Tricyclic Fused Lactams by Mukaiyama Cyclisation of Phthalimides and Evaluation of their Biological Activity
Abstract
1. Introduction
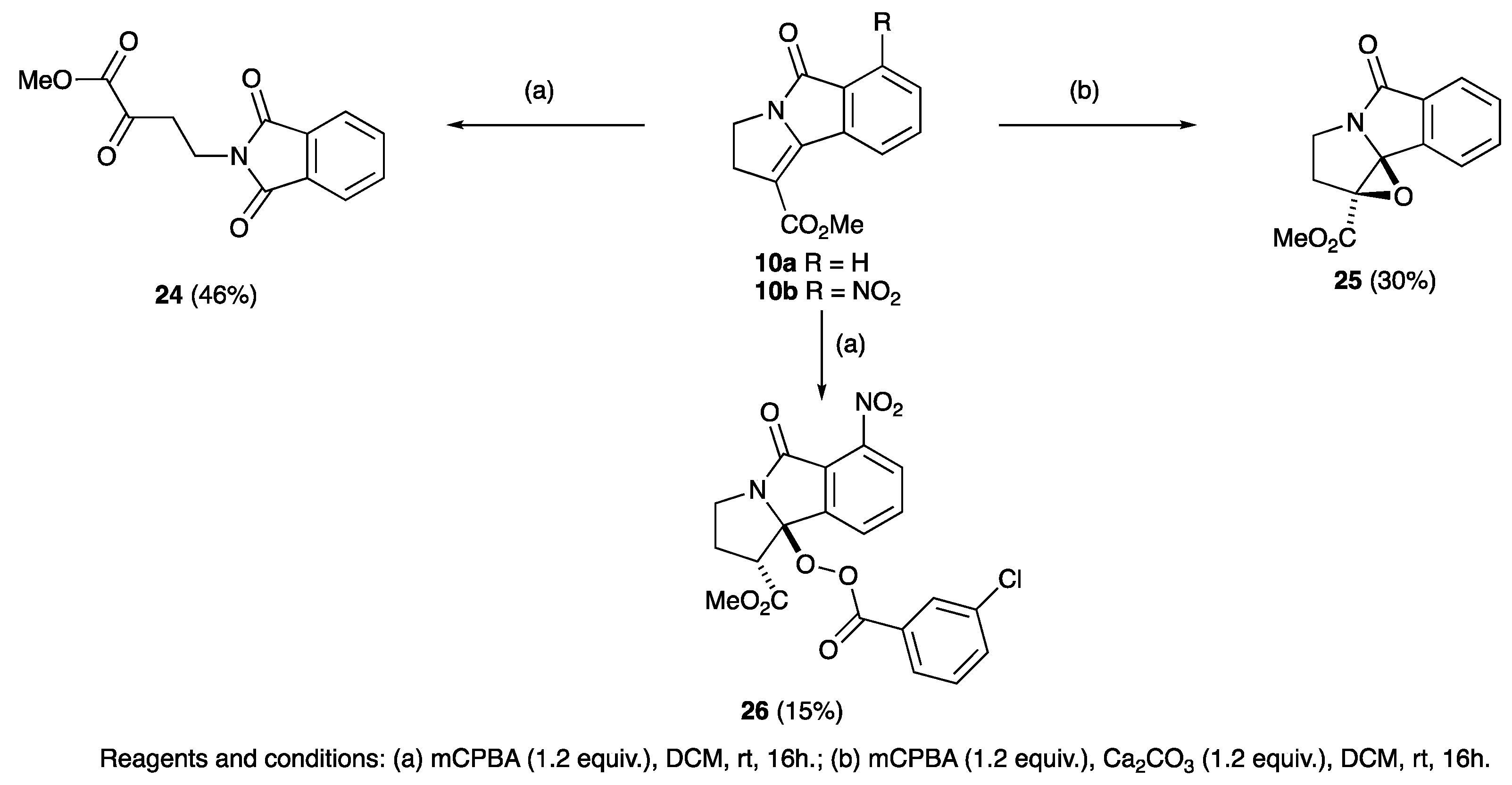
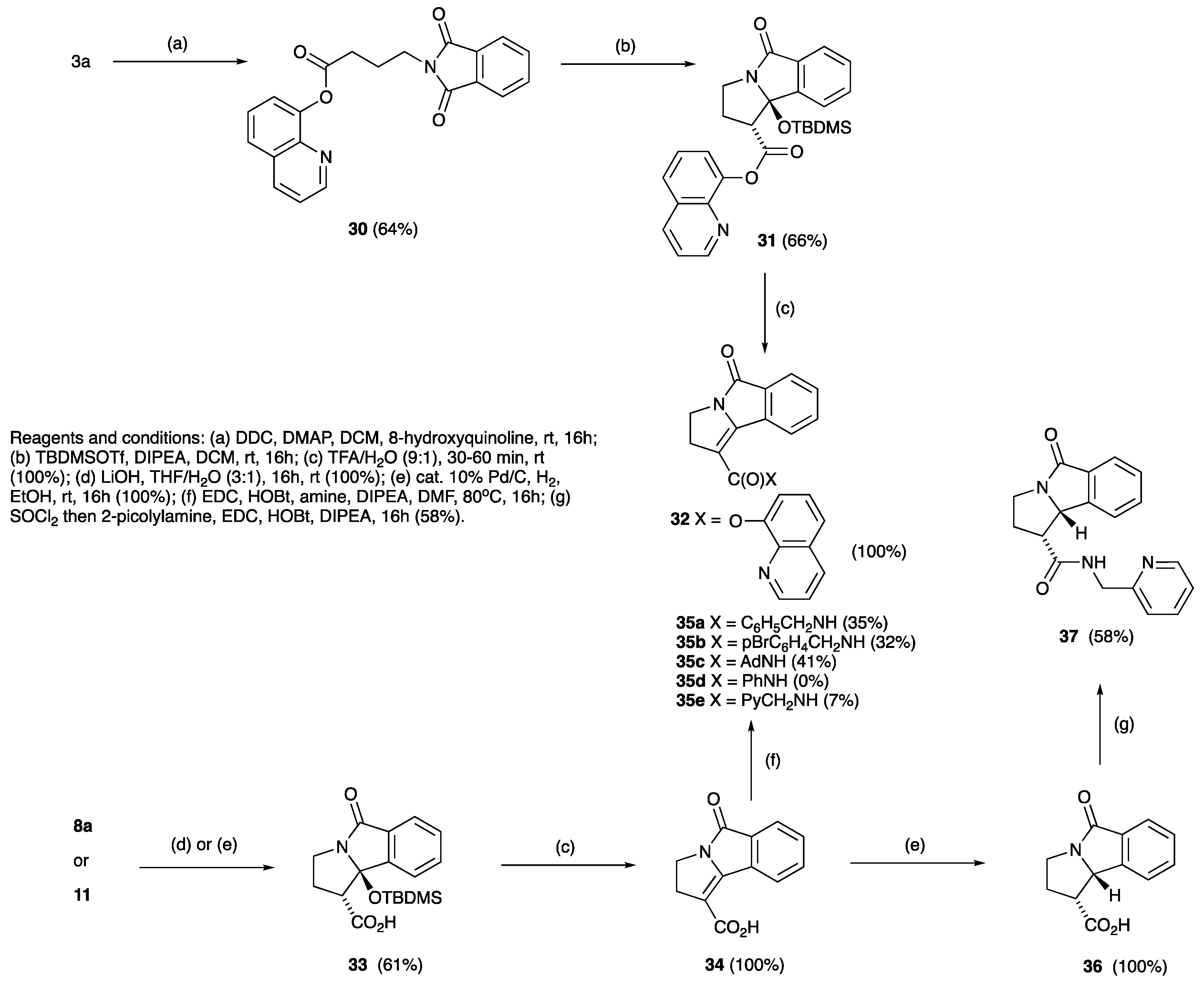
2. Results and Discussion
3. Bioassays
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References and Notes
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the 30 years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Benjamin, D.K.; Bradley, J.; Guidos, R.J.; Jones, R.N.; Murray, B.E.; Bonomo, R.A.; Gilbert, D. 10 × ’20 Progress—Development of new drugs active against gram-negative bacilli: An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, 1685–1694. [Google Scholar] [CrossRef] [PubMed]
- Butler, M.S.; Cooper, M.A. Antibiotics in the clinical pipeline in 2011. J. Antibiot. 2011, 64, 413–425. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; Review on Antimicrobial Resistance: London, UK, 2014. [Google Scholar]
- Singh, S.B. Confronting the challenges of discovery of novel antibacterial agents. Biorg. Med. Chem. Lett. 2014, 24, 3683–3689. [Google Scholar] [CrossRef] [PubMed]
- Silver, L.L. Challenges of antibacterial discovery. Clin. Microbiol. Rev. 2011, 24, 71–109. [Google Scholar] [CrossRef]
- Dick, T.; Young, D. How antibacterials really work: Impact on drug discovery. Future Microbiol. 2011, 6, 603–604. [Google Scholar] [CrossRef]
- Gwynn, M.N.; Portnoy, A.; Rittenhouse, S.F.; Payne, D.J. Challenges of antibacterial discovery revisited. Ann. N. Y. Acad. Sci. 2010, 1213, 5–19. [Google Scholar] [CrossRef]
- Walsh, C.T.; Wencewicz, T.A. Prospects for new antibiotics: A molecule-centered perspective. J. Antibiot. 2014, 67, 7–22. [Google Scholar] [CrossRef]
- Wencewicz, T.A. New antibiotics from Nature’s chemical inventory. Bioorg. Med. Chem. Lett. 2016, 24, 6227–6252. [Google Scholar] [CrossRef]
- Payne, D.J.; Gwynn, M.N.; Holmes, D.J.; Pompliano, D.L. Drugs for bad bugs: Confronting the challenges of antibacterial discovery. Nat. Rev. Drug Discov. 2007, 6, 29–40. [Google Scholar] [CrossRef]
- Cragg, G.M.; Grothaus, P.G.; Newman, D.J. Impact of natural products on developing new anti-cancer agents. Chem. Rev. 2009, 109, 3012–3043. [Google Scholar] [CrossRef] [PubMed]
- Walsh, D.P.; Chang, Y.-T. Chemical genetics. Chem. Rev. 2006, 106, 2476–2530. [Google Scholar] [CrossRef] [PubMed]
- Morel, C.; Mossialos, E. Stoking the antibiotic pipeline. Br. Med. J. 2010, 340, 1115–1118. [Google Scholar] [CrossRef] [PubMed]
- So, A.D.; Gupta, N.; Cars, O. Tackling antibiotic resistance. Br. Med. J. 2010, 340, 1091–1092. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.A.; Waldmann, H. Protein structure similarity clustering and natural product structure as guiding principles in drug discovery. Drug Discov. Today 2005, 10, 471–483. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, R.; Dekker, F.J.; Waldmann, H. Design of compound libraries based on natural product scaffolds and protein structure similarity clustering (PSSC). Mol. BioSyst. 2005, 1, 36–45. [Google Scholar] [CrossRef]
