Abstract
The aim of this study was to determine the types of UV filters used in adult and children’s sunscreen products sold in Poland (part of the EU market) and their frequency of use. The INCI compositions of sunscreen products were collected and analyzed for the presence of UV filters. The study included 150 randomly selected preparations for adults (from 71 brands) and 50 for children (from 33 brands). The survey concerned the UV filters listed in Annex VI to Regulation (EC) No 1223/2009 of the European Parliament and Council of 30 November 2009 on cosmetic products. The most frequently used UV filters in the child sunscreens were triazine derivatives: bis-ethylhexyloxyphenol methoxyphenyl triazine (60.0%) and ethylhexyl triazone (52.0%), and ethylhexyl salicylate (46.0%), a derivative of salicylic acid. The most common in adult sunscreens were butyl methoxydibenzoylmethane (56.0%), a dibenzoylmethane derivative, followed by the salicylic acid derivative ethylhexyl salicylate (54.7%) and the triazine derivatives bis-ethylhexyloxyphenol methoxyphenyl triazine (54.7%) and ethylhexyl triazone (50.0%). Physical filters, including their nano and non-nano forms, were more popular in sunscreens for children, i.e., 50.0% (TiO2) and 22.0% (ZnO), than for adults: 21.3% (TiO2) and 6.7% (ZnO). For both adults and children, many cosmetic products contained four or five UV filters per preparation; however, the child preparations often used two UV filters. To summarize, the following UV filters dominate in photoprotectors for both adults and children: butyl methoxydibenzoylmethane, bis-ethylhexyloxyphenol methoxyphenyl triazine, ethylhexyl triazone, ethylhexyl salicylate, and diethylamino hydroxybenzoyl hexyl benzoate.
1. Introduction
UV filters are added to preparations to protect the skin by absorbing or blocking UV light. Sunscreen preparations with UV filters help protect against acute effects of UVR exposure, like tanning, erythema, and immunosuppression, and help prevent phototoxic damage resulting from chronic exposure, such as premature skin aging, pigmentation, collagen degradation, and skin cancer [1,2,3].
UVA rays (320–400 nm) can penetrate skin deeper than UVB rays, and are responsible for skin carcinogenesis, immunosuppression, hyperpigmentation, and skin aging [3,4,5]. In turn, UVB rays (290–320 nm) have higher photon energy, and hence can induce potentially mutagenic DNA photoproducts and contribute to the formation of erythema, skin pigmentation, photoimmunosuppression, and skin cancers [6,7,8].
However, solar radiation is also beneficial for living organisms. It exerts a positive effect on human health, inter alia by activating 7-dehydrocholesterol to synthesize Vitamin D in the human skin epidermis or lowering blood pressure through release of nitric oxide. Moreover, exposure to UV rays can improve mood by inducing endorphin release [9,10].
UV filters can be organic agents, often called “chemical”, that can absorb UV rays and release thermal energy, or inorganic (mineral) agents, sometimes called “physical”, that scatter and reflect UV rays. Organic filters can be divided into the following groups of derivatives: para-aminobenzoic acid esters, salicylic acid derivatives, cinnamic acid derivatives, benzylidenecamphor derivatives, benzophenone derivatives, dibenzoylmethane derivatives, benzimidazole and benzotriazole derivatives, triazine derivatives, and various others, such as Polysilicone-15 [11]. The presence of chromophores with a conjugated system of π- bonds in their molecules is typical for organic filters, most often an aromatic ring bonded to a carbonyl group or connected by a carbon-carbon double bond [11].
The list of UV filters approved for use in cosmetics in the EU (32 entries) is stated in Annex VI to the Cosmetics Regulation (Regulation (EC) No 1223/2009), which also includes their maximum allowed concentrations [12].
Recently, much attention has been paid to the safety of UV filters for humans and the environment. Some UV filters, e.g., avobenzone or ethylhexyl dimethyl PABA, under UV radiation, generate photodegradation products and reactive oxygen species (ROS), causing phototoxicity and/or photoallergic processes in the skin [13,14,15]. Although some research suggests that homosalate may act as an endocrine disruptor, the SCCS states homosalate is safe for consumers when used in the final product at concentrations up to 7.34% [16]. The SCCS has raised concerns about the endocrine-disrupting properties of 4-methylbenzylidene camphor (4-MBC), including both the thyroid and estrogen systems [17]. The SCCS needs further research to finally determine the safety of benzophenone-3 for the endocrine system, and recommends its use as a UV filter at concentration up to 6% in face cream, hand cream, and lipsticks [18]. In addition, the use of some UV filters, e.g., benzophenone-3 (oxybenzone) or octocrylene, may be associated with adverse effects, including allergic and photoallergic contact dermatitis [18,19,20,21,22]. Photocontact allergy to octocrylene may occur in patients with previous photoallergy to topically applied ketoprofen, but in general, contact allergy attributed to octocrylene appears very rarely [20]. The FDA have highlighted the need for additional safety data on several filters, including octisalate, homosalate, octocrylene, oxybenzone, octinoxate, and avobenzone [23].
The combination of sunscreens with antioxidant and/or anti-inflammatory agents may lower the risk of skin cancer or other skin damage (e.g., sunburn, erythema, inflammation). As such, it has been proposed that some natural products, such as flavonoids, phenolic acids, anthocyanins or carotenoids, or seaweed and plant extracts, may also be used as skin care against UV radiation [24,25].
More recently, contamination from sunscreen products has been found to pose a threat to coastal ecosystems, as they enter the marine environment through direct contact with beachgoers. High concentrations of benzophenone-3 (BP3) and 4-methylbenzylidene camphor (4-MBC), as well as TiO2 and ZnO, in the surface microlayer were reported in the southern Mediterranean Sea during summer [26,27,28,29]. It is believed that sunscreen ingredients may cause bleaching on coral reefs; to counter this, Hawaii, the U.S. Virgin Islands, and Palau took precautionary measures in this regard and withdrew the use of preparations containing benzophenone-3 (BP3, oxybenzone) and ethylhexyl methoxycinnamate (EHMC, octinoxate) [30,31].
The aim of the study was to gain knowledge about the UV filters selected by manufacturers in sunscreen preparations for adults and for children. Learning about the current trends and frequency of use of photoprotective substances on the Polish market (part of the EU market) may be the opportunity to take a closer look at the safety of the most popular UV filters used in sunscreen preparations.
2. Results
The compositions of 150 sunscreen preparations for adults and 50 for children were analyzed for the presence of UV-photoprotective ingredients. Briefly, the labels of the product were searched and the identified products were classified into the appropriate group of derivatives. The analysis also included the number of UV filters per preparation.
