Changes in Polyphenolic Profile and Antioxidant Properties of Selected Raw and Processed Vegetables Under Different Cooking Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Vegetable Samples
2.2. Vegetable Extracts Preparation
2.3. Determination of Phenolic Compounds
2.3.1. Total Phenolic Content (TPC) Analysis
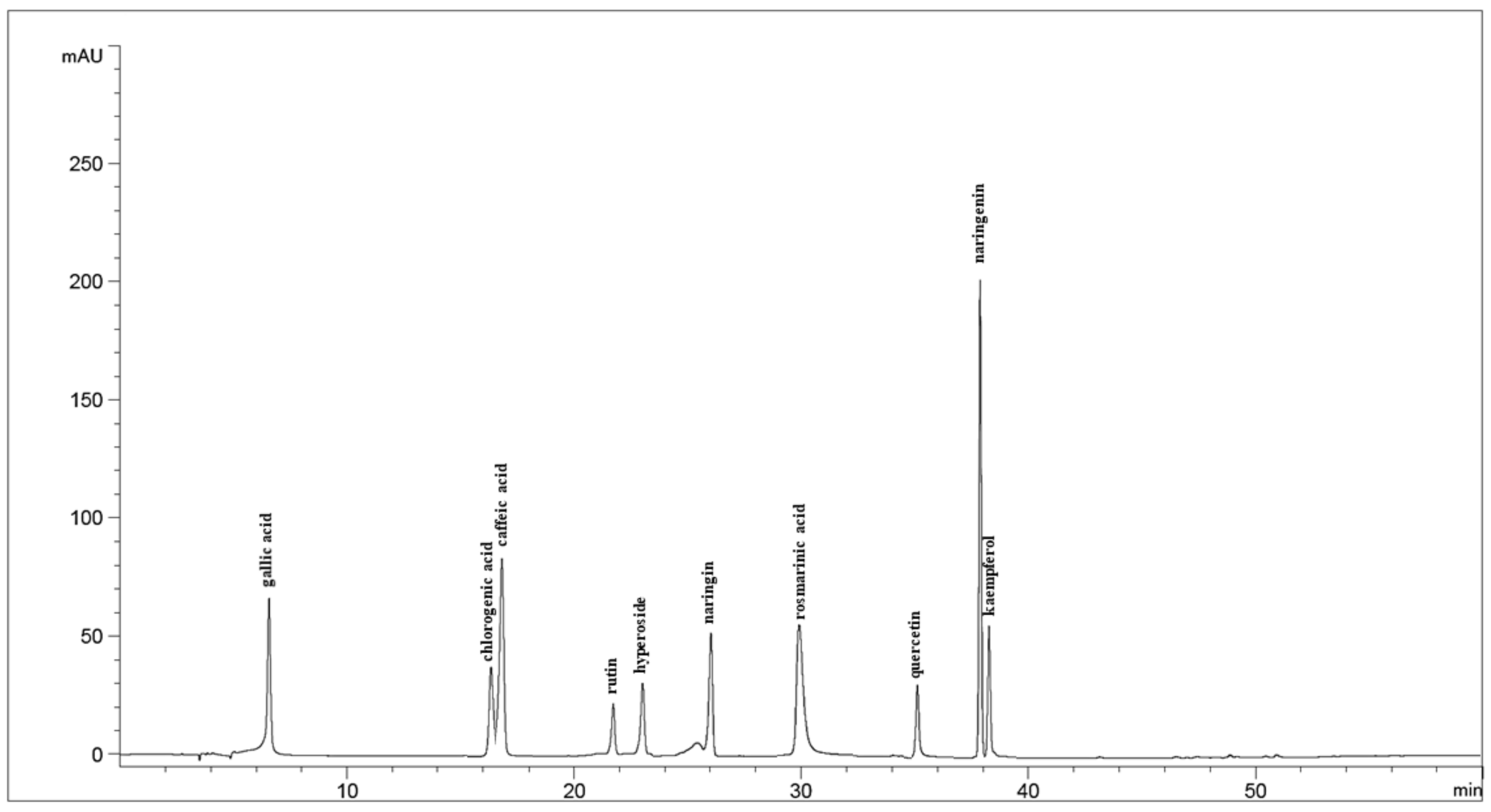
2.3.2. Phenolic Profile Analysis by HPLC–UV–VIS
2.4. Determination of Antioxidant Capacity
2.5. Chemicals and Reagents
2.6. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic and Profile of Phenolic Compounds
3.1.1. Total Phenolic Content
3.1.2. Phenolic Acids Profile
3.1.3. Flavonoid Glycosides and Aglycones Profile
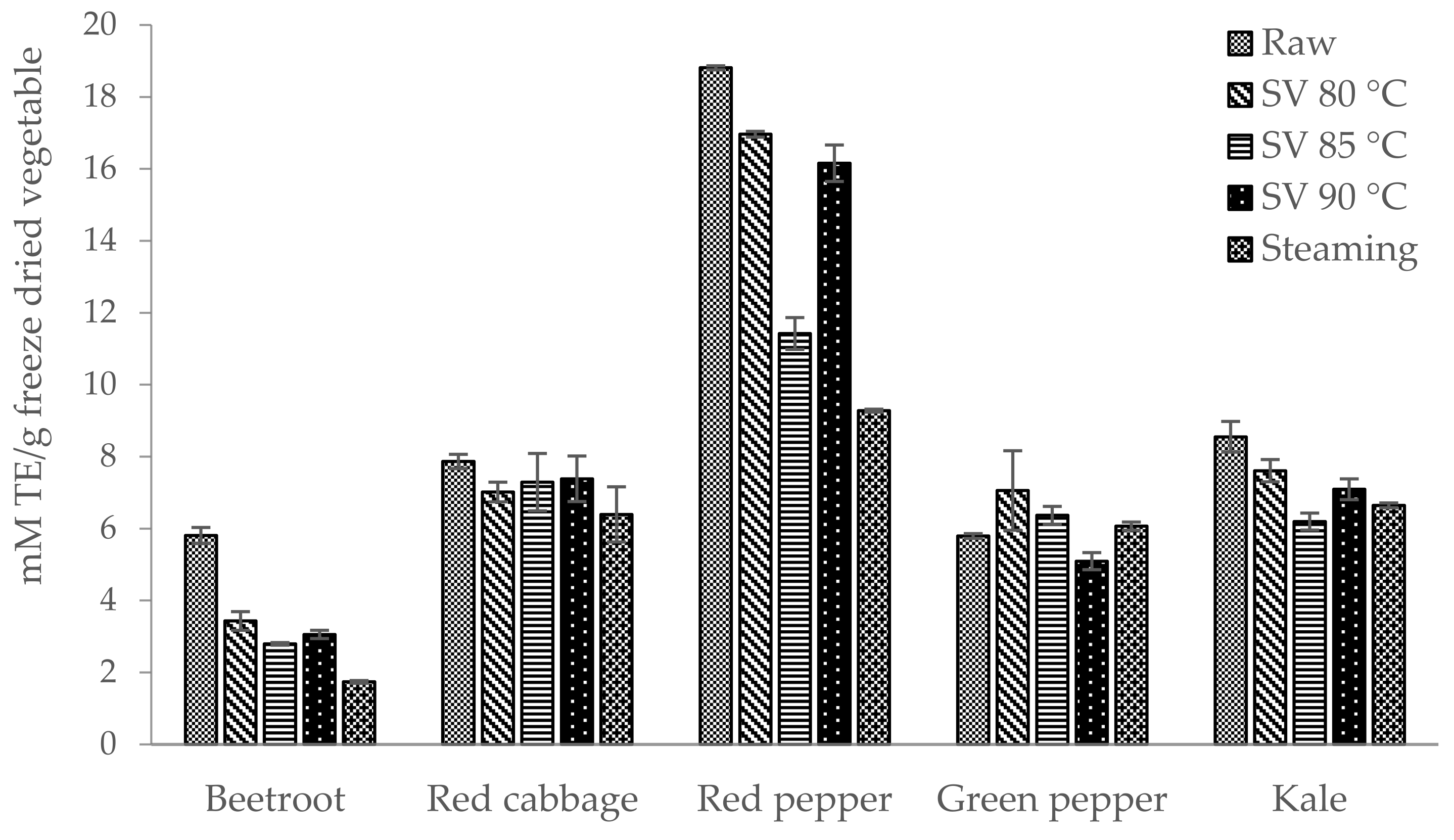
3.2. Biological Activity
3.3. Correlation Between the Total Polyphenol Content (TPC) and Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DNA | Deoxyribonucleic acid |
| DPPH | 2,2-Diphenyl-1-picrylhydrazyl |
| GAE | Gallic acid equivalent |
| HPLC–UV–VIS | High performance liquid chromatography ultraviolet detector |
| ICH | International Conference of Harmonisation |
| LOD | Limit of detection |
| LOQ | Limit of quantification |
| n.d. | No detected |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| SD | Standard deviation |
| SV | Sous-vide |
| TE | Trolox equivalent |
| TEAC | Trolox equivalent antioxidant capacity |
| TPC | Total phenolic content |
| WHO | World Health Organisation |
References
- ABC Żywienia. Available online: https://ncez.pzh.gov.pl/abc-zywienia/ (accessed on 17 January 2025).
