Evaluation of an Ozone-Induced Free Radical Solution’s Characteristics and Its Efficacy as an Alternative Pest Control Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
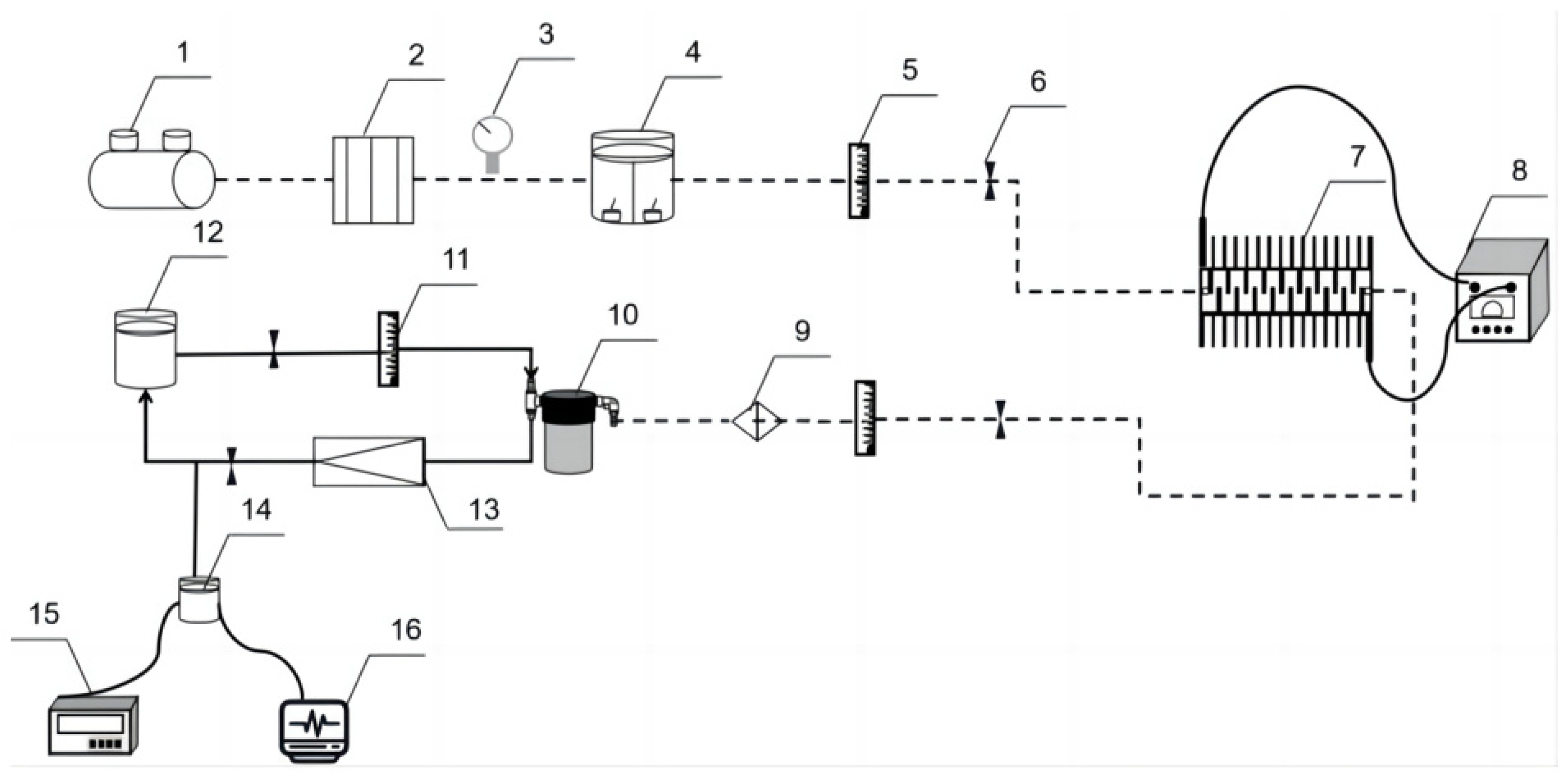
2.2. Instruments and Equipment
2.3. Test Sites and Varieties
2.4. Methods
2.4.1. Preparation of Ozone-Induced Free Radical Solution
2.4.2. Analysis of Ozone Radical Solution Oxidation and Stability
2.4.3. Detection of Free Radical Species in Ozone-Induced Free Radical Solution
- (1)
- Detection of hydroxyl radical (·OH) [19,20,21,22]: Terephthalic acid (TA) undergoes a reaction with hydroxyl radicals, resulting in the formation of 2-hydroxyterephthalic acid (TAOH), with the reaction equation illustrated in Figure 2. A total of 166 mg of TA reagent was dissolved in 100 mL of 1 mol/L NaOH solution (referred to as TA mother liquor), from which 0.1 mL was further diluted to 50 mL to obtain a 10 mmol/L TA solution. A total of 3 mL of the 10 mmol/L TA solution was mixed with 5 mL of the free radical solution, and the emission peak was measured by a fluorescence spectrophotometer at an excitation wavelength of 315 nm and emission of 425 nm.
