Establishment of a Periprosthetic Acetabular Bone Defect in an In Vivo Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model
2.2. Livestock Farming
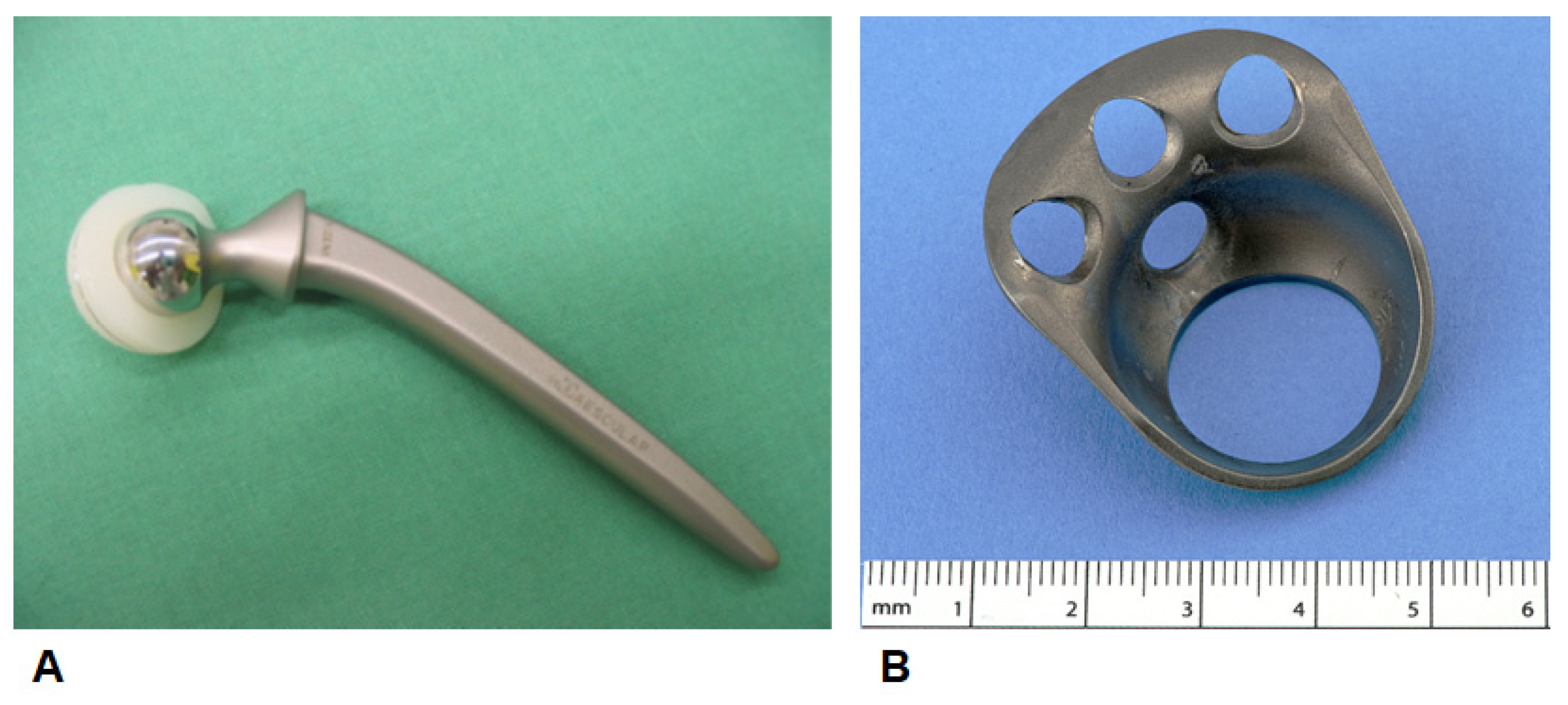
2.3. Implants/Bone Substitutes
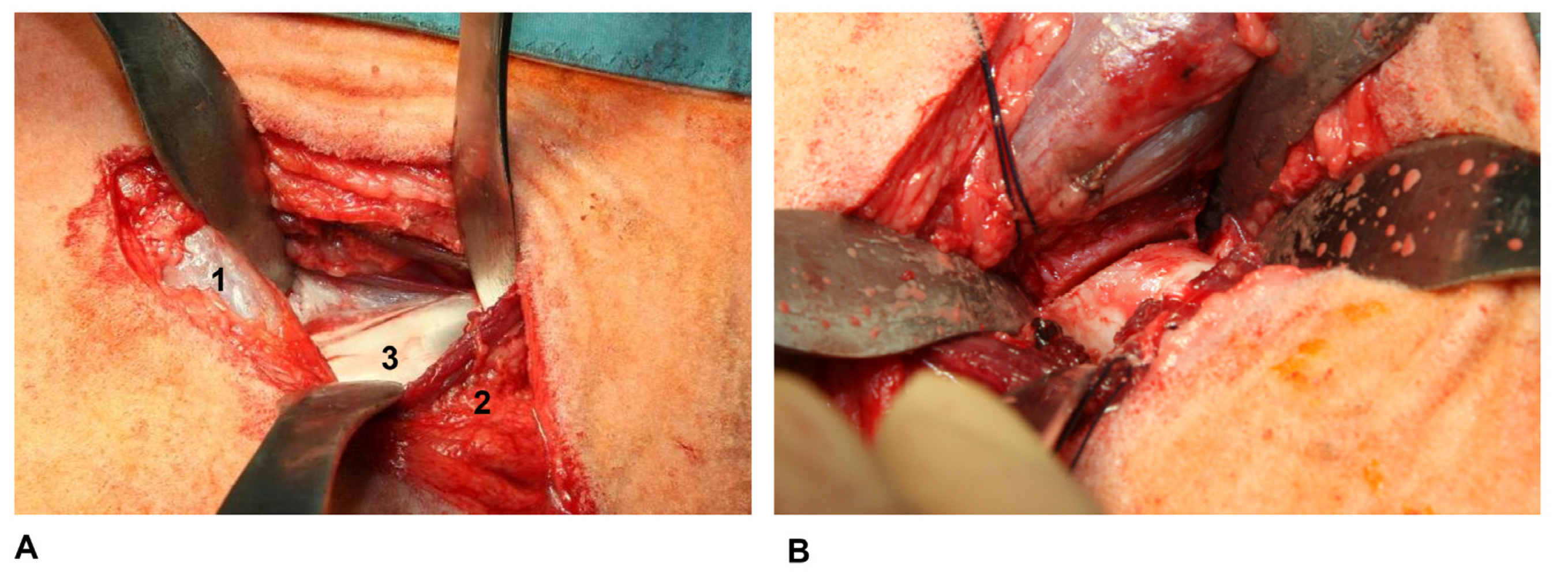
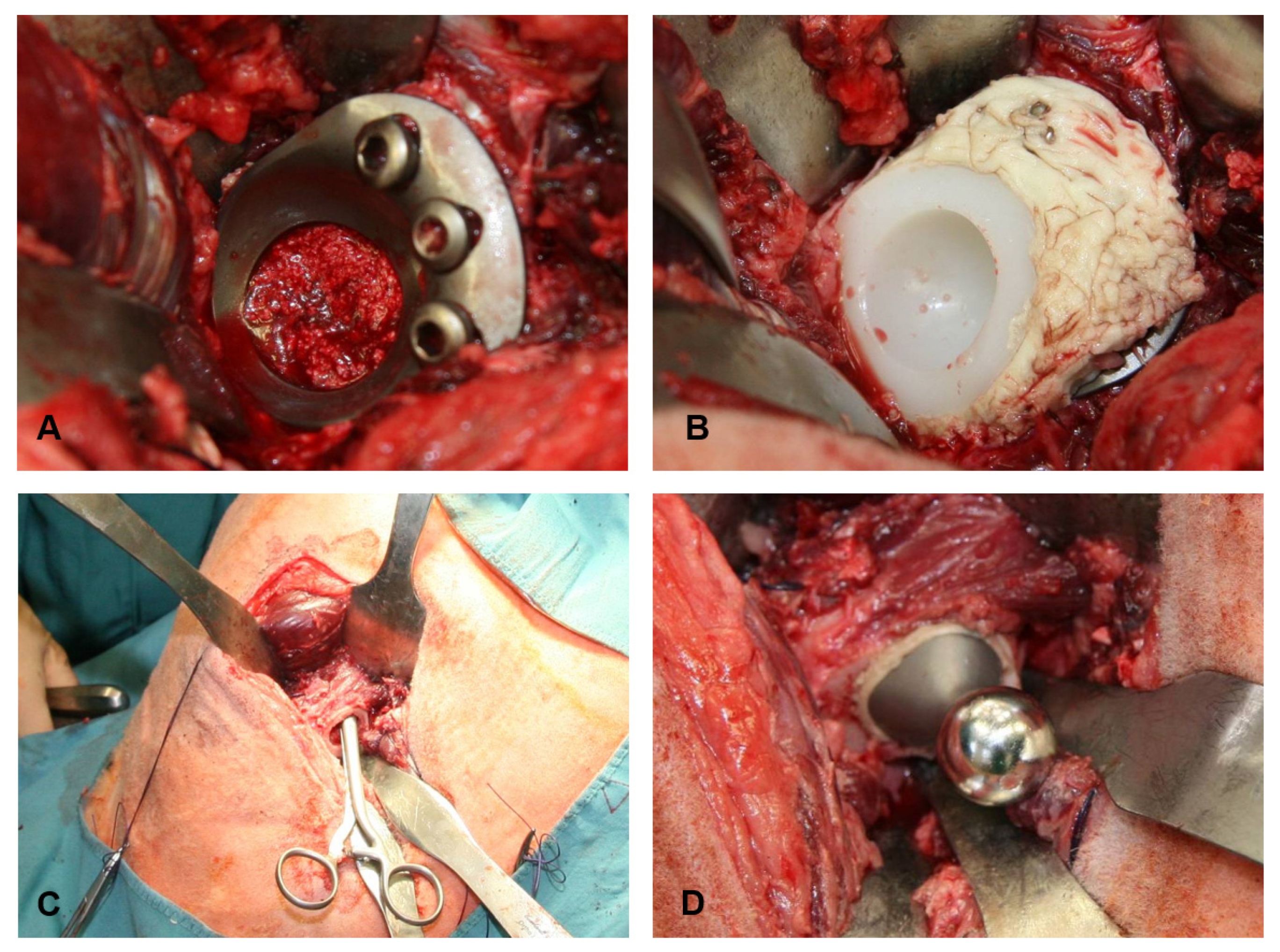
2.4. Anaesthesia and Surgical Technique
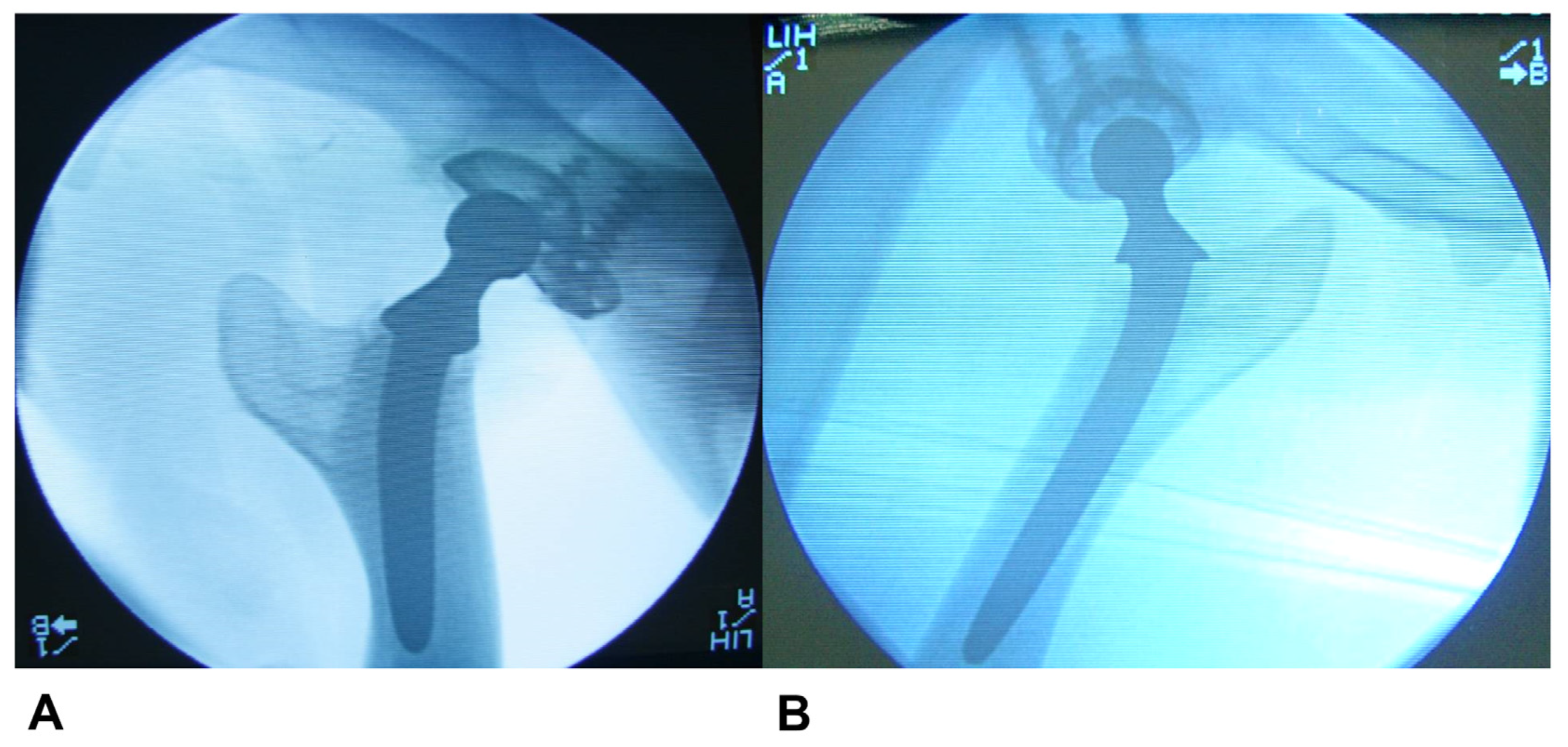
2.5. Sample Retrieval and Radiological, Macroscopic and Microscopic Evaluation
2.6. Statistical Analysis
3. Results
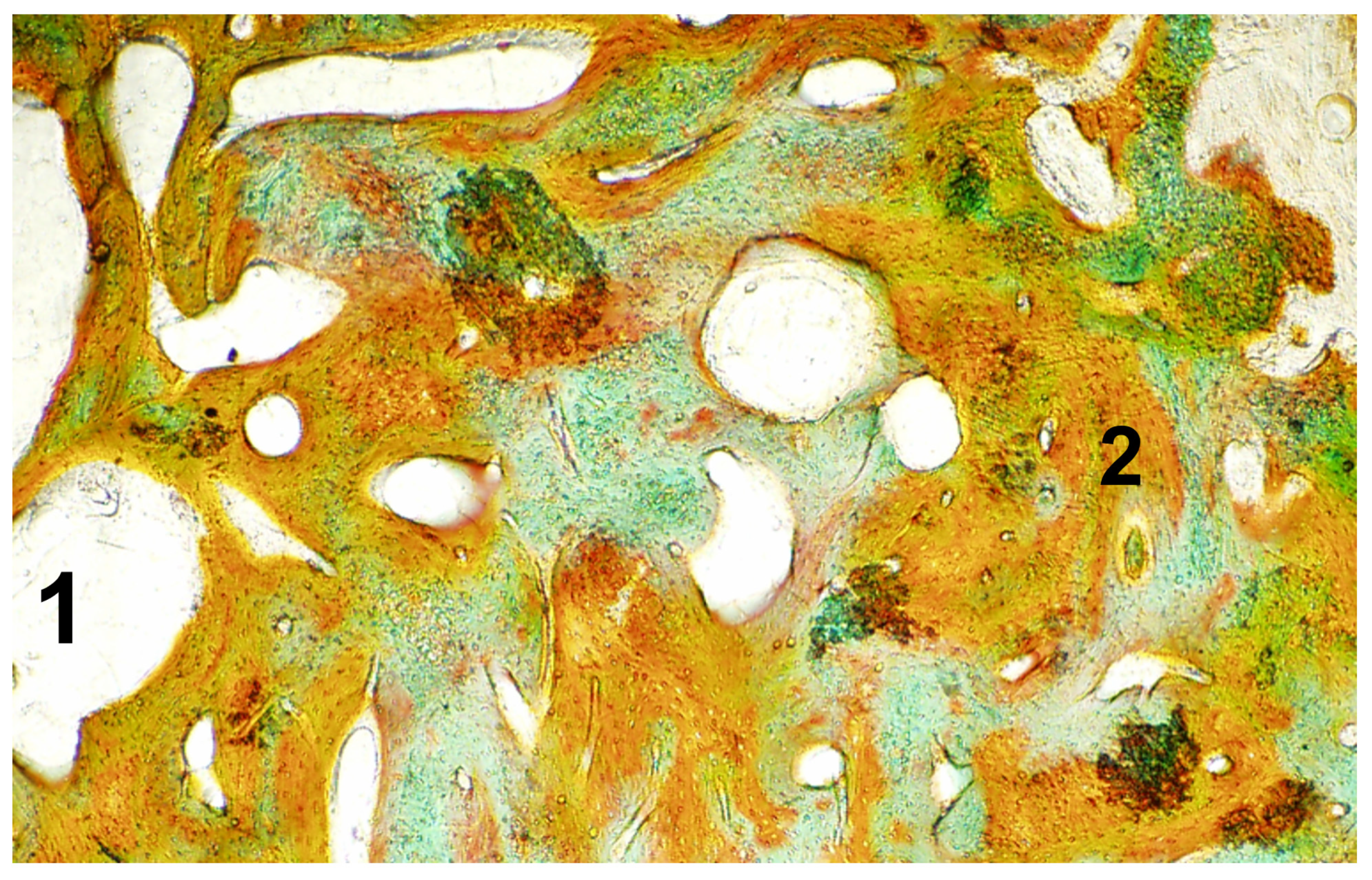
Macroscopic, Microscopic, and Radiological Evaluation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Oonishi, H.; Iwaki, Y.; Kin, N.; Kushitani, S.; Murata, N.; Wakitani, S.; Imoto, K. Hydroxyapatite in Revision of Total Hip Replacements with Massive Acetabular Defects: 4- to 10-Year Clinical Results. J. Bone Joint Surg. Br. 1997, 79, 87–92. [Google Scholar] [CrossRef]
- Tanaka, C.; Shikata, J.; Ikenaga, M.; Takahashi, M. Acetabular Reconstruction Using a Kerboull-Type Acetabular Reinforcement Device and Hydroxyapatite Granules. J. Arthroplasty 2003, 18, 719–725. [Google Scholar] [CrossRef]
