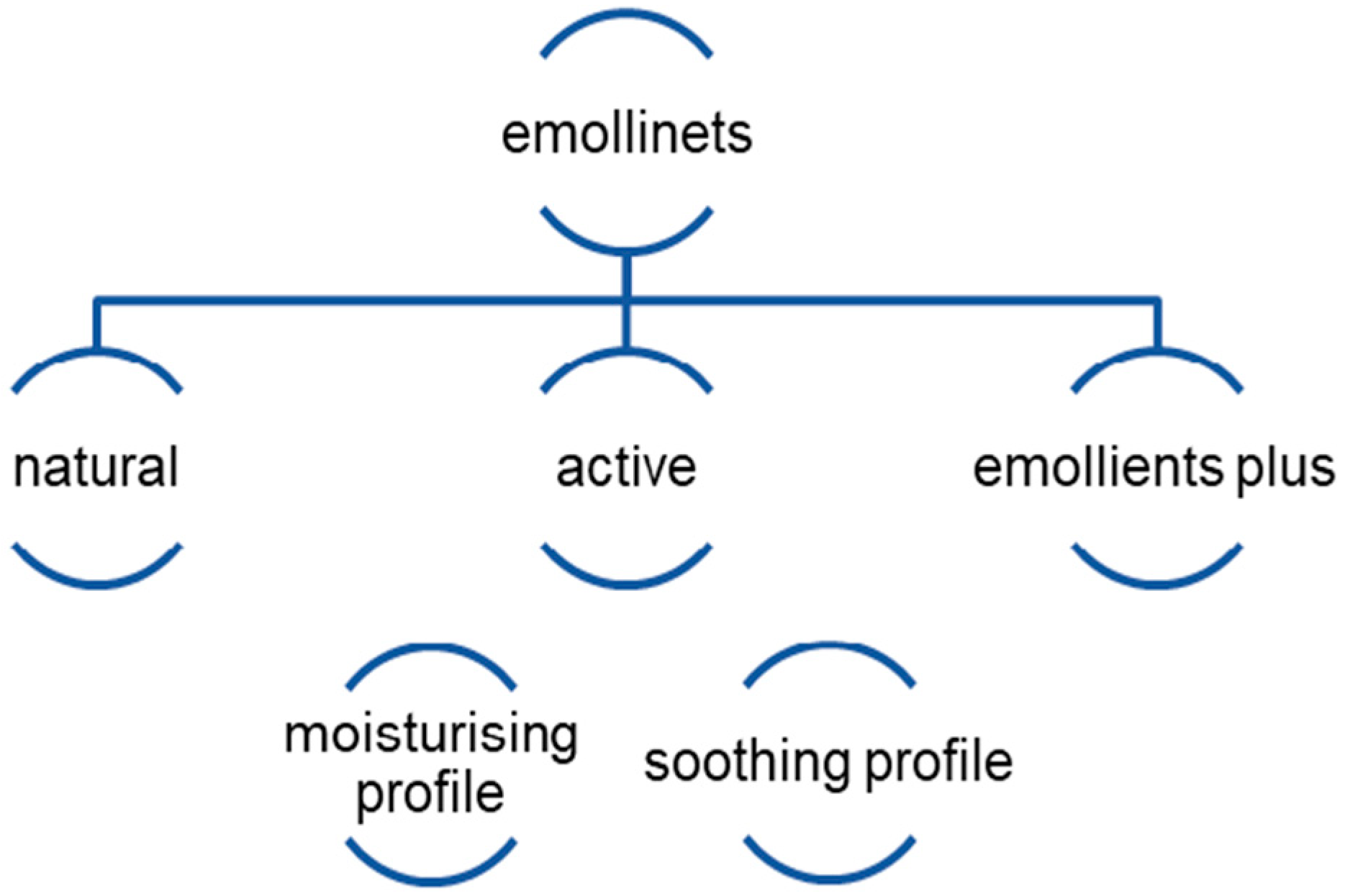
4. Components of Emollients and Their Effect on the Changes That Occur in Atopic Dermatitis
5. Use of Emollients in the Care of Skin with Atopic Dermatitis among Children and Adults
6. Effect of Emollients on the Prevention of AD among Newborns and Children
7. Recommendations in the Use of Emollients in Atopic Dermatitis and Their Synergism with Other Behaviors and Therapies
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kamińska, E. Rola Emolientów W Atopowym Zapaleniu Skóry U Dzieci. Dev. Period Med. 2018, 22, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Garcia Gatmaitan, J.; Lee, J.H. Challenges and Future Trends in Atopic Dermatitis. Int. J. Mol. Sci. 2023, 24, 11380. [Google Scholar] [CrossRef] [PubMed]
- Torres, T.; Ferreira, E.O.; Gonçalo, M.; Mendes-Bastos, P.; Selores, M.; Filipe, P. Update on Atopic Dermatitis. Acta Médica Port. 2019, 32, 606. [Google Scholar] [CrossRef] [PubMed]
- Salvati, L.; Cosmi, L.; Annunziato, F. From Emollients to Biologicals: Targeting Atopic Dermatitis. Int. J. Mol. Sci. 2021, 22, 10981. [Google Scholar] [CrossRef]
- Jaworek, A.K.; Wojas-Pelc, A. History of Atopic Dermatitis—A Short Review from Ancient to Modern Medicine. Dermatol. Rev. 2017, 104, 636–647. [Google Scholar] [CrossRef]
- Anania, C.; Brindisi, G.; Martinelli, I.; Bonucci, E.; D’Orsi, M.; Ialongo, S.; Nyffenegger, A.; Raso, T.; Spatuzzo, M.; De Castro, G.; et al. Probiotics Function in Preventing Atopic Dermatitis in Children. Int. J. Mol. Sci. 2022, 23, 5409. [Google Scholar] [CrossRef]
- Trzeciak, M.; Zysk, W.; Wolańska-Buzalska, D. “Emollients Plus” with Vitreoscilla Filiformis in Monotherapy and Adjunctive Therapy in Skin Diseases in Children. Dermatol. Rev. 2023, 110, 602–607. [Google Scholar] [CrossRef]
- Kim, J.; Kim, B.E.; Leung, D.Y.M. Pathophysiology of Atopic Dermatitis: Clinical Implications. Allergy Asthma Proc. 2019, 40, 84–92. [Google Scholar] [CrossRef]
- Moosbrugger-Martinz, V.; Leprince, C.; Méchin, M.-C.; Simon, M.; Blunder, S.; Gruber, R.; Dubrac, S. Revisiting the Roles of Filaggrin in Atopic Dermatitis. Int. J. Mol. Sci. 2022, 23, 5318. [Google Scholar] [CrossRef]
- Nowicki, R.; Grubska-Suchanek, E.; Trzeciak, M.; Wilkowska, A. Current Options and Perspectives for the Topical Treatment of Atopic Dermatitis. Dermatol. Rev. 2021, 108, 117–125. [Google Scholar] [CrossRef]
- Kanabaj, K.; Shawkat, S.; Kuźniak, A.; Adamski, Z.; Jenerowicz, D. Selected Clinical and Therapeutic Aspects of Atopic and Contact Dermatitis. Przegląd Dermatol. 2021, 108, 394–406. [Google Scholar] [CrossRef]
- Arents, B.; van Zuuren, E.; Fedorowicz, Z.; Hughes, O. Global Report on Atopic Dermatitis 2022 Atopicdermatitisatlas.org. 2022. Available online: https://www.eczemacouncil.org/assets/docs/global-report-on-atopic-dermatitis-2022.pdf (accessed on 17 October 2022).
