Heart Rate and Rhythm Changes in Dogs Treated in a Hyperbaric Oxygen Chamber
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Selection
2.2. Treatment Procedure
2.3. Statistical Analysis
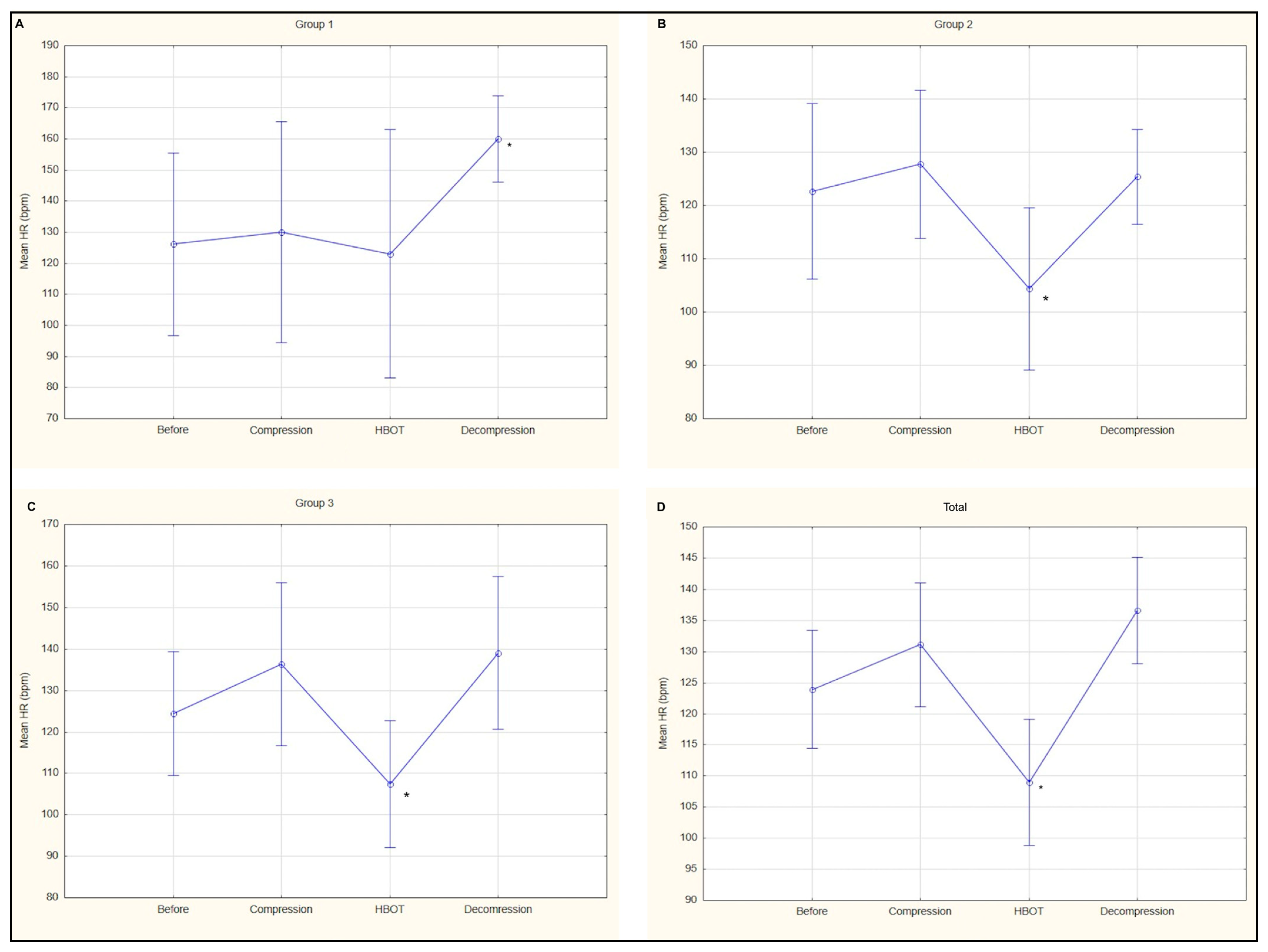
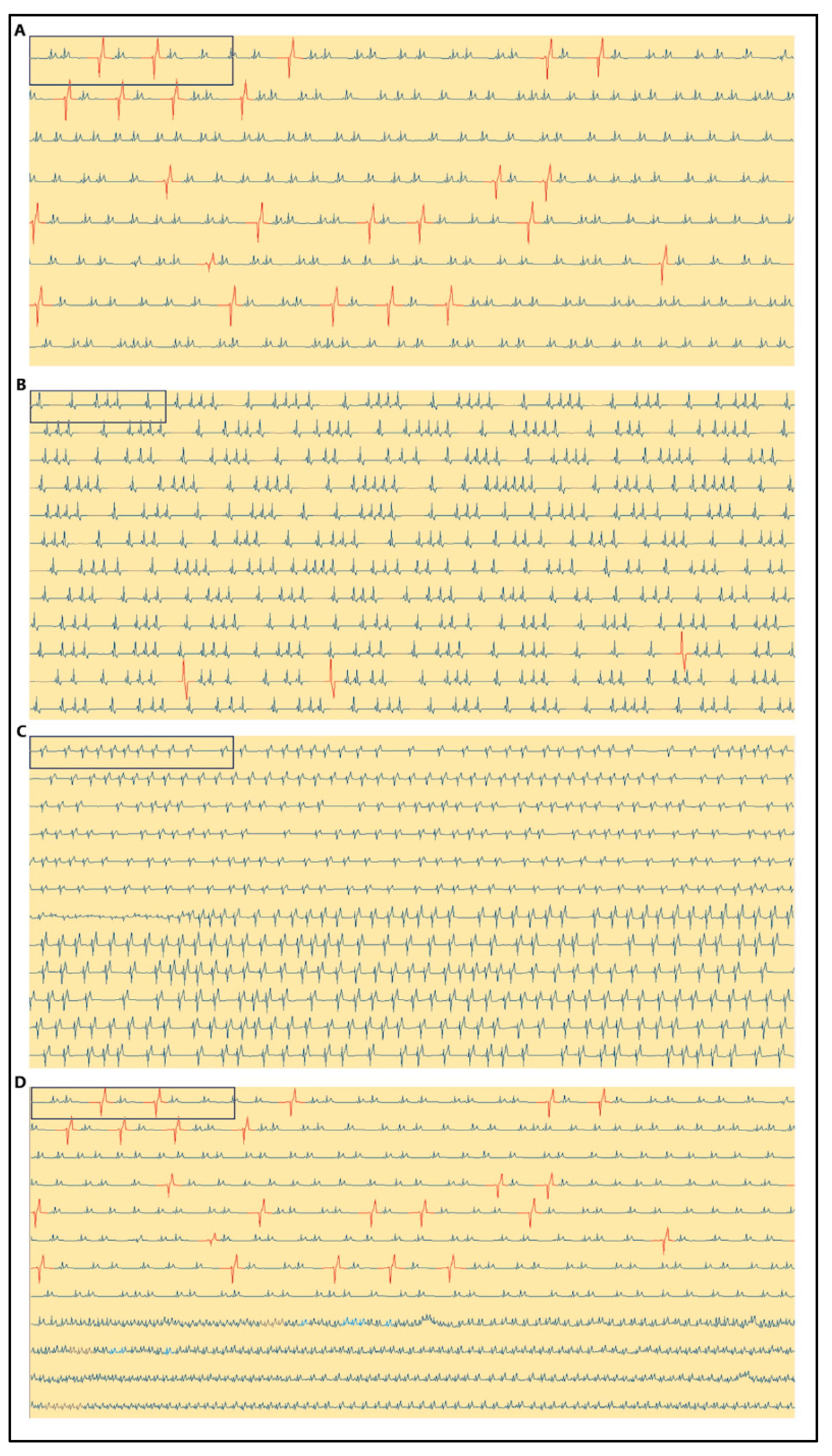
3. Results
4. Discussion
5. Conclusions
6. Limitations and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cowley, R.A.; Attar, S.; Blair, E.; Esmond, W.G.; Michaelis, M.; Ollodart, R. Prevention and treatment of shock by hyperbaric oxygenation. Ann. N. Y. Acad. Sci. 1965, 117, 673–683. [Google Scholar] [CrossRef] [PubMed]
- Weglicki, W.B.; Whalen, R.E.; Thompson, H.K.; McIntosh, H.D. Effects of Hyperbaric Oxygenation on Excess Lactate Production in Exercising Dogs. Am. J. Physiol. Leg. Content 1966, 210, 473–477. [Google Scholar] [CrossRef]
- Winter, P.M.; Gupta, R.K.; Michalski, A.H.; Lanphier, E.H. Modification of Hyperbaric Oxygen Toxicity by Experimental Venous Admixture. J. Appl. Physiol. 1967, 23, 954–963. [Google Scholar] [CrossRef]
- Whalen, R.E.; Saltzman, H.A. Hyperbaric Oxygenation in the Treatment of Acute Myocardial Infarction. Prog. Cardiovasc. Dis. 1968, 10, 575–583. [Google Scholar] [CrossRef]
- Edwards, M.L. Hyperbaric Oxygen Therapy. Part 1: History and Principles. J. Vet. Emerg. Crit. Care 2010, 20, 284–288. [Google Scholar] [CrossRef] [PubMed]
- Edwards, M.L. Hyperbaric Oxygen Therapy. Part 2: Application in Disease. J. Vet. Emerg. Crit. Care 2010, 20, 289–297. [Google Scholar] [CrossRef]
- Hosgood, G.; Kerwin, S.C.; Lewis, D.D.; Strain, G.S.; Hedlund, C.S.; Elkins, A.D. Clinical Review of the Mechanism and Applications of Hyperbaric Oxygen Therapy in Small Animal Surgery. Vet. Comp. Orthop. Traumatol. 1992, 5, 31–39. [Google Scholar] [CrossRef]
- Crowe, D.T., Jr. Hyperbaric oxygen therapy in veterinary medicine: A case series at Carson-Tahoe veterinary hospital. Hyperb. Med. Today 2000, 1, 13–15. [Google Scholar]
- Shmalberg, J.; Davies, W.; Lopez, S.; Shmalberg, D.; Zilberschtein, J. Rectal Temperature Changes and Oxygen Toxicity in Dogs Treated in a Monoplace Chamber. Hyperb. Med. 2015, 42, 95. [Google Scholar]