- Danishefsky, S. On the potential of natural products in the discovery of pharma leads: A case for reassessment. Nat. Prod. Rep. 2010, 27, 1114–1116. [Google Scholar] [CrossRef]
- Khan, M.K.; Wang, D.; Moloney, M.G. Functionalised Nitrogen Heterocycles and the Search for New Antibacterials and Bioactives. Synthesis 2020, 52, 1602–1616. [Google Scholar] [CrossRef]
- Singh, S.B.; Zink, D.L.; Goetz, M.A.; Dombrowski, A.W.; Polishook, J.D.; Hazuda, D.J. Equisetin and a novel opposite stereochemical homolog phomasetin, two fungal metabolites as inhibitors of HIV-1 integrase. Tetrahedron Lett. 1998, 39, 2243–2246. [Google Scholar] [CrossRef]
- Ganzle, M.G. Reutericyclin: Biological activity, mode of action, and potential applications. Appl. Microbiol. Biotechnol. 2004, 64, 326–332. [Google Scholar] [CrossRef]
- Phillips, J.W.; Goetz, M.; Smith, S.; Zink, D.; Polishook, J.; Onishi, R.; Salowe, S.; Wiltsie, J.; Allocco, J.; Sigmund, J.; et al. Discovery of kibdelomycin, a potent new class of bacterial type II topoisomerase inhibitor by chemical-genetic profiling in Staphylococcus aureus. Chem. Biol. 2011, 18, 955–965. [Google Scholar] [CrossRef] [PubMed]
- Tuske, S.; Sarafianos, S.G.; Wang, X.; Hudson, B.; Sineva, E.; Mukhopadhyay, J.; Birktoft, J.J.; Leroy, O.; Ismail, S.; Clark, A.D.; et al. Inhibition of Bacterial RNA Polymerase by Streptolydigin: Stabilization of a Straight-Bridge-Helix Active-Center Conformation. Cell 2005, 122, 541–552. [Google Scholar] [CrossRef] [PubMed]
- Moloney, M.G.; Trippier, P.C.; Yaqoob, M.; Wang, Z. The Oxazolomycins: A Structurally Novel Class of Bioactive Compounds. Curr. Drug Discov. Technol. 2004, 1, 181–199. [Google Scholar] [CrossRef]
- Schwartz, R.E.; Helms, G.L.; Bolessa, E.A.; Wilson, K.E.; Giacobbe, R.A.; Tkacz, J.S.; Bills, G.F.; Liesch, J.M.; Zink, D.L.; Curotto, J.E.; et al. Pramanicin, a novel antimicrobial agent from a fungal fermentation. Tetrahedron 1994, 50, 1675–1686. [Google Scholar] [CrossRef]
- Jeong, Y.-C.; Moloney, M.G. Tetramic Acids as Scaffolds: Synthesis, Tautomeric and Antibacterial Behaviour. Synlett 2009, 2487–2491. [Google Scholar] [CrossRef]
- Nogawa, T.; Kawatani, M.; Uramoto, M.; Okano, A.; Aono, H.; Futamura, Y.; Koshino, H.; Takahashi, S.; Osada, H. Pyrrolizilactone, a new pyrrolizidinone metabolite produced by a fungus. J. Antibiot. 2013, 66, 621–623. [Google Scholar] [CrossRef]
- Nakai, R.; Ishida, H.; Asai, A.; Ogawa, H.; Yamamoto, Y.; Kawasaki, H.; Akinaga, S.; Mizukami, T.; Yamashita, Y. Telomerase inhibitors identified by a forward chemical genetics approach using a yeast strain with shortened telomere length. Chem. Biol. 2006, 13, 183–190. [Google Scholar] [CrossRef]
- Agatsuma, T.; Akama, T.; Nara, S. Matsumiya, S.; Nakai, R.; Ogawa, H.; Otaki, S.; Ikeda, S.-I.; Saitoh, Y.; Kanda, Y. UCS1025A and B, new antitumor antibiotics from the fungus Acremonium species. Org. Lett. 2002, 4, 4387–4390. [Google Scholar] [CrossRef]