- Clebak, K.T.; Helm, L.; Uppal, P.; Davis, C.R.; Helm, M.F. Atopic Dermatitis. Prim. Care Clin. Off. Pract. 2023, 50, 191–203. [Google Scholar] [CrossRef] [PubMed]
- Silverberg, N. Atopic Dermatitis Prevention and Treatment. Cutis 2017, 100, 173–177. [Google Scholar]
- Bartoszak, L.; Czarnecka-Operacz, M. Contact Allergy in Children with Atopic Dermatitis. Adv. Dermatol. Allergol. 2007, 24, 120–126. [Google Scholar]
- Murphy, P.B.; Hooten, J.N.; Atwater, A.R.; Gossman, W. Allergic Contact Dermatitis; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Abramovits, W.; Hung, P.; Tong, K.B. Efficacy and Economics of Topical Calcineurin Inhibitors for the Treatment of Atopic Dermatitis. Am. J. Clin. Dermatol. 2006, 7, 213–222. [Google Scholar] [CrossRef]
- Czarnecka-Operacz, M. Pielęgnacja Skóry Chorego Na Atopowe Zapalenie Skóry W Świetle Nowoczesnej Wiedzy Medycznej. Alergia 2015, 3, 24–28. [Google Scholar]
- Jurzak, M.; Rudyk, A. Składniki Aktywne Kosmetyków I Dermokosmetyków Stosowane W Pielęgnacji Skóry Z Atopowym Zapaleniem; Oficyna Wydawnicza: Warsaw, Poland, 2012. [Google Scholar]
- Zelenkova, H.; Kerob, D.; Salah, S.; Demessant-Flavigny, A.-L. Impact of Daily Use of Emollient “Plus” on Corticosteroid Consumption in Patients with Atopic Dermatitis: An Open, Randomized Controlled Study. J. Eur. Acad. Dermatol. Venereol. 2023, 37, 27–34. [Google Scholar] [CrossRef]
- Hlela, C.; Osei-Sekyere, B.; Yeboah Senyah, A. Emollients—Latest and Greatest Uses in Atopic Dermatitis. Curr. Allergy Clin. Immunol. 2022, 35, 2–5. [Google Scholar]
- Nola, I.; Kostović, K.; Kotrulja, L.; Lugović, L. The Use of Emollients as Sophisticated Therapy in Dermatology. Acta Dermatovenerol. Croat. 2003, 11, 80–87. [Google Scholar]
- Dębowska, D.; Monika Pasikowska-Piwko, N.; Katarzyna, N.; Kisiel, A. Terapia Emolientowa W Przebiegu Atopowego Zapalenia Skóry. Emollient Therapy in the Treatment of Atopic Dermatitis. Klin. Pediatryczna 2021, 29, 5028–5032. [Google Scholar]
- Wollenberg, A.; Christen-Zäch, S.; Taieb, A.; Paul, C.; Thyssen, J.P.; Bruin-Weller, M.; Vestergaard, C.; Seneschal, J.; Werfel, T.; Cork, M.J.; et al. ETFAD/EADV Eczema Task Force 2020 Position Paper on Diagnosis and Treatment of Atopic Dermatitis in Adults and Children. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2717–2744. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Chalmers, J.R.; Hanifin, J.M.; Thomas, K.S.; Cork, M.J.; McLean, W.H.I.; Brown, S.J.; Chen, Z.; Chen, Y.; Williams, H.C. Emollient Enhancement of the Skin Barrier from Birth Offers Effective Atopic Dermatitis Prevention. J. Allergy Clin. Immunol. 2014, 134, 818–823. [Google Scholar] [CrossRef] [PubMed]
- Simpson, E.L.; Berry, T.M.; Brown, P.A.; Hanifin, J.M. A Pilot Study of Emollient Therapy for the Primary Prevention of Atopic Dermatitis. J. Am. Acad. Dermatol. 2010, 63, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Horimukai, K.; Morita, K.; Narita, M.; Kondo, M.; Kitazawa, H.; Nozaki, M.; Shigematsu, Y.; Yoshida, K.; Niizeki, H.; Motomura, K.; et al. Application of Moisturizer to Neonates Prevents Development of Atopic Dermatitis. J. Allergy Clin. Immunol. 2014, 134, 824–830.e6. [Google Scholar] [CrossRef]
- Glatz, M.; Jo, J.-H.; Kennedy, E.A.; Polley, E.C.; Segre, J.A.; Simpson, E.L.; Kong, H.H. Emollient Use Alters Skin Barrier and Microbes in Infants at Risk for Developing Atopic Dermatitis. PLoS ONE 2018, 13, e0192443. [Google Scholar] [CrossRef]
- Rippke, F.; Schreiner, V.; Doering, T.; Maibach, H.I. Stratum Corneum PH in Atopic Dermatitis. Am. J. Clin. Dermatol. 2004, 5, 217–223. [Google Scholar] [CrossRef]
- Capone, K.; Kirchner, F.; Klein, S.; Tierney, N. Effects of Colloidal Oatmeal Topical Atopic Dermatitis Cream on Skin Microbiome and Skin Barrier Properties. J. Drugs Dermatol. 2020, 19, 524–531. [Google Scholar] [CrossRef]
- Chandrashekar, B.S.; Luger, T.; Rajendran, S.C.; Parathasaradhi, A.; Thomas, J.; Ganjoo, A.; Sharma, D.; Damishetty, R.; Ruby, N.; Sujay, V.; et al. Role of Actives in Emollients in Atopic Dermatitis. J. Dermatol. Ski. Sci. 2024, 6, 16–23. [Google Scholar] [CrossRef]
- Tokura, Y.; Hayano, S. Subtypes of Atopic Dermatitis: From Phenotype to Endotype. Allergol. Int. 2021, 71, 14–24. [Google Scholar] [CrossRef]
- Marciniak, A.; Hasse-Cieślińska, M.; Jenerowicz, D.; Czarnecka-Operacz, M. The Role of Minor Hanifin and Rajka Criteria in Diagnosis of Atopic Dermatitis Patients. Post. Derm. Alerg. 2008, 25, 55–60. [Google Scholar]
- Brenninkmeyer, E.E.A. Atopic and Atopiform Dermatitis; University of Amsterdam: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Fal, A. Alergia, Choroby Alergiczne, Astma; Medycyna Praktyczna: Cholerzyn, Poland, 2011. [Google Scholar]
- Bożek, A.; Reich, A. Assessment of the Severity of Atopic Dermatitis. Dermatol. Rev. 2016, 6, 479–485. [Google Scholar] [CrossRef]
- Oranje, A.P.; Glazenburg, E.J.; Wolkerstorfer, A.; de Waard-van der Spek, F.B. Practical Issues on Interpretation of Scoring Atopic Dermatitis: The SCORAD Index, Objective SCORAD and the Three-Item Severity Score. Br. J. Dermatol. 2007, 157, 645–648. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Langan, S.; Deckert, S.; Svensson, A.; von Kobyletzki, L.; Thomas, K.; Spuls, P. Assessment of Clinical Signs of Atopic Dermatitis: A Systematic Review and Recommendation. J. Allergy Clin. Immunol. 2013, 132, 1337–1347. [Google Scholar] [CrossRef] [PubMed]