- (2)
- Detection of superoxide radicals (·O2−) [23]: Nitroblue tetrazolium chloride (NBT) reacts with superoxide radicals to produce formazan, with the reaction equation depicted in Figure 3. A total of 817.6 mg of NBT was dissolved in 100 mL of pure water to create the mother liquor, from which 0.1 mL was subsequently diluted to 50 mL to achieve a 10 mmol/L NBT solution. A total of 3 mL of the 10 mmol/L NBT solution was mixed with 5 mL of the ozone-induced free radical solution, and the NBT absorption peak at approximately 259 nm was measured using an ultraviolet–visible spectrophotometer. A lower absorption peak compared to the original solution indicates the presence of superoxide radicals.
- (3)
- Detection of singlet oxygen (1O2) [24,25]: 1,3-Diphenylisobenzofuran (DPBF) specifically reacts with singlet oxygen, with the reaction equation presented in Figure 4. A total of 0.27 g of DPBF was dissolved in 100 mL of pure water to form the mother liquor, from which 0.1 mL was diluted to 50 mL, yielding a 10 mmol/L DPBF solution. A total of 3 mL of the 10 mmol/L DPBF solution was mixed with 5 mL of the ozone-induced free radical solution, and the absorbance at a wavelength of 410 nm was measured using an ultraviolet–visible spectrophotometer, followed by analysis of the absorbance change. A decrease in absorbance indicates the presence of singlet oxygen.
2.4.4. Efficacy Test of Ozone-Induced Free Radical Solution in Controlling Typical Pathogens
- (1)
- Inhibitory Effect of ozone-induced free radical solution on Bacillus subtilis
- (2)
- Analysis of the Effect of ozone-induced free radical solution on the Structure of Bacillus subtilis
- (3)
- Pest Control Efficacy in Greengrocery
2.4.5. Statistical Analysis Methods
3. Results
3.1. Oxidation and Stability Analysis of Ozone-Induced Free Radical Solution
3.1.1. Effect of pH on Oxidizability and Stability
3.1.2. Effect of Air Intake on Oxidizability and Stability
3.1.3. Effect of Liquid Volume on Oxidation and Stability
3.2. Detection of Free Radical Species
3.2.1. Hydroxyl Radical
3.2.2. Superoxide Radical
3.2.3. Singlet Oxygen
3.3. Effect of Ozone-Induced Free Radical Solution on Microbial Killing Mechanism and Disease Control
3.3.1. Inhibitory Effect of Ozone-Induced Free Radical Solution on Bacillus subtilis
3.3.2. Analysis of the Effect of the Ozone-Induced Free Radical Solution on the Structure of Bacillus subtilis
3.3.3. Pest Control Efficacy in Greengrocery
4. Discussion
4.1. Oxidation and Stability Analysis of Ozone-Induced Free Radical Solution
4.2. Detection of Free Radical Species
4.3. Effect of Ozone-Induced Free Radical Solution on Microbial Killing Mechanism and Disease Control
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jing, Q.; Liu, J.; Chen, A.; Chen, C.; Liu, J. The Spatial–Temporal Chemical Footprint of Pesticides in China from 1999 to 2018. Environ. Sci. Pollut. Res. 2022, 29, 75539–75549. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Li, C.; Wang, L. Have Pesticides and Fertilizers Improved Agricultural Development? The Threshold Effect Based on China’s Agricultural Film Usage. Appl. Sci. 2023, 14, 6. [Google Scholar] [CrossRef]
- Arab, A.; Mostafalou, S. Neurotoxicity of Pesticides in the Context of CNS Chronic Diseases. Int. J. Environ. Health Res. 2022, 32, 2718–2755. [Google Scholar] [CrossRef]
- Pascale, A.; Laborde, A. Impact of Pesticide Exposure in Childhood. Rev. Environ. Health 2020, 35, 221–227. [Google Scholar] [CrossRef]