- Smith, B.A.; Hosgood, G.; Hedlund, C.S. Omental Pedicle Used to Manage a Large Dorsal Wound in a Dog. J. Small Anim. Pract. 1995, 36, 267–270. [Google Scholar] [CrossRef]
- Birnie, G.L.; Fry, D.R.; Best, M.P. Safety and Tolerability of Hyperbaric Oxygen Therapy in Cats and Dogs. J. Am. Anim. Hosp. Assoc. 2018, 54, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Montalbano, C.; Kiorpes, C.; Elam, L.; Miscioscia, E.; Shmalberg, J. Common Uses and Adverse Effects of Hyperbaric Oxygen Therapy in a Cohort of Small Animal Patients: A Retrospective Analysis of 2,792 Treatment Sessions. Front. Vet. Sci. 2021, 8, 764002. [Google Scholar] [CrossRef] [PubMed]
- Gautier, A.; Graff, E.C.; Bacek, L.; Fish, E.J.; White, A.; Palmer, L.; Kuo, K. Effects of Ovariohysterectomy and Hyperbaric Oxygen Therapy on Systemic Inflammation and Oxidation in Dogs. Front. Vet. Sci. 2020, 6, 506210. [Google Scholar] [CrossRef]
- Latimer, C.R.; Lux, C.N.; Roberts, S.; Drum, M.G.; Braswell, C.; Sula, M.J.M. Effects of Hyperbaric Oxygen Therapy on Uncomplicated Incisional and Open Wound Healing in Dogs. Vet. Surg. 2018, 47, 827–836. [Google Scholar] [CrossRef]
- Demchenko, I.T.; Zhilyaev, S.Y.; Moskvin, A.N.; Krivchenko, A.I.; Piantadosi, C.A.; Allen, B.W. Baroreflex-Mediated Cardiovascular Responses to Hyperbaric Oxygen. J. Appl. Physiol. 2013, 115, 819–828. [Google Scholar] [CrossRef]
- Graff, B.; Szyndler, A.; Czechowicz, K.; Kucharska, W.; Graff, G.; Boutouyrie, P.; Laurent, S.; Narkiewicz, K. Relationship between Heart Rate Variability, Blood Pressure and Arterial Wall Properties during Air and Oxygen Breathing in Healthy Subjects. Auton. Neurosci. 2013, 178, 60–66. [Google Scholar] [CrossRef]
- Mathieu, D.; Favory, R.; Collet, F.; Linke, J.C.; Wattel, F. Physiologic Effects of Hyperbaric Oxygen on Hemodynamics and Microcirculation. In Handbook on Hyperbaric Medicine, 1st ed.; Springer: New York City, NY, USA, 2006; Volume 7, pp. 75–101. [Google Scholar] [CrossRef]
- Baisan, R.A.; Vulpe, V.; Ohad, D.G. Short-Term Heart Rate Variability in Healthy Dogs and Dogs in Various Stages of Degenerative Mitral Valve Disease Evaluated before Pharmacotherapy. Vet. J. 2021, 274, 105704. [Google Scholar] [CrossRef]