- Rehal, B.; Armstrong, A. Health Outcome Measures in Atopic Dermatitis: A Systematic Review of Trends in Disease Severity and Quality-of-Life Instruments 1985–2010. PLoS ONE 2011, 6, e17520. [Google Scholar] [CrossRef]
- Czarnowicki, T.; He, H.; Krueger, J.G.; Guttman-Yassky, E. Atopic Dermatitis Endotypes and Implications for Targeted Therapeutics. J. Allergy Clin. Immunol. 2019, 143, 1–11. [Google Scholar] [CrossRef]
- Kim, J.S. Pediatric Atopic Dermatitis: The Importance of Food Allergens. Semin. Cutan. Med. Surg. 2008, 27, 156–160. [Google Scholar] [CrossRef]
- Koga, C.; Kabashima, K.; Shiraishi, N.; Kobayashi, M.; Tokura, Y. Possible Pathogenic Role of Th17 Cells for Atopic Dermatitis. J. Investig. Dermatol. 2008, 128, 2625–2630. [Google Scholar] [CrossRef]
- Yosipovitch, G.; Papoiu, A.D.P. What Causes Itch in Atopic Dermatitis? Curr. Allergy Asthma Rep. 2008, 8, 306–311. [Google Scholar] [CrossRef]
- Hong, J.; Buddenkotte, J.; Berger, T.G.; Steinhoff, M. Management of Itch in Atopic Dermatitis. Semin. Cutan. Med. Surg. 2011, 30, 71–86. [Google Scholar] [CrossRef]
- Blume-Peytavi, U.; Metz, M. Atopic Dermatitis in Children: Management of Pruritus. J. Eur. Acad. Dermatol. Venereol. 2012, 26, 2–8. [Google Scholar] [CrossRef]
- Ramírez-Marín, H.A.; Silverberg, J.I. Differences between Pediatric and Adult Atopic Dermatitis. Pediatr. Dermatol. 2022, 39, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Brunner, P.M.; Israel, A.; Zhang, N.; Leonard, A.; Wen, H.-C.; Huynh, T.; Tran, G.; Lyon, S.; Rodriguez, G.; Immaneni, S.; et al. Early-Onset Pediatric Atopic Dermatitis Is Characterized by TH2/TH17/TH22-Centered Inflammation and Lipid Alterations. J. Allergy Clin. Immunol. 2018, 141, 2094–2106. [Google Scholar] [CrossRef] [PubMed]
- Avena-Woods, C. Overview of Atopic Dermatitis. Am. J. Manag. Care 2017, 23, S115–S123. [Google Scholar] [PubMed]
- Kowalska-Olędzka, E.; Czarnecka, M.; Baran, A. Epidemiology of Atopic Dermatitis in Europe. J. Drug Assess. 2019, 8, 126–128. [Google Scholar] [CrossRef] [PubMed]
- Esaki, H.; Czarnowicki, T.; Gonzalez, J.; Oliva, M.; Talasila, S.; Haugh, I.; Rodriguez, G.; Becker, L.; Krueger, J.G.; Guttman-Yassky, E.; et al. Accelerated T-Cell Activation and Differentiation of Polar Subsets Characterizes Early Atopic Dermatitis Development. J. Allergy Clin. Immunol. 2016, 138, 1473–1477.e5. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Leonard, A.; Pavel, A.B.; Malik, K.; Raja, A.; Glickman, J.; Estrada, Y.D.; Peng, X.; del Duca, E.; Sanz-Cabanillas, J.; et al. Age-Specific Changes in the Molecular Phenotype of Patients with Moderate-To-Severe Atopic Dermatitis. J. Allergy Clin. Immunol. 2019, 144, 144–156. [Google Scholar] [CrossRef]
- Śliwa, K.; Sikora, E.; Ogonowski, J. Kosmetyki Do Pielęgnacji Skóry Atopowej. Wiadomości Chem. 2011, 65, 651–673. [Google Scholar]
- Elias, P.M.; Wakefield, J.S.; Man, M.-Q. Moisturizers versus Current and Next-Generation Barrier Repair Therapy for the Management of Atopic Dermatitis. Ski. Pharmacol. Physiol. 2018, 32, 1–7. [Google Scholar] [CrossRef]
- Grześk-Kaczyńska, M.; Petrus-Halicka, J.; Kaczyński, S.; Bartuzi, Z.; Ukleja-Sokołowska, N. Should Emollients Be Recommended for the Prevention of Atopic Dermatitis?—New Evidence and Current State of Knowledge. J. Clin. Med. 2024, 13, 863. [Google Scholar] [CrossRef]
- Fowler, J.F.; Nebus, J.; Wallo, W.; Eichenfield, L.F. Colloidal Oatmeal Formulations as Adjunct Treatments in Atopic Dermatitis. J. Drugs Dermatol. 2012, 11, 804–807. [Google Scholar]
- Fowler, J.; Silverberg, N. Active Naturals Have a Key Role in Atopic Dermatitis. Semin. Cutan. Med. Surg. 2008, 27, 8–10. [Google Scholar] [CrossRef] [PubMed]
- Gueniche, A.; Liboutet, M.; Cheilian, S.; Fagot, D.; Juchaux, F.; Breton, L. Vitreoscilla Filiformis Extract for Topical Skin Care: A Review. Front. Cell. Infect. Microbiol. 2021, 11, 747663. [Google Scholar] [CrossRef] [PubMed]
- Seite, S.; Zelenkova, H.; Martin, R. Clinical Efficacy of Emollients in Atopic Dermatitis Patients—Relationship with the Skin Microbiota Modification. Clin. Cosmet. Investig. Dermatol. 2017, 10, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Hon, K.L.; Kung, J.S.C.; Ng, W.G.G.; Leung, T.F. Emollient Treatment of Atopic Dermatitis: Latest Evidence and Clinical Considerations. Drugs Context 2018, 7, 1–14. [Google Scholar] [CrossRef]
- Lin, T.-K.; Zhong, L.; Santiago, J. Anti-Inflammatory and Skin Barrier Repair Effects of Topical Application of Some Plant Oils. Int. J. Mol. Sci. 2017, 19, 70. [Google Scholar] [CrossRef]
- Wyszkowska-Kolatko, M.; Koczurkiewicz, P.; Wójcik, K.; Pękala, E. Medicinal Plants Used in Skin Diseases Treatment. Postępy Fitoter. 2015, 3, 184–192. [Google Scholar]
- Shadi, T.Z.; Talal, A.Z. A Review of Four Common Medicinal Plants Used to Treat Eczema. J. Med. Plants Res. 2015, 9, 702–711. [Google Scholar] [CrossRef]
- Varma, S.R.; Sivaprakasam, T.O.; Arumugam, I.; Dilip, N.; Raghuraman, M.; Pavan, K.B.; Rafiq, M.; Paramesh, R. In Vitro Anti-Inflammatory and Skin Protective Properties of Virgin Coconut Oil. J. Tradit. Complement. Med. 2019, 9, 5–14. [Google Scholar] [CrossRef]
- Verallo-Rowell, V.M.; Dillague, K.M.; Syah-Tjundawan, B.S. Novel Antibacterial and Emollient Effects of Coconut and Virgin Olive Oils in Adult Atopic Dermatitis. Dermat. Contact Atopic Occup. Drug 2008, 19, 308–315. [Google Scholar] [CrossRef]