- Nakai, R.; Ogawa, H.; Asai, A.; Ando, K.; Agaisuma, T.; Maisumiya, S.; Akinaga, S.; Yamashita, Y.; Mizukami, T. UCS1025A, a Novel Antibiotic Produced by Acremonium sp. J. Antibiot. 2000, 53, 294–296. [Google Scholar] [CrossRef]
- Sugie, Y.; Hirai, H.; Kachi-Tonai, H.; Kim, Y.-J.; Kojima, Y.; Shiomi, Y.; Sugiura, A.; Suzuki, Y.; Yoshikawa, N.; Brennan, L.; et al. New Pyrrolizidinone Antibiotics CJ-16, 264 and CJ-16, 367. J. Antibiot. 2001, 54, 917–925. [Google Scholar] [CrossRef]
- Li, L.; Tang, M.C.; Tang, S.; Gao, S.; Soliman, S.; Hang, L.; Xu, W.; Ye, T.; Watanabe, K.; Tang, Y. Genome Mining and Assembly-Line Biosynthesis of the UCS1025A Pyrrolizidinone Family of Fungal Alkaloids. J. Am. Chem. Soc. 2018, 140, 2067–2071. [Google Scholar] [CrossRef] [PubMed]
- De Figueiredo, R.M.; Fröhlich, R.; Christmann, M. Efficient Synthesis and Resolution of Pyrrolizidines. Angew. Chem. Int. Ed. 2007, 46, 2883–2886. [Google Scholar] [CrossRef] [PubMed]
- Lambert, T.H.; Danishefsky, S.J. Total Synthesis of UCS1025A. J. Am. Chem. Soc. 2006, 128, 426–427. [Google Scholar] [CrossRef] [PubMed]
- Hoye, T.R.; Dvornikovs, V. Comparative Diels−Alder Reactivities within a Family of Valence Bond Isomers: A Biomimetic Total Synthesis of (±)-UCS1025A. J. Am. Chem. Soc. 2006, 128, 2550–2551. [Google Scholar] [CrossRef]
- Nozaki, K.; Oshima, K.; Utimoto, K. Trialkylborane as an initiator and terminator of free radical reactions. Facile routes to boron enolates via α-carbonyl radicals and aldol reaction of boron enolates. Bull. Chem. Soc. Jpn. 1991, 64, 403–409. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Pulukuri, K.K.; Rigol, S.; Buchman, M.; Shah, A.A.; Cen, N.; McCurry, M.D.; Beabout, K.; Shamoo, Y. Enantioselective Total Synthesis of Antibiotic CJ-16,264, Synthesis and Biological Evaluation of Designed Analogues, and Discovery of Highly Potent and Simpler Antibacterial Agents. J. Am. Chem. Soc. 2017, 139, 15868–15877. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Rigol, S.; Yu, R. Total Synthesis Endeavors and Their Contributions to Science and Society: A Personal Account. CCS Chem. 2019, 1, 3–37. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Rigol, S. A brief history of antibiotics and select advances in their synthesis. J. Antibiot. 2017, 71, 153–184. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Shah, A.A.; Korman, H.; Khan, T.; Shi, L.; Worawalai, W.; Theodorakis, E.A. Total Synthesis and Structural Revision of Antibiotic CJ-16,264. Angew. Chem. Int. Ed. 2015, 54, 9203–9208. [Google Scholar] [CrossRef]
- Lambert, T.H.; Danishefsky, S.J. Synthesis of UCS1025A. Synfacts 2006, 0536. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Shi, L.; Lu, M.; Pattanayak, M.R.; Shah, A.A.; Ioannidou, H.A.; Lamani, M. Total Synthesis of Myceliothermophins C, D, and E. Angew. Chem. Int. Ed. 2014, 126, 11150–11154. [Google Scholar] [CrossRef]