- Mit, N.; Cherednichenko, O.; Mussayeva, A.; Khamdiyeva, O.; Amirgalieva, A.; Begmanova, M.; Tolebaeva, A.; Koishekenova, G.; Zaypanova, S.; Pilyugina, A.; et al. Ecological Risk Assessment and Long-Term Environmental Pollution Caused by Obsolete Undisposed Organochlorine Pesticides. J. Environ. Sci. Health Part B 2021, 56, 490–502. [Google Scholar] [CrossRef] [PubMed]
- Ojija, F.; Bacaro, G. Characterization of Insect–Pollinator Biodiversity in Agrochemical-Contaminated Agricultural Habitats. Diversity 2024, 16, 33. [Google Scholar] [CrossRef]
- Romeo-Oliván, A.; Pagès, M.; Breton, C.; Lagarde, F.; Cros, H.; Yobrégat, O.; Violleau, F.; Jacques, A. Ozone Dissolved in Water: An Innovative Tool for the Production of Young Plants in Grapevine Nurseries? Ozone Sci. Eng. 2022, 44, 521–535. [Google Scholar] [CrossRef]
- Da Costa, A.R.; Faroni, L.R.D.; Salomão, L.C.C.; Cecon, P.R.; De Alencar, E.R. Use of Ozonized Water to Control Anthracnose in Papaya and Its Effect on the Quality of the Fruits. Ozone Sci. Eng. 2021, 43, 384–393. [Google Scholar] [CrossRef]
- Zou, L.; Wang, Y.; Huang, C.; Li, B.; Lyu, J.; Wang, S.; Lu, H.; Li, J. Meta-Cresol Degradation by Persulfate through UV/O3 Synergistic Activation: Contribution of Free Radicals and Degradation Pathway. Sci. Total Environ. 2021, 754, 142219. [Google Scholar] [CrossRef]
- Cheng, G.; Li, Z.; Sun, L.; Li, Y.; Fu, J. Application of Microwave/Electrodeless Discharge Ultraviolet/Ozone Sterilization Technology in Water Reclamation. Process Saf. Environ. Prot. 2020, 138, 148–156. [Google Scholar] [CrossRef]
- Hardison, D.R.; Cooper, W.J.; Mezyk, S.P.; Bartels, D.M. The Free Radical Chemistry of Tert-Butyl Formate: Rate Constants for Hydroxyl Radical, Hydrated Electron and Hydrogen Atom Reaction in Aqueous Solution. Radiat. Phys. Chem. 2002, 65, 309–315. [Google Scholar] [CrossRef]
- Fan, X.; Song, Y. Advanced Oxidation Process as a Postharvest Decontamination Technology to Improve Microbial Safety of Fresh Produce. J. Agric. Food Chem. 2020, 68, 12916–12926. [Google Scholar] [CrossRef]
- Terao, D.; De Lima Nechet, K.; Shiraishi Frighetto, R.T.; Cerqueira Sasaki, F.F. Ozonated Water Combined with Heat Treatment to Control the Stem-End Rot of Papaya. Sci. Hortic. 2019, 257, 108722. [Google Scholar] [CrossRef]
- Drogoudi, P.; Pantelidis, G.; Thomidis, T. Impact of Ozonated Water on Brown Rot Development and Storage Potential of Nectarine and Plum. Ozone Sci. Eng. 2023, 45, 410–418. [Google Scholar] [CrossRef]
- Abreu, M.R.; Delalibera, I.; Pereira, N.R.C.; Camargo-Mathias, M.I. Morphophysiological Analysis of the Salivary Glands of Rhipicephalus sanguineus Sensu Lato (Acari: Ixodidae) Exposed to Ozonated Water: A Control Strategy. Med. Vet. Entomol. 2021, 35, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Chebbah, A.; Hadjeri, S.; Nemmich, S.; Nassour, K.; Zouzou, N.; Tilmatine, A. Development and Experimental Analysis of a New “Serpentine-Shape” Surface-DBD Ozone Generator—Comparison with a Cylindrical Volume-DBD Ozone Generator. Ozone Sci. Eng. 2017, 39, 209–216. [Google Scholar] [CrossRef]
- Park, J.; Park, J.; Lee, J.; Jeong, B. Space Sterilization Effect through High-Density Plasma Ozone Using DBD Device. J. Electr. Eng. Technol. 2022, 17, 2771–2778. [Google Scholar] [CrossRef]
