Feasibility Study of Native Ureolytic Bacteria for Biocementation Towards Coastal Erosion Protection by MICP Method
Abstract
:1. Introduction
2. Methodology
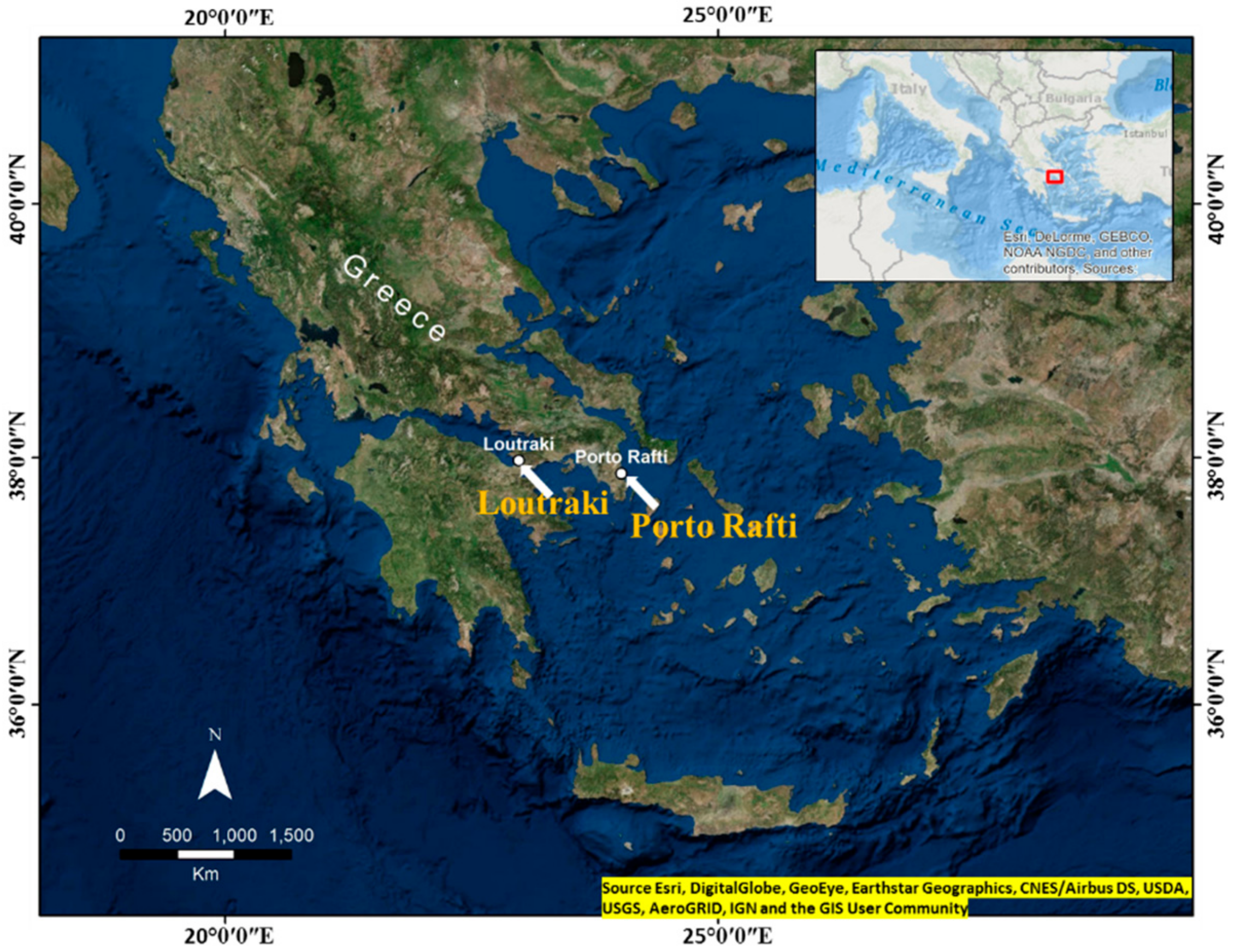
2.1. Isolation and Bacteria Cell Culture
2.2. Assessment of Urease Activity
2.3. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) Analysis
2.4. Microbial CaCO3 Precipitation Test
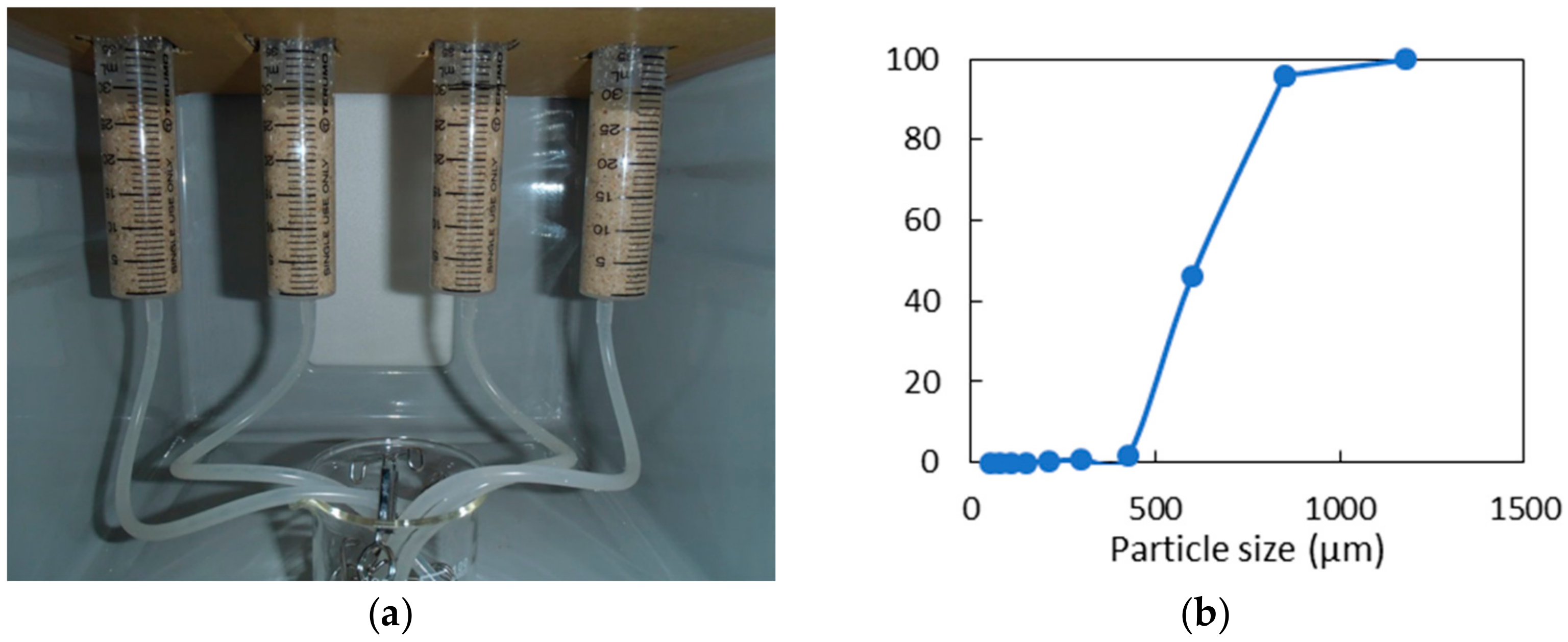
2.5. Sand Solidification Test
3. Results and Discussion
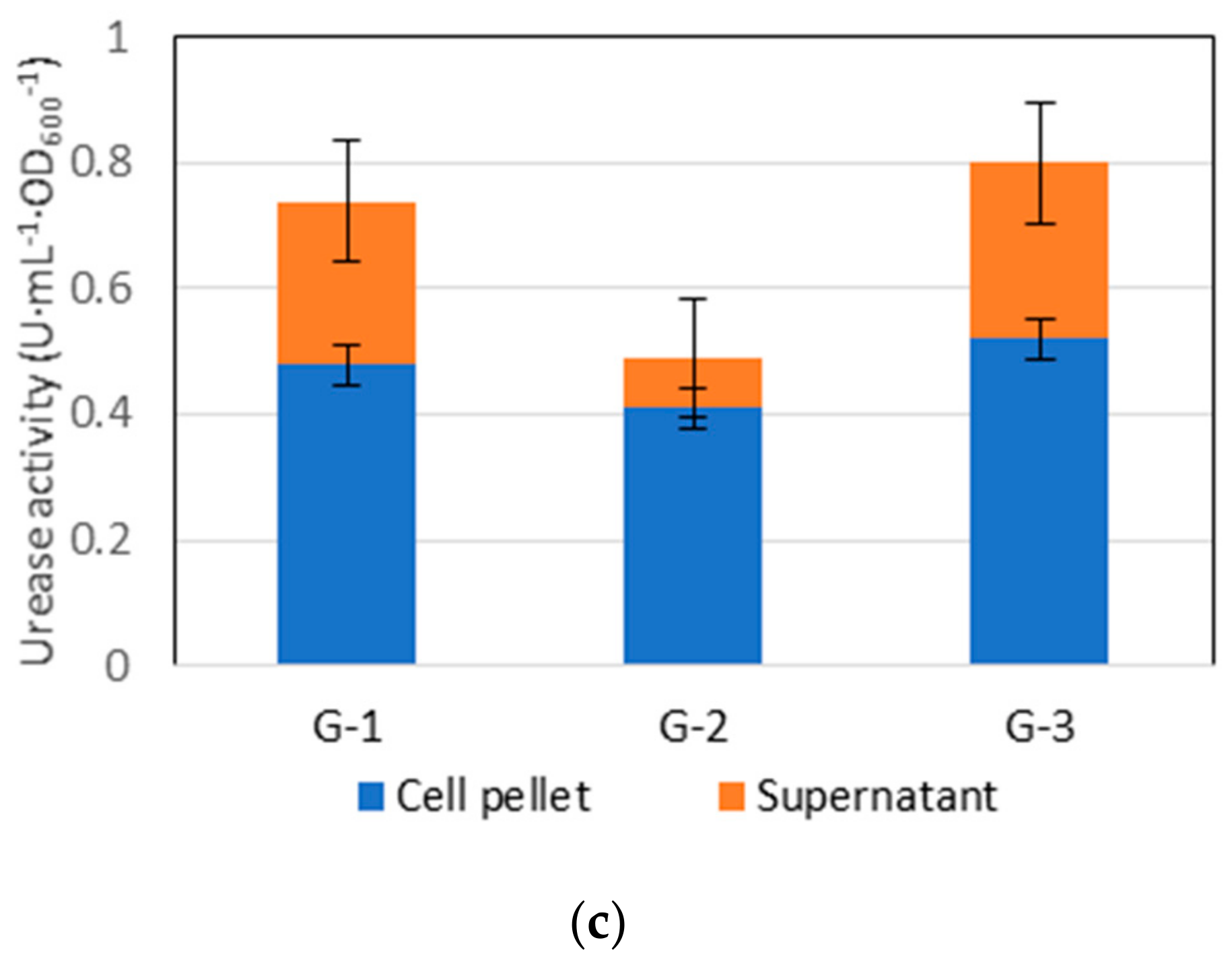
3.1. Cell Concentration (Isolated Species) and Urease Activity
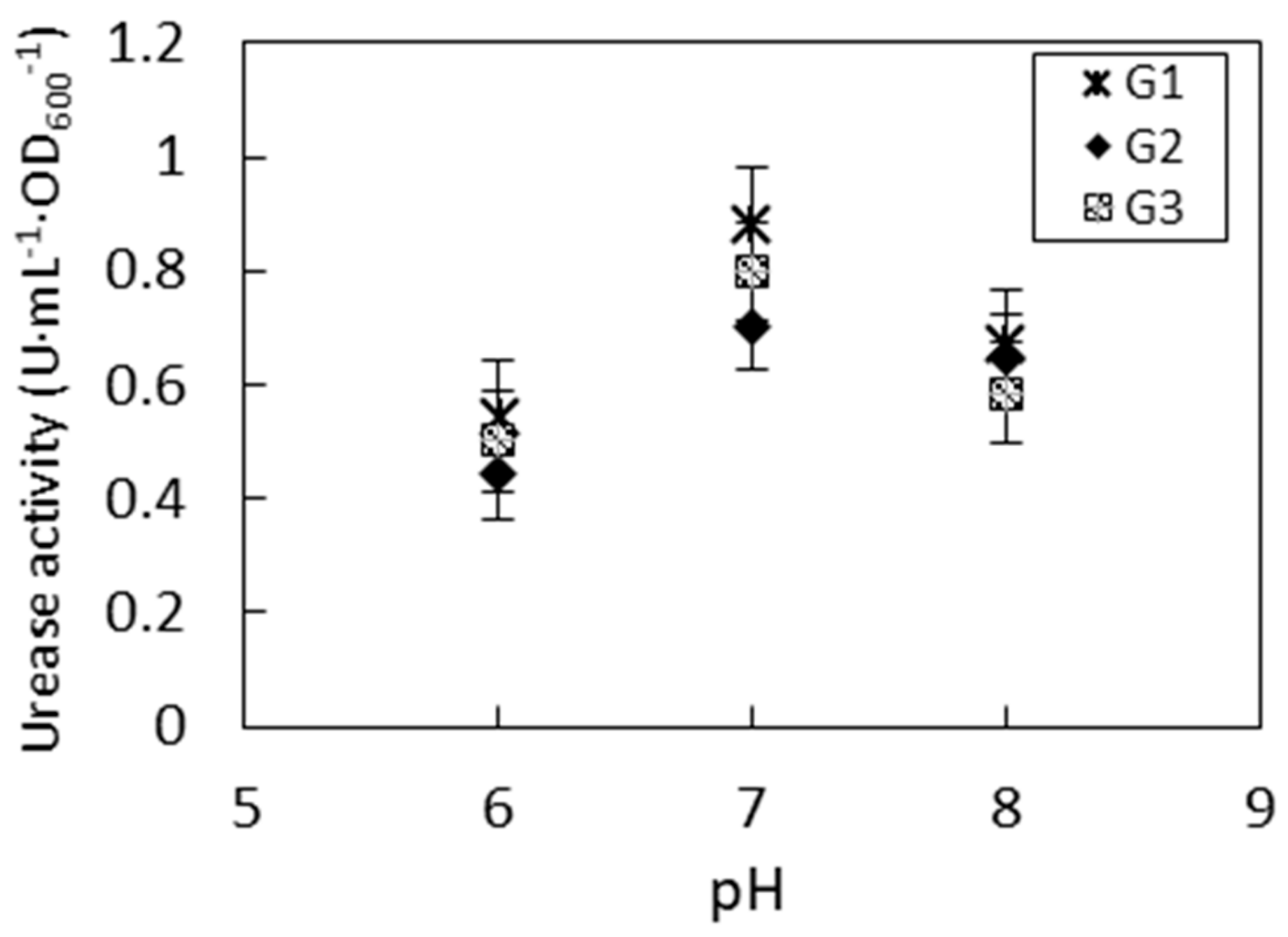
3.2. Impact of pH to Urease Activity
3.3. Impact of Temperature to Urease Activity
3.4. SDS-PAGE Analysis
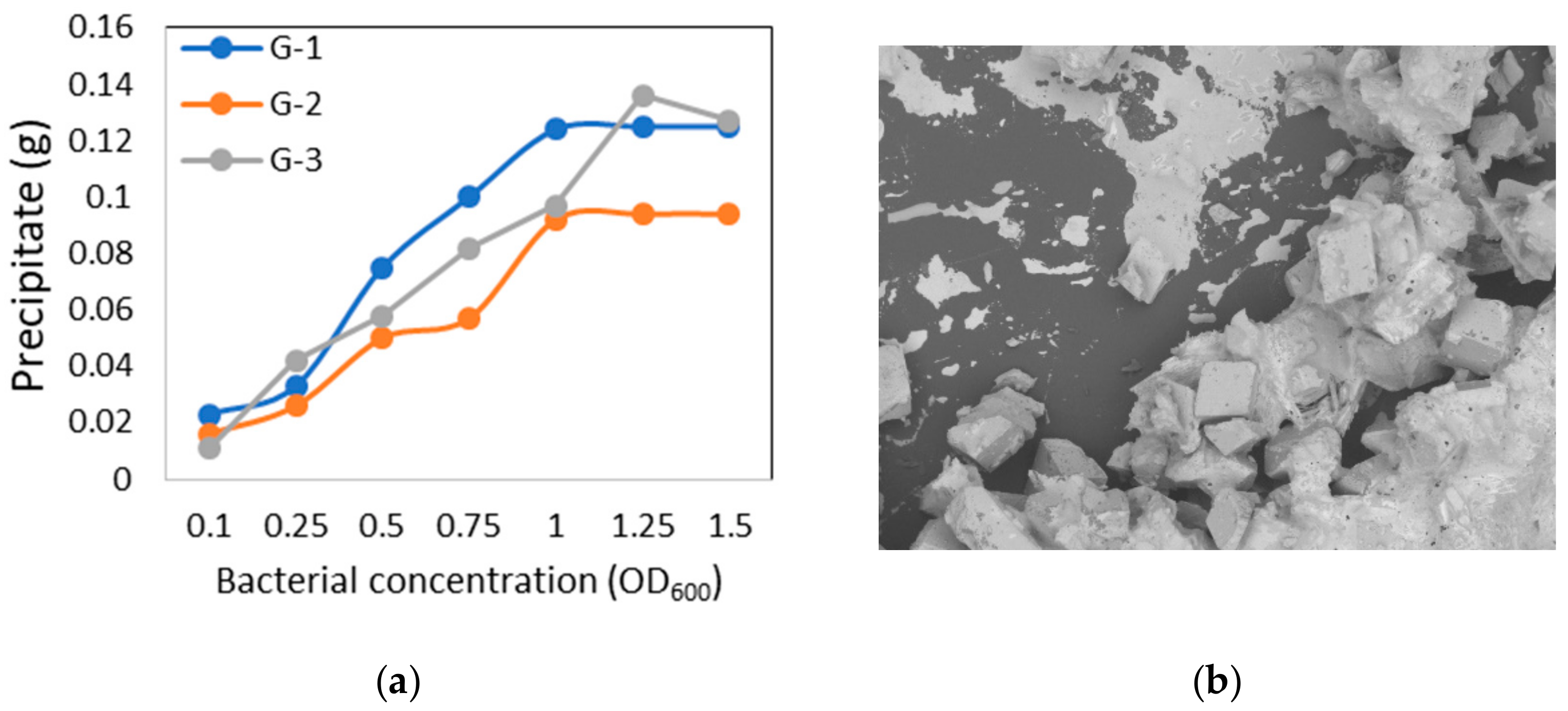
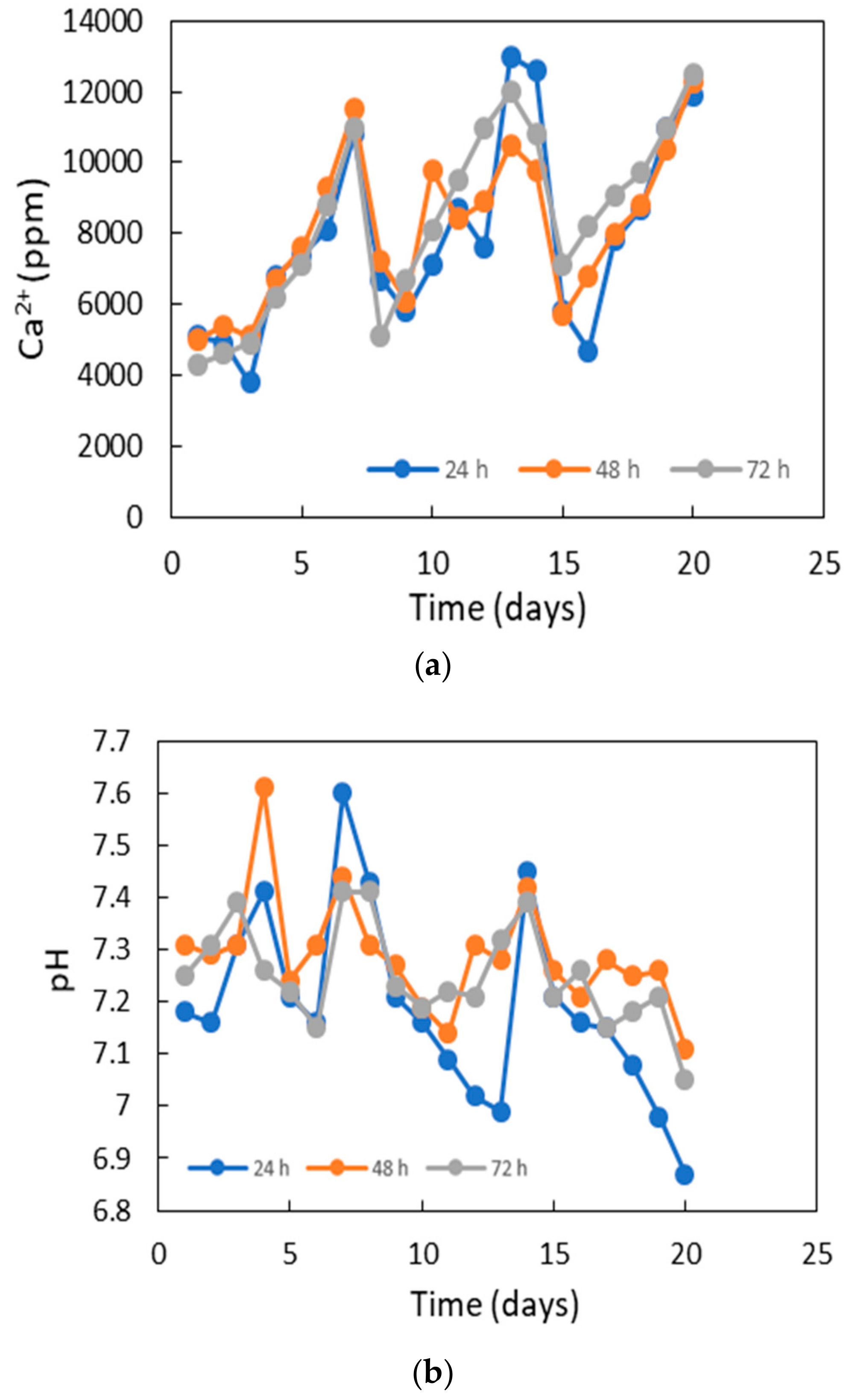
3.5. Microbial CaCO3 Precipitation Test
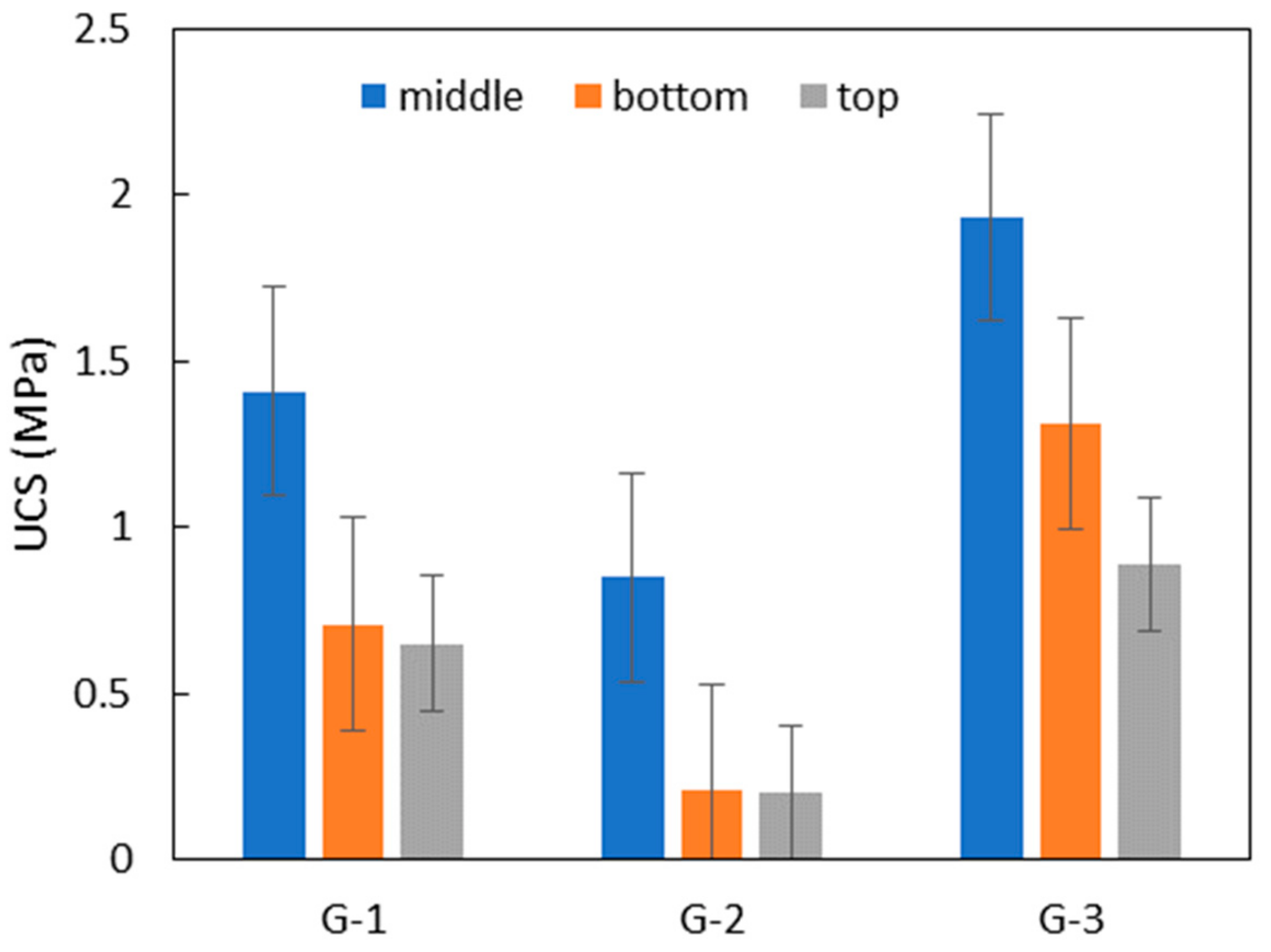
3.6. Potentiality for Sand Solidification and Design for the Application
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Salifu, E.; MacLachlan, E.; Iyer, K.R.; Knapp, C.W.; Tarantino, A. Application of microbially induced calcite precipitation in erosion mitigation and stabilisation of sandy soil foreshore slopes: A preliminary investigation. Eng. Geol. 2016, 201, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Evelpidou, N.; Vassilopoulos, A.; Leonidopoulou, D.; Poulos, S. AN INVESTIGATION OF THE COASTAL EROSION CAUSES IN SAMOS ISLAND, EASTERN AEGEAN SEA. Tájökológiai Lapok 2008, 6, 295–310. [Google Scholar]