- Mohammad Salamatullah, A.; Hayat, K.; Mabood Husain, F.; Asif Ahmed, M.; Arzoo, S.; Musaad Althbiti, M.; Alzahrani, A.; Al-Zaied, B.A.M.; Kahlil Alyahya, H.; Albader, N.; et al. Effects of Different Solvents Extractions on Total Polyphenol Content, HPLC Analysis, Antioxidant Capacity, and Antimicrobial Properties of Peppers (Red, Yellow, and Green (Capsicum annum L.). Evid. Based Complement. Alternat. Med. 2022, 19, 7372101. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Qi, Y.; Zheng, H. Dietary Polyphenol, Gut Microbiota, and Health Benefits. Antioxidants 2022, 11, 1212. [Google Scholar] [CrossRef] [PubMed]
- Parcheta, M.; Świsłocka, R.; Orzechowska, S.; Akimowicz, M.; Choińska, R.; Lewandowski, W. Recent Developments in Effective Antioxidants: The Structure and Antioxidant Properties. Materials 2021, 14, 1984. [Google Scholar] [CrossRef] [PubMed]
- Mostafavi Abdolmaleky, H.; Zhou, J.-R. Gut Microbiota Dysbiosis, Oxidative Stress, Inflammation, and Epigenetic Alterations in Metabolic Diseases. Antioxidants 2024, 13, 985. [Google Scholar] [CrossRef]
- Mithul Aravind, S.; Wichienchot, S.; Tsao, R.; Ramakrishnan, S.; Chakkaravarthi, S. Role of Dietary Polyphenols on Gut Microbiota, Their Metabolites and Health Benefits. Food Res. Int. 2021, 142, 110189. [Google Scholar] [CrossRef]
- Sejbuk, M.; Mirończuk-Chodakowska, I.; Karav, S.; Witkowska, A.M. Dietary Polyphenols, Food Processing and Gut Microbiome: Recent Findings on Bioavailability, Bioactivity, and Gut Microbiome Interplay. Antioxidants 2024, 13, 1220. [Google Scholar] [CrossRef]
- Baldwin, D.E. Sous vide cooking: A Review. Int. J. Gastron. Food Sci. 2012, 1, 15–30. [Google Scholar]
- Kathuria, D.; Dhiman, A.K.; Attri, S. Sous vide, a culinary technique for improving quality of food products: A Review. Trends Food Sci. Technol. 2022, 119, 57–68. [Google Scholar] [CrossRef]
- Michalak-Majewska, M.; Stanikowski, P.; Gustaw, W.; Sławińska, A.; Radzki, W.; Skrzypczak, K.; Jabłońska-Ryś, E. Technologia sous-vide—Innowacyjny sposób Obróbki Cieplnej żywności. Żywn. Nauka Technol. Jakość/Food Sci. Technol. Qual. 2018, 116, 34–44. [Google Scholar] [CrossRef]
- Creed, P.G. The sensory and nutritional quality of ‘sous vide’ foods. Food Control 1995, 6, 45–52. [Google Scholar] [CrossRef]
- Yıkmış, S.; Aksu, H.; Çöl, B.G.; Demirçakmak, İ.L. Evaluation of sous-vide technology in gastronomy. Int. J. Agric. Life Sci. 2018, 4, 226–231. [Google Scholar]
- Amoroso, L.; Rizzo, V.; Muratore, G. Nutritional values of potato slices added with rosemary essential oil cooked in sous vide bags. Int. J. Gastron. Food Sci. 2019, 15, 1–5. [Google Scholar] [CrossRef]
- Yılmaz, S.N.; İlyasoğlu, H. Comparision of the effect of Sous vide with traditional cooking methods on color properties and anti-oxidant capacity of zucchini and Green Bean. Food Health 2023, 9, 37–42. [Google Scholar] [CrossRef]