- Sheffield, P.J.; Desautels, D.A. Hyperbaric and Hypobaric Chamber Fires: A 73-Year Analysis. Undersea Hyperb. Med. 1997, 24, 153–164. [Google Scholar]
- Petrie, J.P. Practical Application of Holter Monitoring in Dogs and Cats. Clin. Tech. Small Anim. Pract. 2005, 20, 173–181. [Google Scholar] [CrossRef]
- Ostrowski, R.P.; Colohan, A.R.T.; Zhang, J.H. Mechanism of Hyperbaric Oxygen-Induced Neuroprotection in a Rat Model of Subarachnoid Hemorrhage. J. Cereb. Blood Flow Metab. 2005, 25, 554–571. [Google Scholar] [CrossRef]
- Weinstein, P.R.; Anderson, G.G.; Telles, D.A. Results of Hyperbaric Oxygen Therapy during Temporary Middle Cerebral Artery Occlusion in Unanesthetized Cats. Neurosurgery 1987, 20, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Van Hulst, R.A.; Drenthen, J.; Haitsma, J.J.; Lameris, T.W.; Visser, G.H.; Klein, J.; Lachmann, B. Effects of Hyperbaric Treatment in Cerebral Air Embolism on Intracranial Pressure, Brain Oxygenation, and Brain Glucose Metabolism in the Pig. Crit. Care Med. 2005, 33, 841–846. [Google Scholar] [CrossRef] [PubMed]
- Shoshani, O.; Shupak, A.; Barak, A.; Ullman, Y.; Ramon, Y.; Lindenbaum, E.; Peled, Y. Hyperbaric Oxygen Therapy for Deep Second Degree Burns: An Experimental Studyin the Guinea Pig. Br. J. Plast. Surg. 1998, 51, 67–73. [Google Scholar] [CrossRef]
- Fries, R.B.; Wallace, W.A.; Roy, S.; Kuppusamy, P.; Bergdall, V.; Gordillo, G.M.; Melvin, W.S.; Sen, C.K. Dermal Excisional Wound Healing in Pigs Following Treatment with Topically Applied Pure Oxygen. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2005, 579, 172–181. [Google Scholar] [CrossRef]
- Pellitteri, P.K.; Kennedy, T.L.; Youn, B.A. The Influence of Intensive Hyperbaric Oxygen Therapy on Skin Flap Survival in a Swine Model. Arch. Otolaryngol. Head Neck Surg. 1992, 118, 1050–1054. [Google Scholar] [CrossRef]
- Amaral, B.P.; Krause, A.; Pasini, J.S.; Silva, J.C.; Inkelmann, M.A.; Müller, D.C.M. Hyperbaric Oxygen Therapy in Wound Healing in Mice. Arq. Bras. Med. Vet. Zootec. 2021, 73, 361–366. [Google Scholar] [CrossRef]
- Van Neck, J.W.; Tuk, B.; Fijneman, E.M.G.; Redeker, J.J.; Talahatu, E.M.; Tong, M. Hyperbaric Oxygen Therapy for Wound Healing in Diabetic Rats: Varying Efficacy after a Clinically-Based Protocol. PLoS ONE 2017, 12, e0177766. [Google Scholar] [CrossRef]