- Casetti, F.; Wölfle, U.; Gehring, W.; Schempp, C.M. Dermocosmetics for Dry Skin: A New Role for Botanical Extracts. Ski. Pharmacol. Physiol. 2011, 24, 289–293. [Google Scholar] [CrossRef]
- Danby, S.G.; AlEnezi, T.; Sultan, A.; Lavender, T.; Chittock, J.; Brown, K.; Cork, M.J. Effect of Olive and Sunflower Seed Oil on the Adult Skin Barrier: Implications for Neonatal Skin Care. Pediatr. Dermatol. 2012, 30, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Nadora, D.; Burney, W.; Chaudhuri, R.K.; Galati, A.; Min, M.; Fong, S.; Lo, K.; Chambers, C.J.; Sivamani, R.K. Prospective Randomized Double-Blind Vehicle-Controlled Study of Topical Coconut and Sunflower Seed Oil-Derived Isosorbide Diesters on Atopic Dermatitis. Dermatitis 2024, 35, S62–S69. [Google Scholar] [CrossRef] [PubMed]
- Eichenfield, L.F.; McCollum, A.; Msika, P. The Benefits of Sunflower Oleodistillate (SOD) in Pediatric Dermatology. Pediatr. Dermatol. 2009, 26, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; Choi, D.-K.; Lee, S.-S.; Choi, S.J.; Kim, C.D.; Yoon, T.-J.; Lee, J.-H. Enhancement of Keratinocyte Differentiation by Rose Absolute Oil. Ann. Dermatol. 2010, 22, 255. [Google Scholar] [CrossRef]
- Puglia, C.; Bonina, F. In Vivo Spectrophotometric Evaluation of Skin Barrier Recovery after Topical Application of Soybean Phytosterols. J. Cosmet. Sci. 2008, 59, 217–224. [Google Scholar]
- Walczak-Zeidler, K.; Felczak-Guzik, A.; Nowak, I. Oleje Roślinne Stosowane Jako Surowce Kosmetyczne—Leksykon; Cursiva: Warsaw, Poland, 2013. [Google Scholar]
- Schäfer, N.; Sobczyk, M.; Burczyk, D.; Balwierz, R.; Skotnicka-Graca, U. Possibilities of Using Vegetable Oils in Acne Skin Care. Aesthetic Cosmetol. Med. 2022, 11, 49–54. [Google Scholar] [CrossRef]
- Awazuhara, H.; Kawai, H.; Baba, M.; Matsui, T.; Komiyama, A. Antigenicity of the Proteins in Soy Lecithin and Soy Oil in Soybean Allergy. Clin. Exp. Allergy 1998, 28, 1559–1564. [Google Scholar] [CrossRef]
- Bettzuege-Pfaff, B.I.; Melzer, A. Treating Dry Skin and Pruritus with a Bath Oil Containing Soya Oil and Lauromacrogols. Curr. Med. Res. Opin. 2005, 21, 1735–1739. [Google Scholar] [CrossRef]
- Kim, J.N.; Han, S.N.; Ha, T.J.; Kim, H.-K. Black Soybean Anthocyanins Attenuate Inflammatory Responses by Suppressing Reactive Oxygen Species Production and Mitogen Activated Protein Kinases Signaling in Lipopolysaccharide-Stimulated Macrophages. Nutr. Res. Pract. 2017, 11, 357. [Google Scholar] [CrossRef]
- Zielińska, A.; Nowak, I. Abundance of Active Ingredients in Sea-Buckthorn Oil. Lipids Health Dis. 2017, 16, 95. [Google Scholar] [CrossRef]
- Resich-Kozieł, L.; Niemyska, K. Zastosowanie Oleju Z Rokitnika W Kosmetologii. Kosmetol. Estet. 2020, 9, 187–191. [Google Scholar]
- Koskovac, M.; Cupara, S.; Kipic, M.; Barjaktarevic, A.; Milovanovic, O.; Kojicic, K.; Markovic, M. Sea Buckthorn Oil—A Valuable Source for Cosmeceuticals. Cosmetics 2017, 4, 40. [Google Scholar] [CrossRef]
- Kurek-Górecka, A.; Balwierz, R.; Mizera, P.; Nowak, M.; Żurawska-Płaksej, E. Znaczenie Terapeutyczne I Kosmetyczne Oleju Konopnego. Farm. Pol. 2018, 74, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Wydro, D. The Effect of Oral Supplementation with Omega-3 and 6 Acids on Skin Condition—Pilot Study. Acad. Aesthet. Anti-Aging Med. 2012, 4, 46–54. [Google Scholar]
- Callaway, J.; Schwab, U.; Harvima, I.; Halonen, P.; Mykkänen, O.; Hyvönen, P.; Järvinen, T. Efficacy of Dietary Hempseed Oil in Patients with Atopic Dermatitis. J. Dermatol. Treat. 2005, 16, 87–94. [Google Scholar] [CrossRef]
- Zielińska, A.; Nowak, I. Kwasy Tłuszczowe W Olejach Roślinnych I Ich Znaczenie W Kosmetyce. Chemik 2014, 68, 103–110. [Google Scholar]
- Chrząstek, L.; Dondela, B.; Deska, M. Safe Components of Cosmetics Lipids and Derivative. Pr. Nauk. Akad. Im Jana Długosza W Częstochowie Technol. Inform. Inżynieria Bezpieczeństwa 2015, 3, 9–27. [Google Scholar] [CrossRef]
- Lee, C.; Eom, Y.; Yang, H.; Jang, M.; Jung, S.; Park, Y.; Lee, S.; Jung, H. Skin Barrier Restoration and Moisturization Using Horse Oil-Loaded Dissolving Microneedle Patches. Ski. Pharmacol. Physiol. 2018, 31, 163–171. [Google Scholar] [CrossRef]
- Cristiano, L.; Guagni, M. Zooceuticals and Cosmetic Ingredients Derived from Animals. Cosmetics 2022, 9, 13. [Google Scholar] [CrossRef]
- Kiechl-Kohlendorfer, U.; Berger, C.; Inzinger, R. The Effect of Daily Treatment with an Olive Oil/Lanolin Emollient on Skin Integrity in Preterm Infants: A Randomized Controlled Trial. Pediatr. Dermatol. 2008, 25, 174–178. [Google Scholar] [CrossRef]
- Żmudzińska, M.; Czarnecka-Operacz, M. The Lanolin Paradox Phenomenon. Adv. Dermatol. Allergol. 2008, 25, 66–68. [Google Scholar]
- Kowalska, M.; Woźniak, M.; Paździor, M. Assessment of the Sensory and Moisturizing Properties of Emulsions with Hemp Oil. Acta Polytech. Hung. 2017, 14, 183–195. [Google Scholar] [CrossRef]
- Kowalska, M.; Mendrycka, M.; Zbikowska, A.; Kowalska, D. Assessment of a Stable Cosmetic Preparation Based on Enzymatic Interesterified Fat, Proposed in the Prevention of Atopic Dermatitis. Acta Pol. Pharm. 2017, 74, 465–476. [Google Scholar] [PubMed]
- Kowalska, M.; Żbikowska, A.; Woźniak, M.; Amanowicz, A. Quality of Emulsions Based on Modified Watermelon Seed Oil, Stabilized with Orange Fibres. Molecules 2022, 27, 513. [Google Scholar] [CrossRef] [PubMed]