- Mujah, D.; Shahin, M.A.; Cheng, L. State-of-the-Art Review of Biocementation by Microbially Induced Calcite Precipitation (MICP) for Soil Stabilization. Geomicrobiol. J. 2017, 34, 524–537. [Google Scholar] [CrossRef]
- Danjo, T.; Kawasaki, S. Formation mechanisms of beachrocks in Okinawa and Ishikawa, Japan, with a Focus on Cements. Mater. Trans. 2014, 55, 493–500. [Google Scholar] [CrossRef]
- Danjo, T.; Kawasaki, S. Microbially Induced Sand Cementation Method Using Pararhodobacter sp. Strain SO1, Inspired by Beachrock Formation Mechanism. Mater. Trans. 2016, 57, 428–437. [Google Scholar] [CrossRef] [Green Version]
- Sheehan, C.; Harrington, J. An environmental and economic analysis for geotube coastal structures retaining dredge material. Resour. Conserv. Recycl. 2012, 61, 91–102. [Google Scholar] [CrossRef]
- Ashis, M. Application of geotextiles in Coastal Protection and Coastal Engineering Works: An overview. Int. Res. J. Environ. Sci. 2015, 4, 96–103. [Google Scholar]
- Lee, S.C.; Hashim, R.; Motamedi, S.; Song, K.-I. Utilization of geotextile tube for sandy and muddy coastal management: A review. Sci. World J. 2014, 2014, 494020. [Google Scholar] [CrossRef]
