Synthesis and Characterization of a Photocatalytic Material from TiO2 Nanoparticles Supported on Zeolite Obtained from Ignimbrite Residue Used in Decolorization of Methyl Orange
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Obtaining Zeolite from Ignimbrite Residue
2.3. Synthesis of TiO2 Nanoparticles and the TiO2/Zeolite Material
2.4. Characterization
2.5. Photocatalytic Activity
3. Results and Discussion
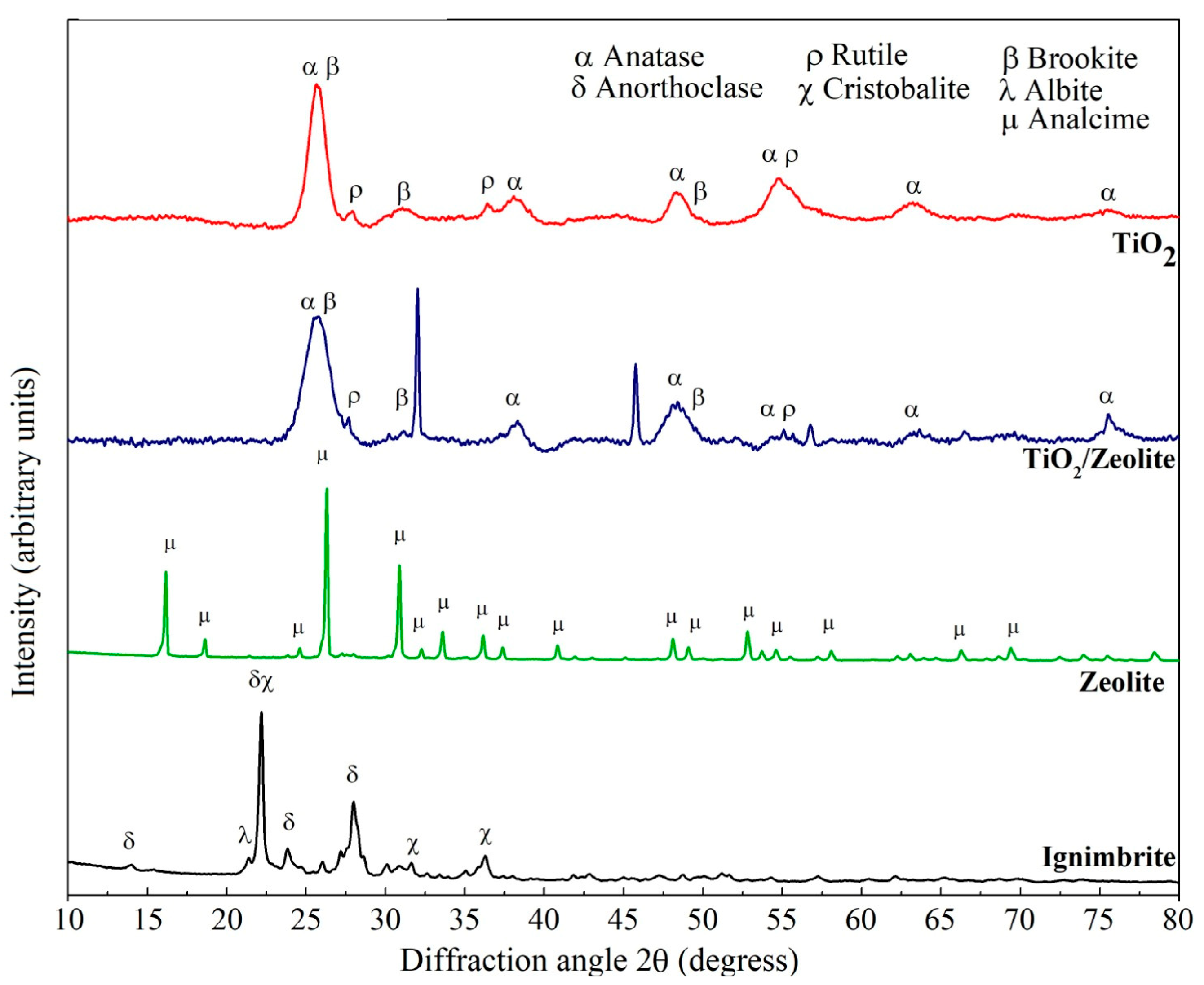
3.1. XRD Analysis
3.2. SEM Analysis
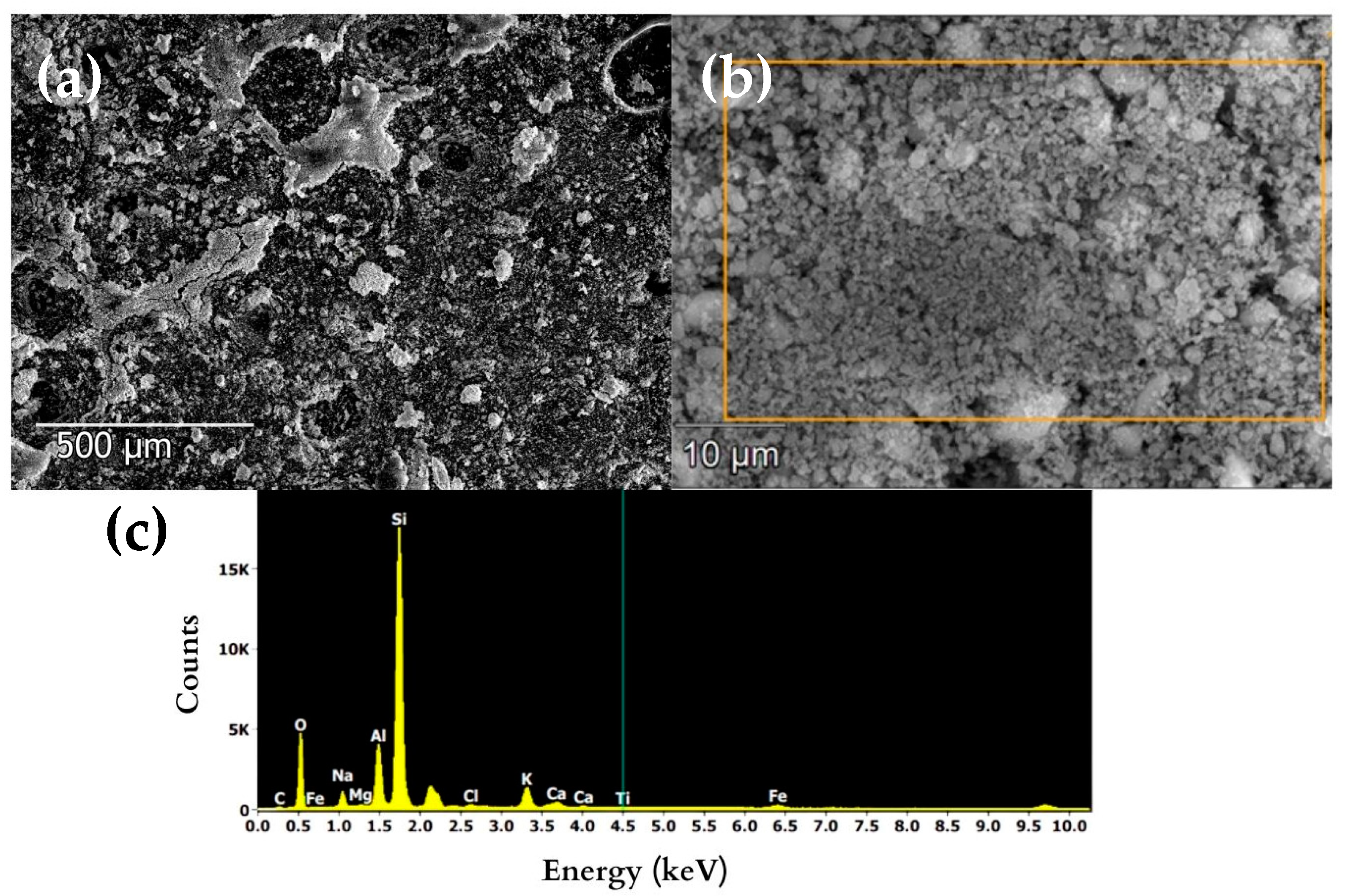
3.2.1. Ignimbrite
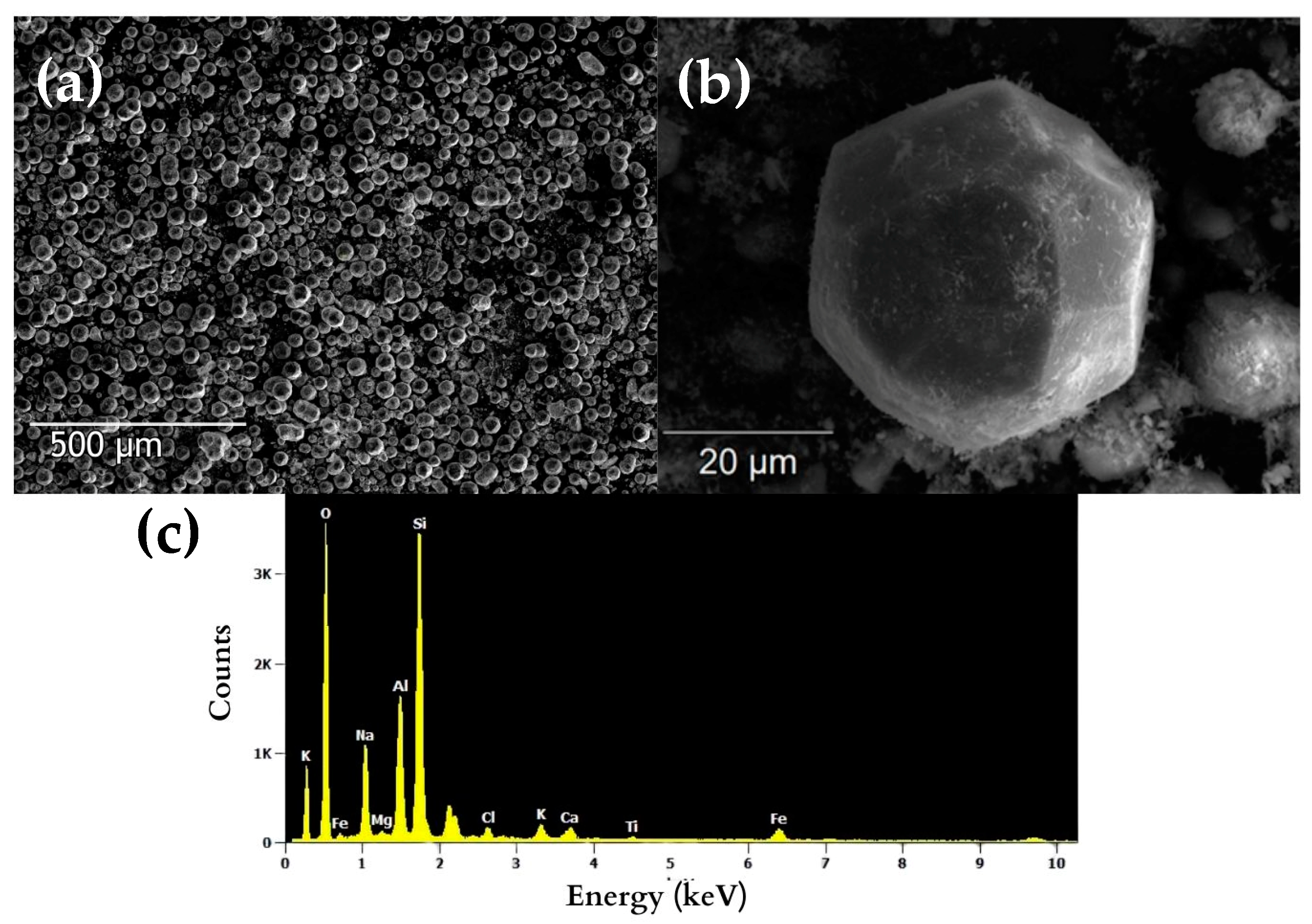
3.2.2. Zeolite
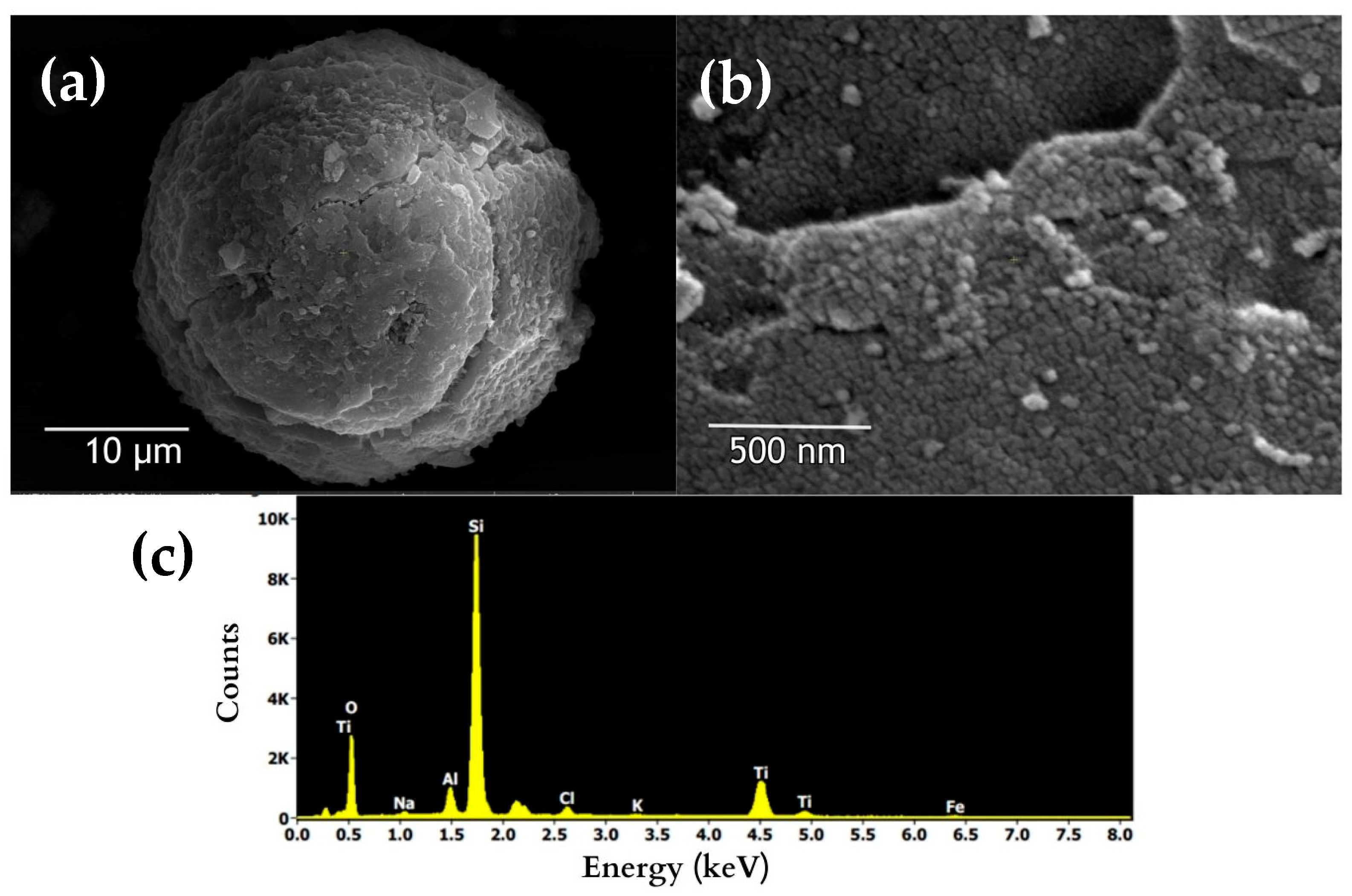
3.2.3. Synthesized TiO2 Nanoparticles
3.2.4. TiO2/Zeolite Photocatalytic Material
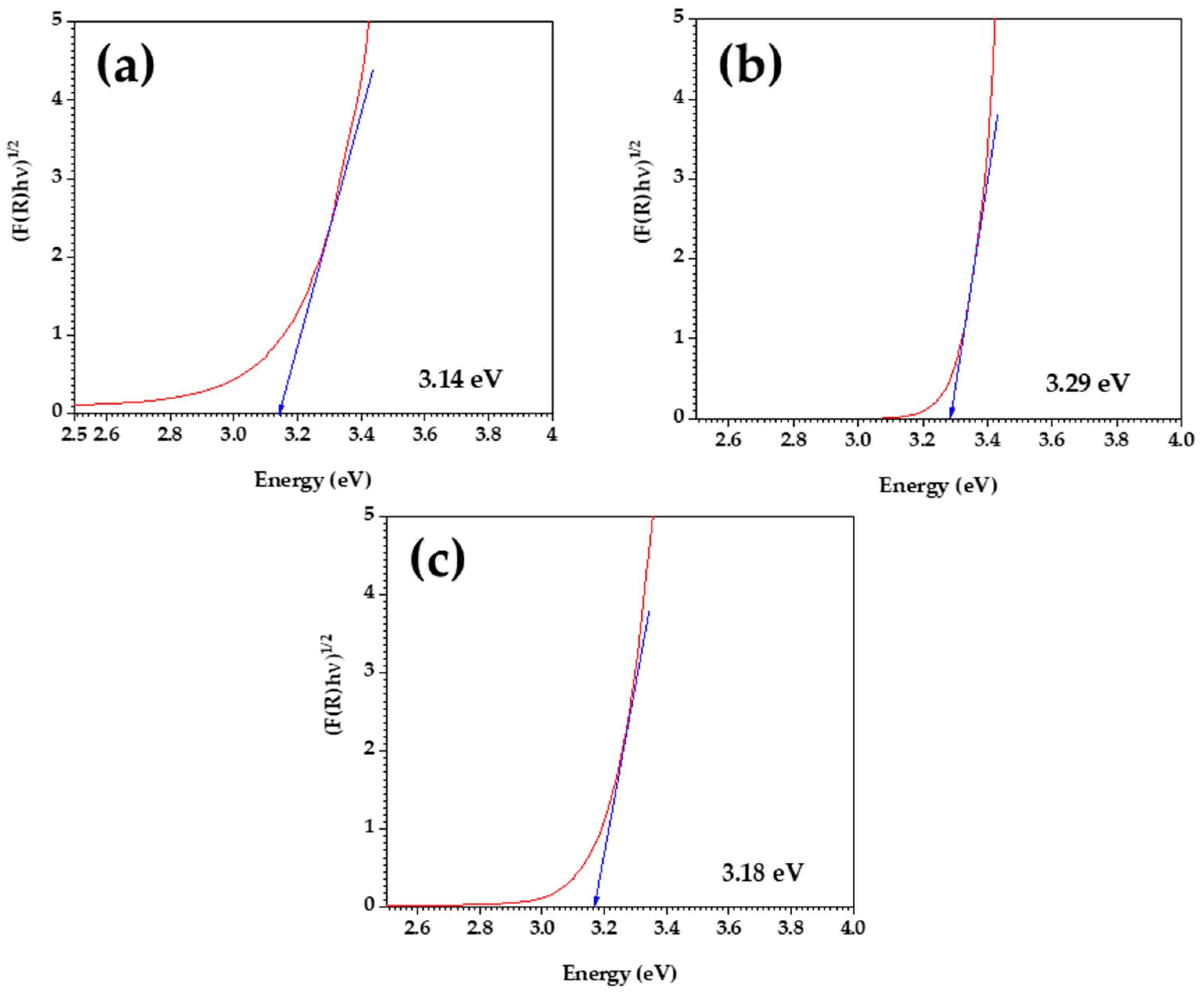
3.3. UV–Visible Spectroscopy
3.4. Physisorption Test Parameters
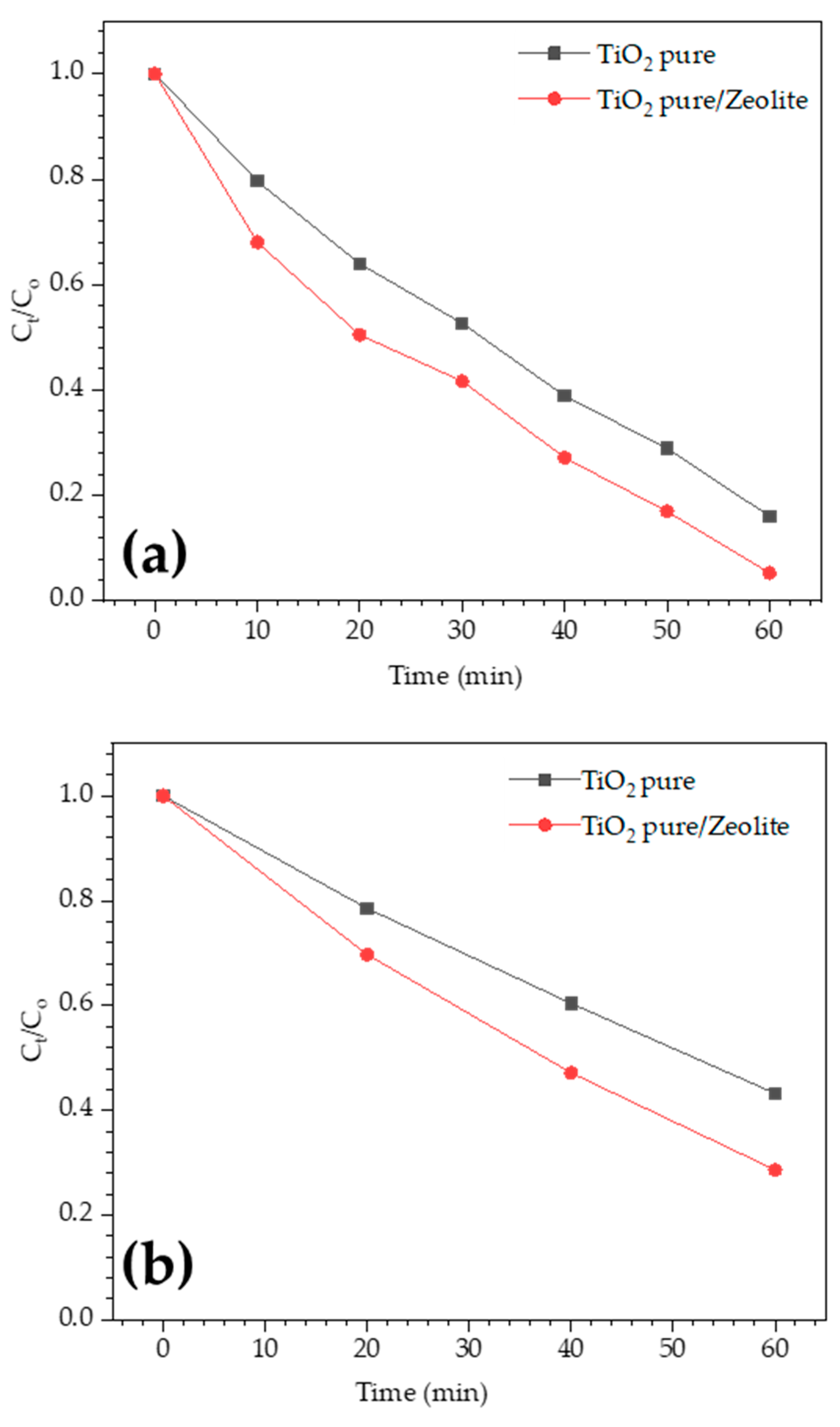
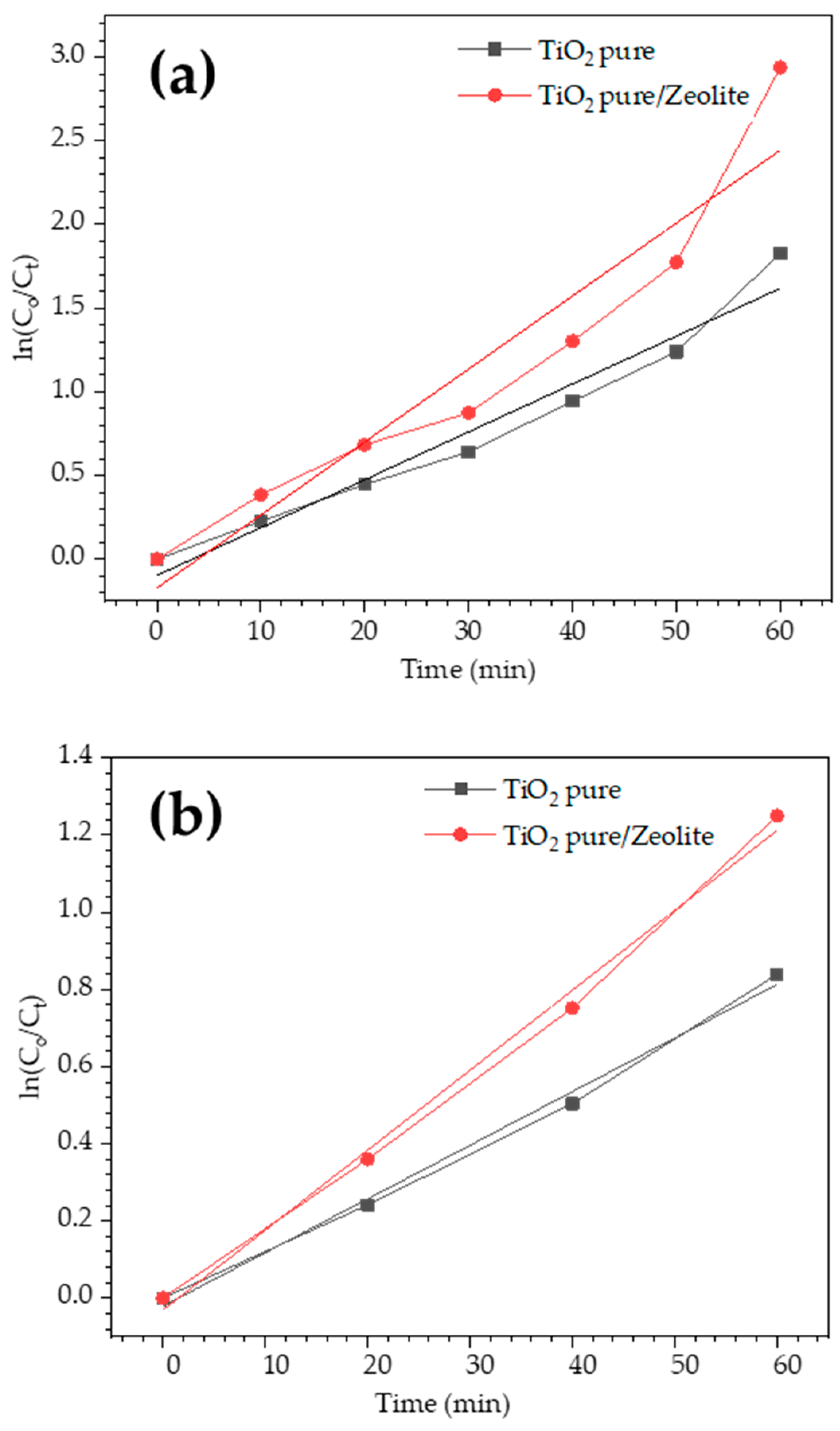
3.5. Photocatalytic Performance Results
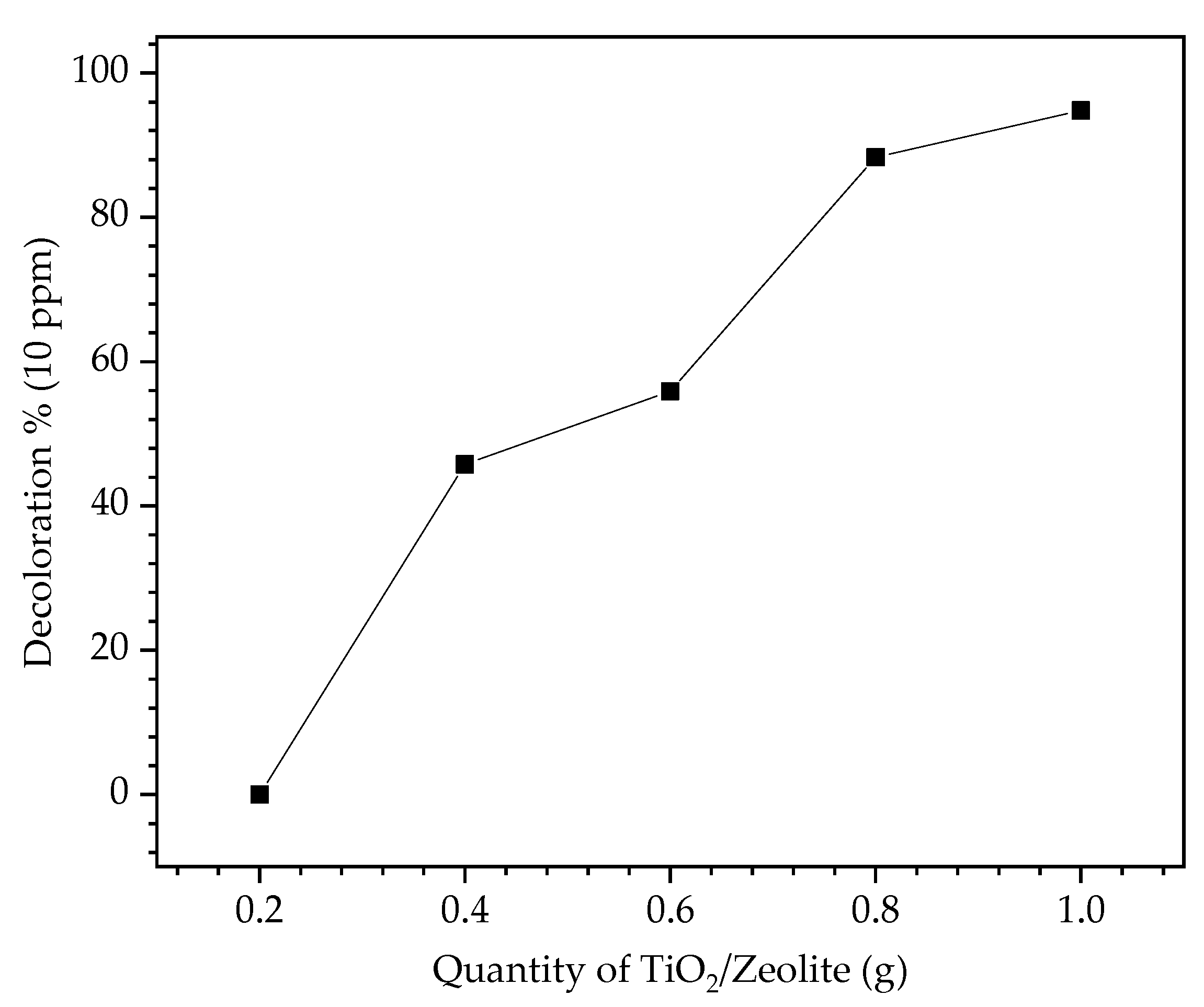
3.5.1. Effect of Catalyst Loading
3.5.2. The Reusability Study
3.5.3. Comparative Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Crini, G.; Lichtfouse, E. Wastewater Treatment: An Overview. In Green Adsorbents for Pollutant Removal; Springer: Berlin, Germany, 2018; pp. 1–21. [Google Scholar] [CrossRef]
- Abdellah, M.H.; Nosier, S.A.; El-Shazly, A.H.; Mubarak, A.A. Photocatalytic Decolorization of Methylene Blue Using TiO2/UV System Enhanced by Air Sparging. Alex. Eng. J. 2018, 57, 3727–3735. [Google Scholar] [CrossRef]
- Khataee, A.R.; Pons, M.N.; Zahraa, O. Photocatalytic Degradation of Three Azo Dyes Using Immobilized TiO2 Nanoparticles on Glass Plates Activated by UV Light Irradiation: Influence of Dye Molecular Structure. J. Hazard. Mater. 2009, 168, 451–457. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Guo, J. Removal of chromium from wastewater by membrane filtration, chemical precipitation, ion exchange, adsorption electrocoagulation, electrochemical reduction, electrodialysis, electrodeionization, photocatalysis and nanotechnology: A review. Environ. Chem. Lett. 2020, 18, 2055–2068. [Google Scholar] [CrossRef]
- Ahmad, A.; Mohd-Setapar, S.H.; Chuong, C.S.; Khatoon, A.; Wani, W.A.; Kumar, R.; Rafatullah, M. Recent advances in new generation dye removal technologies: Novel search for approaches to reprocess wastewater. RSC Adv. 2015, 5, 30801–30818. [Google Scholar] [CrossRef]