- Kowalska, M.; Woźniak, M.; Turek, P.; Żbikowska, A. New Fat Bases in Model Emulsion Systems in Physicochemical and Consumer Evaluation. Appl. Sci. 2024, 14, 3553. [Google Scholar] [CrossRef]
- Ab Hadi, H. Honey, a Gift from Nature to Health and Beauty: A Review. Br. J. Pharm. 2016, 1, 46–54. [Google Scholar] [CrossRef]
- Burlando, B.; Cornara, L. Honey in Dermatology and Skin Care: A Review. J. Cosmet. Dermatol. 2013, 12, 306–313. [Google Scholar] [CrossRef]
- Koppes, S.; Charles, F.; Lammers, L.; Frings-Dresen, M.; Kezic, S.; Rustemeyer, T. Efficacy of a Cream Containing Ceramides and Magnesium in the Treatment of Mild to Moderate Atopic Dermatitis: A Randomized, Double-Blind, Emollient- and Hydrocortisone-Controlled Trial. Acta Derm. Venereol. 2016, 96, 948–953. [Google Scholar] [CrossRef]
- Simpson, E.; Böhling, A.; Bielfeldt, S.; Bosc, C.; Kerrouche, N. Improvement of Skin Barrier Function in Atopic Dermatitis Patients with a New Moisturizer Containing a Ceramide Precursor. J. Dermatol. Treat. 2013, 24, 122–125. [Google Scholar] [CrossRef]
- Gasparri, F. Effects of a Novel Emollient Cream on Skin Moisture, Epidermal Barrier Function and Atopic Dermatitis Signs and Symptoms: Results from a Clinical Study. In Proceedings of the 24th World Congress of Dermatology, Milan, Italy, 10–15 June 2019. [Google Scholar]
- Dinani, N.; Patel, M.; George, S. ‘Dermatologically Tested’: What Does It Really Mean? Br. J. Dermatol. 2017, 177, 176. [Google Scholar]
- van Zuuren, E.J.; Fedorowicz, Z.; Lavrijsen, A.; Christensen, R.; Arents, B. Emollients and Moisturisers for Eczema. Cochrane DatabaseSyst.Rev. 2016, 2. [Google Scholar] [CrossRef]
- Li, Z.; Hu, L.; Elias, P.M.; Man, M.-Q. Skin Care Products Can Aggravate Epidermal Function: Studies in a Murine Model Suggest a Pathogenic Role in Sensitive Skin. Contact Dermat. 2017, 78, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, R.; Trzeciak, M.; Kaczmarski, M.; Wilkowska, A.; Czarnecka-Operacz, M.; Kowalewski, C.; Rudnicka, L.; Kulus, M.; Mastalerz-Migas, A.; Peregud-Pogorzelski, J.; et al. Atopic Dermatitis. Interdisciplinary Diagnostic and Therapeutic Recommendations of the Polish Dermatological Society, Polish Society of Allergology, Polish Pediatric Society and Polish Society of Family Medicine. Part I. Prophylaxis, Topical Treatment and Phototherapy. Alergol. Pol. Pol. J. Allergol. 2019, 6, 69–80. [Google Scholar] [CrossRef]
- Hamelmann, E.; Herz, U.; Holt, P.; Host, A.; Lauener, R.P.; Matricardi, P.M.; Wahn, U.; Wickman, M. New Visions for Basic Research and Primary Prevention of Pediatric Allergy: An IPAC Summary and Future Trends. Pediatr. Allergy Immunol. 2008, 19, 4–16. [Google Scholar] [CrossRef]
- Kaci, G.; Goudercourt, D.; Dennin, V.; Pot, B.; Doré, J.; Ehrlich, S.D.; Renault, P.; Blottière, H.M.; Daniel, C.; Delorme, C. Anti-Inflammatory Properties of Streptococcus Salivarius, a Commensal Bacterium of the Oral Cavity and Digestive Tract. Appl. Environ. Microbiol. 2014, 80, 928–934. [Google Scholar] [CrossRef]
- Eyerich, K.; Novak, N. Immunology of Atopic Eczema: Overcoming the Th1/Th2 Paradigm. Allergy 2013, 68, 974–982. [Google Scholar] [CrossRef]
- Bradshaw, L.E.; Wyatt, L.A.; Brown, S.J.; Haines, R.H.; Montgomery, A.A.; Perkin, M.R.; Lawton, S.; Sach, T.H.; Chalmers, J.R.; Ridd, M.J.; et al. Emollients for Prevention of Atopic Dermatitis: 5-Year Findings from the BEEP Randomized Trial. Allergy 2022, 78, 995–1006. [Google Scholar] [CrossRef]
- Skjerven, H.O.; Rehbinder, E.M.; Vettukattil, R.; LeBlanc, M.; Granum, B.; Haugen, G.; Hedlin, G.; Landrø, L.; Marsland, B.J.; Rudi, K.; et al. Skin Emollient and Early Complementary Feeding to Prevent Infant Atopic Dermatitis (PreventADALL): A Factorial, Multicentre, Cluster-Randomised Trial. Lancet 2020, 395, 951–961. [Google Scholar] [CrossRef]
- Czarnecka-Operacz, M.; Sadowska-Przytocka, A. The Updated Knowlegde on Proper Skin Care Modalities in Patients with Atopic Dermatitis. Alergia 2015, 3, 24–26. [Google Scholar]
- Katibi, O.S.; Cork, M.J.; Flohr, C.; Danby, S.G. Moisturizer Therapy in Prevention of Atopic Dermatitis and Food Allergy: To Use or Disuse? Ann. Allergy Asthma Immunol. 2022, 128, 512–525. [Google Scholar] [CrossRef]
- Koutroulis, I.; Petrova, K.; Kratimenos, P.; Gaughan, J. Frequency of Bathing in the Management of Atopic Dermatitis. Clin. Pediatr. 2014, 53, 677–681. [Google Scholar] [CrossRef] [PubMed]
- Tarr, A.; Iheanacho, I. Should We Use Bath Emollients for Atopic Eczema? BMJ 2009, 339, b4273. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gallard, S. Emollient Ingredients: Making the Best Clinical Choice for Older Patients. Dermatol. Nurs. 2022, 21, 27–30. [Google Scholar]
- Hon, K.L.E.; Leung, T.F.; Wong, Y.; So, H.K.; Li, A.M.; Fok, T.F. A Survey of Bathing and Showering Practices in Children with Atopic Eczema. Clin. Exp. Dermatol. 2005, 30, 351–354. [Google Scholar] [CrossRef]
- Wollenberg, A.; Kinberger, M.; Arents, B.; Aszodi, N.; Avila Valle, G.; Barbarot, S.; Bieber, T.; Brough, H.A.; Calzavara Pinton, P.; Christen-Zäch, S.; et al. European Guideline (EuroGuiDerm) on Atopic Eczema: Part I—Systemic Therapy. J. Eur. Acad. Dermatol. Venereol. 2022, 36, 1409–1431. [Google Scholar] [CrossRef]
| Treatment In Atopic Dermatitis: | |
|---|---|