- Kubo, R.; Kawasaki, S.; Suzuki, K.; Yamaguchi, S.; Hata, T. Geological Exploration of Beachrock through Geophysical Surveying on Yagaji Island, Okinawa, Japan. Mater. Trans. 2013, 55, 342–350. [Google Scholar] [CrossRef]
- Neumeier, U. Experimental modelling of beachrock cementation under microbial influence. Sediment. Geol. 1999, 126, 35–46. [Google Scholar] [CrossRef]
- Whiffin, V.S.; van Paassen, L.A.; Harkes, M.P. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Sarayu, K.; Iyer, N.R.; Murthy, A.R. Exploration on the biotechnological aspect of the ureolytic bacteria for the production of the cementitious materials—A review. Appl. Biochem. Biotechnol. 2014, 172, 2308–2323. [Google Scholar] [CrossRef] [PubMed]
- Tiano, P.; Biagiotti, L.; Mastromei, G. Bacterial bio-mediated calcite precipitation for monumental stones conservation: Methods of evaluation. J. Microbiol. Methods 1999, 36, 139–145. [Google Scholar] [CrossRef]
- Achal, V.; Mukherjee, A.; Basu, P.C.; Reddy, M.S. Strain improvement of Sporosarcina pasteurii for enhanced urease and calcite production. J. Ind. Microbiol. Biotechnol. 2009, 36, 981–988. [Google Scholar] [CrossRef]
- Jonkers, H.M.; Thijssen, A.; Muyzer, G.; Copuroglu, O.; Schlangen, E. Application of bacteria as self-healing agent for the development of sustainable concrete. Ecol. Eng. 2010, 36, 230–235. [Google Scholar] [CrossRef]