2.1. Types of UV Filters and Their Frequency of Use in Preparations
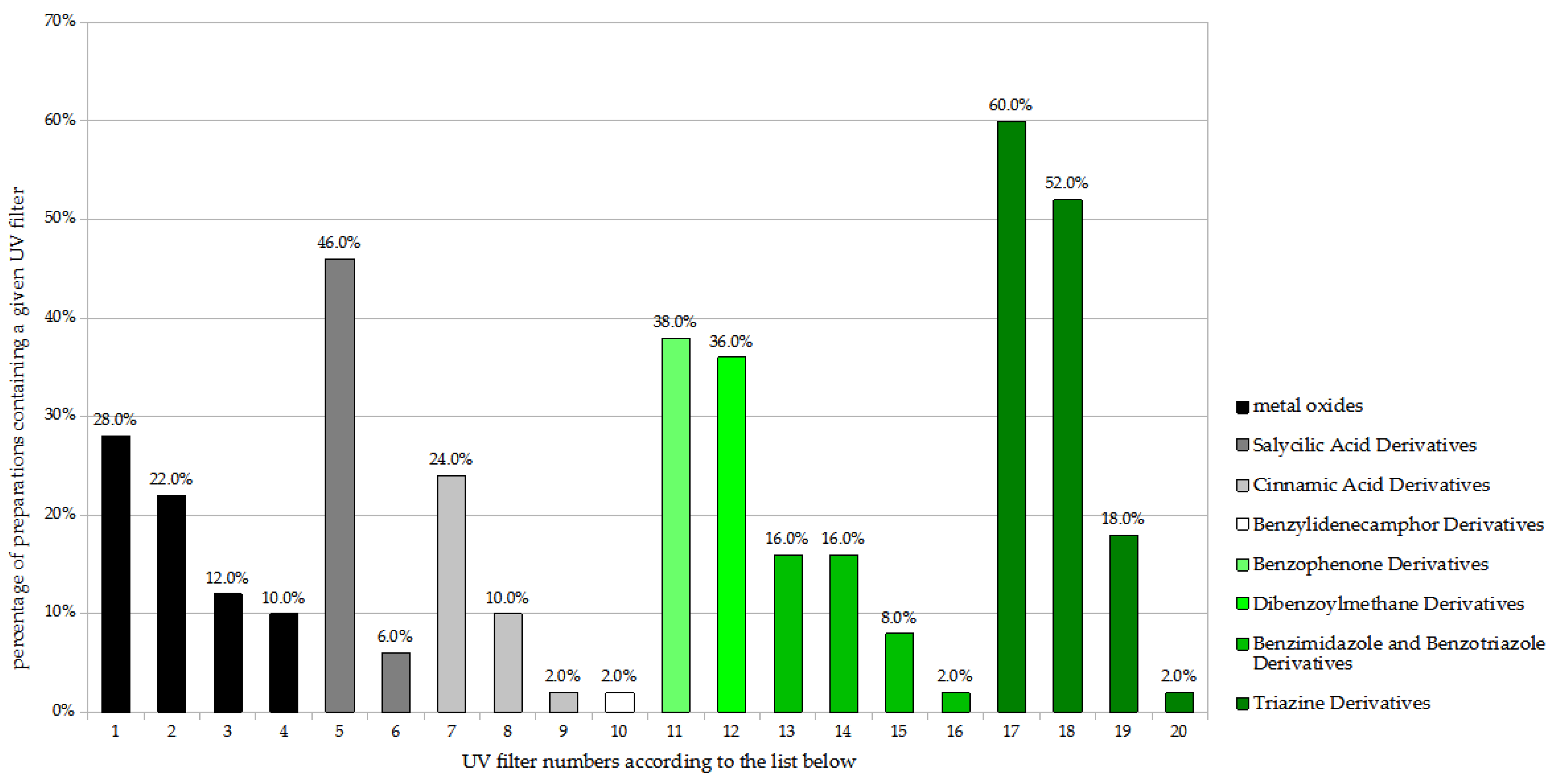
A detailed analysis of the UV filters found in the preparations for children (n = 50) is shown in Figure 1. The most popular substances were two triazine derivatives: bis-ethylhexyloxyphenol methoxyphenyl triazine (BEMT; Tinosorb S), found in 60.0% of the analyzed preparations, and ethylhexyl triazone (EHT), found in 52.0%. These were followed by the salicylic acid derivative—ethylhexyl salicylate (EHS)—present in 46.0%. Finally, the benzophenone derivative diethylamino hydroxybenzoyl hexyl benzoate (DHHB), the dibenzoylmethane derivative butyl methoxydibenzoylmethane (BMBM; Avobenzone), the physical filters titanium dioxide (non-nano) and titanium dioxide (nano), and the cinnamic acid derivative octocrylene (OC) were all found in 20% to 40%.
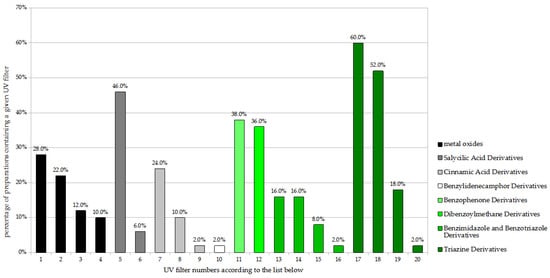
Figure 1.
Frequency of use of UV filters in children sunscreen products (n = 50). 1. Titanium dioxide; 2. Titanium dioxide (nano); 3. Zinc oxide; 4. Zinc oxide (nano); 5. Ethylhexyl salicylate (EHS); 6. Homosalate; 7. Octocrylene (OC); 8. Ethylhexyl methoxycinnamate (EHMC); 9. Isoamyl p-methoxycinnamate; 10. Terephthalylidene dicamphor sulfonic acid; 11. Diethylamino hydroksybenzoyl hexyl benzoate (DHHB); 12. Butyl methoxydibenzoylmethane (BMBM; Avobenzone); 13. Phenylbenzimidazole sulfonic acid; 14. Methylene bis-benzotriazolyl tetramethylbutylphenol (nano); 15. Drometrizole trisiloxane; 16. Methylene bis-benzotriazolyl tetramethylbutylphenol; 17. Bis-ethylhexyloxyphenol methoxyphenyl triazine (BEMT; Tinosorb S); 18. Ethylhexyl triazone (EHT); 19. Diethylhexyl butamino triazone; 20. Tris-biphenyl triazine (nano).
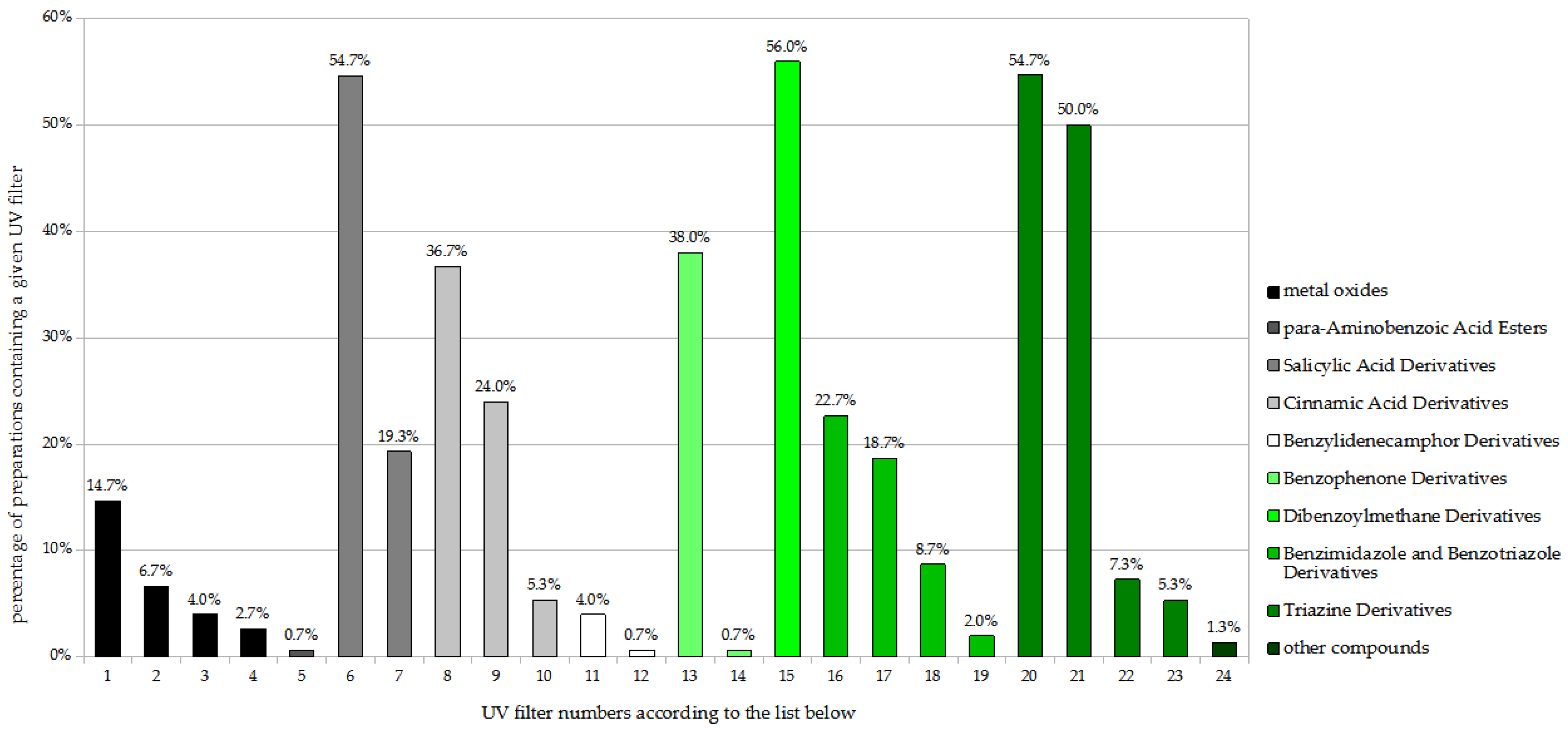
Among the adult sunscreens (Figure 2), the largest share was demonstrated by the dibenzoylmethane derivative butyl methoxydibenzoylmethane (BMBM; Avobenzone) with 56.0% of preparations, followed by the salicylic acid derivative ethylhexyl salicylate (EHS) and the triazine derivative bis-ethylhexyloxyphenol methoxyphenyl triazine (BEMT; Tinosorb S), each present in 54.7%, and then a triazine derivative, ethylhexyl triazone (EHT), with 50.0%. The following also had significant percentage shares, ranging from 20% to 40%: diethylamino hydroxybenzoyl hexyl benzoate (DHHB), the cinnamic acid derivatives octocrylene (OC) and ethylhexyl methoxycinnamate (EHMC), and phenylbenzimidazole sulfonic acid (PBSA).