- Zavadlav, S.; Blažić, M.; Van de Velde, F.; Vignatti, C.; Fenoglio, C.; Piagentini, A.M.; Pirovani, M.E.; Perotti, C.M.; Bursać Kovačević, D.; Putnik, P. Sous-vide as a technique for preparing healthy and high-quality vegetable and seafood products. Foods 2020, 9, 1537. [Google Scholar] [CrossRef]
- Iborra-Bernad, C.; García-Segovia, P.; Martínez-Monzó, J. Physico-chemical and structural characteristics of vegetables cooked under sous-vide, Cook-vide, and conventional boiling. J. Food Sci. 2015, 80, E1725–E1734. [Google Scholar] [CrossRef]
- Muñoz, S.; Achaerandio, I.; Yang, Y.; Pujolà, M. Sous vide processing as an alternative to common cooking treatments: Impact on the starch profile, color, and shear force of potato (Solanum tuberosum L.). Food Bioprocess Technol. 2017, 10, 759–769. [Google Scholar] [CrossRef]
- Clausen, M.P.; Christensen, M.; Djurhuus, T.H.; Duelund, L.; Mouritsen, O.G. The quest for umami: Can Sous vide contribute? Int. J. Gastron. Food Sci. 2018, 13, 129–133. [Google Scholar] [CrossRef]
- Bayomy, H.M. Effects of culinary treatments on the physicochemical properties of Ulva lactuca collected from Tabuk coast of Red Sea in Saudi Arabia. Saudi J. Biol. Sci. 2022, 29, 2355–2362. [Google Scholar] [CrossRef]
- dos Reis, L.C.; de Oliveira, V.R.; Hagen, M.E.; Jablonski, A.; Flôres, S.H.; de Oliveira Rios, A. Effect of cooking on the concentration of bioactive compounds in broccoli (Brassica oleracea var. avenger) and cauliflower (Brassica oleracea var. Alphina F1) grown in an organic system. Food Chem. 2015, 172, 770–777. [Google Scholar] [CrossRef]
- Martínez, S.; Armesto, J.; Gómez-Limia, L.; Carballo, J. Impact of processing and storage on the nutritional and sensory properties and bioactive components of Brassica spp. A Review. Food Chem. 2020, 313, 126065. [Google Scholar] [CrossRef]
- Narwojsz, A.; Borowska, E.J.; Polak-Śliwińska, M.; Danowska-Oziewicz, M. Effect of Different Methods of Thermal Treatment on Starch and Bioactive Compounds of Potato. Plant Foods Hum. Nutr. 2020, 75, 98–304. [Google Scholar] [CrossRef] [PubMed]
- Roascio-Albistur, A.; Gámbaro, A. Consumer perception of a non-traditional market on sous-vide dishes. Int. J. Gastron. Food Sci. 2018, 11, 20–24. [Google Scholar] [CrossRef]
- Lafarga, T.; Viñas, I.; Bobo, G.; Simó, J.; Aguiló-Aguayo, I. Effect of steaming and Sous vide processing on the total phenolic content, vitamin C and antioxidant potential of the genus Brassica. Innov. Food Sci. Emerg. Technol. 2018, 47, 412–420. [Google Scholar] [CrossRef]
- Ávila, S.; Zalamanski, S.; Tanikawa, L.M.; Kruger, C.C.H.; Ferreira, S.M.R. Influence of Cooking Methods on In Vitro Bioaccessibility of Phenolics, Flavonoids, and Antioxidant Activity of Red Cabbage. Plant Foods Hum. Nutr. 2023, 78, 124–131. [Google Scholar] [CrossRef]
- Studzińska-Sroka, E.; Majchrzak-Celińska, A.; Bańdurska, M.; Rosiak, N.; Szwajgier, D.; Baranowska-Wójcik, E.; Szymański, M.; Gruszka, W.; Cielecka-Piontek, J. Is Caperatic Acid the Only Compound Responsible for Activity of Lichen Platismatia glauca within the Nervous System? Antioxidants 2022, 11, 2069. [Google Scholar] [CrossRef]