- Levitan, D.M.; Hitt, M.; Geiser, D.R.; Lyman, R. Rationale for Hyperbaric Oxygen Therapy in Traumatic Injury and Wound Care in Small Animal Veterinary Practice. J. Small Anim. Pract. 2021, 62, 719–729. [Google Scholar] [CrossRef]
- Gouveia, D.; Bimbarra, S.; Carvalho, C.; Cardoso, A.; Gamboa, Ó.; Teixeira, R.; Ferreira, A.; Martins, Â. Effects of Hyperbaric Oxygen Therapy on Wound Healing in Veterinary Medicine: A Pilot Study. Open Vet. J. 2021, 11, 544–554. [Google Scholar] [CrossRef]
- Amora, L.D.A.S.; Murashima, A.D.A.B.; Rossato, M.; Moreira, M.B.; Hyppolito, M.Â.; Fagundes, D.J. The Effects of Hyperbaric Oxygen Therapy upon Ototoxic Injuries Produced by Amikacin in Guinea Pigs. Braz. J. Otorhinolaryngol. 2013, 79, 342–348. [Google Scholar] [CrossRef]
- Baumwart, C.A.; Doherty, T.J.; Schumacher, J.; Willis, R.S.; Adair, H.S.; Rohrbach, B.W. Effects of Hyperbaric Oxygen Treatment on Horses with Experimentally Induced Endotoxemia. Am. J. Vet. Res. 2011, 72, 1266–1275. [Google Scholar] [CrossRef] [PubMed]
- Tousoulis, D.; Kampoli, A.-M.; Tentolouris Nikolaos Papageorgiou, C.; Stefanadis, C. The Role of Nitric Oxide on Endothelial Function. Curr. Vasc. Pharmacol. 2011, 10, 4–18. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, A.; Tesselaar, E.; Henricson, J.; Sjöberg, F. Prostaglandins and Radical Oxygen Species Are Involved in Microvascular Effects of Hyperoxia. J. Vasc. Res. 2010, 47, 441–450. [Google Scholar] [CrossRef]
- Dallinger, S.; Dorner, G.T.; Wenzel, R.; Graselli, U.; Findl, O.; Eichler, H.G.; Woltz, M.; Schmetterer, L. Endothelin-1 contributes to hyperoxia-induced vasoconstriction in the human retina. Investig. Ophthalmol. Vis. Sci. 2000, 41, 864–869. [Google Scholar]
- Takagi, C.; King, G.L.; Takagi, H.; Lin, Y.-W.; Clermont, A.C.; Bursell, S.-E. Endothelin-1 Action via Endothelin Receptors Is a Primary Mechanism Modulating Retinal Circulatory Response to Hyperoxia. Investig. Ophthalmol. Vis. Sci. 1996, 37, 2099–2109. [Google Scholar]
- Kenmure, A.C.; Murdoch, W.R.; Hutton, I.; Cameron, A.J. Hemodynamic Effects of Oxygen at 1 and 2 Ata Pressure in Healthy Subjects. J. Appl. Physiol. 1972, 32, 223–226. [Google Scholar] [CrossRef]