- Jodzis, S.; Barczyński, T. Ozone Synthesis and Decomposition in Oxygen-Fed Pulsed DBD System: Effect of Ozone Concentration, Power Density, and Residence Time. Ozone Sci. Eng. 2019, 41, 69–79. [Google Scholar] [CrossRef]
- Masek, A.; Chrzescijanska, E.; Latos-Brozio, M.; Zaborski, M. Characteristics of Juglone (5-Hydroxy-1,4,-Naphthoquinone) Using Voltammetry and Spectrophotometric Methods. Food Che. 2019, 301, 125279. [Google Scholar] [CrossRef]
- Yu, Y.X.; Bai, M.D.; Yang, X.T.; Ji, Z.X.; Li, J.; Yao, L. Hydroxyl radical degradation of norfloxacin in high algae drinking water system. China’s Environ. Sci. 2018, 38, 4545–4550. [Google Scholar] [CrossRef]
- Khan, Z.U.H.; Gul, N.S.; Sabahat, S.; Sun, J.; Tahir, K.; Shah, N.S.; Muhammad, N.; Rahim, A.; Imran, M.; Iqbal, J.; et al. Removal of Organic Pollutants through Hydroxyl Radical-Based Advanced Oxidation Processes. Ecotoxicol. Environ. Saf. 2023, 267, 115564. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Shi, H.; Hu, X.; Liu, E.; Fan, J. Fabricating CsPbX3/CN Heterostructures with Enhanced Photocatalytic Activity for Penicillins 6-APA Degradation. Chem. Eng. J. 2020, 381, 122692. [Google Scholar] [CrossRef]
- Zhao, Y.; Liang, X.; Shi, H.; Wang, Y.; Ren, Y.; Liu, E.; Zhang, X.; Fan, J.; Hu, X. Photocatalytic Activity Enhanced by Synergistic Effects of Nano-Silver and ZnSe Quantum Dots Co-Loaded with Bulk g-C3N4 for Ceftriaxone Sodium Degradation in Aquatic Environment. Chem. Eng. J. 2018, 353, 56–68. [Google Scholar] [CrossRef]
- Entradas, T.; Waldron, S.; Volk, M. The Detection Sensitivity of Commonly Used Singlet Oxygen Probes in Aqueous Environments. J. Photochem. Photobiol. B Biol. 2020, 204, 111787. [Google Scholar] [CrossRef] [PubMed]
- Tian, N.; Zhang, Y.; Li, X.; Xiao, K.; Du, X.; Dong, F.; Waterhouse, G.I.N.; Zhang, T.; Huang, H. Precursor-Reforming Protocol to 3D Mesoporous g-C 3 N 4 Established by Ultrathin Self-Doped Nanosheets for Superior Hydrogen Evolution. Nano Energy 2017, 38, 72–81. [Google Scholar] [CrossRef]
- Pang, B.; Huang, L.; Teng, J.; Zhang, J.; Xia, N.; Wei, B. Effect of Pile Fermentation on the Cells of Chinese Liupao Tea: The First Record of Cell Wall of Liupao Tea on Transmission Electron Microscope. Food Chem. 2021, 361, 130034. [Google Scholar] [CrossRef] [PubMed]
- Hickey, W.J.; Shetty, A.R.; Massey, R.J.; Toso, D.B.; Austin, J. Three-dimensional Bright-field Scanning Transmission Electron Microscopy Elucidate Novel Nanostructure in Microbial Biofilms. J. Microsc. 2017, 265, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Malinga, L.N.; Laing, M.D. Efficacy of Three Biopesticides against Cotton Pests under Field Conditions in South Africa. Crop Prot. 2021, 145, 105578. [Google Scholar] [CrossRef]
- Kamakshi, N.; Neelima, S.; Venkataramanamma, K.; Kalyani, D.L. Efficacy of Insecticides Against Thrips, Leafhoppers and Whiteflies in Sunflower. Indian J. Entomol. 2021, 83, 595–597. [Google Scholar] [CrossRef]
- Egel, D.S.; Hoagland, L.; Davis, J.; Marchino, C.; Bloomquist, M. Efficacy of Organic Disease Control Products on Common Foliar Diseases of Tomato in Field and Greenhouse Trials. Crop Prot. 2019, 122, 90–97. [Google Scholar] [CrossRef]
- Buchan, K.A.H.; Martin-Robichaud, D.J.; Benfey, T.J. Measurement of Dissolved Ozone in Sea Water: A Comparison of Methods. Aquac. Eng. 2005, 33, 225–231. [Google Scholar] [CrossRef]
