Carbon Spheres as CO2 Sorbents
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Characterization
3. Results and Discussion
3.1. Samples’ Morphology
3.2. Surface Chemistry
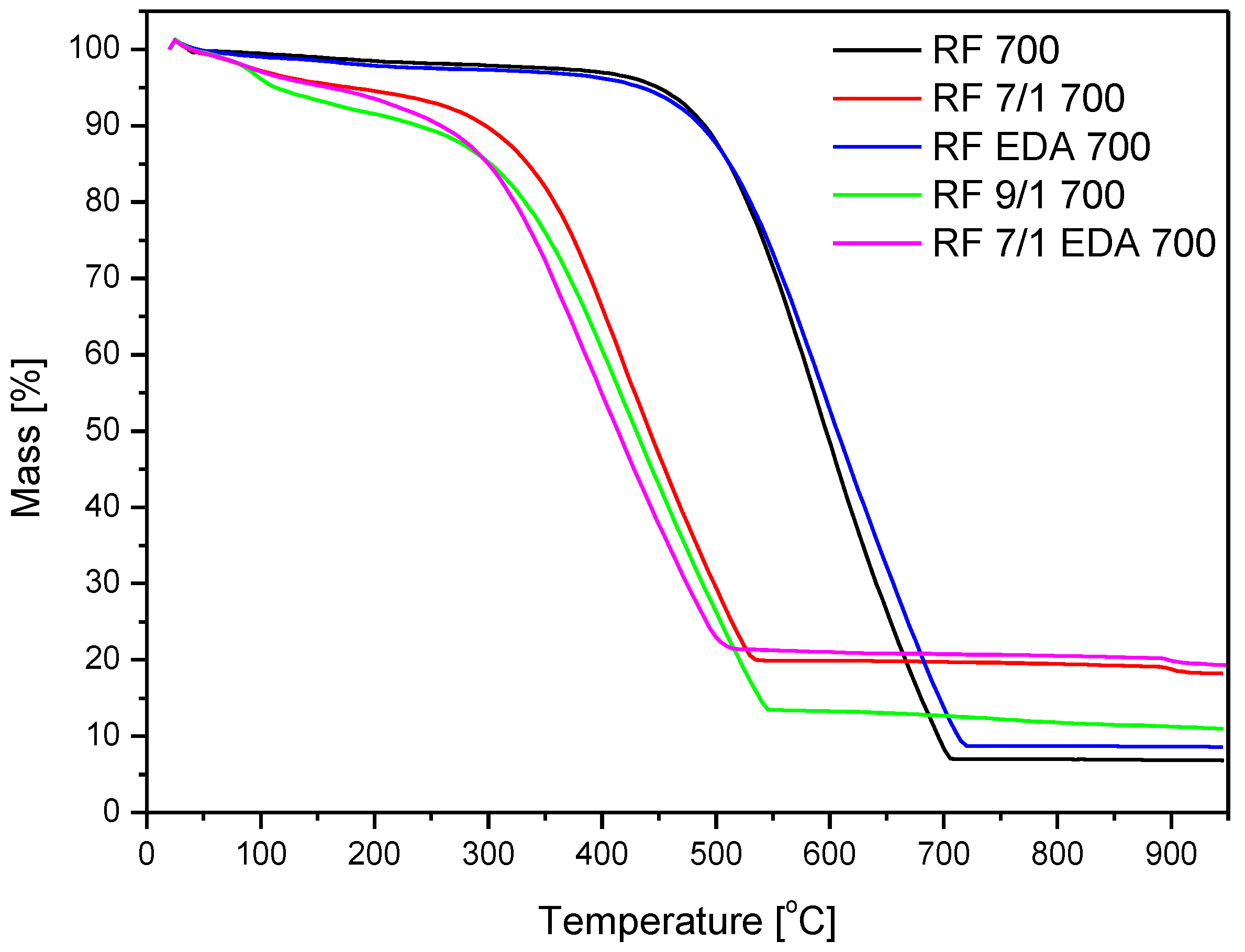
3.3. Thermogravimetric Analysis
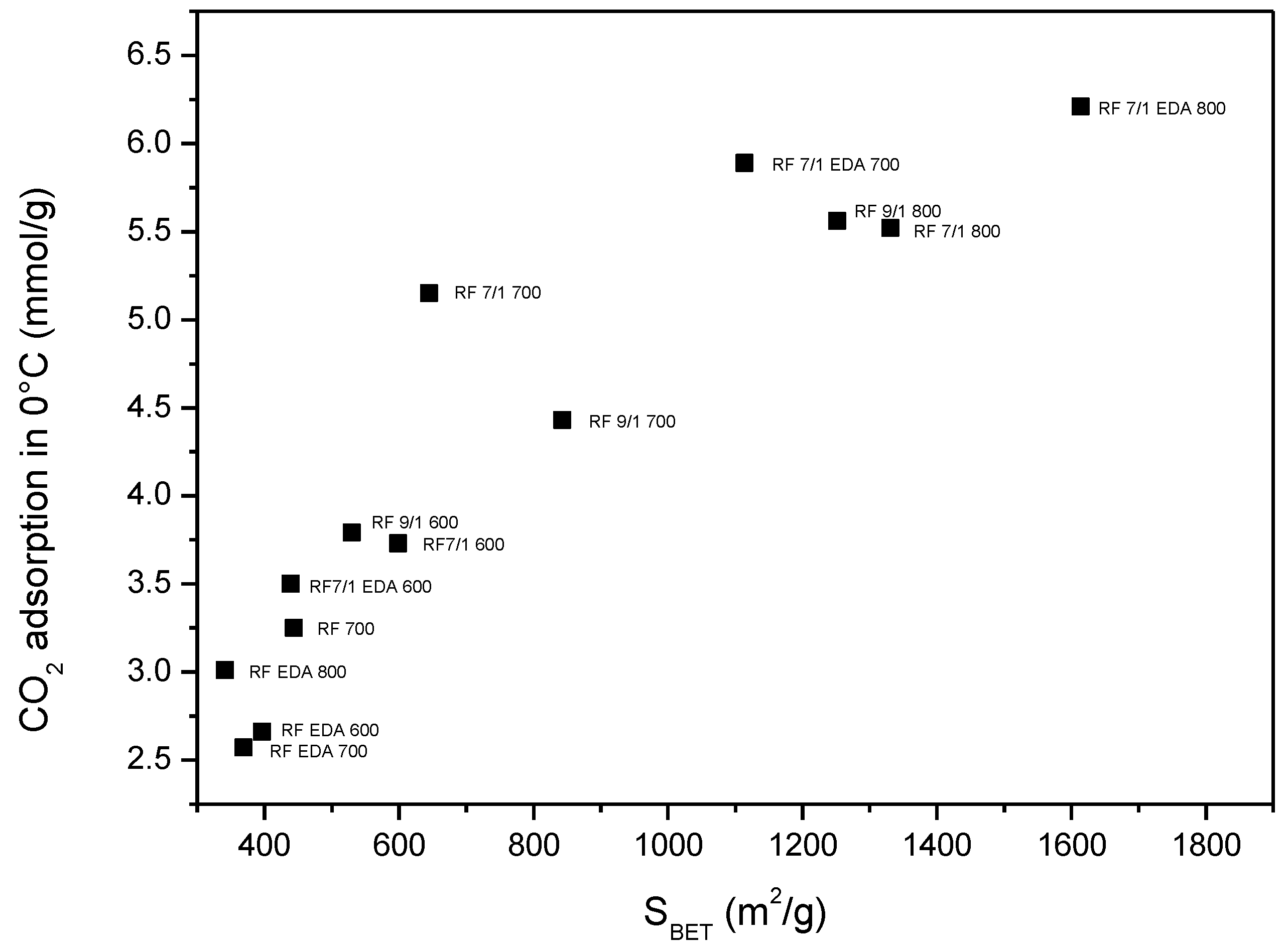
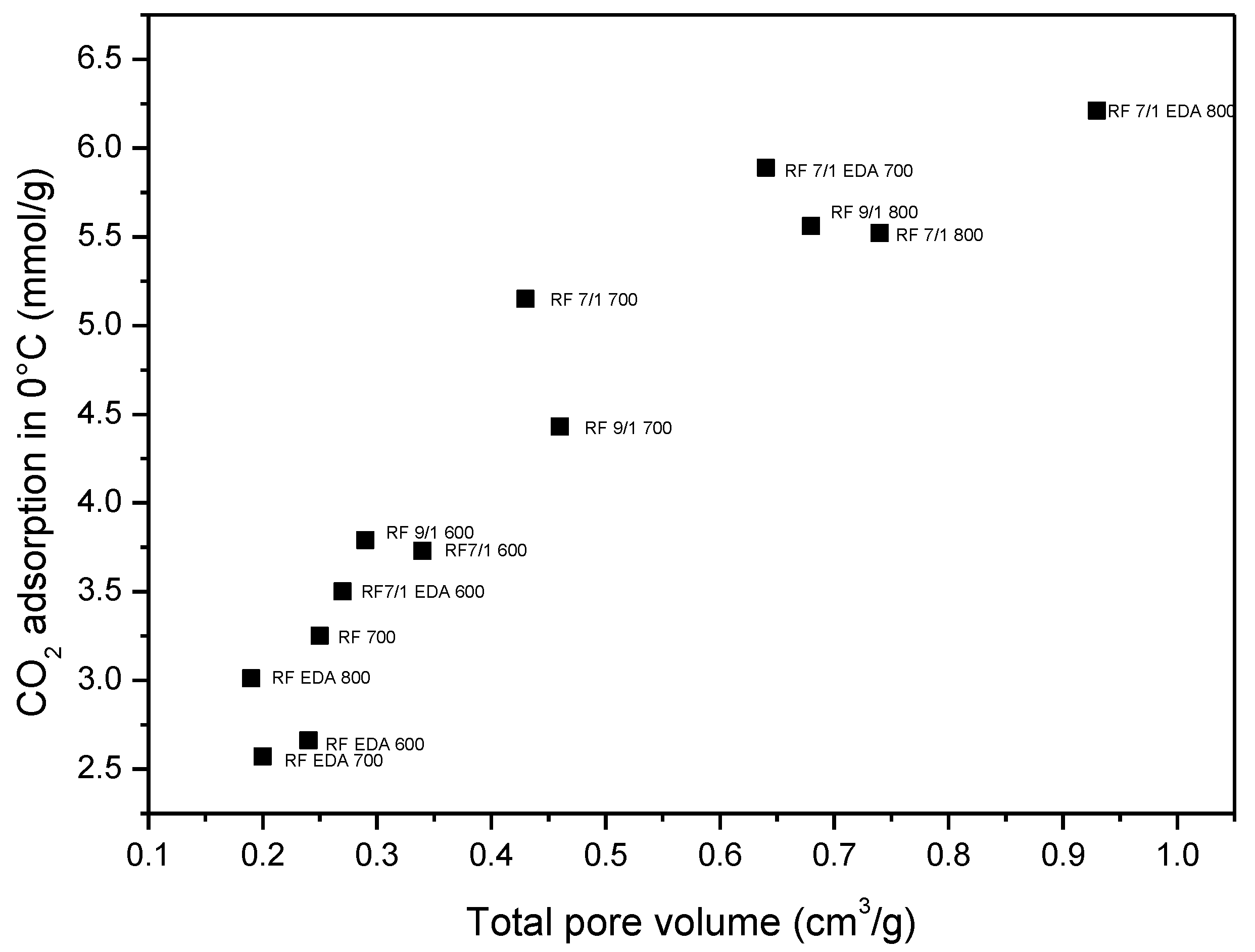