- Van Paassen, L.A.; Ghose, R.; van der Linden, T.J.M.; van der Star, W.R.L. Quantifying Biomediated Ground Improvement by Ureolysis: Large-Scale Biogrout Experiment. J. Geotech. Geoenviron. Eng. 2010, 136, 1721–1728. [Google Scholar] [CrossRef]
- Harkes, M.P.; van Paassen, L.A.; Booster, J.L.; Whiffin, V.S.; van Loosdrecht, M.C.M. Fixation and distribution of bacterial activity in sand to induce carbonate precipitation for ground reinforcement. Ecol. Eng. 2010, 36, 112–117. [Google Scholar] [CrossRef]
- Omoregie, A.I.; Ngu, L.H.; Ong, D.E.L.; Nissom, P.M. Low-cost cultivation of Sporosarcina pasteurii strain in food-grade yeast extract medium for microbially induced carbonate precipitation (MICP) application. Biocatal. Agric. Biotechnol. 2019, 17, 247–255. [Google Scholar] [CrossRef]
- Lee, C.; Lee, H.; Kim, O.B. Biocement fabrication and design application for a sustainable urban area. Sustainability 2018, 10, 4079. [Google Scholar] [CrossRef]
- Ge, P.; Nayanthara, N.; Buddhika, A.; Dassanayake, N.; Nakashima, K.; Kawasaki, S.; Lanka, S.; Author, C. Biocementation of Sri Lankan Beach Sand Using Locally Isolated Bacteria: A Baseline Study on The. Int. J. GEOMATE 2019, 17, 55–62. [Google Scholar]