- Nghi, N.H.; Cuong, L.C.; Dieu, T.V.; Oanh, D.T.Y. Study of Water Disinfection by Analyzing the Ozone Decomposition Process. Vietnam J. Chem. 2018, 56, 591–595. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, B.; Li, W.; Li, B.; Han, Z.; Zhang, Y.; Ding, A.; Wang, S.; Ma, J.; He, X. The Catalytic Oxidation Process of Atrazine by Ozone Microbubbles: Bubble Formation, Ozone Mass Transfer and Hydroxyl Radical Generation. Chemosphere 2023, 325, 138361. [Google Scholar] [CrossRef] [PubMed]
- Pituco, M.M.; Marrocos, P.H.; Santos, R.J.; Dias, M.M.; Lopes, J.C.B.; Moreira, F.C.; Vilar, V.J.P. NETmix Technology as Ozone Gas Injection System: Assessment of the Gas-Liquid Mass Transfer. Chem. Eng. Process. Process Intensif. 2023, 194, 109566. [Google Scholar] [CrossRef]
- De Rezende, A.J.; De Alencar, E.R.; Ferreira, M.D.A.; Ferreira, W.F.D.S. Control of Listeria monocytogenes in Refrigerated Ozonated Water. Ozone Sci. Eng. 2022, 44, 281–290. [Google Scholar] [CrossRef]
- Wang, B.; Xiong, X.; Shui, Y.; Huang, Z.; Tian, K. A Systematic Study of Enhanced Ozone Mass Transfer for Ultrasonic-Assisted PTFE Hollow Fiber Membrane Aeration Process. Chem. Eng. J. 2019, 357, 678–688. [Google Scholar] [CrossRef]
- Prada-Vásquez, M.A.; Pituco, M.M.; Caixeta, M.P.; Cardona Gallo, S.A.; Botero-Coy, A.M.; Hernández, F.; Torres-Palma, R.A.; Vilar, V.J.P. Ozonation Using a Stainless-Steel Membrane Contactor: Gas-Liquid Mass Transfer and Pharmaceuticals Removal from Secondary-Treated Municipal Wastewater. Chemosphere 2024, 349, 140888. [Google Scholar] [CrossRef] [PubMed]
- Piskarev, I.M. The Formation of Ozone-Hydroxyl Mixture in Corona Discharge and Lifetime of Hydroxyl Radicals. IEEE Trans. Plasma Sci. 2021, 49, 1363–1372. [Google Scholar] [CrossRef]
- Von Sonntag, C.; Dowideit, P.; Xingwang, F.; Mertens, R.; Xianming, P.; Schuchmann, M.N.; Schuchmann, H.-P. The Fate of Peroxyl Radicals in Aqueous Solution. Water Sci. Technol. 1997, 35, 9–15. [Google Scholar] [CrossRef]
- Tang, L.; Zhou, S.; Li, F.; Sun, L.; Lu, H. Ozone Micronano-Bubble-Enhanced Selective Degradation of Oxytetracycline from Production Wastewater: The Overlooked Singlet Oxygen Oxidation. Environ. Sci. Technol. 2023, 57, 18550–18562. [Google Scholar] [CrossRef]
- Cao, Y.; Xie, D.; Huang, Y.; Huang, C.; Zhang, K.; Zhang, X.; Wang, S. Investigation of Hydroxyl Radical Yield in an Impact-Jet Hydraulic Cavitator. Processes 2022, 10, 2194. [Google Scholar] [CrossRef]
- Tesio, A.Y.; Torres, W.; Villalba, M.; Davia, F.; Del Pozo, M.; Córdoba, D.; Williams, F.J.; Calvo, E.J. Role of Superoxide and Singlet Oxygen on the Oxygen Reduction Pathways in Li−O2 Cathodes at Different Li + Ion Concentration**. ChemElectroChem 2022, 9, e202201037. [Google Scholar] [CrossRef]
- Tampieri, F.; Ginebra, M.-P.; Canal, C. Quantification of Plasma-Produced Hydroxyl Radicals in Solution and Their Dependence on the pH. Anal. Chem. 2021, 93, 3666–3670. [Google Scholar] [CrossRef] [PubMed]
- Lan, W.; Chen, X.; Zhao, Y.; Xie, J. Insights into the Antibacterial Mechanism of Ozone Water Combined with Tea Polyphenols against Shewanella Putrefaciens: Membrane Disruption and Oxidative Stress. Int. J. Food Sci. Technol. 2022, 57, 7423–7433. [Google Scholar] [CrossRef]
- Xu, H.; Yi, L.; Li, C.; Sun, Y.; Hou, L.; Bai, J.; Kong, F.; Han, X.; Lan, Y. Design and Experiment of Ecological Plant Protection UAV Based on Ozonated Water Spraying. Drones 2023, 7, 291. [Google Scholar] [CrossRef]
- Landa Fernández, I.A.; Monje-Ramirez, I.; Orta Ledesma De Velásquez, M.T. Tomato Crop Improvement Using Ozone Disinfection of Irrigation Water. Ozone Sci. Eng. 2019, 41, 398–403. [Google Scholar] [CrossRef]