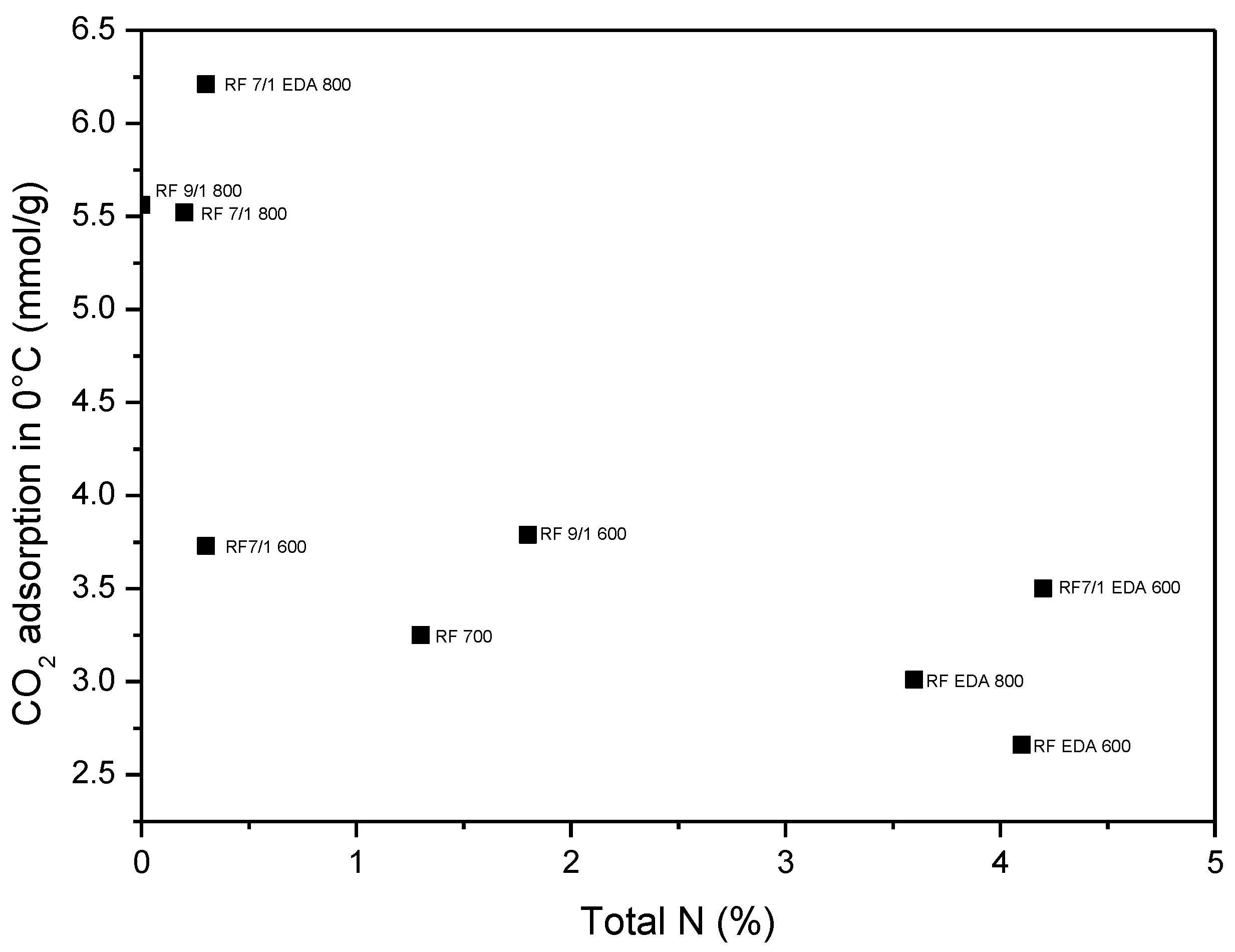
3.4. Adsorption Studies
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Attahiru, Y.B.; Aziz, M.M.A.; Kassim, K.A.; Shahid, S.; Wan Abu Bakar, W.A.; NSashruddin, T.F.; Rahman, F.A.; Ahamed, M.I. A review on green economy and development of green roads and highways using carbon neutral materials. Renew. Sus. Energy Rev. 2019, 101, 600–613. [Google Scholar] [CrossRef]
- Zhang, L.L.; Long, R.Y.; Chen, H.; Geng, J.C. A review of China’s road traffic carbon emissions. J. Clean. Prod. 2019, 207, 569–581. [Google Scholar] [CrossRef]
- Zhu, X.; Baran, S.; Cel, W.; Cao, Y. Sustainable approach to mitigation of CO2 emission. Ecol. Chem. Eng. S 2015, 21, 617–622. [Google Scholar] [CrossRef]
- D’Alessandro, D.M.; Smit, B.; Long, J.R. Carbon dioxide capture: Prospects for new materials. Angew. Chem. Int. Ed. 2010, 49, 6058–6082. [Google Scholar] [CrossRef] [PubMed]
- OECD/IEA. CO2 Emissions From Fuel Combustion Highlights (2017 Edition); OECD/IEA: Paris, France. Available online: www.iea.org (accessed on 15 April 2019).