- Wei, S.; Cui, H.; Jiang, Z.; Liu, H.; He, H.; Fang, N. Biomineralization processes of calcite induced by bacteria isolated from marine sediments. Braz. J. Microbiol. 2015, 46, 455–464. [Google Scholar] [CrossRef] [PubMed]
- Novitsky, J.A. Calcium carbonate precipitation by marine bacteria. Geomicrobiol. J. 1981, 2, 375–388. [Google Scholar] [CrossRef]
- Natarajan, K.R. Kinetic Study of the Enzyme Urease from Dolichos biflorus. J. Chem. Educ. 2009, 72, 556. [Google Scholar] [CrossRef]
- Mortensen, B.M.; Haber, M.J.; Dejong, J.T.; Caslake, L.F.; Nelson, D.C. Effects of environmental factors on microbial induced calcium carbonate precipitation. J. Appl. Microbiol. 2011, 111, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Ferris, F.G.; Phoenix, V.; Fujita, Y.; Smith, R.W. Kinetics of calcite precipitation induced by ureolytic bacteria at 10 to 20°C in artificial groundwater. Geochim. Cosmochim. Acta 2004, 68, 1701–1710. [Google Scholar] [CrossRef]
- Fujita, M.; Nakashima, K.; Achal, V.; Kawasaki, S. Whole-cell evaluation of urease activity of Pararhodobacter sp. isolated from peripheral beachrock. Biochem. Eng. J. 2017, 124, 1–5. [Google Scholar] [CrossRef]
- Zhu, T.; Dittrich, M.; Zhang, W.; Bhubalan, K.; Dittrich, M.; Zhu, T. Carbonate Precipitation through Microbial Activities in Natural Environment, and Their Potential in Biotechnology: A Review. Front. Bioeng. Biotechnol. 2016, 4, 4. [Google Scholar] [CrossRef] [Green Version]