- Schulz, K.S. Application of Arthroplasty Principles to Canine Cemented Total Hip Replacement. Vet. Surg. 2000, 29, 578–593. [Google Scholar] [CrossRef]
- Hummel, D. Zurich Cementless Total Hip Replacement. Vet. Clin. North Am. Small Anim. Pract. 2017, 47, 917–934. [Google Scholar] [CrossRef]
- Lelgemann, B.V. Optimierung der Verbundfestigkeit Zwischen Knochenzement und Azetabulärem Knochen bei Künstlichem Hüftgelenkersatz; Aachener Beiträge zur Medizin: Aachen, Germany, 2006; ISBN 3-86130-696-4. [Google Scholar]
- Mumme, T. Verbesserung der Verbundfestigkeit Zwischen Hydrophobem Knochenzement und Hydrophilem Knochen Durch Einen Amphiphilen Knochenhaftvermittler—Am Beispiel der Zementierten Hüftgelenksendoprothetik; Wissenschaftsverlag Mainz: Aachen, Germany, 2007; Volume 46. [Google Scholar]
- Gabriele Sommer, N.; Hahn, D.; Okutan, B.; Marek, R.; Weinberg, A.-M. Animal Models in Orthopedic Research: The Proper Animal Model to Answer Fundamental Questions on Bone Healing Depending on Pathology and Implant Material. In Animal Models in Medicine and Biology; Tvrdá, E., Chandra Yenisetti, S., Eds.; IntechOpen: Rijeka, Croatia, 2020; ISBN 978-1-83880-011-6. [Google Scholar]