- Szumigaj-Tarnowska, J.; Szafranek, P.; Uliński, Z.; Ślusarski, C. Efficiency of Gaseous Ozone in Disinfection of Mushroom Growing Rooms. J. Hortic. Res. 2020, 28, 91–100. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Aguayo, E. Effect of Irrigation with Ozonated Water on the Quality of Capsicum Seedlings Grown in the Nursery. Agric. Water Manag. 2019, 221, 547–555. [Google Scholar] [CrossRef]














| Solution Concentration (mg/L) | Mean Number of Colonies (cfu/mL) | |
|---|---|---|
| Bacillus subtilis | Germicidal Ratio (%) | |
| Control group | 2.95 × 105 | 0 |
| 0.3 | 1.43 × 105 | 51.53 |
| 0.5 | 3.65 × 104 | 87.63 |
| 0.8 | 1.8 × 104 | 93.9 |
| 1.2 | 15 | 99.99 |
| Treatment Time (min) | Mean Number of Colonies (cfu/mL) | |
|---|---|---|
| Bacillus subtilis | Germicidal Ratio (%) | |
| Control group | 2.45 × 104 | 0 |
| 1 | 3.65 × 104 | 85.1 |
| 3 | 2.2 × 104 | 91.02 |
| 5 | 1.95 × 104 | 92.04 |
| 10 | 5.5 × 103 | 97.76 |
| Treatment Group | Description of the Situation | Control Effect Diagram |
|---|---|---|
| (A) | Pest grade: Level 1 Description: This group served as the control group, with treatments limited to water spraying. Each Chinese cabbage leaf exhibited 26 wormholes on its surface. The prevalence of wormholes was significant, and the extent of leaf area consumed was considerable, indicating a severe infestation. |  |
| (B) | Pest grade: Level 2 Description: A 0.5 mg/L ozone-induced free radical solution was applied to this group. The leaf surface of Chinese cabbage in this group exhibited fewer wormholes compared to control group A, totaling 14. While some damage to the leaf surface was observed, it was markedly less severe than in the control group, indicating a degree of pest control. |  |
| (C) | Pest grade: Level 3 Description: The group received a 1.5 mg/L ozone-induced free radical solution spray. The figure illustrates a reduction in the number of wormholes on the surface of Chinese cabbage leaves, totaling 9. This suggests that a high concentration of ozone-induced free radical solution effectively inhibits the disease. |  |
| (D) | Pest grade: Level 4 Description: This group was treated with 5% imidacloprid for disease control. The leaves exhibited 6 wormholes, each with a small area. |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Tang, P.; Cao, A.; Ni, P.; Zhang, B.; Chang, Z. Evaluation of an Ozone-Induced Free Radical Solution’s Characteristics and Its Efficacy as an Alternative Pest Control Method. Appl. Sci. 2024, 14, 3111. https://doi.org/10.3390/app14073111
Wu C, Tang P, Cao A, Ni P, Zhang B, Chang Z. Evaluation of an Ozone-Induced Free Radical Solution’s Characteristics and Its Efficacy as an Alternative Pest Control Method. Applied Sciences. 2024; 14(7):3111. https://doi.org/10.3390/app14073111
Chicago/Turabian StyleWu, Chundu, Peng Tang, Aineng Cao, Pengfei Ni, Bo Zhang, and Zhongwei Chang. 2024. "Evaluation of an Ozone-Induced Free Radical Solution’s Characteristics and Its Efficacy as an Alternative Pest Control Method" Applied Sciences 14, no. 7: 3111. https://doi.org/10.3390/app14073111
APA StyleWu, C., Tang, P., Cao, A., Ni, P., Zhang, B., & Chang, Z. (2024). Evaluation of an Ozone-Induced Free Radical Solution’s Characteristics and Its Efficacy as an Alternative Pest Control Method. Applied Sciences, 14(7), 3111. https://doi.org/10.3390/app14073111