- Martinez, S.T.; Belouezzane, C.; Pinto, A.C.; Glasnov, T. Synthetic strategies towards the azabicyclo 3.3.0-octane core of natural pyrrolizidine alkaloids. an overview. Org. Prep. Proc. Int. 2016, 48, 223–253. [Google Scholar] [CrossRef]
- Uchida, K.; Ogawa, T.; Yasuda, Y.; Mimura, H.; Fujimoto, T.; Fukuyama, T.; Wakimoto, T.; Asakawa, T.; Hamashima, Y.; Kan, T. Stereocontrolled Total Synthesis of (+)-UCS1025A. Chem. Int. Ed. 2012, 51, 12850–12853. [Google Scholar] [CrossRef] [PubMed]
- Hoye, T.R.; Dvornikovs, V.; Sizova, E. Silylative Dieckmann-Like Cyclizations of Ester-Imides (and Diesters). Org. Lett. 2006, 8, 5191–5194. [Google Scholar] [CrossRef]
- De Figueiredo, R.M.; Oczipka, P.; Fröhlich, R.; Christmann, M. Synthesis of 4-Maleimidobutyric Acid and Related Maleimides. Synthesis 2008, 1316–1318. [Google Scholar] [CrossRef]
- De Figueiredo, R.M.; Voith, M.; Fröhlich, R.; Christmann, M. Synthesis of a Malimide Analogue of the Telomerase Inhibitor UCS1025A Using a Dianionic Aldol Strategy. Synlett 2007, 3, 391–394. [Google Scholar] [CrossRef]
- Ibbotson, L.T.; Christensen, K.E.; Genov, M.; Pretsch, A.; Pretsch, D.; Moloney, M.G. Skeletal Analogues of UCS1025A and B by Cyclization of Maleimides: Synthesis and Biological Activity. Synlett 2022, 33, 396–400. [Google Scholar] [CrossRef]
- Guénin, E.; Monteil, M.; Bouchemal, N.; Prangé, T.; Lecouvey, M. Syntheses of phosphonic esters of alendronate, pamidronate and neridronate. Eur. J. Org. Chem. 2007, 20, 3380–3391. [Google Scholar] [CrossRef]
- Wu, H.; Wu, J.; Zhang, W.; Li, J.; Fang, J.; Lian, X.; Qin, T.; Hao, J.; Zhou, Q.; Wu, S. Discovery and structure-activity relationship study of phthalimide-phenylpyridine conjugate as inhibitor of Wnt pathway. Bioorg. Med. Chem. Lett. 2019, 29, 870–872. [Google Scholar] [CrossRef]
- Dato, F.M.; Sheikh, M.; Uhl, R.Z.; Schüller, A.W.; Steinkrüger, M.; Koch, P.; Neudörfl, J.M.; Gütschow, M.; Goldfuss, B.; Pietsch, M. ω-Phthalimidoalkyl Aryl Ureas as Potent and Selective Inhibitors of Cholesterol Esterase. ChemMedChem 2018, 13, 1833–1847. [Google Scholar] [CrossRef]
- Gabbasov, T.M.; Tsyrlina, E.M.; Spirikhin, L.V.; Yunusov, M.S. Amides of N-Deacetyllappaconitine and Amino Acids. Chem. Nat. Compd. 2018, 54, 951–955. [Google Scholar] [CrossRef]
- Griesbeck, A.G.; Henz, A.; Kramer, W.; Lex, J.; Nerowski, F.; Oelgemöller, M.; Peters, K.; Peters, E.M. Synthesis of Medium-and Large-Ring Compounds Initiated by Photochemical Decarboxylation of ω-Phthalimidoalkanoates. Helv. Chim. Acta 1997, 80, 912–933. [Google Scholar] [CrossRef]
- Low temperature single crystal X-ray diffraction data for 6b, 8a, 8b, 8c, 12a, 21, 16b, 23, 27f and 27b were collected using a Rigaku Oxford SuperNova diffractometer and data for 9 were collected at Diamond Light Source, Beamline I19-1. Raw frame data were reduced using CrysAlisPro and the structures were solved using ‘Superflip’ before refinement with CRYSTALS as per the CIF. Full refinement details are given in the Supporting Information (CIF); Crystallographic data have been deposited with the Cambridge Crystallographic Data Centre (CCDC 2160046-56).