- Gerber, T.; Traykova, T. Development and In Vivo Test of Sol-Gel Derived Bone Grafting Materials. J. Sol-Gel Sci. Technol. 2003, 26, 1173–1178. [Google Scholar] [CrossRef]
- Traykova, T.; Bötcher, R.; Neumann, H.G.; Henkel, K.-O.; Bienengräber, V.; Gerber, T. Silica/Calcium Phosphate Sol-Gel Derived Bone Grafting Material—From Animal Tests to First Clinical Experience. Key Eng. Mater. 2003, 254–256, 679–682. [Google Scholar] [CrossRef]
- Henkel, K.-O.; Lenz, J.-H.; Gerber, T.; Bienengräber, V. Ein qualitativ neuartiges Knochenaufbaumaterial auf Hydroxylapatit-Xerogel-Basis. ZWR Dtsch. Zahnärztebl. 2005, 114, 416–418. [Google Scholar] [CrossRef]
- Müller-Rath, R.; Wirtz, D.C.; Siebert, C.H.; Andereya, S.; Gravius, S.; Hermanns-Sachweh, B.; Marx, R.; Mumme, T. Amphiphilic Bonder Improves Adhesion at the Acrylic Bone Cement–Bone Interface of Cemented Acetabular Components in Total Hip Arthroplasty: In Vivo Tests in an Ovine Model. Arch. Orthop. Trauma Surg. 2008, 128, 701–707. [Google Scholar] [CrossRef]
- Schreurs, B.W.; Slooff, T.J.; Buma, P.; Verdonschot, N. Basic Science of Bone Impaction Grafting. Instr. Course Lect. 2001, 50, 211–220. [Google Scholar] [PubMed]