- Luis, P. Use of monoethanolamine (MEA) for CO2 capture in a global scenario: Consequences and alternatives. Desalination 2016, 380, 93–99. [Google Scholar] [CrossRef]
- Sema, T.; Naami, A.; Usubharatana, P.; Wang, X.; Gao, R.; Liang, Z.; Idem, R.; Tontiwachwuthikul, P. Mass transfer of CO2 absorption in hybrid MEA-methanol solvents in packed column. Energy Proc. 2013, 37, 883–889. [Google Scholar] [CrossRef]
- Samanta, A.; Zhao, A.; Shimizu, G.K.H.; Sarkar, P.; Gupta, R. Post-combustion CO2 capture using solid sorbents: A review. Ind. Eng. Chem. Res. 2012, 51, 1438–1463. [Google Scholar] [CrossRef]
- Hauchhum, L.; Mahanta, P. Carbon dioxide adsorption on zeolites and activated carbon by pressure swing adsorption in a fixed bed. Int. J. Energy Environ. Eng. 2014, 5, 349–356. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Bai, H.; Su, F.; Chen, W.; Hwang, J.F.; Lee, H.-H. Adsorption of Carbon Dioxide from Gas Streams via Mesoporous Spherical-Silica Particles. J. Air Waste Manag. Assoc. 2010, 60, 489–496. [Google Scholar] [CrossRef]
- Lu, W.; Sculley, J.P.; Yuan, D.; Krishna, R.; Wei, Z.; Zhou, H.C. Polyamine-tethered porous polymer networks for carbon dioxide capture from flue gas. Angew. Chem. Int. Ed. 2012, 51, 7480–7484. [Google Scholar] [CrossRef]
- Li, J.R.; Ma, Y.; McCarthy, M.C.; Sculley, J.; Yu, J.; Jeong, H.K.; Balbuena, P.B.; Zhou, H.C. Carbon dioxide capture-related gas adsorption and separation in metal-organic frameworks. Coord. Chem. Rev. 2011, 255, 1791–1823. [Google Scholar] [CrossRef]
- Gui, M.M.; Yap, Y.X.; Chai, S.P.; Mohamed, A.R. Multi-walled carbon nanotubes modified with (3-aminopropyl)triethoxysilane for effective carbon dioxide adsorption. Int. J. Green. Gas Control 2013, 14, 65–73. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. A general and facile synthesis strategy towards highly porous carbons: Carbonization of organic salts. J. Mater. Chem. A 2013, 1, 13738. [Google Scholar] [CrossRef]
- Xu, B.; Zheng, D.; Jia, M.; Cao, G.; Yang, Y. Nitrogen-doped porous carbon simply prepared by pyrolyzing a nitrogen-containing organic salt for supercapacitors. Electrochim. Acta 2013, 98, 176–182. [Google Scholar] [CrossRef]
- Zhang, X.; Li, W.; Lu, A. Designed porous carbon materials for efficient CO2 adsorption and separation. New Carbon Mater. 2015, 30, 481–501. [Google Scholar] [CrossRef]
- Wang, R.; Lang, J.; Yan, X. Effect of surface area and heteroatom of porous carbon materials on electrochemical capacitance in aqueous and organic electrolytes. Sci. China Chem. 2014, 57, 1570–1578. [Google Scholar] [CrossRef]
- Choma, J.; Kloske, M.; Dziura, A.; Stachurska, K.; Jaroniec, M. Preparation and Studies of Adsorption Properties of Microporous Carbon Spheres. Eng. Prot. Environ. 2016, 19, 169–182. [Google Scholar] [CrossRef]