| Dependent on the severity of AD | During exacerbations—topical application of corticosteroids (hydrocortisone, prednisolone) and calcineurin inhibitors (pimecrolimus and tacrolimus) |
| Maintenance (for chronic lesions or periodic exacerbations) | Periodic use of topical corticosteroids and calcineurin inhibitors (to prevent recurrence of skin inflammation) |
| Severe forms | Treatment of the severe form, which is refractory to classical therapeutic strategies—phototherapy, high-potency topical corticosteroids, oral steroid therapy, biologic drugs (Dupilumab), immunosuppressive drugs such as azathioprine, cyclosporine A, mycophenolate mofetil (MMF), psychotherapy |
| Accompanying | Avoidance of exacerbating factors (desensitization may be attempted), antibiotics (in case of bacterial superinfections, usually S. aureus), antivirals (acyclovir or valacyclovir during Herpes simplex virus superinfections in the form of herpetic eczema), antifungals, antihistamines and sedatives (in case of high intensity of pruritus), phototherapy, psychological help |
| Supplementary | Continuous use of emollients, even if there are no obvious inflammatory changes on the skin (during periods of remission) |
| “GREATER” CRITERIA | “LESSER” CRITERIA |
|---|---|
| Itching of the skin | Dryness of the skin |
| Chronic and recurrent course of the disease | Positive results of point skin tests |
| Typical location of skin lesions | Fish scale or follicular keratosis |
| Atopy in the patient or family history | Dennie and Morgan’s sign (eye fold) |
| Darkening around the eyes | |
| Increased IgE levels | |
| Early age of onset of lesions | |
| Recurrent skin infections | |
| Non-specific hand and/or foot eczema | |
| Nipple eczema | |
| Cheilitis | |
| Recurrent conjunctivitis | |
| Corneal cone | |
| Subcapsular cataract | |
| Anterior cervical fold | |
| White dermographism | |
| Food intolerance | |
| Itching of the skin after sweating | |
| Wool intolerance | |
| White dandruff | |
| Highlighting of hair follicles | |
| Facial erythema | |
| Exacerbation after stress |
| AZS Feature | Children | Adults |
|---|---|---|
| Distribution of changes | Face, torso, upper and lower limb extensor area [40,51] Diaper dermatitis is common in infants [19]. | All body areas up to 30–40 years of age predominance of lesions in the folds and skin folds [19] |
| Morphology of the lesions | Inflammation [40,51,52] | Lichenoid, dry lesions [40,51,52] |
| TH2/TH1 severity | High levels of TH2 in blood and skin, low TH1 [40,51] | TH2 lymphocytes predominate in the early stages of the disease, while TH1/TH17 increases with age [40,51]. |
| Epithelium and lipid barrier | Decreased function, pronounced barrier defect [40,51] | Retained function, less pronounced defect [40,51] |
| Therapeutic strategy | Therapies that are used in psoriasis can be effective [40,51]. | Therapy targeting the skin barrier in earlier stages [40,51] |
| Active Ingredient of Plant Origin | Properties of the Ingredient | Selected Studies Conducted on a Particular Ingredient towards Confirmation of Beneficial Effects on AD | Confirmation of the Properties and Validity of the Indicated Ingredient in AD—Conclusions of the Study |
|---|---|---|---|
| Aloes (Aloe vera) | It has moisturizing, antibacterial and antifungal properties, which can prevent the superinfection of skin lesions in patients with AD [59]. According to the authors of [61], saponins are responsible for the strong antiseptic properties of aloe vera gel. In addition, the presence of salicylic acid shows anti-inflammatory and antibacterial effects. The presence of magnesium lactate in aloe vera gel is responsible for inhibiting the activity of histidine decarboxylase, which reduces the synthesis of histamine (responsible for allergic reactions, pain and itching) [61]. | In 2010 and 2015, two animal model studies were conducted during which the effects of aloe vera on IgE levels were examined in animal models of atopic dermatitis. In a group of Balb/c mice with atopic dermatitis that received topical application of aloe vera extract for 10 days, a reduction in serum IgE levels was noted compared to the control group [59]. | The results of the study confirm that the medicinal properties of aloe vera may vary depending on its form (fresh aloe vera, gel form, etc.) [59,62]. Freshly prepared aloe vera is more beneficial, while the gel form is enzymatically or microbiologically sensitive [59,62]. |
| Coconut oil (Cocos nucifera L.) | The properties of this oil depend on the pressing method. Virgin coconut oil is considered a better form because the fatty acids are not lost through the processing [59]. Coconut virgin oil is extracted from the fresh and ripe flesh of coconut palm nuts [63]. The authors [63] confirm that virgin coconut oil exhibits anticancer, antimicrobial or anti-inflammatory properties in in vivo studies. | Two clinical trials have been conducted to evaluate the effects of virgin coconut oil on patients with atopic dermatitis [64]. In an in vitro study [63], the effect of virgin coconut oil on reconstructed human epidermal cells was evaluated. The above study showed that topical application of virgin coconut oil had an anti-inflammatory effect (inhibited the activation of pro-inflammatory cytokines such as IL-6, TNF-alpha and IFN-gamma), The authors [63] also confirmed the effect of virgin coconut oil on improving the skin barrier by increasing the synthesis of filaggrin, among other things. | Both studies confirmed the effect of coconut oil on reducing the severity of eczematous lesions in AD [64]. A study [63] confirmed the softening and moisturizing effects of coconut oil for mild to moderate dry skin in patients with AD. The authors of the above study proved that the use of virgin coconut oil is effective in the treatment of skin diseases with dermal–epidermal barrier dysfunction and reduced the expression of structural proteins. |