- Walter, S.G.; Randau, T.M.; Gravius, N.; Gravius, S.; Fröschen, F.S. Monoflanged Custom-Made Acetabular Components Promote Biomechanical Restoration of Severe Acetabular Bone Defects by Metallic Defect Reconstruction. J. Arthroplasty 2019, 35, 831–835. [Google Scholar] [CrossRef] [PubMed]
- Fröschen, F.S.; Randau, T.M.; Hischebeth, G.T.R.; Gravius, N.; Gravius, S.; Walter, S.G. Mid-Term Results after Revision Total Hip Arthroplasty with Custom-Made Acetabular Implants in Patients with Paprosky III Acetabular Bone Loss. Arch. Orthop. Trauma Surg. 2020, 140, 263–273. [Google Scholar] [CrossRef]
- Kavanagh, B.F.; Ilstrup, D.M.; Fitzgerald, R.H. Revision Total Hip Arthroplasty. J. Bone Joint Surg. Am. 1985, 67, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Gruen, T.A.; McNeice, G.M.; Amstutz, H.C. “Modes of Failure” of Cemented Stem-Type Femoral Components: A Radiographic Analysis of Loosening. Clin. Orthop. 1979, 141, 17–27. [Google Scholar] [CrossRef]
- Brooker, A.F.; Bowerman, J.W.; Robinson, R.A.; Riley, L.H. Ectopic Ossification Following Total Hip Replacement. Incidence and a Method of Classification. J. Bone Joint Surg. Am. 1973, 55, 1629–1632. [Google Scholar] [CrossRef]
- Engh, C.; Griffin, W.; Marx, C. Cementless Acetabular Components. J. Bone Joint Surg. Br. 1990, 72, 53–59. [Google Scholar] [CrossRef]