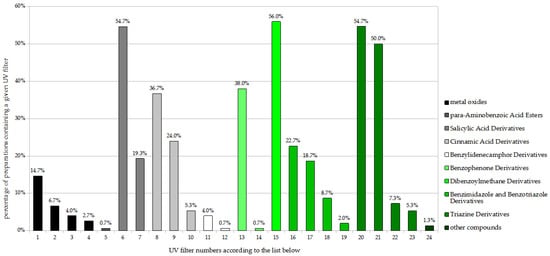
Figure 2.
Frequency of use of UV filters in adult sunscreen products (n = 150). 1. Titanium dioxide; 2. Titanium dioxide (nano); 3. Zinc oxide; 4. Zinc oxide (nano); 5. Ethylhexyl dimethyl PABA; 6. Ethylhexyl salicylate (EHS); 7. Homosalate; 8. Octocrylene (OC); 9. Ethylhexyl methoxycinnamate (EHMC); 10. Isoamyl p-methoxycinnamate; 11. Terephthalylidene dicamphor sulfonic acid; 12. 4-Methylbenzylidene camphor; 13. Diethylamino hydroxybenzoyl hexyl benzoate (DHHB); 14. Benzophenone-3; 15. Butyl methoxydibenzoylmethane (BMBM; Avobenzone); 16. Phenylbenzimidazole sulfonic acid (PBSA); 17. Methylene bis-benzotriazolyl tetramethylbutylphenol (nano); 18. Drometrizole trisiloxane; 19. Methylene bis-benzotriazolyl tetramethylbutylphenol; 20. Bis-ethylhexyloxyphenol methoxyphenyl triazine (BEMT; Tinosorb S); 21. Ethylhexyl triazone (EHT); 22. Diethylhexyl butamino tria one; 23. Tris-biphenyl triazine (nano); 24. Polysilicone-15.
Hence, the most common sunscreen substances in the analyzed cosmetics, both for children and adults, were the organic UV filters from the group of triazine derivatives, salicylic acid derivatives, and dibenzoylmethane derivatives: butyl methoxydibenzoylmethane (BMBM; Avobenzone); bis-ethylhexyloxyphenol methoxyphenyl triazine (BEMT; Tinosorb S);. ethylhexyl salicylate (EHS); ethylhexyl triazone (EHT) (Table 1). Physical filters were more common in preparations for children than for adults. Titanium dioxide (including its nano form) was present in 50.0% of child products and 21.3% of adult products, and zinc oxide (including its nano form) was found in 22.0% of child products but only 6.7% of adult products.

Table 1.
A profile of the most frequently used UV filters in the analyzed cosmetics.
Some of the identified UV filters were found to be relatively rare, appearing in fewer than 5% of sunscreen products. In the preparations for children, these included isoamyl p-methoxycinnamate, terephthalylidene dicamphor sulfonic acid, methylene bis-benzotriazolyl tetramethylbutylphenol, and tris-biphenyl triazine (nano). For adults, these included zinc oxide, zinc oxide (nano), ethylhexyl dimethyl PABA, terephthalylidene dicamphor sulfonic acid, 4-methylbenzylidene camphor, benzophenone-3, methylen bis-benzotriazolyl tetramethylbutylphenol, and polysilicone-15.
Approximately 30% of the UV filters listed in Annex VI of the Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on cosmetic products were not used in any of the tested sunscreen preparations: benzalkonium methosulfate, benzylidene camphor sulfonic acid, polyacrylamidomethyl benzylidene camphor, PEG-25 PABA, benzophenone-4, benzophenone-5, disodium phenyl dibenzimidazole tetrasulfonate, phenylene bis-diphenyltriazine, methoxypropylamino cyclohexenylidene ethoxyethylcyanoacetate, bis-(diethylaminohydroxybenzoyl benzoyl) piperazine, bis-(diethylaminohydroxybenzoyl benzoyl) piperazine (nano), and tris-biphenyl triazine (non-nano form).
2.2. Composition Complexity of the Analyzed Preparations
The complexity of composition, i.e., the number of UV filters per single photoprotective formulation, was recorded. For the sake of brevity, ingredients that do not have a photoprotective function were not included in the list.
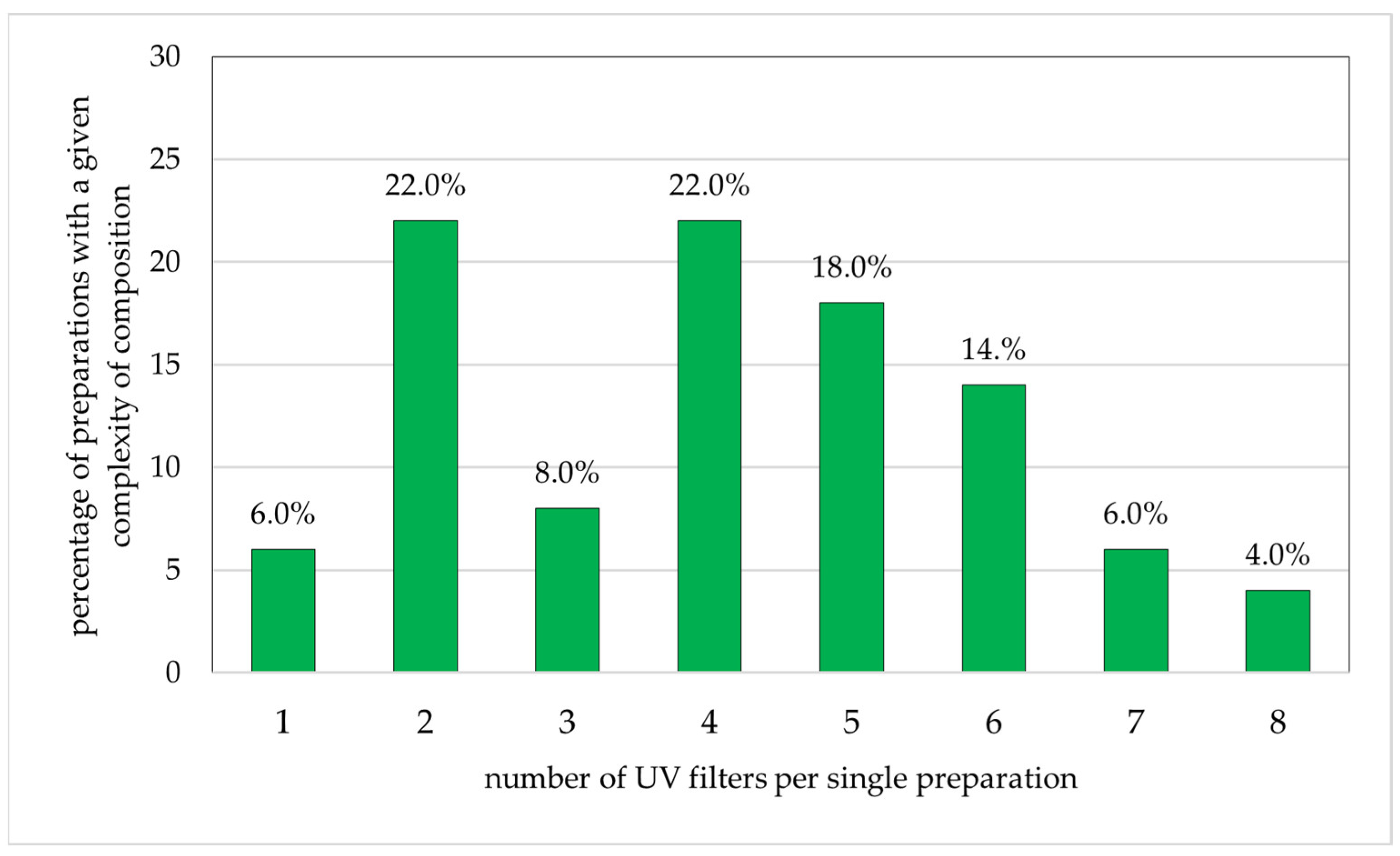
The components of the cosmetic products for children are given in Figure 3. Among the 50 analyzed preparations, the largest single group comprised products with two or four components (each 22.0% of all products). In addition, 18.0% of products contained five UV-protective substances in a single preparation, and 14.0% contained six. Products containing three, one, seven, and eight UV filters in one preparation accounted for only 8.0%, 6.0%, 6.0%, and 4.0% of the study group, respectively.