- Ludwinowicz, J.; Jaroniec, M. Potassium salt-assisted synthesis of highly microporous carbon spheres for CO2 adsorption. Carbon 2015, 82, 297–303. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, J.; Xing, W.; Liu, B.; Zhang, J.; Lin, H.; Cui, H.; Zhuo, S. Resorcinol–formaldehyde resin-based porous carbon spheres with high CO2 capture capacities. J. Energy Chem. 2017, 26, 1007–1013. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Xu, J.; Wang, M.; Zhu, L.; Dai, L.; Jaroniec, M. Nitrogen enriched porous carbon spheres: Attractive materials for supercapacitor L. electrodes and CO2 adsorption. Chem. Mater. 2014, 26, 2820–2828. [Google Scholar] [CrossRef]
- Sevilla, M.; Fuertes, A.B. CO2 adsorption by activated templated carbons. J. Colloid Interface Sci. 2012, 366, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.-C.; Yeh, C.-Y.; Weng, C.-H. Carbon Dioxide Adsorption on Porous and Functionalized Activated Carbon Fibers. Appl. Sci. 2019, 9, 1977. [Google Scholar] [CrossRef]
- Li, Y.; Xu, R.; Wang, B.; Wei, J.; Wang, L.; Shen, M.; Yang, J. Enhanced N-doped Porous Carbon Derived from KOH-Activated Waste Wool: A Promising Material for Selective Adsorption of CO2/CH4 and CH4/N2. Nanomaterials 2019, 9, 266. [Google Scholar] [CrossRef] [PubMed]
- Sevilla, M.; Fuertes, A.B. Sustainable porous carbons with a superior performance for CO2 capture. Energy Environ. Sci. 2011, 4, 1765–1771. [Google Scholar] [CrossRef]
- Liu, J.; Qiao, S.Z.; Liu, H.; Chen, J.; Orpe, A.; Zhao, D.; Lu, G.Q. Extension of the Stöber method to the preparation of monodisperse resorcinol-formaldehyde resin polymer and carbon spheres. Angew. Chem. Int. Ed. 2011, 50, 5947–5951. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Liu, J.; O’Donnell, K.; Liu, T.; Liu, X.; Yan, Z.; Liu, S.; Jaroniec, M. Revisiting the Stöber method: Design of nitrogen-doped porous carbon spheres from molecular precursors of different chemical structures. J. Colloid Interface Sci. 2016, 476, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Niu, W.; Zhang, L.; Cai, H.; Han, M.; Yuan, Y.; Majeed, S.; Anjum, S.; Xu, G. A template-free and surfactant-free method for high-yield synthesis of highly monodisperse 3-aminophenol-formaldehyde resin and carbon nano/microspheres. Macromolecules 2013, 46, 140–145. [Google Scholar] [CrossRef]
- Wickramaratne, N.P.; Jaroniec, M. Activated carbon spheres for CO2 adsorption. ACS Appl. Mater. Interfaces 2013, 5, 1849–1855. [Google Scholar] [CrossRef] [PubMed]
- Pari, G.; Darmawan, S.; Prihandoko, B. Porous Carbon Spheres from Hydrothermal Carbonization and KOH Activation on Cassava and Tapioca Flour Raw Material. Procedia Environ. Sci. 2014, 20, 342–351. [Google Scholar] [CrossRef] [Green Version]
- Wickramaratne, N.P.; Jaroniec, M. Importance of small micropores in CO2 capture by phenolic resin-based activated carbon spheres. J. Mater. Chem. A 2013, 1, 112–116. [Google Scholar] [CrossRef]
- Okunev, A.G.; Sharonov, V.E.; Aristov, Y.I.; Parmon, V.N. Sorption of carbon dioxide from wet gases by K2CO3-in-porous matrix: Influence of the matrix nature. React. Kinet. Catal. Lett. 2000, 71, 355–362. [Google Scholar] [CrossRef]