- Molénat, F.; Boussuges, A.; Grandfond, A.; Rostain, J.C.; Sainty, J.M.; Robinet, C.; Galland, F.; Meliet, J.L. Haemodynamic Effects of Hyperbaric Hyperoxia in Healthy Volunteers: An Echocardiographic and Doppler Study. Clin. Sci. 2004, 106, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, B.; Tetzlaff, K.; Staschen, C.M.; Bettinghausen, E. Cardiac Output Changes during Hyperbaric Hyperoxia. Int. Arch. Occup. Environ. Health 2001, 74, 119–122. [Google Scholar] [CrossRef]
- Naraki, N.; Tomizawa, G.; Mohri, M. Heart Rate in Hyperbaric Environment after Autonomic Blockade. Appl. Hum. Sci. 1973, 9, 260–264. [Google Scholar] [CrossRef]
- Howley, E.T.; Cox, R.H.; Welch, H.G.; Adams, R.P. Effect of Hyperoxia on Metabolic and Catecholamine Responses to Prolonged Exercise. J. Appl. Physiol. 1983, 54, 59–63. [Google Scholar] [CrossRef]
- Fukuda, Y.; Sato, A.; Suzuki, A.; Trzebski, A. Autonomic Nerve and Cardiovascular Responses to Changing Blood Oxygen and Carbon Dioxide Levels in the Rat. J. Auton. Nerv. Syst. 1989, 28, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.M.; Doursout, M.F.; Butler, B.D. Effects of Hyperbaric Hyperoxia on Cardiac and Regional Hemodynamics in Conscious Dogs. Aviat. Space Environ. Med. 1998, 69, 761–765. [Google Scholar]
- Perdrizet, G.A. Principles and practice of hyperbaric medicine: A medical practitioner’s primer, part I. Conn. Med. 2014, 78, 325. [Google Scholar] [PubMed]
- Perdrizet, G.A. Principles and practice of hyperbaric medicine: A medical practitioner’s primer, part II. Conn. Med. 2014, 78, 389. [Google Scholar] [PubMed]
- Leveque, C.; Mrakic Sposta, S.; Theunissen, S.; Germonpré, P.; Lambrechts, K.; Vezzoli, A.; Bosco, G.; Lévénez, M.; Lafère, P.; Guerrero, F.; et al. Oxidative Stress Response Kinetics after 60 Minutes at Different (1.4 ATA and 2.5 ATA) Hyperbaric Hyperoxia Exposures. Int. J. Mol. Sci. 2023, 24, 12361. [Google Scholar] [CrossRef]
- MacLaughlin, K.J.; Barton, G.P.; Braun, R.K.; MacLaughlin, J.E.; Lamers, J.J.; Marcou, M.D.; Eldridge, M.W. Hyperbaric Air Mobilizes Stem Cells in Humans; a New Perspective on the Hormetic Dose Curve. Front. Neurol. 2023, 14, 1192793. [Google Scholar] [CrossRef]