- DeLee, J.G.; Charnley, J. Radiological Demarcation of Cemented Sockets in Total Hip Replacement. Clin. Orthop. 1976, 121, 20–32. [Google Scholar] [CrossRef]
- Charan, J.; Kantharia, N. How to Calculate Sample Size in Animal Studies? J. Pharmacol. Pharmacother. 2013, 4, 303–306. [Google Scholar] [CrossRef]
- Pearce, A.; Richards, R.; Milz, S.; Schneider, E.; Pearce, S. Animal Models for Implant Biomaterial Research in Bone: A Review. Eur. Cell. Mater. 2007, 13, 1–10. [Google Scholar] [CrossRef]
- Martini, L.; Fini, M.; Giavaresi, G.; Giardino, R. Sheep Model in Orthopedic Research: A Literature Review. Comp. Med. 2001, 51, 292–299. [Google Scholar]
- Animal Models in Orthopaedic Research; An, Y.H., Friedman, R.J., Eds.; CRC Press: Boca Raton, FL, USA, 1999; ISBN 978-0-8493-2115-3. [Google Scholar]
- Phillips, T.W.; Johnston, G.; Wood, P. Selection of an Animal Model for Resurfacing Hip Arthroplasty. J. Arthroplasty 1987, 2, 111–117. [Google Scholar] [CrossRef]
- David, A.; Eitenmüller, J.; Muhr, G.; Pommer, A.; Bär, H.F.; Ostermann, P.A.W.; Schildhauer, T.A. Mechanical and Histological Evaluation of Hydroxyapatite-Coated, Titanium-Coated and Grit-Blasted Surfaces under Weight-Bearing Conditions. Arch. Orthop. Trauma Surg. 1995, 114, 112–118. [Google Scholar] [CrossRef]