- Palatinus, L.; Chapuis, G.J. SUPERFLIP—A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Parois, P.; Cooper, R.I.; Thompson, A.L. Crystal structures of increasingly large molecules: Meeting the challenges with CRYSTALS software. Chem. Cent. J. 2015, 9, 30. [Google Scholar] [CrossRef] [PubMed]
- Cooper, R.I.; Thompson, A.L.; Watkin, D.J. CRYSTALS enhancements: Dealing with hydrogen atoms in refinement. J. Appl. Cryst. 2010, 43, 1100–1107. [Google Scholar] [CrossRef]
- Yoon, U.C.; Lee, C.W.; Oh, S.W.; Mariano, P.S. Exploratory studies probing the intermediacy of azomethine ylides in the photochemistry of N-phthaloyl derivatives of α-amino acids and β-amino alcohols. Tetrahedron 1999, 55, 11997–12008. [Google Scholar] [CrossRef]
- Takahashi, Y.; Miyashi, T.; Yoon, U.C.; Oh, S.W.; Mancheno, M.; Su, Z.; Falvey, D.F.; Mariano, P.S. Mechanistic Studies of the Azomethine Ylide-Forming Photoreactions of N-(Silylmethyl)phthalimides and N-Phthaloylglycine. J. Am. Chem. Soc. 1999, 121, 3926–3932. [Google Scholar] [CrossRef]
- Yoon, U.C.; Kim, D.U.; Lee, Y.J.; Choi, Y.S.; Lee, Y.-J.; Ammon, H.L.; Mariano, P.S. Novel and efficient azomethine ylide forming photoreactions of N-(silylmethyl) phthalimides and related acid and alcohol derivative. J. Am. Chem. Soc. 1995, 117, 2698–2710. [Google Scholar] [CrossRef]
- Muchowski, J.M.; Nelson, P.H. The reaction of carboalkyxyclopropyltriphenylphosphonium salts with imide anions: A three-step synthesis of ±isoretronecanol. Tetrahedron Lett. 1980, 21, 4585–4588. [Google Scholar] [CrossRef]
- Fuchs, P.L. Carboethoxycyclopropyltriphenylphosphonium fluoroborate. Reagent for the facile cycloalkenylation of carbonyl groups J. Am. Chem. Soc. 1974, 96, 1607–1609. [Google Scholar]
- Maury, J.; Mouysset, D.; Feray, L.; Marque, S.R.A.; Siri, D.; Bertrand, M.P. Aminomethylation of Michael Acceptors: Complementary Radical and Polar Approaches Mediated by Dialkylzincs. Chem.—Eur. J. 2012, 18, 3241–3247. [Google Scholar] [CrossRef] [PubMed]
- Border, S.E.; Pavlović, R.Z.; Lei, Z.; Gunther, M.J.; Wang, H.; Cui, H.; Badjić, J.D. Light Triggered Transformation of Molecular Baskets into Organic Nanoparticles. Chem.—Eur. J. 2019, 25, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Jamel, N.M.; Alheety, K.A.; Ahmed, B.J. Methods of Synthesis Phthalimide Derivatives and Biological Activity—Review. J. Pharm. Sci. Res. 2019, 11, 3348–3354. [Google Scholar]
- Wang, W.; Ding, J.; Xiao, C.; Tang, Z.; Li, D.; Chen, J.; Zhuang, X.; Chen, X. Synthesis of amphiphilic alternating polyesters with oligo (ethylene glycol) side chains and potential use for sustained release drug delivery. Biomacromolecules 2011, 12, 2466–2474. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.H.; Goel, O.P.; Magano, J.; Rubin, J.R.; Company, W.-L.; Arbor, A. An efficient stereoselective synthesis of [3S(1S,9S)]-3-[[[9-(benzoylamino)octahydro-6,10-dioxo-6H-pyridazino-(1,2-a)(1,2)-diazepin-1-yl]-carbonyl]amino]-4-oxobutanoic acid, an interleukin converting enzyme (ICE) inhibitor. Bioorg. Med. Chem. Lett. 1999, 9, 1587–1592. [Google Scholar] [CrossRef]