- Yang, X.-J.; Dang, B.; Fan, M.-T. Free and Bound Phenolic Compound Content and Antioxidant Activity of Different Cultivated Blue Highland Barley Varieties from the Qinghai-Tibet Plateau. Molecules 2018, 23, 879. [Google Scholar] [CrossRef]
- ICH. Validation of analytical procedures Q2 (R2). In Proceedings of the International Conference of Harmonisation, Geneva, Switzerland, 14 November 2018. [Google Scholar]
- Norma, P.M.; Virginia, N.-M.; Ral, R.-H.; Jos, C.E.; Cristbal, N.A. A Microassay for Quantification of 2,2-Diphenyl-1-Picrylhydracyl (DPPH) Free Radical Scavenging. Afr. J. Biochem. Res. 2014, 8, 14–18. [Google Scholar] [CrossRef]
- Florkiewicz, A.; Socha, R.; Filipiak-Florkiewicz, A.; Topolska, K. Sous-vide technique as an alternative to traditional cooking methods in the context of antioxidant properties of Brassica vegetables. J. Sci. Food Agric. 2019, 99, 173–182. [Google Scholar] [CrossRef]
- Misu, G.A.; Canja, C.M.; Lupu, M.; Matei, F. Advances and Drawbacks of Sous-Vide Technique—A Critical Review. Foods 2024, 13, 2217. [Google Scholar] [CrossRef]
- Farrés-Cebrián, M.; Seró, R.; Saurina, J.; Núñez, O. HPLC-UV Polyphenolic Profiles in the Classification of Olive Oils and Other Vegetable Oils via Principal Component Analysis. Separations 2016, 3, 33. [Google Scholar] [CrossRef]
- Rinaldi, M.; Santi, S.; Paciulli, M.; Ganino, T.; Pellegrini, N.; Visconti, A.; Vitaglione, P.; Barbanti, D.; Chiavaro, E. Comparison of physical, microstructural and antioxidative properties of pumpkin cubes cooked by conventional, vacuum cooking and sous vide methods. J. Sci. Food Agric. 2020, 101, 2534–2541. [Google Scholar] [CrossRef] [PubMed]
- Guillén, S.; Mir-Bel, J.; Oria, R.; Salvador, M.L. Influence of cooking conditions on organoleptic and health-related properties of artichokes, green beans, Broccoli and carrots. Food Chem. 2017, 217, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Koç, M.; Baysan, U.; Devseren, E.; Okut, D.; Atak, Z.; Karataş, H.; Kaymak-Ertekin, F. Effects of different cooking methods on the chemical and physical properties of carrots and Green Peas. Innov. Food Sci. Emerg. Technol. 2017, 42, 109–119. [Google Scholar] [CrossRef]
- Vega-Galvez, A.; Gomez-Perez, L.S.; Zepeda, F.; Vidal, R.L.; Grunenwald, F.; Mejías, N.; Pasten, A.; Araya, M.; Ah-Hen, K.S. Assessment of Bio-Compounds Content, Antioxidant Activity, and Neuroprotective Effect of Red Cabbage (Brassica oleracea var. Capitata rubra) Processed by Convective Drying at Different Temperatures. Antioxidants 2023, 12, 1789. [Google Scholar] [CrossRef]
- Satheesh, N.; Workneh Fanta, S. Kale: Review on nutritional composition, bio-active compounds, anti-nutritional factors, health beneficial properties and value-added products. Cogent Food Agric. 2020, 6, 1811048. [Google Scholar] [CrossRef]