- Ameta, R.; Solanki, M.S.; Benjamin, S.; Ameta, S.C. Photocatalysis. In Advanced Oxidation Processes for Wastewater Treatment: Emerging Green Chemical Technology; Elsevier Inc.: Amsterdam, The Netherlands, 2018; pp. 135–175. [Google Scholar] [CrossRef]
- Ramos, P.G.; Espinoza, J.; Sánchez, L.A.; Rodriguez, J.M. Enhanced photocatalytic degradation of Rhodamine B employing transition metal (Fe, Cu, Co) doped ZnO/rGO nanostructures synthesized by electrospinning-hydrothermal technique. J. Alloys Compd. 2023, 966, 171559. [Google Scholar] [CrossRef]
- Ângelo, J.; Magalhães, P.; Andrade, L.; Mendes, A. Characterization of TiO2-based semiconductors for photocatalysis by electrochemical impedance spectroscopy. Appl. Surf. Sci. 2016, 387, 183–189. [Google Scholar] [CrossRef]
- Florez, D.M.M.G.; Ale, R.B.G.; Idme, A.F.H.; Alarcon, L.A.L.; Huallpa, E.A.; Castro, Y.; Apestegui, P.G.R.; Rodriguez, J.M.R. SiO2-TiO2 Films Supported on Ignimbrite by Spray Coating for the Photocatalytic Degradation of NOx Gas and Methyl Orange Dye. Int. J. Photoenergy 2020, 2020, 4756952. [Google Scholar] [CrossRef]
- Nakata, K.; Fujishima, A. TiO2 Photocatalysis: Design and Applications. J. Photochem. Photobiol. C Photochem. Rev. 2012, 13, 169–189. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, 1901997. [Google Scholar] [CrossRef]
- Sarkar, A.K.; Saha, A.; Tarafder, A.; Panda, A.B.; Pal, S. Efficient Removal of Toxic Dyes via Simultaneous Adsorption and Solar Light Driven Photodegradation Using Recyclable Functionalized Amylopectin-TiO2-Au Nanocomposite. ACS Sustain. Chem. Eng. 2016, 4, 1679–1688. [Google Scholar] [CrossRef]
- Zhang, G.; Song, A.; Duan, Y.; Zheng, S. Enhanced Photocatalytic Activity of TiO2/Zeolite Composite for Abatement of Pollutants. Microporous Mesoporous Mater. 2018, 255, 61–68. [Google Scholar] [CrossRef]
- Fu, X.; Yang, H.; Lu, G.; Tu, Y.; Wu, J. Improved performance of surface functionalized TiO2/activated carbon for adsorption–photocatalytic reduction of Cr(VI) in aqueous solution. Mater. Sci. Semicond. Process 2015, 39, 362–370. [Google Scholar] [CrossRef]
- Martins, A.C.; Cazetta, A.L.; Pezoti, O.; Souza, J.R.B.; Zhang, T.; Pilau, E.J.; Asefa, T.; Almeida, V.C. Sol-gel synthesis of new TiO2/activated carbon photocatalyst and its application for degradation of tetracycline. Ceram. Int. 2017, 43, 4411–4418. [Google Scholar] [CrossRef]