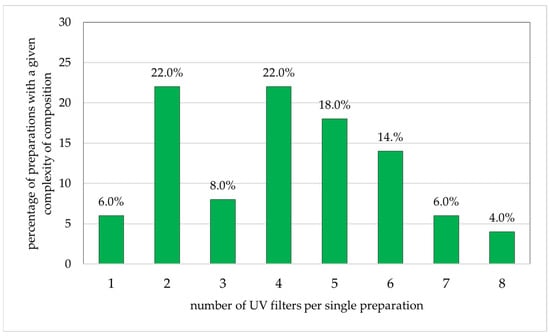
Figure 3.
Percentage of preparations for children (n = 50) with specific numbers of sunscreen ingredients (percentages add up to 100%).
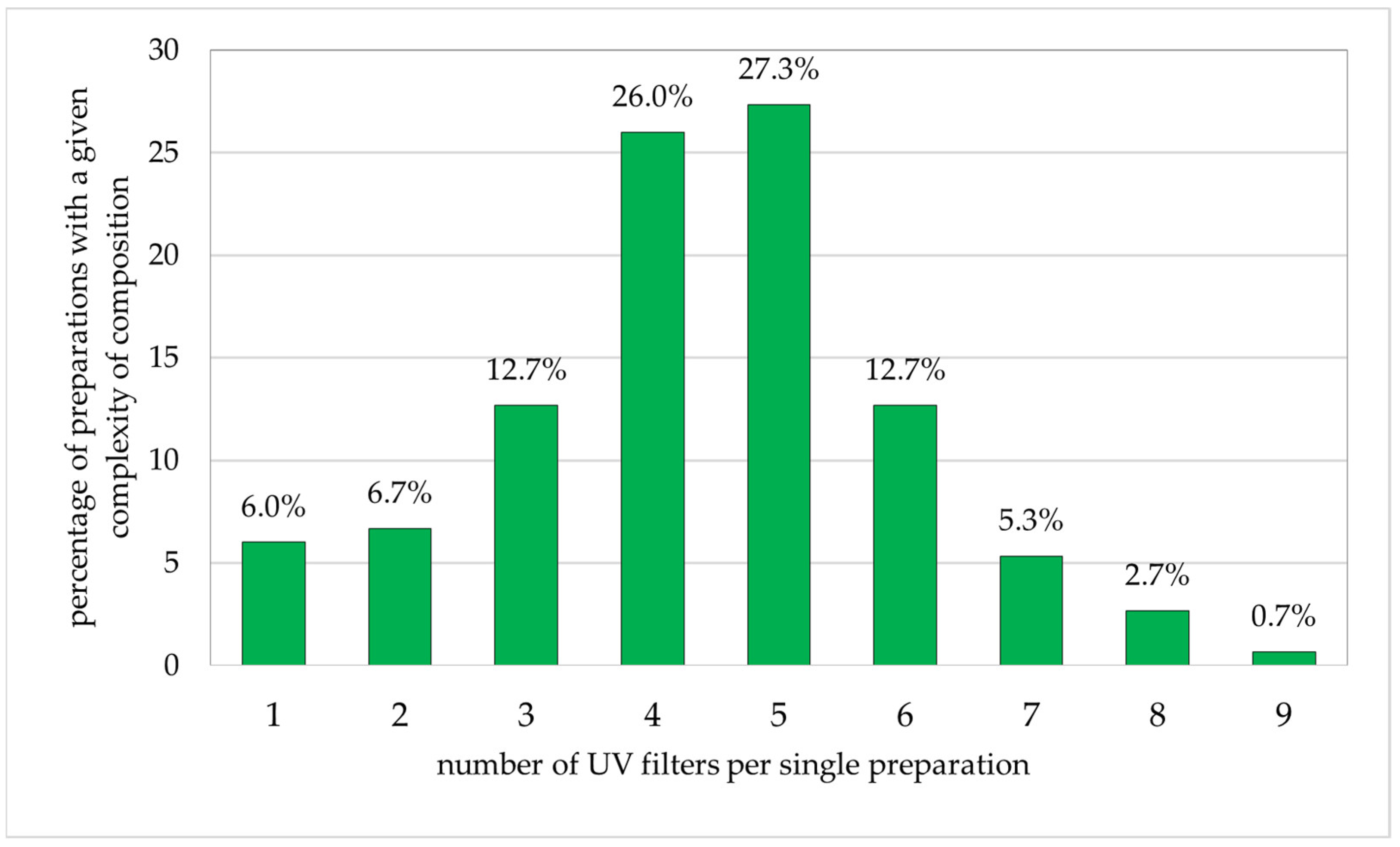
Among the adult preparations (Figure 4), the largest single groups of cosmetics included five (27.0%) or four (26.0%) photoprotective substances in a single preparation. Products with three or six components each accounted for 12.7%. The smallest groups included two, one, eight, or nine products, constituting 6.7%, 6.0%, 5.3%, and 0.7%, respectively.
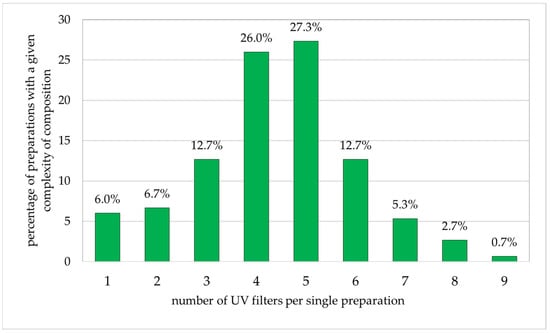
Figure 4.
Percentage of preparations for adults (n = 150) with specific numbers of sunscreen ingredients (percentages add up to 100%).
Hence, for both adults and children, the largest group of products include four and five UV-protective substances, while those for children have two components.
Among the single-component preparations, the most common components were physical UV filters (Table 2). Titanium oxide (nano form) was dominant (100%) in child formulations (n = 3), while TiO2 (55.6%), ZnO (11.1%), TiO2 (nano) (11.1%), octocrylene (11.1%), and ethylhexyl methoxycinnamate (11.1%) were found in the adult preparations (n = 9).

Table 2.
The preparations containing one or two sunscreen components, with the percentage share of individual substances in a given group.
The physical UV filters also dominated in the two-component preparations in both study groups (Table 2). In the group of preparations for children (n = 11), the most common were TiO2 (63.6%), ZnO (45.5%), TiO2 (nano) (36.4%), and ZnO (nano) (27.3%). Two chemical filters, octocrylene and bis-ethylhexyloxyphenol methoxyphenyl triazine, were present but below the value of 20%. Among those for adults (n = 10), the most prevalent were ZnO (40.0%) and TiO2 (30.0%), followed by nanometric scale forms of ZnO and TiO2, and the chemical filters phenylbenzimidazole sulfonic acid, butyl methoxydibenzoylmethane, ethylhexyl salicylate, diethylamino hydroxybenzoyl hexyl benzoate, and octocrylene, all of which were present in 10% to 20% of samples.
Hence, it can be seen that most single- and two-component preparations tended to use physical filters as sunscreen ingredients, and among these, the non-nano form is more common than the nano form.
The complexity of the composition of sunscreen preparations is related to the scope of UV protection of individual filters. When properly selected, the UV filters in a given preparation should fully protect skin against both UVA and UVB radiation. A commonly used combinations of filters that absorb a wide range of UV rays are, e.g., the mixture of Avobenzone (BMBM) with octocrylene (OC) and/or ethylhexyl salicylate (EHS) or the fusion of Avobenzone (BMBM) with Tinosrob S (BEMT), and/or ethylhexyl salicylate (EHS) and/or diethylamino hydroxybenzoyl hexyl benzoate (DHHB). Avobenzone (BMBM) and DHHB provide protection against UVA radiation. Tinosrob S (BEMT) is broad-spectrum filter (UVA and UVB). In turn, the most popular UVB filters include EHT, EHS, OC, EHMC, and PBSA [10,11,19].