- King, F.E.; Clark-Lewis, J.W.; Wade, R.; Swindin, W.A. Syntheses from phthalimido-acids. Part VII. Oxazolones and other intermediates in the synthesis of phthalylpeptides, and an investigation on maleic acid. J. Chem. Soc. 1957, 166, 873–880. [Google Scholar] [CrossRef]
- McKay, A.F.; Garmaise, D.L.; Gaudry, R.; Baker, H.A.; Paris, G.Y.; Kay, R.W.; Just, G.E.; Schwartz, R. Bacteriostats. II.1 The Chemical and Bacteriostatic Properties of Isothiocyanates and their Derivatives. J. Am. Chem. Soc. 1959, 81, 4328–4335. [Google Scholar] [CrossRef]
- Schlessinger, R.H.; Poss, M.A.; Richardson, S. Total synthesis of (+)-rosaramicin aglycone and its diacetate. J. Am. Chem. Soc. 1986, 108, 3112–3114. [Google Scholar] [CrossRef]
- Baker, R.; Cummings, W.J.; Hayes, J.F.; Kumar, A. Enantiospecific synthesis of the C-9 to C-18 fragment of macbecins I and II. J. Chem. Soc. Chem. Commun. 1986, 1, 1237–1239. [Google Scholar] [CrossRef]
- Robins, M.J.; Samano, V.; Johnson, M.D. Nucleic acid-related compounds. 58. Periodinane oxidation, selective primary deprotection, and remarkably stereoselective reduction of tert-butyldimethylsilyl-protected ribonucleosides. Synthesis of 9-(.beta.-D-xylofuranosyl)adenine or 3’-deuterioadenosine from adenosine. J. Org. Chem. 1990, 55, 410–412. [Google Scholar] [CrossRef]
- Martin, S.F.; Dodge, J.A.; Burgess, L.E.; Hartmann, M. A formal total synthesis of (+)-macbecin I. J. Org. Chem. 1992, 57, 1070–1072. [Google Scholar] [CrossRef]
- Mukaiyama, T.; Kobayashi, S. Tin(II) Enolates in the Aldol, Michael, and Related Reactions. Org. React. 1994, 46, 1–103. [Google Scholar]
- Banno, K. New Cross Aldol Reactions. Titanium Tetrachloride-promoted Reactions of Silyl Enol Ethers with Carbonyl Compounds Containing A Functional Group. Bull. Chem. Soc. Jpn. 1976, 49, 2284–2291. [Google Scholar] [CrossRef]
- Kalita, H.R.; Borah, A.J.; Phukan, P. Efficient allylation of aldehydes with allyltributylstannane catalyzed by CuI. Tetrahedron Lett. 2007, 48, 5047–5049. [Google Scholar] [CrossRef]
- Downey, C.W.; Johnson, M.W. A tandem enol silane formation-Mukaiyama aldol reaction mediated by TMSOTf. Tetrahedron Lett. 2007, 48, 3559–3562. [Google Scholar] [CrossRef]
- Mahrwald, R. Diastereoselection in Lewis-acid-mediated aldol additions. Chem. Rev. 1999, 99, 1095–1120. [Google Scholar] [CrossRef] [PubMed]
- Phukan, P. Mukaiyama aldol reactions of silyl enolates catalyzed by iodine. Syn. Commun. 2004, 34, 1065–1070. [Google Scholar] [CrossRef]
- Han, J.H.; Kim, S.B.; Mukaiyama, T. A New Catalyst System for the Aldol Type Condensation of Silyl Enol Ethers and Ketene Silyl Acetals. Bull. Korean Chem. Soc. 1994, 15, 529–531. [Google Scholar] [CrossRef]