- Bouarioua, A.; Zerdaoui, M. Photocatalytic activities of TiO2 layers immobilized on glass substrates by dip-coating technique toward the decolorization of methyl orange as a model organic pollutant. J. Environ. Chem. Eng. 2017, 5, 1565–1574. [Google Scholar] [CrossRef]
- Kuo, H.P.; Yao, S.W.; Huang, A.N.; Hsu, W.Y. Photocatalytic degradation of toluene in a staged fluidized bed reactor using TiO2/silica gel. Korean J. Chem. Eng. 2017, 34, 73–80. [Google Scholar] [CrossRef]
- Kusiak-Nejman, E.; Morawski, A.W. TiO2/graphene-based nanocomposites for water treatment: A brief overview of charge carrier transfer, antimicrobial and photocatalytic performance. Appl. Catal. B 2019, 253, 179–186. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Lu, S.; Guo, W.; Xi, B. Performance and mechanism into TiO2/Zeolite composites for sulfadiazine adsorption and photodegradation. Chem. Eng. J. 2018, 350, 131–147. [Google Scholar] [CrossRef]
- Badvi, K.; Javanbakht, V. Enhanced photocatalytic degradation of dye contaminants with TiO2 immobilized on ZSM-5 zeolite modified with nickel nanoparticles. J. Clean. Prod. 2021, 280, 124518. [Google Scholar] [CrossRef]
- Setthaya, N.; Chindaprasirt, P.; Yin, S.; Pimraksa, K. TiO2-zeolite photocatalysts made of metakaolin and rice husk ash for removal of methylene blue dye. Powder Technol. 2017, 313, 417–426. [Google Scholar] [CrossRef]
- Chong, M.N.; Tneu, Z.Y.; Poh, P.E.; Jin, B.; Aryal, R. Synthesis, characterisation and application of TiO2–zeolite nanocomposites for the advanced treatment of industrial dye wastewater. J. Taiwan. Inst. Chem. Eng. 2015, 50, 288–296. [Google Scholar] [CrossRef]
- Aziztyana, A.P.; Wardhani, S.; Prananto, Y.P.; Purwonugroho, D.; Darjito. Optimisation of Methyl Orange Photodegradation Using TiO2-Zeolite Photocatalyst and H2O2 in Acid Condition. IOP Conf. Ser. Mater. Sci. Eng. 2019, 546, 042047. [Google Scholar] [CrossRef]
- Rahman, A.; Nurjayadi, M.; Wartilah, R.; Kusrini, E.; Prasetyanto, E.A.; Degermenci, V. Enhanced Activity of TiO2/Natural Zeolite Composite for Degradation of Methyl Orange under Visible Light Irradiation. Int. J. Technol. 2018, 9, 1159–1167. [Google Scholar] [CrossRef]