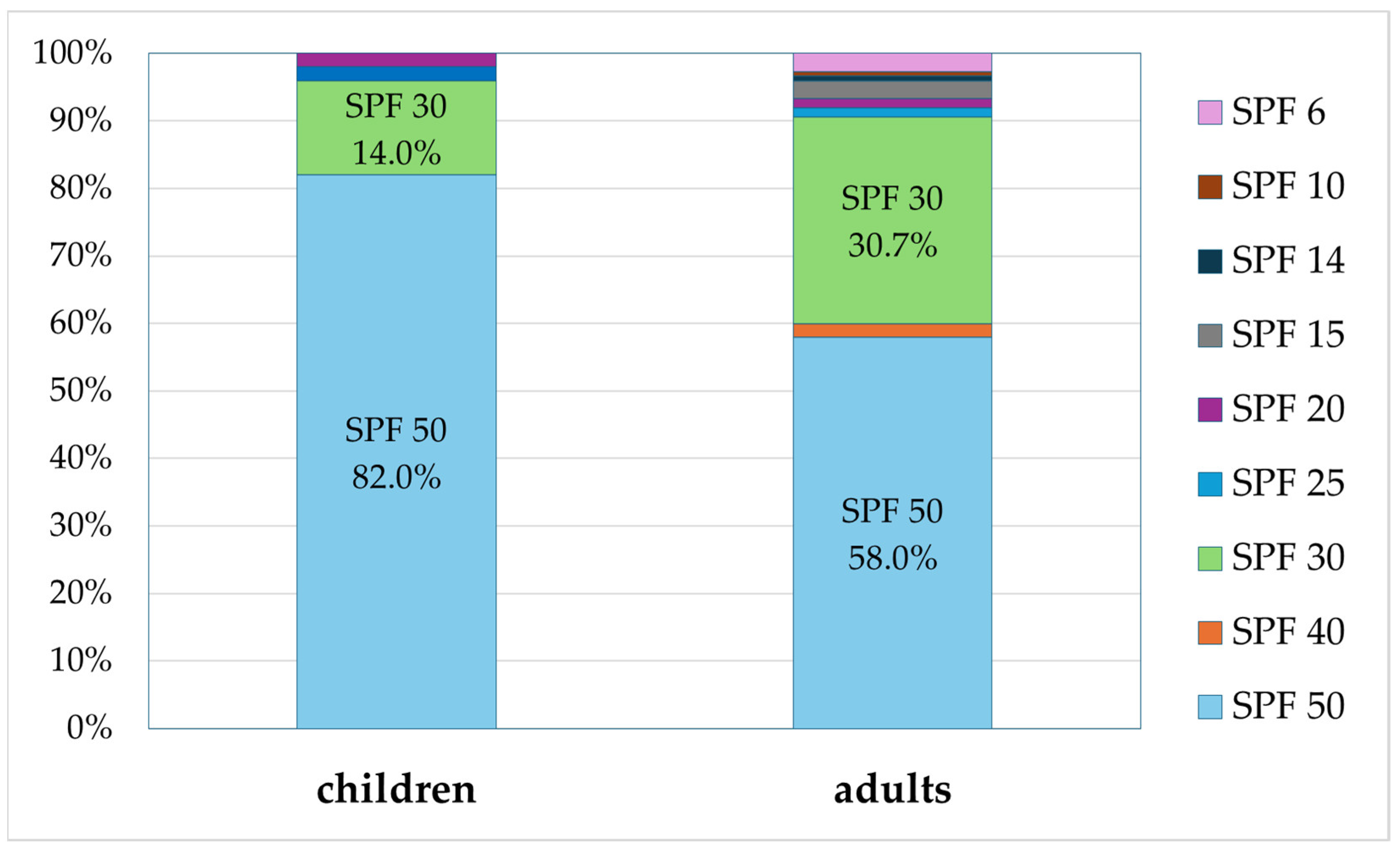
2.3. SPF Values of the Analyzed Preparations
The analyzed sunscreen preparations were also reviewed for their SPF value (Figure 5). Among the preparations for children, those with higher SPF values clearly dominated. As many as 82.0% of preparations for children were those with SPF 50 and 14.0% were those with SPF 30. The SPF of the value 20 was the lowest among preparations for children (2.0%). The reviewed adult sunscreens mostly provide high protection of SPF value greater than or equal to 30, with the following distribution: SPF 50 (50.0%), SPF 40 (2.0%), and SPF 30 (30.7%). The remaining adult preparations, with SPF between 6 and 25, accounted for only 9.3%.
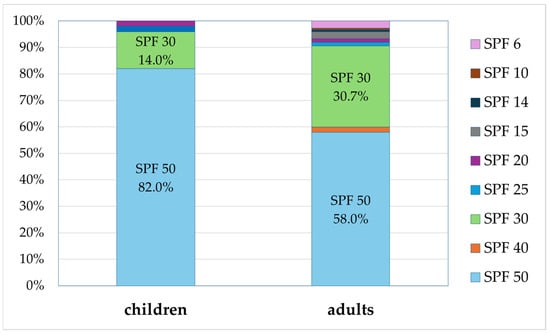
Figure 5.
Percentage of preparations for children and adults with a specific SPF value (percentages add up to 100%).
3. Discussion
Our findings indicate that the most popular photoprotective components, both for children and adults, were butyl methoxydibenzoylmethane (BMBM; Avobenzone), bis-ethylhexyloxyphenol methoxyphenyl triazine (BEMT; Tinosorb S), ethylhexyl salicylate (EHS), and ethylhexyl triazone (EHT), and that most sunscreens in Poland have five or four UV filters in a single preparation.
Two surveys of UV filters contained in sunscreen products for children and adults were conducted in Thailand. The first overview (n = 246) was made between December 2017 and March 2018 [32], and the second (n = 312) in April 2020 [33]. While they yielded similar results for the adult products, the reviews of products for children were very divergent; however, this may be due to them being based on small numbers of products, viz. n = 20 [32] and n = 15 [33].
The first overview (n = 226), performed from December 2017 to March 2018 [32], found the most common UV filter for adult to be ethylhexyl methoxycinnamate (EHMC) (62.8%), followed by titanium dioxide, octocrylene (OC), and butyl methoxydibenzoylmethane (BMBM) in 54.9%, 45.1%, and 44.2%, respectively. The most frequently used UV filter for children (n = 20) was butyl methoxydibenzoylmethane (BMBM) (65.0%). The mineral filter TiO2 (60.0%) was also commonly used, followed by octocrylene (45.0%) and bis-ethylhexyloxyphenol methoxyphenyl triazine (BEMT) (40.0%). Ethylhexyl methoxycinnamate (EHMC) was found less frequently, i.e., only 30.0% [32].
The second survey (n = 297), performed in April 2020 [33], found the most common UV filter for adults to be titanium dioxide (68.0%), followed by ethylhexyl methoxycinnamate (EHMC) (57.2%) and butyl methoxydibenzoylmethane (BMBM) (42.1%), while the most common for children (n = 15) was bis-ethylhexyloxyphenol methoxyphenyl triazine (BEMT) (53.3%), followed by butyl methoxydibenzoylmethane (BMBM) and ethylhexyl salicylate (EHS) (46.7% each). The share for titanium dioxide in this group was 40.0%. [33]. However, these findings cannot be compared with the present study as the filters are intended for people with different skin phototypes from regions with different degrees of sunlight exposure and a different legal environment. Nevertheless, it is worth noting that our results differed meaningfully from the study conducted by Chaiyabutr et al. [33]. The most prevalent filters were 4.5 times (TiO2) and 2.5 times (EHMC) more prevalent than in our present study; in turn, our predominant filter for adults was butyl methoxydibenzoylmethane (BMBM) (56.0%).