- Ishibashi, M.; Hayashi, A.; Akiyoshi, H.; Ohashi, F. The Influences of Hyperbaric Oxygen Therapy with a Lower Pressure and Oxygen Concentration than Previous Methods on Physiological Mechanisms in Dogs. J. Vet. Med. Sci. 2015, 77, 297–304. [Google Scholar] [CrossRef]
- Malpas, S.C. Sympathetic Nervous System Overactivity and Its Role in the Development of Cardiovascular Disease. Physiol. Rev. 2010, 90, 513–557. [Google Scholar] [CrossRef]
- Palatini, P.; Julius, S. The Role of Cardiac Autonomic Function in Hypertension and Cardiovascular Disease. Curr. Hypertens. Rep. 2009, 11, 199–205. [Google Scholar] [CrossRef]
- Borchard, U. The Role of the Sympathetic Nervous System in Cardiovascular Disease. J. Clin. Basic Cardiol. 2001, 4, 175–177. [Google Scholar]
- Oyama, M.A. Neurohormonal Activation in Canine Degenerative Mitral Valve Disease: Implications on Pathophysiology and Treatment. J. Small Anim. Pract. 2009, 50, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Meredith, I.T.; Eisenhofer, G.; Lambert, G.W.; Dewar, E.M.; Jennings, G.L.; Esler, M.D. Cardiac Sympathetic Nervous Activity in Congestive Heart Failure. Evidence for Increased Neuronal Norepinephrine Release and Preserved Neuronal Uptake. Circulation 1993, 88, 136–145. [Google Scholar] [CrossRef]
- Reardon, M.; Malik, M. Changes in Heart Rate Variability with Age. Pacing Clin. Electrophysiol. 1996, 19, 1863–1866. [Google Scholar] [CrossRef] [PubMed]
- Shannon, D.C.; Carley, D.W.; Benson, H. Aging of Modulation of Heart Rate. Am. J. Physiol. Circ. Physiol. 1987, 253, H874–H877. [Google Scholar] [CrossRef]
- Lund, V.E.; Kentala, E.; Scheinin, H.; Klossner, J.; Helenius, H.; Sariola-Heinonen, K.; Jalonen, J. Heart Rate Variability in Healthy Volunteers during Normobaric and Hyperbaric Hyperoxia. Acta Physiol. Scand. 1999, 167, 29–35. [Google Scholar] [CrossRef]
- Stuhr, L.E.B.; Bergo, G.W.; Tyssebotn, I. Systemic Hemodynamics during Hyperbaric Oxygen Exposure in Rats. Aviat. Space Environ. Med. 1994, 65, 531–538. [Google Scholar] [PubMed]
- Simon, A.J.; Torbati, D. Effects of Hyperbaric Oxygen on Heart, Brain, and Lung Functions in Rat. Undersea Biomed. Res. 1982, 9, 263–275. [Google Scholar]