- Ortega-Hernández, E.; Antunes-Ricardo, M.; Jacobo-Velázquez, D.A. Improving the Health-Benefits of Kales (Brassica oleracea L. var. acephala DC) through the Application of Controlled Abiotic Stresses: A Review. Plants 2021, 10, 2629. [Google Scholar] [CrossRef]
- Vučetić, A.; Šovljanski, O.; Pezo, L.; Gligorijević, N.; Kostić, S.; Vulić, J.; Čanadanović-Brunet, J. A Comprehensive Antioxidant and Nutritional Profiling of Brassicaceae Microgreens. Antioxidants 2025, 14, 191. [Google Scholar] [CrossRef]

| Vegetable | Time of Technological Processes [min] | |||
|---|---|---|---|---|
| Sous-Vide | Steaming | |||
| 80.0 °C | 85.0 °C | 90.0 °C | 100 °C | |
| Beetroot | 96.0 | 69.0 | 45.0 | 20.0 |
| Red cabbage | 61.0 | 42.0 | 35.0 | 10.0 |
| Red pepper | 53.0 | 32.0 | 28.0 | 14.0 |
| Green pepper | 43.0 | 34.0 | 32.0 | 8.00 |
| Kale | 14.0 | 17.0 | 6.00 | 13.0 |
| Technological Processes | Beetroot | Red Cabbage | Red Pepper | Green Pepper | Kale |
|---|---|---|---|---|---|
| Raw | 526 ± 2.88 a | 109 ± 0.33 e | 597 ± 8.52 a | 91.9 ± 1.84 e | 555 ± 5.23 c |
| SV 80 °C | 361 ± 3.66 d | 217 ± 1.37 c | 422 ± 12.3 c | 596 ± 15.9 c | 402 ± 5.17 e |
| SV 85 °C | 476 ± 13.4 b | 600 ± 2.11 a | 439 ± 9.40 b | 737 ± 1.38 a | 613 ± 6.33 a |
| SV 90 °C | 444 ± 7.98 c | 576 ± 6.33 b | 434 ± 2.32 b,c | 142 ± 0.28 d | 600 ± 10.8 b |
| Steaming | 293 ± 1.60 e | 187 ± 1.91 d | 303 ± 5.49 d | 718 ± 11.5 b | 461 ± 1.28 d |
| Compound | Retention Time (min) | Regression Equation | Correlation Coefficient (r) | Linearity Range (μg/mL) | LOD (μg/mL) | LOQ (μg/mL) |
|---|---|---|---|---|---|---|
| Gallic acid | 6.95 | y = 58.4571x − 297.0195 | 1.0000 | 10–80 | 1.00 | 3.04 |
| Chlorogenic acid | 16.73 | y = 27.5882x | 0.9999 | 10–160 | 2.68 | 8.13 |
| Caffeic acid | 17.66 | y = 72.5182x | 0.9996 | 10–80 | 2.93 | 8.87 |
| Rutin | 21.72 | y = 11.6193x | 1.0000 | 2.5–40 | 0.44 | 1.34 |
| Hyperoside | 23.05 | y = 17.9836x | 0.9999 | 2.5–40 | 0.80 | 2.41 |
| Naringin | 26.07 | y = 29.5348x + 11.9937 | 1.0000 | 2.5–40 | 0.32 | 0.97 |
| Rosmarinic acid | 30.01 | y = 31.2072x | 1.0000 | 10–160 | 2.05 | 6.23 |
| Quercetin | 35.12 | y = 16.0402x + 4.8604 | 1.0000 | 2.5–40 | 0.46 | 1.40 |
| Naringenin | 37.38 | y = 86.6723x | 0.9999 | 2.5–40 | 0.65 | 1.96 |
| Kaempferol | 38.51 | y = 22.7602x + 14.4550 | 1.0000 | 2.5–40 | 0.32 | 0.98 |
| Vegetables | Technological Processes | Gallic Acid | Chlorogenic Acid | Caffeic Acid | Rosmarinic Acid | Sum of Phenolic Acids |
|---|---|---|---|---|---|---|
| Beetroot | Raw | 27.0 ± 0.23 d | 9.51 ± 0.67 c | 1.58 ± 0.44 d | n.d. | 38.1 ± 0.45 d |
| SV 80 °C | 28.3 ± 0.42 c | 26.5 ± 1.22 b | 2.96 ± 0.11 c | n.d. | 57.8 ± 0.58 b | |
| SV 85 °C | 32.3 ± 0.50 b | 44.1 ± 1.73 a | 6.29 ± 0.05 a | n.d. | 82.7 ± 0.76 a | |
| SV 90 °C | 34.6 ± 0.08 a | 6.23 ± 0.24 d | 4.33 ± 0.38 b | n.d. | 45.2 ± 0.23 c | |
| Steaming | 28.1 ± 0.27 c | 5.40 ± 0.41 d | 1.97 ± 0.14 d | n.d. | 57.8 ± 0.27 b | |
| Red cabbage | Raw | 27.2 ± 1.53 b | 20.9 ± 0.97 a | 2.43 ± 0.21 b | 6.89 ± 0.25 b | 57.4 ± 0.74 c |
| SV 80 °C | 30.0 ± 0.31 a | 13.2 ± 1.33 b | 9.95 ± 0.39 a | 9.26 ± 0.58 a | 62.4 ± 0.65 a | |