A similar survey of randomly selected preparations from several dozen brands was carried out in Portugal in 2021 [34]. The most popular UV filters for adults (n = 379), i.e., with a percentage share above 35%, included butyl methoxydibenzoylmethane (BMBM) (73.9%), octocrylene (OC) (51.7%), bis-ethylhexyloxyphenol methoxyphenyl triazine (BEMT) (47.5%), ethylhexyl triazone (38.0%), and ethylhexyl salicylate (35.9%). In comparison, in the present study, the prevalence of methoxydibenzoylmethane (BMBM) was 17.9 p.p. lower, with 56.0%; this was followed by bis-ethylhexyloxyphenol methoxyphenyl triazone (BEMT; 54.7%), ethylhexyl salicylate (EHS; 54.7%), and ethylhexyl triazine (EHT; 50.0%). These components also occupied the top positions in the Portuguese study, but with lower shares by 7.2 p.p., 18.8 p.p., and 12.0 p.p., respectively. While octocrylene (OC) was the second most common filter in the Portuguese study (51.7%), it was in sixth place in the present study (36.7%) [34].
Clear differences were also observed regarding child sunscreens between the Polish (n = 50) and Portuguese (n = 65). The predominant component in Poland, bis-ethylhexyloxyphenol methoxyphenyl triazine (BEMT; 60.0%), was only in fifth place in the Portuguese study, with a share of approximately 30%. Conversely, the predominant component in the Portuguese study was butyl methoxydibenzoylmethane (BMBM; approximately 60%), which was only fifth on the list in the present study (36.0%). Both studies had similar prevalence values for other popular filters, i.e., ethylhexyl salicylate (EHS) and ethylhexyl triazone (EHT). In addition, similar values were noted for titanium dioxide (non-nano form) in child formulas in the Portuguese study (35.4%) and the present study (28.0%). Interestingly, the benzophenone derivative DHHB, present in our study in preparations for adults and children with a share of as much as 38%, had, in the Portuguese study, a lower share by approximately 18 p.p. and 13 p.p. [34].
The availability of UV filters varies over time and undoubtedly depends on market forces and legal regulations. A 2014 survey of sunscreen products in the UK (n = 337) found the most common UV filters to be butyl methoxydibenzoylmethane (BMBM) and octocrylene (OC), which were present in 96.4% and 90.5% of sunscreen products. Bis-ethylhexyloxyphenol methoxyphenyl triazine (BEMT) was found in 58.5% of products. Ethylhexyl salicylate (EHT), diethylhexyl butamido triazone, and methylene bis-benzotriazolyl tetramethylbutylphenol were found in around 32% each [35].
In the present study, the most frequently used UVA filter in adult sunscreens was butyl methoxydibenzoylmethane (BMBM; Avobenzone) (56.0%). Its frequency of use in child preparations was 36.0%, putting it in fifth position. Avobenzone is considered one of the most common allergenic and photoallergenic [36], and highly photolabile, UV filters [37]. Photopatch testing has found the reactivity rate of avobenzone to be 1.3–1.7% [38,39]. It loses between 50% and 60% of its protective efficacy after one hour of exposure to UV radiation [40]. Avobenzone undergoes keto-enol tautomerism, and its keto form can easily photodegrade into 4-tert-butyl benzoic acid and 4-methoxy benzoic acid, which are responsible for its photoallergic and phototoxic reactions [11,41].
Photodegradation of unstable UV filters such as avobenzone can be prevented by the use of photostabilizers, encapsulation, antioxidants, and the application of quenchers [42]. In addition, combining avobenzone with other photostable filters such as octocrylene and Tinosorb S can also prevent photodegradation [43,44]. Its photostability can also be improved by loading in cyclodextrin, e.g., beta-cyclodextrin polymers (pbCD) cross-linked by epichlorohydrin (pbCDE), or liposome lipid nanoparticles, microparticles, and polymeric nanoparticles [42,45].
A test of avobenzone and five other organic filters (octisalate, homosalate, octocrylene, oxybenzone, octinoxate) using the ToxCast/Tox21 database found all apart from oxybenzone to have low intrinsic biological activity and a low risk of toxicity, including endocrine disruption, in humans [46].
In the present study, bis-ethylhexyloxyphenol methoxyphenyl triazine (BEMT; Tinosorb S) was the most frequently used UV filter (60.0%) for children and the second most common (54.7%) for adults. Unlike avobenzone, BEMT is photostable. It is a broad absorption spectrum filter (UVB, UVA1, and UVA2) with minimal skin penetration and does not disrupt the functioning of the endocrine system [47,48]. In 1999, SCCS confirmed that there was no evidence that this compound was toxic or allergenic [49].
Ethylhexyl salicylate (EHS) was found to be one of the most popular UV filters in adult and child sunscreens, used in 54.7% and 46.0% of the studied samples. It demonstrates photodegradation and can induce some environmental toxicity [19,34]. Clinical trial data indicates that it is systemically absorbed, resulting in plasma concentrations higher than the FDA systemic exposure threshold (0.5 ng/mL) [50].
Two cinnamic acid derivatives, octocrylene (OC) and ethylhexyl methoxycinnamate (EHMC), were also quite popular UV filters in the present study. They were present in 36.6% (OC) and 24.0% (EHMC) of adult preparations, and 24.0% (OC) and 10.0% (EHMC) of child preparations. Unlike EHMC, octocrylene does not exhibit any endocrine disruption potential nor is it photodegradable [19,20]. OC rarely causes skin irritation reactions (0.6% for n = 1031 [20]. The number of reported cases of allergic contact dermatitis after the use of octocrylene seems to be irrelevant considering the widespread use of it in cosmetic products. Photocontact allergy to octocrylene may occur in patients with previous photoallergy to topically applied ketoprofen [20,51]. Both OC and EHMC demonstrate high bioaccumulation rates, passing into breast milk [19].
Ethylhexyl triazone (EHT), was found to be the second most common UV filter in child sunscreens (52.0%) and the fourth in adults (50.0%). It does not show skin penetration [52] and has good photostability [34]. EHT releases free radicals in contact with sunlight [47].
Titanium dioxide (TiO2) is an inorganic UV filter present as both nano (22.0%) and micro (28%) forms in child sunscreens. Titanium oxide is considered a more photochemically stable and less skin irritating filter than most organic filters, but it can generate reactive oxygen species (ROS) when exposed to UV radiation, leading to potential adverse effects [53,54,55]. Therefore, since 2019, titanium dioxide can only be used as a nanomaterial when coated with inert shells like silica, hydrated silica, alumina, aluminium hydroxide, aluminium stearate, stearic acid, trimethoxycaprylylsilane, dimethicone or simethicone, or with some combinations thereof (Annex VI, Regulation No. 1223/2009). TiO2 (nano) is not allowed in applications that may lead to exposure by inhalation [12].
4. Materials and Methods
From October to December 2023, the INCI compositions of sunscreen products available online on the Polish market were collected. The survey involved 150 randomly chosen preparations with UV filters for adults and 50 for children. The adult products included 71 international brands, with one to eight products from each brand, while the child products were obtained from 33 international brands, with one to three products per brand.
The searched UV filters were listed in Annex VI to Regulation (EC) No 1223/2009 of the European Parliament and Council of 30 November 2009 on cosmetic products.
The collected data were processed using Microsoft Excel. The data collected from the INCI compositions of preparations are series of dichotomies that determine for each preparation and filter whether the filter is included in the preparation or not. There is no numerical measure here, such as concentration or dose. Quantitative analysis of such data encounters great difficulties, so only basic statistical analyses were performed on them.
5. Conclusions
Several popular UV filters are present in sunscreen products on the Polish market, i.e., within the EU. The most common organic UV filters found in child sunscreen products, with a frequency of use above 30%, are bis-ethylhexyloxyphenol methoxyphenyl triazine (BEMT; Tinosorb S)—60.0%, ethylhexyl triazone (EHT)—52.0%, ethylhexyl salicylate (EHS)—46.0%, diethylamino hydroxybenzoyl hexyl benzoate (DHHB)—38.0%, and butyl methoxydibenzoylmethane (BMBM; Avobenzone)—36.0%. The most common adult photoprotectors were butyl methoxydibenzoylmethane (BMBM; Avobenzone)—56.0%, bis-ethylhexyloxyphenol methoxyphenyl triazine (BEMT; Tinosorb S)—54.7%, ethylhexyl salicylate (EHS)—54.7%, ethylhexyl triazone (EHT)—50.0%, diethylamino hydroxybenzoyl hexyl benzoate (DHHB)—38.0%, and octocrylene (OC)—36.7%. The most common organic compounds were triazine, salicylic acid, dibenzoylmethane, and benzophenone derivatives.