- Thomason, J.D.; Kraus, M.S.; Surdyk, K.K.; Fallaw, T.; Calvert, C.A. Bradycardia-Associated Syncope in 7 Boxers with Ventricular Tachycardia (2002–2005). J. Vet. Intern. Med. 2008, 22, 931–936. [Google Scholar] [CrossRef]
- Scherlag, B.J.; Kabell, G.; Harrison, L.; Lazzara, R. Mechanisms of Bradycardia-Induced Ventricular Arrhythmias in Myocardial Ischemia and Infarction. Circulation 1982, 65, 1429–1434. [Google Scholar] [CrossRef]
- Eckenhoff, R.G.; Knight, D.R. Cardiac Arrhythmias and Heart Rate Changes in Prolonged Hyperbaric Air Exposures. Undersea Biomed. Res. 1984, 11, 355–367. [Google Scholar]
- Lund; Kentala; Scheinin; Klossner; Sariola-Heinonen; Jalonen. Hyperbaric Oxygen Increases Parasympathetic Activity in Professional Divers. Acta Physiol. Scand. 2000, 170, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Oreto, G.; Luzza, F.; Badessa, F.; Calabrò, M.P.; Mazzone, P.; Carerj, S.; Saporito, F.; Pappone, C. QRS Complex Voltage Changes Associated with Supraventricular Tachycardia. J. Cardiovasc. Electrophysiol. 2001, 12, 1358–1362. [Google Scholar] [CrossRef] [PubMed]
- Hiroko Wakimoto; Naomi Izumida; Asano, Y.; Hiraoka, M.; Kawara, T.; Hiejima, K.; Hirao, K.; Suzuki, F. Augmentation of QRS Wave Amplitudes in the Precordial Leads During Narrow QRS Tachycardia. J. Cardiovasc. Electrophysiol. 2000, 11, 52–60. [Google Scholar] [CrossRef]
| Group 1 | Group 2 | Group 3 | |
|---|---|---|---|
| N | 6 | 15 | 11 |
| Number of different breeds | 2 | 7 | 6 |
| Age (years) | 13 (4–22) | 9.58 (3–14) | 9.6 (4–14) |
| Body weight (kg) | 14.8 (4–27) | 26.2 (4.4–44) | 17.3 (8–23) |
| Temperature | 38.1 (37.5–38.7) | 38.6 (37.8–39.5) | 38.5 (37.9–39.2) |
| Sex (female/male) | 3:3 | 8:7 | 6:5 |
| Health condition | Degenerative mitral valve disease (6) | Elbow joint dysplasia n = 1 Hip joint dysplasia n = 2 Hip joint degeneration n = 2 Cruciate ligament rupture n = 2 Spondylarthrosis n = 5 Ischemic stroke n = 3 | Healthy |
| Variable | Group 1 n = 6 | Group 2 n = 15 | Group 3 n = 11 |
|---|---|---|---|
| Number of QRS bases (median, IQR) | 4061.5 (3588–4350) | 3603 (2796–4559) | 3973 (3444–4358) |
| Min HR base (median, IQR) | 87 (75–100) | 62 (51–94) | 66 (55–88) |