- Lebti, P.P.; Thouret, J.C.; Wörner, G.; Fornari, M. Neogene and Quaternary ignimbrites in the area of Arequipa, Southern Peru: Stratigraphical and petrological correlations. J. Volcanol. Geotherm. Res. 2006, 154, 251–275. [Google Scholar] [CrossRef]
- Huanca, P.K.; Corrales, L.T.; Paredes, B.; Almirón, J.J.; Gonzales, D.P. Estudio de la modificación hidrotermal de un mineral Ignimbrítico para obtener zeolita sintética de alta capacidad de intercambio catiónico. Rev. Bol. Quim 2019, 36, 173–180. [Google Scholar] [CrossRef]
- Waghchaure, R.H.; Adole, V.A.; Jagdale, B.S. Photocatalytic degradation of methylene blue, rhodamine B, methyl orange and Eriochrome black T dyes by modified ZnO nanocatalysts: A concise review. Inorg. Chem. Commun. 2022, 143, 109764. [Google Scholar] [CrossRef]
- Hosseini, M.S.; Sadeghi, M.T.; Khazaei, M. Wettability alteration from superhydrophobic to superhydrophilic via synthesized stable nano-coating. Surf. Coat. Technol. 2017, 326, 79–86. [Google Scholar] [CrossRef]
- Mosquera, E.; Rosas, N.; Debut, A.; Guerrero, V.H. Síntesis y caracterización de nanopartículas de dióxido de titanio obtenidas por el método de sol-gel. Rev. Politécnica 2015, 36, 7. [Google Scholar]
- Lamastra, F.R.; Grilli, M.L.; Leahu, G.; Belardini, A.; Li Voti, R.; Sibilia, C.; Salvatori, D.; Cacciotti, I.; Nanni, F. Photoacoustic Spectroscopy Investigation of Zinc Oxide/Diatom Frustules Hybrid Powders. Int. J. Thermophys. 2018, 39, 110. [Google Scholar] [CrossRef]
- Lamastra, F.R.; Grilli, M.L.; Leahu, G.; Belardini, A.; Li Voti, R.; Sibilia, C.; Salvatori, D.; Cacciotti, I.; Nanni, F. Diatom frustules decorated with zinc oxide nanoparticles for enhanced optical properties. Nanotechnology 2017, 28, 375704. [Google Scholar] [CrossRef]
- Li Voti, R.; Leahu, G.; Sibilia, C.; Matassa, R.; Familiari, G.; Cerra, S.; Salamone, T.A.; Fratoddi, I. Photoacoustics for listening to metal nanoparticle super-aggregates. Nanoscale Adv. 2021, 3, 4692–4701. [Google Scholar] [CrossRef]
- Landi, S.; Segundo, I.R.; Freitas, E.; Vasilevskiy, M.; Carneiro, J.; Tavares, C.J. Use and misuse of the Kubelka-Munk function to obtain the band gap energy from diffuse reflectance measurements. Solid. State Commun. 2022, 341, 114573. [Google Scholar] [CrossRef]
- López, R.; Gómez, R. Band-gap energy estimation from diffuse reflectance measurements on sol–gel and commercial TiO2: A comparative study. J. Sol-Gel Sci. Technol. 2012, 61, 1–7. [Google Scholar] [CrossRef]