- Jahns, T. Urea uptake by the marine bacterium Deleya venusta HG1. J. Gen. Microbiol. 2009, 138, 1815–1820. [Google Scholar] [CrossRef]
- Adams, G.O.; Fufeyin, P.T.; Okoro, S.E.; Ehinomen, I. Bioremediation, Biostimulation and Bioaugmentation: A Review. Int. J. Environ. Bioremediat. Biodegrad. 2015, 3, 28–39. [Google Scholar]
- Clemens, D.L.; Lee, B.Y.; Horwitz, M.A. Purification, characterization, and genetic analysis of Mycobacterium tuberculosis urease, a potentially critical determinant of host-pathogen interaction. J. Bacteriol. 1995, 177, 5644–5652. [Google Scholar] [CrossRef] [Green Version]
- Silva-Castro, G.A.; Uad, I.; Rivadeneyra, A.; Vilchez, J.I.; Martin-Ramos, D.; González-López, J.; Rivadeneyra, M.A. Carbonate Precipitation of Bacterial Strains Isolated from Sediments and Seawater: Formation Mechanisms. Geomicrobiol. J. 2013, 30, 840–850. [Google Scholar] [CrossRef]
- Deng, W.; Wang, Y. Investigating the Factors Affecting the Properties of Coral Sand Treated with Microbially Induced Calcite Precipitation. Adv. Civ. Eng. 2018, 2018, 9590653. [Google Scholar] [CrossRef]
- Kadhim, F.J.; Zheng, J.J. Review of the Factors That Influence on the Microbial Induced Calcite Precipitation. Civ. Environ. Res. 2016, 8, 69–76. [Google Scholar]