- Wade Downey, C.; Ingersoll, J.A.; Glist, H.M.; Dombrowski, C.M.; Barnett, A.T. One-Pot Silyl Ketene Acetal-Formation Mukaiyama–Mannich Additions to Imines Mediated by Trimethylsilyl Trifluoromethanesulfonate. Eur. J. Org. Chem. 2015, 2015, 7287–7291. [Google Scholar] [CrossRef]
- Cottrell, I.F.; Davis, P.J.; Moloney, M.G. Stereoselective oxygenation of bicyclic lactams. Tetrahedron Asymmetry 2004, 15, 1239–1242. [Google Scholar] [CrossRef]
- Tan, S.W.B.; Chai, C.L.L.; Moloney, M.G. Mimics of pramanicin derived from pyroglutamic acid and their antibacterial activity. Org. Biomol. Chem. 2017, 15, 1889–1912. [Google Scholar] [CrossRef] [PubMed]
- Verho, O.; Maetani, M.; Melillo, B.; Zoller, J.; Schreiber, S.L. Stereospecific palladium-catalyzed C–H arylation of pyroglutamic acid derivatives at the C3 position enabled by 8-aminoquinoline as a directing group. Org. Lett. 2017, 19, 4424–4427. [Google Scholar] [CrossRef]
- Rej, S.; Ano, Y.; Chatani, N. Bidentate directing groups: An efficient tool in C–H bond functionalization chemistry for the expedient construction of C–C bonds. Chem. Rev. 2020, 120, 1788–1887. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wang, Y.; Wang, Z.; Zeng, T.; Liu, P.; Engle, K.M. Catalytic intermolecular carboamination of unactivated alkenes via directed aminopalladation. J. Am. Chem. Soc. 2017, 139, 11261–11270. [Google Scholar] [CrossRef] [PubMed]
- Corbet, M.; De Campo, F. 8-Aminoquinoline: A Powerful Directing Group in Metal-Catalyzed Direct Functionalization of C-H Bonds. Angew. Chem.—Int. Ed. 2013, 52, 9896–9898. [Google Scholar] [CrossRef] [PubMed]
- Ko, K.S.; Park, G.; Yu, Y.; Pohl, N.L. Protecting-Group-Based Colorimetric Monitoring of Fluorous-Phase and Solid-Phase Synthesis of Oligoglucosamines. Org. Lett. 2008, 10, 5381–5384. [Google Scholar] [CrossRef] [PubMed]
- Caswell, L.R.; Yang, K.C.C. Nitrophthaloyl and aminophthaloyl derivatives of amino acids. J. Chem. Eng. Data 1968, 13, 291–292. [Google Scholar] [CrossRef]
- Staubli, A.; Ron, E.; Langer, R. Hydrolytically degradable amino acid-containing polymers. J. Am. Chem. Soc. 1990, 112, 4419–4424. [Google Scholar] [CrossRef]
- Richards, J.C.; Spenser, I.D. The stereochemistry of the enzymic decarboxylation of L-arginine and of L-ornithine. Can. J. Chem. 1982, 60, 2810–2820. [Google Scholar] [CrossRef]
- Tomar, R.; Bhattacharya, D.; Babu, S.A. Assembling of medium/long chain-based β-arylated unnatural amino acid derivatives via the Pd(II)-catalyzed sp3 β-C-H arylation and a short route for rolipram-type derivatives. Tetrahedron 2019, 75, 2447–2465. [Google Scholar] [CrossRef]
- Vatulina, G.G.; Tuzhilkova, T.N.; Matveeva, T.V.; Krasnov, V.P.; Burde, N.L.; Alekseeva, L.V. Search for radioprotectors in the series of glutamic acid derivatives. Chem. Pharm. J. 1986, 20, 647–653. [Google Scholar] [CrossRef]