Mineral filters, especially titanium dioxide, are much more popular among child sunscreens (TiO2—28.0% and TiO2 nano—22.0%) than for adults (TiO2—14.7% and TiO2 nano—6.7%). Ten of the UV filters listed in Annex VI to Regulation (EC) No 1223/2009 are not used at all in sunscreen products; these are mostly benzylidenecamphor derivatives, with some benzophenone and triazine derivatives.
Author Contributions
Conceptualization, U.K.-L.; Methodology, A.P.; Formal analysis, A.P.; Investigation, A.P.; Data curation, A.P.; Writing—original draft, A.P.; Writing—review & editing, U.K.-L.; Visualization, A.P.; Supervision, U.K.-L.; Project administration, U.K.-L. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the Medical University of Lodz, grant No. 503/3-066-02/503-31-001.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Acknowledgments
We would like to thank Elżbieta Budzisz for her skillful contribution and support for the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Seité, S.; Colige, A.; Piquemal-Vivenot, P.; Montastier, C.; Fourtanier, A.; Lapière, A.; Nusgens, B. A full-UV spectrum absorbing daily use cream protects human skin against biological changes occurring in photoaging. Photodermatol. Photoimmunol. Photomed. 2000, 16, 147–155. [Google Scholar] [CrossRef] [PubMed]
- Lautenschlager, S.; Wulf, H.C.; Pittelkow, M.R. Photoprotection. Lancet 2007, 370, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Rittié, L.; Fisher, G.J. Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med 2015, 5, a015370. [Google Scholar] [CrossRef] [PubMed]
- Verkouteren, J.A.C.; Ramdas, K.H.R.; Wakkee, M.; Nijsten, T. Epidemiology of basal cell carcinoma: Scholarly review. Br. J. Dermatol. 2017, 177, 359–372. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, N.K.; Norval, M. Photoimmunosuppression: A brief overview. Photodermatol. Photoimmunol. Photomed. 2013, 29, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Rünger, T.M.; Farahvash, B.; Hatvani, Z.; Rees, A. Comparison of DNA damage responses following equimutagenic doses of UVA and UVB: A less effective cell cycle arrest with UVA may render UVA-induced pyrimidine dimers more mutagenic than UVB-induced ones. Photochem. Photobiol. Sci. 2012, 11, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Bernerd, F.; Asselineau, D. Successive alteration and recovery of epidermal differentiation and morphogenesis after specific UVB-damages in skin reconstructed in vitro. Dev. Biol. 1997, 183, 123–138. [Google Scholar] [CrossRef] [PubMed]
- Lavker, R.M.; Gerberick, G.F.; Veres, D.; Irwin, C.J.; Kaidbey, K.H. Cumulative effects from repeated exposures to suberythemal doses of UVB and UVA in human skin. J. Am. Acad. Dermatol. 1995, 32, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Neale, R.E.; Lucas, R.M.; Byrne, S.N.; Hollestein, L.; Rhodes, L.E.; Yazar, S.; Young, A.R.; Berwick, M.; Ireland, R.A.; Olsen, C.M. The effects of exposure to solar radiation on human health. Photochem. Photobiol. Sci. 2023, 22, 1011–1047. [Google Scholar] [CrossRef]
- Jesus, A.; Sousa, A.; Cruz, M.T.; Cidade, H.; Sousa Lobo, J.M.; Almeida, I.F. UV Filters: Challenges and Prospects. Pharmaceuticals 2022, 15, 263. [Google Scholar] [CrossRef]
- Nitulescu, G.; Lupuliasa, D.; Adam-Dima, I.; Nitulescu, G.M. Ultraviolet Filters for Cosmetic Applications. Cosmetics 2023, 10, 101. [Google Scholar] [CrossRef]
- European Commission. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products; Official Journal of the European Union L 342/59; Publications Office of the European Union: Luxembourg, 2009. [Google Scholar]
- Nash, J.F.; Tanner, P.R. Relevance of UV filter/sunscreen product photostability. Photodermatol. Photoimmunol. Photomed. 2014, 30, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, C.M.; Gaspar, L.R. Mangiferin and naringenin affect the photostability and phototoxicity of sunscreens containing avobenzone. J. Photochem. Photobiol. B Biology 2015, 151, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Tarras-Wahlberg, N.; Stenhagen, G.; Larkö, O.; Rosén, A.; Wennberg, A.M.; Wennerström, O. Changes in ultraviolet absorption of sunscreens after ultraviolet irradiation. J. Investig. Dermatol. 1999, 113, 547–553. [Google Scholar] [CrossRef] [PubMed]
- SCCS (Scientific Committee on Consumer Safety). Scientific Advice on the Safety of Homosalate (CAS No 118-56-9, EC No 204-260-8) as a UV-filter in Cosmetic Products in Cosmetic Products; Final Version of 2 December 2021, SCCS/1638/21; European Commission. 2021. Available online: https://inria.hal.science/hal-03464662/ (accessed on 11 April 2024).
- SCCS (Scientific Committee on Consumer Safety). Scientific Opinion on 4-Methylbenzylidene Camphor (4-MBC); Preliminary Version of 22 December, Final Version of 29 April 2022, SCCS/1640/21; European Commission. 2022. Available online: https://health.ec.europa.eu/document/download/bc7fc1c9-9a5e-4f7c-a67f-a03b4dea312b_en?filename=sccs_o_262.pdf (accessed on 11 April 2024).
- SCCS (Scientific Committee on Consumer Safety). Opinion on Benzophenone-3 (CAS No 131-57-7, EC No 205-031-5); Preliminary Version of 15 December 2020, Final Version of 30–31 March 2021, SCCS/1625/20; European Commission. 2021. Available online: https://hal.science/hal-03199396/ (accessed on 11 April 2024).
- Sabzevari, N.; Qiiblawi, S.; Norton, S.A.; Fivenson, D. Sunscreens: UV filters to protect us: Part 1: Changing regulations and choices for optimal sun protection. Int. J. Women’s Dermatol. 2021, 7, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Berardesca, E.; Zuberbier, T.; Sanchez Viera, M.; Marinovich, M. Review of the safety of octocrylene used as an ultravioletfilter in cosmetics. JEADV 2019, 33 (Suppl. 7), 25–33. [Google Scholar] [PubMed]
- Avenel-Audran, M.; Dutartre, H.; Goossens, A.; Jeanmougin, M.; Comte, C.; Bernier, C.; Benkalfate, L.; Michel, M.; Ferrier-Lebouëdec, M.C.; Vigan, M.; et al. Octocrylene, an emerging photoallergen. Arch. Dermatol. 2010, 146, 753–757. [Google Scholar] [CrossRef]
- Bryden, A.M.; Moseley, H.; Ibbotson, S.H.; Chowdhury, M.M.U.; Beck, M.H.; Bourke, J.; English, J.; Farr, P.; Foulds, I.S.; Gawkrodger, D.J.; et al. Photopatch testing of 1155 patients: Results of the U.K. Multicentre photopatch study group. Br. J. Dermatol. 2006, 155, 737–747. [Google Scholar] [CrossRef] [PubMed]
- FDA (Ed.) Sunscreen Drug Products for Over-the-Counter Human Use. Center for Drug Evaluation and Research, U.S. Department of Health and Human Services. 2019. Available online: https://www.federalregister.gov/documents/2019/02/26/2019-03019/sunscreendrug-products-for-over-the-counter-human-use (accessed on 21 March 2024).