- Phillips, T.W.; Gurr, K.R.; Rao, D.R. Hip Implant Evaluation in an Arthritic Animal Model. Arch. Orthop. Trauma Surg. 1990, 109, 194–196. [Google Scholar] [CrossRef]
- Phillips, T.W.; Gurr, K. A Preconditioned Arthritic Hip Model. J. Arthroplasty 1989, 4, 193–200. [Google Scholar] [CrossRef]
- Nunamaker, D.M. Experimental Models of Fracture Repair. Clin. Orthop. 1998, 355, S56–S65. [Google Scholar] [CrossRef]
- Turner, A.S.; Alvis, M.; Myers, W.; Stevens, M.L.; Lundy, M.W. Changes in Bone Mineral Density and Bone-Specific Alkaline Phosphatase in Ovariectomized Ewes. Bone 1995, 17, S395–S402. [Google Scholar] [CrossRef]
- Bergmann, G.; Graichen, F.; Rohlmann, A. Hip Joint Forces in Sheep. J. Biomech. 1999, 32, 769–777. [Google Scholar] [CrossRef]
- Gugala, Z.; Gogolewski, S. Regeneration of Segmental Diaphyseal Defects in Sheep Tibiae Using Resorbable Polymeric Membranes: A Preliminary Study. J. Orthop. Trauma 1999, 13, 187–195. [Google Scholar] [CrossRef]