| Oat (Avena sativa L.) | It soothes feelings of itching and irritation. Due to the presence of avenanthramides, oatmeal exhibits anti-inflammatory and antioxidant effects [62]. | Clinical studies [62] conducted to evaluate the effects of oats on eczematous lesions have shown a reduction in skin redness, dryness, scaling and pruritus after applying oat extracts to the skin. | Oats used in colloidal form as an ingredient in emollients significantly reduced dry skin. According to the authors of [62], colloidal oats reduced the intensity of pruritus. These results confirm the anti-inflammatory and antioxidant effects of colloidal oats in the skin care of patients with AD [62]. |
| Sunflower oil (Helianthus annuus L.) | It contains a high concentration of fatty acids such as linoleic acid, linolenic acid, oleic acid, palmitic acid and stearic acid [65]. According to the authors of [65], refined sunflower oil contains about 90% essential fatty acids (EFAs). According to the authors of [52], EFA deficiency in patients with atopic dermatitis causes an increase in skin barrier permeability, resulting in excessive water loss and loss of Natural Moisturizing Factor. According to studies [60], sunflower seed oil contains higher concentrations of linoleic acid compared to olive oil. Studies [60,65,66] have shown that linoleic acid acted as an agonist of peroxisome proliferator activated receptors (PPAR), which stimulates keratinocyte proliferation and synthesis of lipids, cholesterol or ceramides. Consequently, the skin barrier is strengthened [60,65]. | A randomized trial [67] evaluated the effects of isosorbide diesters derived from sunflower oil and coconut oil and their interaction with colloidal oats on clinical symptoms of AD. In in vivo studies [65,68], a cream that contained 2% refined sunflower oil increased epidermal lipid synthesis and increased skin hydration, and decreased TEWL. | Studies [60,65] have shown improved hydration of adult skin without inducing erythema after using sunflower oil. It was also confirmed that the use of a 2% cream with refined sunflower oil reduced the amount of corticosteroids used in atopic dermatitis [65,68]. Topical application of emollients containing sunflower and coconut oil fatty esters reduced pruritus and reduced the amount of topical corticosteroids used [67]. |
| Rosehip oil (Rosa canina L.) | Rosehip oil contains a large amount of essential fatty acids, including linoleic acid (35.9–54.8%) or alpha-linolenic acid (16.6–26.5%), as well as a large number of antioxidants such as tocopherol and carotenoids, so rosehip oil has anti-inflammatory and regenerative effects [60]. | In vivo application of rose oil (0.1%) reduced TEWL and increased expression of the structural protein filaggrin in the skin of an animal model [69]. Rose oil (obtained by flower extraction) inhibited proliferation and induced expression of involucrin or filaggrin in keratinocytes in vitro [65]. | Research [60] confirms that due to its content of the above components, rosehip oil counteracts the development of inflammation and oxidative stress. |
| Soybean oil (Glycine max L.) | Soybean oil is characterized by the presence of soy phytosterols (such as genistein), which have positive effects on skin barrier regeneration [60,70]. Genistein has a protective effect on keratinocytes, protecting them from the damaging effects of free radicals responsible for aging at the cellular level [71,72]. Vitamin K in soybean oil soothes skin redness, seals the walls of blood vessels and promotes skin regeneration [71,72]. Soybean oil is a source of unsaturated essential fatty acids mainly linoleic (50%), oleic or palmitic acid [23]. | A study [73] related that no increase in IgE and IgG antibodies was observed in people with food allergy to soybeans after soybean oil application to the skin, which indicates that soybean oil applied to the skin has less sensitizing potential. Another study [74] evaluating the effects of soybean oil and anti-itch lauromacrogols contained in a bath lotion in patients with atopic eczema confirmed their efficacy and safety. The above study included 3500 patients, and the average duration of treatment was 42 days. The authors emphasize that the oil was well tolerated and effective in treating dry and itchy skin lesions. | Topical application of soybean oil extracts has been shown to reduce TEWL [60]. Regular use of soybean oil increases skin hydration, relieves inflammation and reduces recurrence [23]. Studies [75] confirm these actions, as anthocyanins from black soybean inhibit the production of reactive oxygen species (ROS) and protein kinases. This has the effect of reducing inflammation [75]. The use of a bath lotion containing soybean oil, among other ingredients, reduced the use of pharmaceuticals, such as steroids, in 60% of patients [74]. |