| SV 85 °C | 27.0 ± 0.16 b | 5.68 ± 0.63 d | 2.14 ± 0.19 b | 4.85 ± 0.22 c | 39.7 ± 0.30 d | |
| SV 90 °C | 30.5 ± 0.13 a | 9.27 ± 1.24 c | 9.67 ± 0.51 a | 10.7 ± 0.83 a | 60.1 ± 0.68 b | |
| Steaming | 26.4 ± 0.26 b | 8.06 ± 0.72 c,d | n.d. | n.d. | 34.5 ± 0.25 e | |
| Red pepper | Raw | 26.9 ± 0.20 a | 19.6 ± 1.53 c | n.d. | 3.28 ± 0.01 c | 49.8 ± 0.58 b |
| SV 80 °C | n.d | 17.6 ± 1.23 c | n.d. | 8.26 ± 0.21 a | 25.9 ± 0.48 e | |
| SV 85 °C | n.d. | 23.2 ± 0.25 b | 4.45 ± 0.37 | 8.43 ± 0.18 a | 36.1 ± 0.20 d | |
| SV 90 °C | 26.5 ± 0.10 a | 13.4 ± 0.36 d | n.d. | 5.11 ± 0.13 b | 45.0 ± 0.20 c | |
| Steaming | 26.8 ± 0.13 a | 54.6 ± 2.36 a | n.d. | 8.61 ± 0.43 a | 90.0 ± 0.97 a | |
| Green pepper | Raw | n.d. | 43.5 ± 3.06 c | 6.23 ± 0.46 b | 43.9 ± 1.06 b | 93.6 ± 1.53 c |
| SV 80 °C | n.d. | 117 ± 3.87 a | 5.61 ± 0.08 b | 91.3 ± 0.88 a | 213 ± 1.61 a | |
| SV 85 °C | 35.4 ± 0.62 | 89.7 ± 6.90 b | 10.3 ± 0.49 a | 8.06 ± 0.60 d | 143 ± 2.15 b | |
| SV 90 °C | n.d | 36.4 ± 2.82 c | 2.51 ± 0.24 d | 5.68 ± 0.34 e | 44.6 ± 1.13 d | |
| Steaming | n.d. | n.d | 3.64 ± 0.00 c | 10.4 ± 0.31 c | 14.0 ± 0.16 e | |
| Kale | Raw | 30.0 ± 0.12 b | 82.9 ± 1.77 a | 10.2 ± 0.85 b | 3.90 ± 0.27 c | 127 ± 0.75 b |
| SV 80 °C | 28.1 ± 0.22 b | 39.8 ± 3.08 c | 5.98 ± 0.43 d | 30.7 ± 2.79 b | 105 ± 1.63 c | |
| SV 85 °C | 78.7 ± 2.26 a | 69.1 ± 2.94 b | 7.78 ± 0.64 c | 40.4 ± 3.48 a | 196 ± 2.33 a | |
| SV 90 °C | n.d. | 68.2 ± 3.84 b | 14.5 ± 0.44 a | 5.51 ± 0.02 c | 88.2 ± 1.43 d | |
| Steaming | n.d. | 61.0 ± 3.35 b | 6.34 ± 0.57 c,d | 39.6 ± 2.99 a | 107 ± 2.30 c |
| Vegetables | Technological Processes | Rutin | Hyperoside | Naringin | Sum of Flavonoid Glycosides | Quercetin | Naringenin | Kaempferol | Sum of Flavonoid Aglycones |
|---|---|---|---|---|---|---|---|---|---|
| Beetroot | Raw | 8.97 ± 0.62 e | n.d. | 16.8 ± 1.13 d | 25.8 ± 0.88 e | n.d. | n.d. | n.d. | n.d. |
| SV 80 °C | 51.0 ± 0.32 c | n.d. | 27.4 ± 0.17 c | 78.4 ± 0.25 c | 7.13 ± 0.56 b | n.d. | n.d. | 7.13 ± 0.56 b | |
| SV 85 °C | 40.0 ± 1.03 d | n.d. | 64.5 ± 0.20 a | 104 ± 0.62 b | 15.4 ± 0.51 a | n.d. | n.d. | 15.4 ± 0.51 a | |
| SV 90 °C | 91.4 ± 3.48 a | n.d. | 38.4 ± 0.91 b | 130 ± 2.20 a | 5.84 ± 0.18 c | n.d. | n.d. | 5.84 ± 0.18 c | |
| Steaming | 61.7 ± 0.12 b | n.d. | 14.1 ± 0.02 e | 75.8 ± 0.07 d | n.d. | n.d. | n.d. | n.d. | |
| Red cabbage | Raw | 16.8 ± 0.57 c | n.d | n.d. | 16.8 ± 0.57 d | 50.7 ± 0.06 a | n.d. | n.d. | 50.7 ± 0.06 a |
| SV 80 °C | 62.5 ± 2.77 a | 16.2 ± 0.89 b | n.d. | 78.7 ± 1.83 a | 4.57 ± 0.53 b | n.d. | n.d. | 4.57 ± 0.53 b | |
| SV 85 °C | n.d. | n.d | n.d | n.d. | n.d. | n.d. | n.d. | n.d. | |
| SV 90 °C | 21.0 ± 1.17 b | 8.17 ± 0.52 c | 2.31 ± 0.53 a | 31.5 ± 0.74 c | n.d. | n.d. | n.d. | n.d. | |
| Steaming | 24.2 ± 1.78 b | 18.5 ± 0.50 a | 1.09 ± 0.15 a | 43.8 ± 0.81 b | 4.49 ± 0.54 b | n.d. | n.d. | 4.49 ± 0.54 b | |
| Red pepper | Raw | 10.3 ± 0.37 b | 6.28 ± 0.58 c | n.d. | 16.6 ± 0.47 d | n.d. | n.d. | n.d | n.d. |