- Sibera, D.; Narkiewicz, U.; Kapica, J.; Serafin, J.; Michalkiewicz, B.; Wróbel, R.J.; Morawski, A.W. Preparation and characterisation of carbon spheres for carbon dioxide capture. J. Por. Mater. 2019, 26, 19–27. [Google Scholar] [CrossRef]
- Sibera, D.; Sreńscek-Nazzal, J.; Morawski, W.A.; Michalkiewicz, B.; Serafin, J.; Wróbel, R.J.; Narkiewicz, U. Microporous carbon spheres modified with EDA used as carbon dioxide sorbents. Adv. Mat. Lett. 2018, 9, 432–435. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef] [Green Version]
- Adinata, D.; Daud, W.M.A.W.; Aroua, M.K. Preparation and characterization of activated carbon from palm shell by chemical activation with K2CO3. Bioresour. Technol. 2007, 98, 145–149. [Google Scholar] [CrossRef]
- Guo, J.; Lua, A.C. Textural and chemical properties of adsorbent prepared from palm shell by phosphoric acid activation. Mater. Chem. Phys. 2003, 80, 114–119. [Google Scholar] [CrossRef]
- Musa, M.S.; Sanagi, M.M.; Nur, H.; Wan Ibrahim, W.A. Understanding pore formation and structural deformation in carbon spheres during KOH activation. Sains Malays 2015, 44, 613–618. [Google Scholar] [CrossRef]
- Chen, Y.D.; Chen, W.Q.; Huang, B.; Huang, M.J. Process optimization of K2C2O4-activated carbon from kenaf core using Box-Behnken design. Chem. Eng. Res. Des. 2013, 91, 1783–1789. [Google Scholar] [CrossRef]
- Mohamed, M.A.; Galwey, A.K.; Halawy, S.A. The activities of some metal oxides in promoting the thermal decomposition of potassium oxalate. Thermochim. Acta 2002, 387, 63–74. [Google Scholar] [CrossRef]
- The Dow Chemical Company. Product Safety Assessment. DOWTM Ethylenediamine, Form No. 233-00318-MM-0515X; The Dow Chemical Company: Midland, MI, USA, 2015. [Google Scholar]
- Ya, T. Kosolapova, Carbides, Properties, Production, and Applications; Plenum Press: New York, NY, USA; London, UK, 1971. [Google Scholar]
- Hu, X.; Radosz, M.; Cychosz, K.A.; Thommes, M. CO2-filling capacity and selectivity of carbon nanopores: Synthesis, texture, and pore-size distribution from quenched-solid density functional theory (QSDFT). Environ. Sci. Technol. 2011, 45, 7068–7074. [Google Scholar] [CrossRef]
- Yin, G.; Liu, Z.; Liu, Q.; Wu, W. The role of different properties of activated carbon in CO2 adsorption. Chem. Eng. J. 2013, 230, 133–140. [Google Scholar] [CrossRef]
- Presser, V.; McDonough, J.; Yeon, S.H.; Gogotsi, Y. Effect of pore size on carbon dioxide sorption by carbide derived carbon. Energy Environ. Sci. 2011, 4, 3059–3066. [Google Scholar] [CrossRef]
- Lee, S.Y.; Park, S.J. Determination of the optimal pore size for improved CO2 adsorption in activated carbon fibers. J. Coll. Interf. Sci. 2013, 389, 230–235. [Google Scholar] [CrossRef] [PubMed]
- Gadkaree, K.P.; Jaroniec, M. Pore structure development in activated carbon honeycombs. Carbon 2000, 38, 983–993. [Google Scholar] [CrossRef]