- Heckman, J.D.; Boyan, B.D.; Aufdemorte, T.B.; Abbott, J.T. The Use of Bone Morphogenetic Protein in the Treatment of Non-Union in a Canine Model. J. Bone Joint Surg. Am. 1991, 73, 750–764. [Google Scholar] [CrossRef]
- den Boer, F.C.; Patka, P.; Bakker, F.C.; Wippermann, B.W.; van Lingen, A.; Vink, G.Q.M.; Boshuizen, K.; Haarman, H.J.T.M. New Segmental Long Bone Defect Model in Sheep: Quantitative Analysis of Healing with Dual Energy X-ray Absorptiometry. J. Orthop. Res. 1999, 17, 654–660. [Google Scholar] [CrossRef]
- Aaron, J.E.; Skerry, T.M. Intramembranous Trabecular Generation in Normal Bone. Bone Miner. 1994, 25, 211–230. [Google Scholar] [CrossRef]
- Nickel, R.; Schummer, A.; Seiferle, E.; Frewein, J.; Nickel, R. Bewegungsapparat; Lehrbuch der Anatomie der Haustiere; Parey [u.a.]: Berlin, Germany, 1992; ISBN 978-3-489-58016-4. [Google Scholar]


| Number of Animals [n] | Groups |
|---|---|
| 5 | Preliminary experiment to optimize the main experiment |
| 9 | Control group: Impaction bone grafting with autologous sheep cancellous bone |
| 9 | Group 1: NanoBone® [Artoss GmbH, Rostock, Germany] |
| 9 | Group 2: Ovine Tutoplast®-processed cancellous bone chips [Tutogen® Medical GmbH, Neunkirchen am Brand, Germany] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fröschen, F.S.; Randau, T.M.; Haddouti, E.