- Müller, B.R. Preparation and characterization of K2CO3-doped powdered activated carbon for effective in-vitro adsorption of deoxynivalenol. Bioresour. Technol. 2021, 15, 100703. [Google Scholar] [CrossRef]
- Klima, K.M.; Schollbach, K.; Brouwers, H.J.H.; Yu, Q. Enhancing the thermal performance of Class F fly ash-based geopolymer by sodalite. Constr. Build. Mater. 2022, 314, 125574. [Google Scholar] [CrossRef]
- Cruz Sánchez, E.; Torres, M.E.; Diaz, C.; Saito, F. Effects of grinding of the feldspar in the sintering using a planetary ball mill. J. Mater. Process. Technol. 2004, 152, 284–290. [Google Scholar] [CrossRef]
- Rajakrishnamoorthy, P.; Karthikeyan, D.; Saravanan, C.G. Emission reduction technique applied in SI engines exhaust by using zsm5 zeolite as catalysts synthesized from coal fly ash. Mater. Today Proc. 2020, 22, 499–506. [Google Scholar] [CrossRef]
- Verrecchia, G.; Cafiero, L.; De Caprariis, B.; Dell’Era, A.; Pettiti, I.; Tuffi, R.; Scarsella, M. Study of the parameters of zeolites synthesis from coal fly ash in order to optimize their CO2 adsorption. Fuel 2020, 276, 118041. [Google Scholar] [CrossRef]
- Churata, R.; Almirón, J.; Vargas, M.; Tupayachy-Quispe, D.; Torres-Almirón, J.; Ortiz-Valdivia, Y.; Velasco, F. Study of Geopolymer Composites Based on Volcanic Ash, Fly Ash, Pozzolan, Metakaolin and Mining Tailing. Buildings 2022, 12, 1118. [Google Scholar] [CrossRef]
- Vichaphund, S.; Aht-Ong, D.; Sricharoenchaikul, V.; Atong, D. Characteristic of fly ash derived-zeolite and its catalytic performance for fast pyrolysis of Jatropha waste. Environ. Technol. 2014, 35, 2254–2261. [Google Scholar] [CrossRef]
- Ojha, K.; Pradhan, N.C.; Samanta, A.N. Zeolite from fly ash: Synthesis and characterization. Bull. Mater. Sci. 2004, 27, 555–564. [Google Scholar] [CrossRef]
- Galindo Valbuena, H.M.; Medina, A.F.; Vargas, J.C.; Hernández Fandiño, O. Synthesis of zeolites Na-A, Na-X, and analcime from crushed stone waste and their applications in heavy metal removal in aqueous media. Chem. Eng. Res. Des. 2023, 197, 159–172. [Google Scholar] [CrossRef]