- Robl, J.A. Peptidomimetic synthesis: Utilization of N-acyliminium ion cyclization chemistry in the generation of 7, 6-and 7, 5-fused bicyclic lactams. Tetrahedron Lett. 1994, 35, 393–396. [Google Scholar] [CrossRef]
- Fife, T.H.; Duddy, N.W. Intramolecular aminolysis of esters. Cyclization of esters of (o-aminophenyl) acetic acid. J. Am. Chem. Soc. 1983, 105, 74–79. [Google Scholar] [CrossRef]
- Kim, G.; Keum, G. A new route to quinolone and indole skeletons via ketone-and ester-imide cyclodehydration reactions. Heterocycles 1997, 45, 1979–1988. [Google Scholar] [CrossRef]
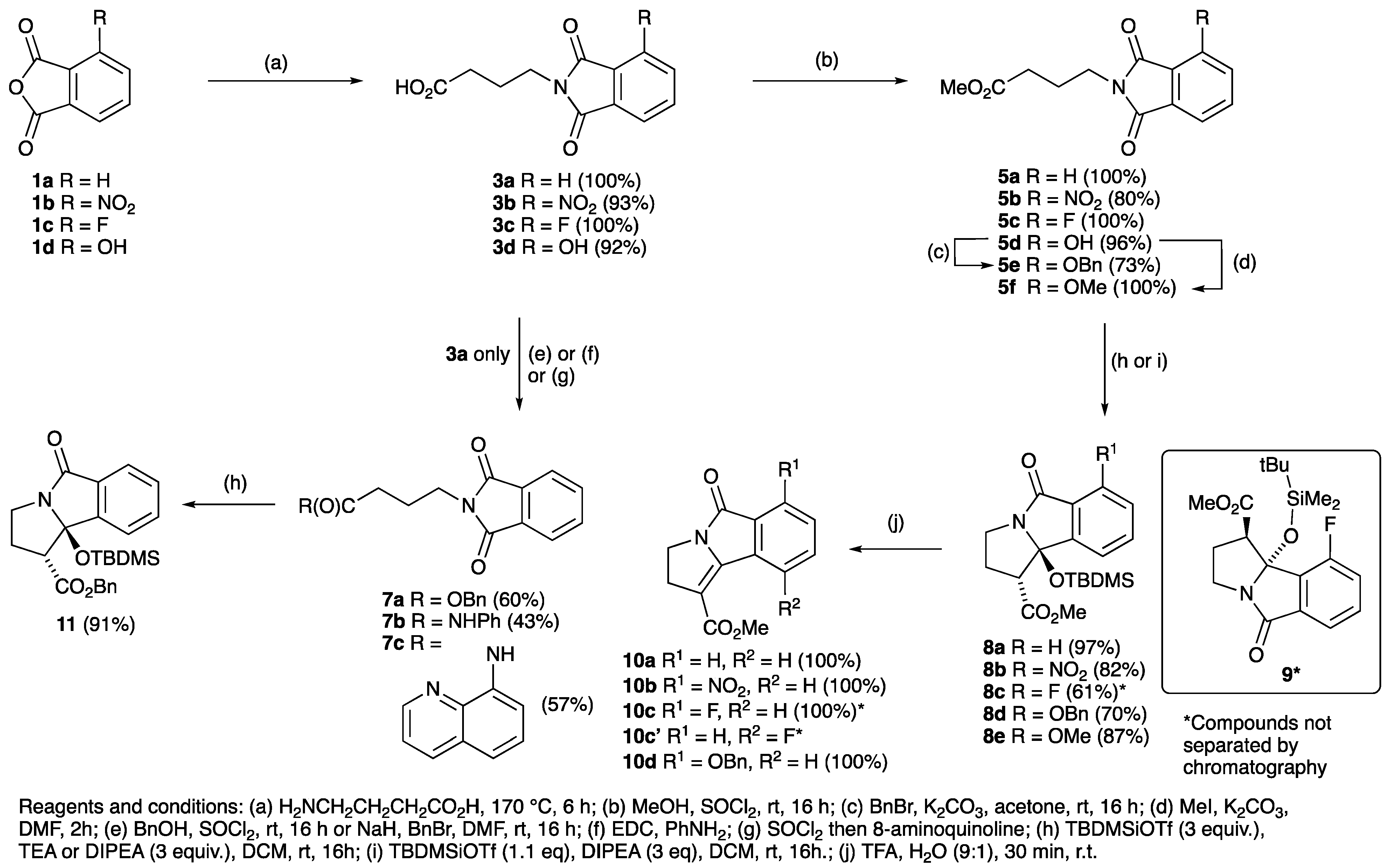
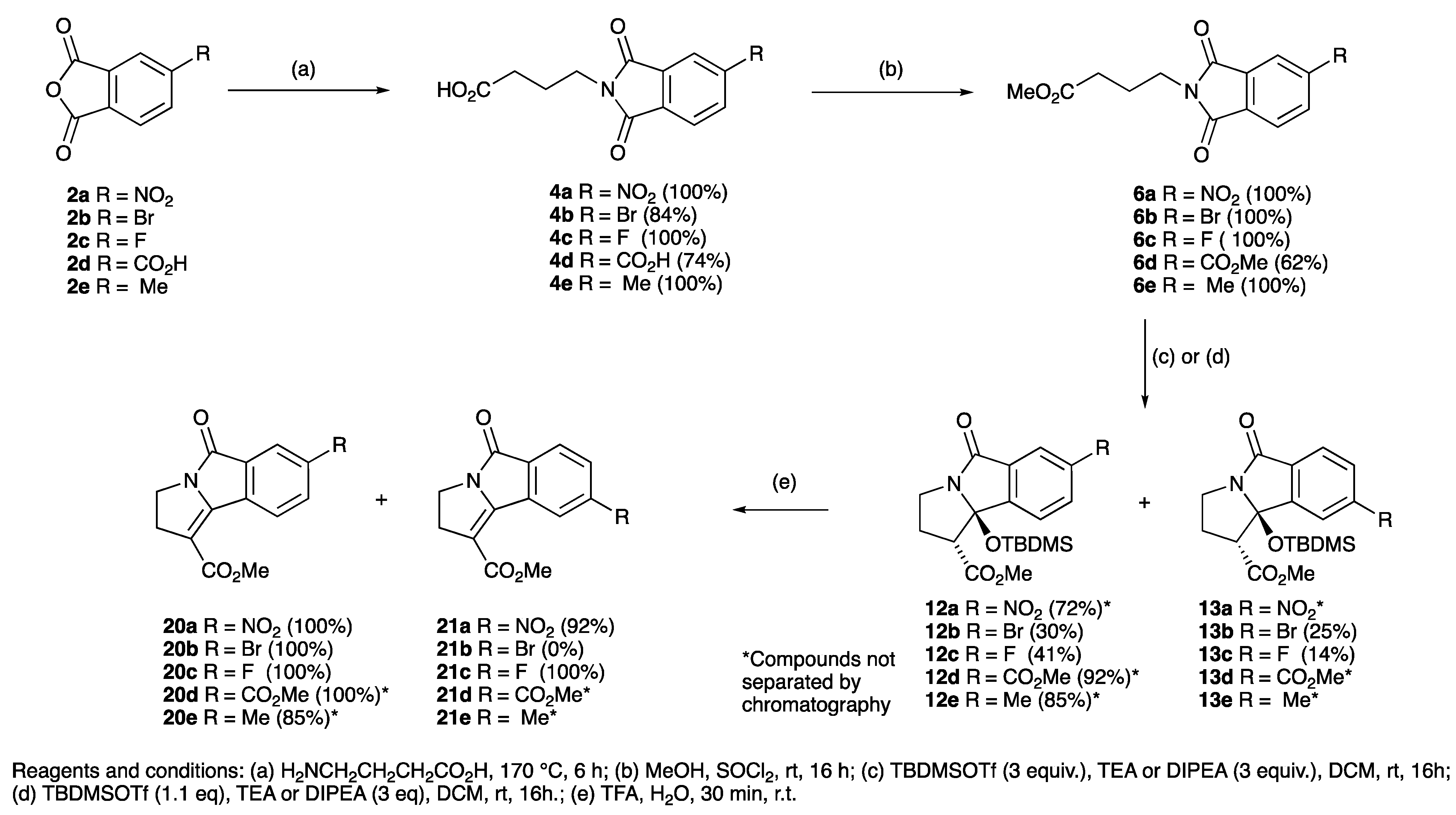
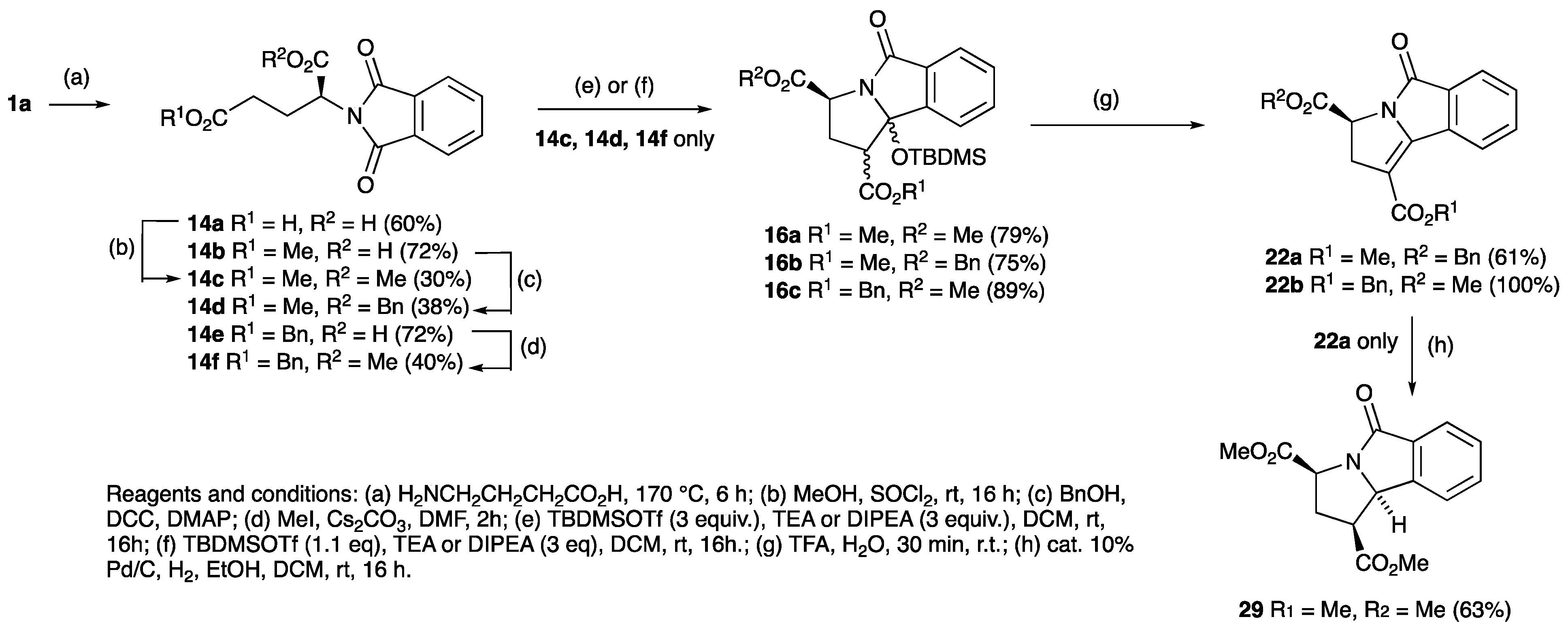
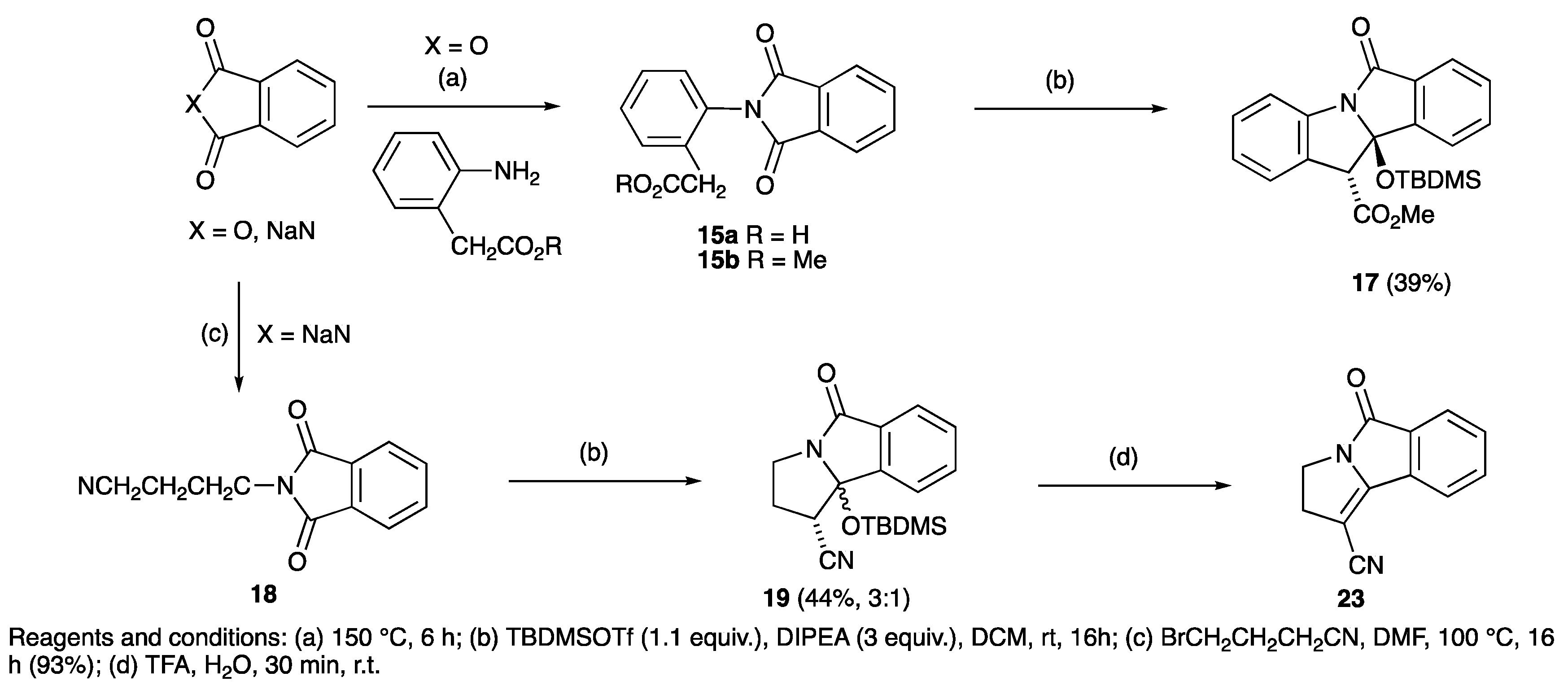



| R | Phthalimide | Temperature (°C) | Yield (%) | Ester | Yield (%) | Lactam | Yield (%) |
|---|---|---|---|---|---|---|---|
| H | 3a | 170 | 100 | 5a | 100 | 8a | 97 |
| NO2 | 3b | 175 | 93 | 5b | 80 | 8b | 82 |
| F | 3c | 170 | 100 | 5c | 100 | 8c/9 | 61 * |
| OH | 3d | 195 | 92 | 5d | 96 | - | - |
| OBn | - | - | - | 5e | 73 | 8d | 70 |
| OMe | - | - | - | 5f | 100 | 8e | 87 |
| NO2 | 4a | 175 | 100 | 6a | 100 | 12a,13a | 72 * |
| Br | 4b | 170 | 84 | 6b | 0 (100) | 12b,13b | 55 * |
| F | 4c | 170 | 100 | 6c | 100 | 12c,13c | 55 * |
| CO2H | 4d | 205 | 74 | - | - | - | - |
| CO2Me | - | - | - | 6d | 62 | 12d,13d | 92 * |
| CH3 | 4e | 170 | 100 | 6e | 100 | 12e,13e | 85 * |
| Substrate | Product | R1 | R2 | R3 | R4 | Yield (%) |
|---|---|---|---|---|---|---|
| 10a | 27a | H | H | H | H | 100 |
| 10b | 27b | NH2 | H | H | H | 100 |
| 20a | 27c | H | NH2 | H | H | 100 |
| 21a | 27d | H | H | NH2 | H | 100 |
| 10c | 27e | F | H | H | H | 50 |
| 10c’ | 27f | H | H | H | F | 14 |
| 20c | 27g | H | F | H | H | 100 |
| 10d | 27h | OH | H | H | H | 86 |
| 20e | 27i | H | Me | H | H | 95 |
| 21e | 27j | H | H | Me | H |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ibbotson, L.T.; Christensen, K.E.; Genov, M.; Pretsch, A.; Pretsch, D.; Moloney, M.G. Tricyclic Fused Lactams by Mukaiyama Cyclisation of Phthalimides and Evaluation of their Biological Activity. Antibiotics 2023, 12, 9. https://doi.org/10.3390/antibiotics12010009
Ibbotson LT, Christensen KE, Genov M, Pretsch A, Pretsch D, Moloney MG. Tricyclic Fused Lactams by Mukaiyama Cyclisation of Phthalimides and Evaluation of their Biological Activity. Antibiotics. 2023; 12(1):9. https://doi.org/10.3390/antibiotics12010009
Chicago/Turabian StyleIbbotson, Lewis T., Kirsten E. Christensen, Miroslav Genov, Alexander Pretsch, Dagmar Pretsch, and Mark G. Moloney. 2023. "Tricyclic Fused Lactams by Mukaiyama Cyclisation of Phthalimides and Evaluation of their Biological Activity" Antibiotics 12, no. 1: 9. https://doi.org/10.3390/antibiotics12010009
APA StyleIbbotson, L. T., Christensen, K. E., Genov, M., Pretsch, A., Pretsch, D., & Moloney, M. G. (2023). Tricyclic Fused Lactams by Mukaiyama Cyclisation of Phthalimides and Evaluation of their Biological Activity. Antibiotics, 12(1), 9. https://doi.org/10.3390/antibiotics12010009