| Sea buckthorn oil (Hippophae rhamnoides L.). | Oil is obtained from the seeds or pulp of sea buckthorn berries. The berries contain large amounts of vitamin C [76]. The authors of [77] report that the vitamin C content, depending on the variety, varies between 28 and 201 mg/100 g of fresh fruit, so the vitamin C content is 15 times higher than in orange fruit. Its content is responsible for the antioxidant effect of sea buckthorn oil, as well as its protective effect against harmful UVA and UVB radiation. Studies [77] report that the vitamin A content of sea buckthorn oil provides regenerative and anti-aging effects. Sea buckthorn oil obtained from the seeds provides large amounts of palmitoleic acid responsible for stimulating skin regeneration and wound healing [78]. The authors of [76] report that sea buckthorn oil contains gamma-linolenic acid, which improves blood circulation, nutrition and oxygenation of the skin, and in its deeper layers is converted to prostaglandins, which neutralize inflammation. | The authors of [78] report that when applied externally, sea buckthorn oil can reduce sores, scars, discoloration and allergic and inflammatory skin lesions. Other studies [78] have shown that a single application of a cream consisting of natural emollients (40% sea buckthorn oil or 40% olive oil) increases skin hydration more than the use of synthetic emollients (for example, isopropyl myristate). | Sea buckthorn oil relieves symptoms of atopic dermatitis, reducing dryness, irritation, flaking and itching [78]. Sea buckthorn oil has been confirmed to be effective in the treatment of skin dermatoses and to promote the granulation process of hard-to-heal wounds [76]. |
| Hemp oil (Cannabis sativa L.) | Produced from hemp seed. The authors [79] report that hemp oil contains terpenes, which have anti-inflammatory and antioxidant properties. The oil is rich in essential fatty acids (linoleic, linolenic, oleic), as well as saturated acids (such as palmitic) responsible for the mechanical barrier of the skin, which protect it from external factors and water loss. Another study [80] shows that the content of essential fatty acids has a rejuvenating effect. The authors of [79] prove that in hemp oil the ratio of omega-6 to omega-3 fatty acids (3:1) has a beneficial effect, as it regulates the metabolic activity of cells and enhances anti-inflammatory effects. The phytosterol content (mainly β- sitosterol at 70%) in hemp oil is responsible for anti-inflammatory and antimicrobial effects, antioxidant activity and stimulation of collagen synthesis [79]. | According to studies conducted in [23,79] to evaluate the effects of hemp oil on patients with AD, it has been proven that its regular use helped reduce pruritus and excessive dry skin. The authors of the above study emphasize that gamma-linolenic acid had an important role in alleviating the symptoms of atopic dermatitis [23,79]. In a study conducted by another group of authors [81], it was noted that the administration of hemp oil orally for a period of 8 weeks to a group of patients with atopic dermatitis resulted in favorable changes in the serum fatty acid profile. The authors of [81] noted an increase in the concentration of gamma-linolenic acid in blood lipids. Based on the survey responses of patients who participated in the study, there was a significant reduction in pruritus and dry skin after hemp oil administration. | All the described studies [23,79,80,81,82,83] confirm the beneficial effects of hemp oil on the skin of patients with atopic dermatitis. The authors of [23] assert that hemp oil increased the hydration of the stratum corneum of the skin, and alleviated inflammation and pruritus. A study [79] confirmed that phytosterols and flavonoids found in hemp oil have anti-allergic and immunostimulating effects on the skin of patients with AD. Other authors [82,83] confirm that essential fatty acids soothe skin irritation and accelerate skin regeneration through anti-inflammatory effects, significantly reducing inflammation in AD. Studies [79] on the effects of phytosterols contained in hemp oil confirm its effect on reducing transepidermal water loss. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kowalska, M.K.; Orłowska, S.M.; Bednarczyk, Ł. Applied Research on Atopic Dermatitis with Special Emphasis on the Role of Emollients in This Disorder: A Review. Appl. Sci. 2024, 14, 8315. https://doi.org/10.3390/app14188315
Kowalska MK, Orłowska SM, Bednarczyk Ł. Applied Research on Atopic Dermatitis with Special Emphasis on the Role of Emollients in This Disorder: A Review. Applied Sciences. 2024; 14(18):8315. https://doi.org/10.3390/app14188315
Chicago/Turabian StyleKowalska, Małgorzata Katarzyna, Sara Małgorzata Orłowska, and Łukasz Bednarczyk. 2024. "Applied Research on Atopic Dermatitis with Special Emphasis on the Role of Emollients in This Disorder: A Review" Applied Sciences 14, no. 18: 8315. https://doi.org/10.3390/app14188315
APA StyleKowalska, M. K., Orłowska, S. M., & Bednarczyk, Ł. (2024). Applied Research on Atopic Dermatitis with Special Emphasis on the Role of Emollients in This Disorder: A Review. Applied Sciences, 14(18), 8315. https://doi.org/10.3390/app14188315