- Jung, Y.J.; Kim, W.G.; Yoon, Y.; Kang, J.W.; Hong, Y.M.; Kim, H.W. Removal of amoxicillin by UV and UV/H2O2 processes. Sci. Total Environ. 2012, 420, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Znad, H.; Abbas, K.; Hena, S.; Awual, M.R. Synthesis a novel multilamellar mesoporous TiO2/ZSM-5 for photo-catalytic degradation of methyl orange dye in aqueous media. J. Environ. Chem. Eng. 2018, 6, 218–227. [Google Scholar] [CrossRef]
- Rodríguez Valdivia, M. Hydrothermal synthesis of zeolites from a volcanic ash by alkaline treatment and their potential application in the removal of NH4+, Pb2+, Zn2+ and Mn2+. Matéria 2022, 27, e13132. [Google Scholar] [CrossRef]
- Novembre, D.; Gimeno, D. Synthesis and characterization of analcime (ANA) zeolite using a kaolinitic rock. Sci. Rep. 2021, 11, 13373. [Google Scholar] [CrossRef] [PubMed]
- Vigil de la Villa Mencía, R.; Goiti, E.; Ocejo, M.; Giménez, R.G. Synthesis of zeolite type analcime from industrial wastes. Microporous Mesoporous Mater. 2020, 293, 109817. [Google Scholar] [CrossRef]
- Setthaya, N.; Pindi, C.; Chindaprasirt, P.; Pimraksa, K. Synthesis of faujasite and analcime using of rice husk ashand metakaolin. Adv. Mater. Res. 2013, 770, 209–212. [Google Scholar] [CrossRef]
- Jin, Y.; Qi, T.; Ge, Y.; Chen, J.; Liang, L.; Ju, J.; Zhao, J. Ultrasensitive electrochemical determination of phosphate in water by using hydrophilic TiO2 modified glassy carbon electrodes. Anal. Methods 2021, 13, 996–1002. [Google Scholar] [CrossRef] [PubMed]
- Jansson, I.; Suárez, S.; Garcia-Garcia, F.J.; Sánchez, B. Zeolite–TiO2 hybrid composites for pollutant degradation in gas phase. Appl. Catal. B 2015, 178, 100–107. [Google Scholar] [CrossRef]
- Katal, R.; Masudy-Panah, S.; Tanhaei, M.; Farahani, M.H.D.A.; Jiangyong, H. A review on the synthesis of the various types of anatase TiO2 facets and their applications for photocatalysis. Chem. Eng. J. 2020, 384, 123384. [Google Scholar] [CrossRef]
- Tayade, R.J.; Surolia, P.K.; Kulkarni, R.G.; Jasra, R.V. Photocatalytic degradation of dyes and organic contaminants in water using nanocrystalline anatase and rutile TiO2. Sci. Technol. Adv. Mater. 2007, 8, 455–462. [Google Scholar] [CrossRef]
- Dette, C.; Pérez-Osorio, M.A.; Kley, C.S.; Punke, P.; Patrick, C.E.; Jacobson, P.; Giustino, F.; Jung, S.J.; Kern, K. TiO2 Anatase with a Bandgap in the Visible Region. Nano Lett. 2014, 14, 6533–6538. [Google Scholar] [CrossRef] [PubMed]