- Keykha, H.A.; Asadi, A.; Zareian, M. Environmental Factors Affecting the Compressive Strength of Microbiologically Induced Calcite Precipitation-Treated Soil. Geomicrobiol. J. 2017, 34, 889–894. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Q.; Zhang, H.; Amini, F.; Li, C. A Full Contact Flexible Mold for Preparing Samples Based on Microbial-Induced Calcite Precipitation Technology. Geotech. Test. J. 2014, 37, 917–921. [Google Scholar]
- Al Imran, M. Combination Technology of Geotextile Tube and Artificial Beachrock for Coastal Protection. Int. J. GEOMATE 2017, 13, 67–72. [Google Scholar] [CrossRef]




| Ureolytic Bacteria | Type | References |
|---|---|---|
| Sporosarcina pasteurii | Land | [1,10,18] |
| Bacillus cohnii | Land | [14] |
| Bacillus subtilis | Land | [12] |
| Micrococcus sp. | Marine | This study |
| Pseudoalteromonas sp. | Marine | This study |
| Virgibacillus sp. | Marine | This study |
| Reagent | Concentration (g/L) |
|---|---|
| MgCl2·6H2O | 222.23 |
| CaCl2·2H2O | 30.7 |
| SrCl2·6H2O | 0.85 |
| KCl | 13.90 |
| KBr | 2.00 |
| H3BO3 | 0.54 |
| NaCl | 490.68 |
| Na2SO4 | 81.88 |
| Composition | Concentration (g/L) |
|---|---|
| CO(NH2)2 | 30.0 |
| CaCl2 | 55.5 |
| Nutrient broth | 3.0 |
| NaHCO3 | 2.12 |
| NH4Cl | 10.0 |
| Test Period | Incubation Time | Temperature | Solidification Solution Concentration | Nutrient Injection Interval | Solidification Solution Injection Interval |