- Li, L.; Chong, L.; Huang, T.; Ma, Y.; Li, Y.; Ding, H. Natural products and extracts from plants as natural UV filters for sunscreens: A review. Anim. Models Exp. Med. 2023, 6, 183–195. [Google Scholar] [CrossRef]
- Soleimani, S.; Yousefzadi, M.; Babaei Mahani Nezhad, S.; Pozharitskaya, O.N.; Shikov, A.N. Potential of the Ethyl Acetate Fraction of Padina boergesenii as a Natural UV Filter in Sunscreen Cream formulation. Life 2023, 13, 239. [Google Scholar] [CrossRef]
- Thallinger, D.; Labille, J.; Milinkovitch, T.; Boudenne, J.L.; Loosli, F.; Slomberg, D.; Angeletti, B.; Lefrançois, C. UV filter occurrence in beach water of the Mediterranean coast—A field survey over 2 years in Palavas-les-Flots. Int. J. Cosmet. Sci. 2023, 45, 67–83. [Google Scholar] [CrossRef] [PubMed]
- Agawin, N.S.R.; Sunyer-Caldú, A.; Díaz-Cruz, M.S.; Frank-Comas, A.; García-Márquez, M.G.; Tovar-Sánchez, A. Mediterranean seagrass Posidonia oceanica accumulates sunscreen filters. Mar. Pollut. Bull. 2022, 176, 113417. [Google Scholar] [CrossRef] [PubMed]
- Tovar-Sánchez, A.; Sánchez-Quiles, D.; Rodríguez-Romero, A. Massive coastal tourism influx to the Mediterranean Sea: The environmental risk of sunscreens. Sci. Total Environ. 2019, 656, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Quiles, D.; Blasco, J.; Tovar-Sánchez, A. Sunscreen components are a new environmental concern in coastal waters: An overview. In Sunscreens in Coastal Ecosystems, The Handbook of Environmental Chemistry; Tovar-Sánchez, A., Sánchez-Quiles, D., Blasco, J., Eds.; Springer: Cham, Switzerland, 2020; Volume 94, pp. 1–14. [Google Scholar]
- Miller, I.B.; Pawlowski, S.; Kellermann, M.Y.; Petersen-Thiery, M.; Moeller, M.; Nietzer, S.; Schupp, P.J. Toxic effects of UV filters from sunscreens on coral reefs revisited: Regulatory aspects for “reef safe” products. Environ. Sci. Eur. 2021, 33, 74. [Google Scholar] [CrossRef]
- Heron, S.F.; Maynard, J.A.; Van Hooidonk, R.; Eakin, C.M. Warming trends and bleaching stress of the world’s coral reefs 1985–2012. Sci. Rep. 2016, 6, 38402. [Google Scholar] [CrossRef] [PubMed]
- Phadungsaksawasdi, P.; Sirithanabadeekul, P. Ultraviolet filters in sunscreen products labeled for use in children and for sensitive skin. Pediatr. Dermatol. 2020, 37, 632–636. [Google Scholar] [CrossRef] [PubMed]
- Chaiyabutr, C.; Sukakul, T.; Kumpangsin, T.; Bunyavaree, M.; Charoenpipatsin, N.; Wongdama, S.; Boonchai, W. Ultraviolet filters in sunscreens and cosmetic products-A market survey. Contact Dermat. 2021, 85, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Jesus, A.; Augusto, I.; Duarte, J.; Sousa, E.; Cidade, H.; Cruz, M.T.; Lobo, J.M.S.; Almeida, I.F. Recent Trends on UV filters. Appl. Sci. 2022, 12, 12003. [Google Scholar] [CrossRef]
- Kerr, A.C. A survey of the availability of sunscreen filters in the UK. Clin. Exp. Dermatol. 2011, 36, 541–543. [Google Scholar] [CrossRef]
- Heurung, A.R.; Raju, S.I.; Warshaw, E.M. Adverse reactions to sunscreen agents: Epidemiology, responsible irritants and allergens, clinical characteristics, and management. Dermatitis 2014, 25, 289–326. [Google Scholar] [CrossRef]
- Sambandan, D.R.; Ratner, D. Sunscreens: An overview and update. J. Am. Acad. Dermatol. 2011, 64, 748–758. [Google Scholar] [CrossRef] [PubMed]
- Kerr, A.C.; Ferguson, J.; Haylett, A.K.; Rhodes, L.E.; Adamski, H.; Alomar, A.; Serra, E.; Antoniou, C.; Aubin, F.; Vigan, M.; et al. A European multicentre photopatch test study. Br. J. Dermatol. 2012, 166, 1002–1009. [Google Scholar]
- Scalf, L.A.; Davis, M.D.P.; Rohlinger, A.L.; Connolly, S.M. Photopatch testing of 182 patients: A 6-year experience at the Mayo Clinic. Dermatitis 2009, 20, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Bouillon, C. Recent advances in sun protection. J. Dermatol. Sci. 2000, 23 (Suppl. 1), 57–61. [Google Scholar] [CrossRef]
- Karlsson, I.; Hillerstrom, L.; Stenfeldt, A.L.; Martensson, J.; Borje, A. Photodegradation of dibenzoylmethanes: Potential cause of photocontact allergy to sunscreens. Chem. Res. Toxicol 2009, 22, 1881–1892. [Google Scholar] [CrossRef] [PubMed]
- Gholap, A.D.; Sayyad, S.F.; Hatvate, N.T.; Dhumal, V.V.; Pardeshi, S.R.; Chavda, V.P.; Vora, L.K. Drug Delivery Strategies for Avobenzone: A Case Study of Photostabilization. Pharmaceutics 2023, 15, 1008. [Google Scholar] [CrossRef] [PubMed]
- Chatelain, E.; Gabard, B. Photostabilization of butyl methoxydibenzoylmethane (Avobenzone) and ethylhexyl methoxycinnamate by bis-ethylhexyloxyphenol methoxyphenyl triazine(Tinosorb S), a new UV broadband filter. Photochem. Photobiol. 2001, 74, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Gaspar, L.R.; Maia Campos, P.M.B.G. Evaluation of the photostability of different UV filter combinations in a sunscreen. Int. J. Pharm. 2006, 307, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Karpkird, T.; Khunsakorn, R.; Noptheeranuphap, C.; Midpanon, S. Inclusion complexes and photostability of UV filters and curcumin with beta-cyclodextrin polymers: Effect on cross-linkers. J. Incl. Phenom. Macrocycl. Chem. 2018, 91, 37–45. [Google Scholar] [CrossRef]
- Onyango, D.O.; Selman, B.G.; Rose, J.L.; Ellison, C.A.; Nash, J.F. Comparison between endocrine activity assessed using ToxCast/Tox21 database and human plasma concentration of sunscreen active ingredients/UV filters. Toxicol. Sci. 2023, 196, 25–37. [Google Scholar] [CrossRef]
- Santander Ballestín, S.; Luesma Bartolomé, M.J. Toxicity of Different Chemical Components in Sun Cream Filters and Their Impact on Human Health: A Review. Appl. Sci. 2023, 13, 712. [Google Scholar] [CrossRef]
- Varrella, S.; Danovaro, R.; Corinaldesi, C. Assessing the eco-compatibility of new generation sunscreen products through a combined microscopic-molecular approach. Environ. Pollut. 2022, 314, 120212. [Google Scholar] [CrossRef] [PubMed]
- Opinion of the Scientific Committee on Cosmetic Products and Non-Food Products Intended for Consumers Concerning 2,4-Bis-{[4-(2-ethyl-hexyloxy)-2-hydroxy]-phenyl}-6-(4-methoxyphenyl)-(1,3,5)-triazine. 17 February 1999. Available online: https://ec.europa.eu/health/archive/ph_risk/committees/sccp/documents/out52_en.pdf (accessed on 20 March 2024).
- Matta, M.K.; Florian, J.; Zusterzeel, R.; Pilli, N.R.; Patel, V.; Volpe, D.A.; Yang, Y.; Oh, L.; Bashaw, E.; Zineh, I.; et al. Effect of Sunscreen Application on Plasma Concentration of Sunscreen Active Ingredients: A Randomized Clinical Trial. JAMA 2020, 323, 256–267. [Google Scholar] [CrossRef] [PubMed]
- SCCS (Scientific Committee on Consumer Safety). Opinion on Octocrylene (CAS No 6197-30-4, EC No 228-250-8); Preliminary Version of 15 January 2021, Final Version of 30–31 March 2021, SCCS/1627/21; European Commission: Brussels, Belgium, 2021. [Google Scholar]
- Souza, C.; Maia Campos, P.M.B.G. Development of a HPLC method for determination of four UV filters in sunscreen and its application to skin penetration studies. Biomed. Chromatogr. 2017, 31, e4029. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Huang, J.; Jiang, X.; Huang, Y.; Zhu, X.; Cai, Z. Environmental Fate and Toxicity of Sunscreen-Derived Inorganic Ultraviolet Filters in Aquatic Environments: A Review. Nanomaterials 2022, 12, 699. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.L.; Lim, H.W. A review of inorganic UV filters zinc oxide and titanium dioxide. Photodermatol. Photoimmunol. Photomed. 2019, 35, 442–446. [Google Scholar] [CrossRef]
- Bartoszewska, M.; Adamska, E.; Kowalska, A.; Grobelna, B. Novelty Cosmetic Filters Based on Nanomaterials Composed of Titanium Dioxide Nanoparticles. Molecules 2023, 28, 645. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).