- Shi, D.; Guo, Z.; Bedford, N. Nanotitanium Oxide as a Photocatalytic Material and its Application. In Nanomaterials and Devices; Elsevier: Amsterdam, The Netherlands, 2015; pp. 161–174. [Google Scholar] [CrossRef]
- Dincer, I.; Zamfirescu, C. Hydrogen Production by Photonic Energy. In Sustainable Hydrogen Production; Elsevier: New York, NY, USA, 2016; pp. 309–391. [Google Scholar] [CrossRef]
- Matsukawa, Y.; Hirata, S.; Inada, M.; Enomoto, N.; Hojo, J.; Hayashi, K. Kinetic effects of polymorphs and surface areas on adsorption and photocatalytic decomposition of acetaldehyde on titania. Chem. Eng. J. 2020, 397, 125422. [Google Scholar] [CrossRef]
- Guo, B.; Shen, H.; Shu, K.; Zeng, Y.; Ning, W. The study of the relationship between pore structure and photocatalysis of mesoporous TiO2. J. Chem. Sci. 2009, 121, 317–321. [Google Scholar] [CrossRef]
- Labhane, P.K.; Patle, L.B.; Huse, V.R.; Sonawane, G.H.; Sonawane, S.H. Synthesis of reduced graphene oxide sheets decorated by zinc oxide nanoparticles: Crystallographic, optical, morphological and photocatalytic study. Chem. Phys. Lett. 2016, 661, 13–19. [Google Scholar] [CrossRef]
- Sulaiman, F.; Sari, D.K.; Kustiningsih, I. The influence of ozone on the photocatalytic degradation of phenol using TiO2 photocatalyst supported by Bayah natural zeolite. AIP Conf. Proc. 2017, 1840, 110014. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.W.; Kim, H. Photocatalytic degradation of tetracycline antibiotics using hydrothermally synthesized two-dimensional molybdenum disulfide/titanium dioxide composites. J. Colloid Interface Sci. 2022, 606, 454–463. [Google Scholar] [CrossRef]

| Weight Percentage % | ||||
|---|---|---|---|---|
| Oxides | Ignimbrite | Zeolite | TiO2 | TiO2/Zeolite |
| SiO2 | 74.97 | 54.72 | ---- | 73.55 |
| Al2O3 | 13.52 | 19.60 | ---- | 5.77 |
| Na2O | 4.38 | 12.98 | ---- | 1.06 |
| TiO2 | 0.18 | 0.52 | 99.52 | 17.54 |
| Cl | 0.37 | 0.83 | 0.48 | 1.18 |
| Others | 6.58 | 11.35 | ---- | 0.90 |
| Total | 100 | 100 | 100 | 100 |
| Sample | Band Gap (eV) |
|---|---|
| TiO2/zeolite | 3.14 |
| TiO2—Sigma Aldrich | 3.29 |
| Synthesized TiO2 | 3.18 |
| Sample | Surface Area [m2/g] | Pore Volume [cm3/g] | Average Pore Diameter [nm] |
|---|---|---|---|
| Zeolite analcime | 1.740 ± 0.005 | 0.000489 | 2.1426 |
| TiO2—Sigma Aldrich | 80.571 ± 0.278 | 0.022379 | 2.1420 |