|---|---|---|---|---|---|
| 21 days | 24 h | 30 °C | 0.5 M | - | - |
| 48 h | 7 days | 1 day | |||
| 72 h | - | - |
| Code Name | Species |
|---|---|
| G1 | Micrococcus sp. |
| G2 | Pseudoalteromonas sp. |
| G3 | Virgibacillus sp. |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Imran, M.A.; Kimura, S.; Nakashima, K.; Evelpidou, N.; Kawasaki, S. Feasibility Study of Native Ureolytic Bacteria for Biocementation Towards Coastal Erosion Protection by MICP Method. Appl. Sci. 2019, 9, 4462. https://doi.org/10.3390/app9204462
Imran MA, Kimura S, Nakashima K, Evelpidou N, Kawasaki S. Feasibility Study of Native Ureolytic Bacteria for Biocementation Towards Coastal Erosion Protection by MICP Method. Applied Sciences. 2019; 9(20):4462. https://doi.org/10.3390/app9204462
Chicago/Turabian StyleImran, Md Al, Shuya Kimura, Kazunori Nakashima, Niki Evelpidou, and Satoru Kawasaki. 2019. "Feasibility Study of Native Ureolytic Bacteria for Biocementation Towards Coastal Erosion Protection by MICP Method" Applied Sciences 9, no. 20: 4462. https://doi.org/10.3390/app9204462
APA StyleImran, M. A., Kimura, S., Nakashima, K., Evelpidou, N., & Kawasaki, S. (2019). Feasibility Study of Native Ureolytic Bacteria for Biocementation Towards Coastal Erosion Protection by MICP Method. Applied Sciences, 9(20), 4462. https://doi.org/10.3390/app9204462