| Max HR (median, IQR) | 206.5 (204–210) | 205 (154–215) | 201 (195–213) |
| Average HR (median, IQR) | 134.5 (112–145) | 121 (101–153) | 128 (104–135) |
| Number of QRS compressions (median, IQR) | 484 (410–602) | 483 (379–547) | 603 (448–624) |
| Min HR compression (median, IQR) | 90.5 (73–114) | 84 (68–110) | 83 (72–97) |
| Max HR compression (median, IQR) | 188 (181–198) | 173 (157–196) | 195 (167–220) |
| Average HR compression (median, IQR) | 130 (103–151) | 131 (107–140) | 127 (119–157) |
| Number of QRS HBOTs (median, IQR) | 1025.5 (7504–12,586) | 8379 (7553–9224) | 8983 (8041–11,236) |
| Min HR HBOT (median, IQR) | 65.5 (61–77) | 50 (42–62) | 53 (39–65) |
| Max HR HBOT (median, IQR) | 208.5 (204–211) | 204 (160–220) | 194 (168–215) |
| Average HR HBOT (median, IQR) | 127 (996–148) | 96 * (86–107) | 107 * (90–134) |
| Number of QRS decompressions (median, IQR) | 127 (96–148) | 131 (71–109) | 147 (136–163) |
| Min HR decompression (median, IQR) | 109 (97–124) | 89 (71–109) | 101 (59–112) |
| Max HR decompression (median, IQR) | 197 (181–209) | 155 (143–191) | 173 (169–191) |
| Average HR decompression (median, IQR) | 157 ^ (149–168) | 123 (115–141) | 142 (118–163) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graczyk, S.; Łunkiewicz, W.; Grzeczka, A.; Zyśko, D.; Pasławski, R.; Pasławska, U. Heart Rate and Rhythm Changes in Dogs Treated in a Hyperbaric Oxygen Chamber. Appl. Sci. 2024, 14, 9963. https://doi.org/10.3390/app14219963
Graczyk S, Łunkiewicz W, Grzeczka A, Zyśko D, Pasławski R, Pasławska U. Heart Rate and Rhythm Changes in Dogs Treated in a Hyperbaric Oxygen Chamber. Applied Sciences. 2024; 14(21):9963. https://doi.org/10.3390/app14219963
Chicago/Turabian StyleGraczyk, Szymon, Wojciech Łunkiewicz, Arkadiusz Grzeczka, Dorota Zyśko, Robert Pasławski, and Urszula Pasławska. 2024. "Heart Rate and Rhythm Changes in Dogs Treated in a Hyperbaric Oxygen Chamber" Applied Sciences 14, no. 21: 9963. https://doi.org/10.3390/app14219963
APA StyleGraczyk, S., Łunkiewicz, W., Grzeczka, A., Zyśko, D., Pasławski, R., & Pasławska, U. (2024). Heart Rate and Rhythm Changes in Dogs Treated in a Hyperbaric Oxygen Chamber. Applied Sciences, 14(21), 9963. https://doi.org/10.3390/app14219963