-M.; Schildberg, F.A.; Müller-Broich, J.D.; Götz, W.; Reimann, S.; Wirtz, D.C.; Gravius, S. Establishment of a Periprosthetic Acetabular Bone Defect in an In Vivo Model. Appl. Sci. 2024, 14, 3375. https://doi.org/10.3390/app14083375
Fröschen FS, Randau TM, Haddouti E-M, Schildberg FA, Müller-Broich JD, Götz W, Reimann S, Wirtz DC, Gravius S. Establishment of a Periprosthetic Acetabular Bone Defect in an In Vivo Model. Applied Sciences. 2024; 14(8):3375. https://doi.org/10.3390/app14083375
Chicago/Turabian StyleFröschen, Frank Sebastian, Thomas Martin Randau, El-Mustapha Haddouti, Frank Alexander Schildberg, Jacques Dominik Müller-Broich, Werner Götz, Susanne Reimann, Dieter Christian Wirtz, and Sascha Gravius. 2024. "Establishment of a Periprosthetic Acetabular Bone Defect in an In Vivo Model" Applied Sciences 14, no. 8: 3375. https://doi.org/10.3390/app14083375
APA StyleFröschen, F. S., Randau, T. M., Haddouti, E.-M., Schildberg, F. A., Müller-Broich, J. D., Götz, W., Reimann, S., Wirtz, D. C., & Gravius, S. (2024). Establishment of a Periprosthetic Acetabular Bone Defect in an In Vivo Model. Applied Sciences, 14(8), 3375. https://doi.org/10.3390/app14083375