| Synthesized TiO2 | 86.951 ± 0.346 | 0.025958 | 2.1505 |
| TiO2/zeolite | 169.079 ± 0.802 | 0.042061 | 2.1411 |
| Sample | Concentration [ppm] | %Ef fdeg |
|---|---|---|
| TiO2/zeolite | 10 | 94.71 |
| Pure synthesized TiO2 | 10 | 83.93 |
| TiO2/zeolite | 20 | 71.36 |
| Pure synthesized TiO2 | 20 | 56.78 |
| Sample | Concentration [ppm] | k (min−1) | R2 |
|---|---|---|---|
| TiO2/zeolite | 10 | 0.0436 | 0.963 |
| Pure synthesized TiO2 | 10 | 0.0286 | 0.917 |
| TiO2/zeolite | 20 | 0.0207 | 0.994 |
| Pure synthesized TiO2 | 20 | 0.0139 | 0.994 |
| Sample | Degradation Efficiency (%) | Concentration |
|---|---|---|
| TiO2/zeolite (First Test) | 92.80 | 10 ppm |
| TiO2/zeolite (Second Test) | 88.15 | |
| TiO2/zeolite (Third Test) | 87.64 | |
| TiO2/zeolite (First Test) | 70.20 | 20 ppm |
| TiO2/zeolite (Second Test) | 67.69 | |
| TiO2/zeolite (Third Test) | 18.06 |
| Sample | Degradation/Time Test | Concentration |
|---|---|---|
| TiO2/zeolite (this work) | 94.71%/60 min | 10 ppm |
| TiO2/zeolite [23] | 77.68%/60 min | 10 ppm |
| TiO2/SDS [50] | 90%/300 min | 30 ppm |
| TiO2 layers on glass substrates—3 layers [16] | 97.6%/180 min | 30 ppm |
| TiO2/zeolite (this work) | 71.36%/60 min | 20 ppm |
| TiO2/zeolite [23] | 68.61%/60 min | 20 ppm |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huayna, G.; Laura, A.; Churata, R.; Lazo, L.; Guzmán, R.; Ramos, P.G.; Rodriguez, J.M. Synthesis and Characterization of a Photocatalytic Material from TiO2 Nanoparticles Supported on Zeolite Obtained from Ignimbrite Residue Used in Decolorization of Methyl Orange. Appl. Sci. 2024, 14, 3146. https://doi.org/10.3390/app14083146
Huayna G, Laura A, Churata R, Lazo L, Guzmán R, Ramos PG, Rodriguez JM. Synthesis and Characterization of a Photocatalytic Material from TiO2 Nanoparticles Supported on Zeolite Obtained from Ignimbrite Residue Used in Decolorization of Methyl Orange. Applied Sciences. 2024; 14(8):3146. https://doi.org/10.3390/app14083146
Chicago/Turabian StyleHuayna, Gianina, Antonio Laura, Rossibel Churata, Luis Lazo, Rivalino Guzmán, Pierre G. Ramos, and Juan M. Rodriguez. 2024. "Synthesis and Characterization of a Photocatalytic Material from TiO2 Nanoparticles Supported on Zeolite Obtained from Ignimbrite Residue Used in Decolorization of Methyl Orange" Applied Sciences 14, no. 8: 3146. https://doi.org/10.3390/app14083146