- Casco, M.E.; Martínez-Escandell, M.J.; Silvestre-Albero, J.F.; Rodríguez-Reinoso, J.F. Effect of the porous structure in carbon materials for CO2 capture at atmospheric and high-pressure. Carbon 2014, 67, 230–235. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Watanabe, T.; Kanoh, H.; Hata, K.; Ohba, T. Cooperative CO2 adsorption promotes high CO2 adsorption density over wide optimal nanopore range. Adsorpt. Sci. Technol. 2018, 36, 625–639. [Google Scholar] [CrossRef]












| Carbon Source | Activator | CO2 Adsorption 0 °C [mmol/g] 1 bar | CO2 Adsorption 25 °C [mmol/g] 1 bar | Reference |
|---|---|---|---|---|
| Resorcinol–formaldehyde resin | K2C2O4 | 7.67 | 4.95 | [18] |
| Resorcinol–formaldehyde resin | K2C2O4 | 6.6 | - | [19] |
| Resorcinol–formaldehyde resin | KOH | 7.34 | 4.83 | [20] |
| Resorcinol–formaldehyde resin/EDA | EDA; CO2 | 6.2 | 4.1 | [21] |
| Furfuryl alcohol | KOH | 5.8 | 3.3 | [22] |
| Polyacrylonitryle | KOH | - | 2.74 | [23] |
| Waste wool | KOH | 3.73 | 2.81 | [24] |
| Starch | KOH | 6.6 | 4.3 | [25] |
| Sample | C | O | N | K |
|---|---|---|---|---|
| at. % | ||||
| RF_700 | 96 | 3 | ~1 | - |
| RF_7_1_600 | 88 | 11 | <1 | ~1 |
| RF_7_1_800 | 92 | 7 | <1 | ~1 |
| RF_7_1_EDA600 | 87 | 8 | 3 | ~2 |
| RF_7_1_EDA800 | 88 | 9 | <1 | ~3 |
| RF_9_1_600 | 76 | 18 | ~2 | ~4 |
| RF_9_1_800 | 85 | 11 | <1 | ~3 |
| RF_EDA600 | 92 | 4 | ~4 | - |
| RF_EDA800 | 93 | 3 | ~4 | - |
| Sample | Start of Mass Loss (°C) | Max of Mass Loss (°C) | End of Mass Loss (°C) | Residue (%) |
|---|---|---|---|---|
| RF 700 | 364 | 600 | 712 | 7.03 |
| RF 7/1 600 | 181 | 420 | 547 | 16.71 |
| RF 7/1 700 | 181 | 420 | 547 | 20 |
| RF 7/1 800 | 181 | 432 | 573 | 14.53 |
| RF + EDA 700 | 364 | 589 | 728 | 8.6 |
| RF 7/1 + EDA 700 | 149 | 391 | 519 | 21 |
| Sample | Density (g/cm3) | SBET (m2/g) | Total Pore Volume (cm3/g) | CO2 Adsorption at 0 °C (mmol/g) | CO2 Adsorption at 25 °C (mmol/g) |
|---|---|---|---|---|---|
| RF 700 | 1.79 | 444 | 0.25 | 3.25 | 2.43 |
| RF 7/1 600 | 1.68 | 599 | 0.34 | 3.73 | 3.41 |
| RF 7/1 700 | 1.97 | 645 | 0.43 | 5.15 | 3.67 |
| RF 7/1 800 | 2.26 | 1331 | 0.74 | 5.52 | 3.96 |
| RF 9/1 600 | 1.75 | 530 | 0.29 | 3.79 | 3.20 |
| RF 9/1 700 | 1.80 | 843 | 0.46 | 4.43 | 3.74 |
| RF 9/1 800 | 1.95 | 1252 | 0.68 | 5.56 | 3.87 |
| RF EDA 600 | 1.59 | 396 | 0.24 | 2.66 | 1.94 |
| RF EDA 700 | 1.67 | 369 | 0.20 | 2.57 | 2.01 |
| RF EDA 800 | 1.72 | 341 | 0.19 | 3.01 | 2.28 |
| RF 7/1 EDA 600 | 1.62 | 439 | 0.27 | 3.50 | 2.51 |
| RF 7/1 EDA 700 | 1.74 | 1114 | 0.64 | 5.89 | 4.60 |
| RF 7/1 EDA 800 | 1.99 | 1614 | 0.93 | 6.21 | 4.02 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Staciwa, P.; Narkiewicz, U.; Sibera, D.; Moszyński, D.; Wróbel, R.J.; Cormia, R.D. Carbon Spheres as CO2 Sorbents. Appl. Sci. 2019, 9, 3349. https://doi.org/10.3390/app9163349
Staciwa P, Narkiewicz U, Sibera D, Moszyński D, Wróbel RJ, Cormia RD. Carbon Spheres as CO2 Sorbents. Applied Sciences. 2019; 9(16):3349. https://doi.org/10.3390/app9163349
Chicago/Turabian StyleStaciwa, P., U. Narkiewicz, D. Sibera, D. Moszyński, R. J. Wróbel, and R. D. Cormia. 2019. "Carbon Spheres as CO2 Sorbents" Applied Sciences 9, no. 16: 3349. https://doi.org/10.3390/app9163349