| SV 80 °C | 8.49 ± 0.79 c | n.d. | 6.25 ± 1.15 c | 14.7 ± 0.97 e | n.d. | 0.84 ± 0.01 b | n.d | 0.84 ± 0.01 c | |
| SV 85 °C | 12.8 ± 0.89 a | 27.7 ± 0.38 a | 10.6 ± 0.41 a | 51.1 ± 0.56 a | n.d. | n.d. | 4.41 ± 0.75 a | 4.41 ± 0.75 b | |
| SV 90 °C | 9.88 ± 0.56 b,c | 5.46 ± 0.70 c | 9.13 ± 0.36 a,b | 24.5 ± 0.54 c | n.d. | n.d. | n.d. | n.d. | |
| Steaming | 11.1 ± 1.05 b | 17.2 ± 1.32 b | 8.12 ± 0.70 b | 36.4 ± 1.02 b | n.d. | 10.5 ± 0.20 a | 0.89 ± 0.17 b | 11.4 ± 0.10 a | |
| Green pepper | Raw | 42.7 ± 1.88 d | n.d. | 6.95 ± 0.71a | 49.7 ± 1.30 d | 10.3 ± 0.23 d | n.d. | 3.60 ± 0.66 d | 13.9 ± 0.45 d |
| SV 80 °C | 159 ± 11.5 a | n.d. | n.d. | 159 ± 11.5 a | 11.9 ± 1.19 c,d | n.d. | n.d. | 11.9 ± 1.19 e | |
| SV 85 °C | 94.6 ± 7.42 b | n.d. | n.d. | 94.6 ± 7.42 b | 16.6. ± 0.13 a | n.d. | 19.4 ± 0.18 a | 36.0 ± 0.16 a | |
| SV 90 °C | 50.7 ± 4.06 d | n.d. | n.d. | 50.7 ± 4.06 d | 12.7 ± 1.34 b,c | n.d. | 9.85 ± 1.01 c | 22.6 ± 1.18 c | |
| Steaming | 70.7 ± 2.02 c | n.d. | 8.83 ± 0.74 a | 79.5 ± 1.38 c | 14.4 ± 1.32 a,b | n.d. | 11.6 ± 0.35 b | 26.0 ± 0.84 b | |
| Kale | Raw | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 4.17 ± 0.38 a | 4.17 ± 0.38 b |
| SV 80 °C | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 2.67 ± 0.10 c | 2.67 ± 0.10 c | |
| SV 85 °C | n.d. | n.d. | n.d. | n.d. | 20.2 ± 1.74 | n.d. | 3.96 ± 0.29 a,b | 24.2 ± 1.02 a | |
| SV 90 °C | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 2.07 ± 0.14 d | 2.07 ± 0.14 c | |
| Steaming | n.d. | n.d. | n.d. | n.d. | n.d. | n.d. | 3.59 ± 0.07 b | 3.59 ± 0.07 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kosewski, G.; Chanaj-Kaczmarek, J.; Dziedzic, K.; Jakubowski, K.; Lisiak, N.; Przysławski, J.; Drzymała-Czyż, S. Changes in Polyphenolic Profile and Antioxidant Properties of Selected Raw and Processed Vegetables Under Different Cooking Methods. Appl. Sci. 2025, 15, 4677. https://doi.org/10.3390/app15094677
Kosewski G, Chanaj-Kaczmarek J, Dziedzic K, Jakubowski K, Lisiak N, Przysławski J, Drzymała-Czyż S. Changes in Polyphenolic Profile and Antioxidant Properties of Selected Raw and Processed Vegetables Under Different Cooking Methods. Applied Sciences. 2025; 15(9):4677. https://doi.org/10.3390/app15094677
Chicago/Turabian StyleKosewski, Grzegorz, Justyna Chanaj-Kaczmarek, Krzysztof Dziedzic, Karol Jakubowski, Natalia Lisiak, Juliusz Przysławski, and Sławomira Drzymała-Czyż. 2025. "Changes in Polyphenolic Profile and Antioxidant Properties of Selected Raw and Processed Vegetables Under Different Cooking Methods" Applied Sciences 15, no. 9: 4677. https://doi.org/10.3390/app15094677
APA StyleKosewski, G., Chanaj-Kaczmarek, J., Dziedzic, K., Jakubowski, K., Lisiak, N., Przysławski, J., & Drzymała-Czyż, S. (2025). Changes in Polyphenolic Profile and Antioxidant Properties of Selected Raw and Processed Vegetables Under Different Cooking Methods. Applied Sciences, 15(9), 4677. https://doi.org/10.3390/app15094677







