Abstract
Flavonoids are secondary metabolites that exhibit remarkable biological activities, including antimicrobial properties against Klebsiella pneumoniae, a pathogen responsible for several serious nosocomial infections. However, oral administration of these compounds faces considerable challenges, such as low bioavailability and chemical instability. Thus, the encapsulation of flavonoids in nanosystems emerges as a promising strategy to mitigate these limitations, offering protection against degradation; greater solubility; and, in some cases, controlled and targeted release. Different types of nanocarriers, such as polymeric nanoparticles, liposomes, and polymeric micelles, among others, have shown potential to increase the antimicrobial efficacy of flavonoids by reducing the therapeutic dose required and minimizing side effects. In addition, advances in nanotechnology enable co-encapsulation with other therapeutic agents and the development of systems responsive to more specific stimuli, optimizing treatment. In this context, the present article provides an updated review of the literature on flavonoids and the main nanocarriers used for delivering flavonoids with antibacterial properties against Klebsiella pneumoniae.
1. Introduction
Klebsiella pneumoniae is a Gram-negative bacterium belonging to the order Enterobacterales, commonly found in the human intestinal microbiota and frequently isolated from various environments, including aquatic bodies, food sources, and sewage systems [1,2,3]. K. pneumoniae can exist as symbionts, conditional pathogens, or outright pathogens, implicated in a range of infections such as urinary tract infections, liver abscesses, bacteremia, and pneumonia [4,5,6,7,8]. Notably, it is recognized as a nosocomial opportunist, particularly afflicting debilitated and immunocompromised patients. Its rapid dissemination within hospital settings underscores its significance as a hospital-acquired pathogen, contributing to considerable morbidity and mortality among hospitalized individuals [9,10].
In addition to these infections, Klebsiella pneumoniae is known for its capacity to develop resistance to antimicrobials, particularly exacerbated by the excessive and inappropriate use of antibiotics. The rising rates of bacterial resistance in K. pneumoniae present a global concern, as therapeutic regimens become less effective and the negative prognosis of patients increases significantly [3,11]. One of the primary antibiotic classes used to treat resistant strains of K. pneumoniae is carbapenems, given that a substantial proportion of resistant strains harbor a chromosomal β-lactamase gene, rendering penicillins such as ampicillin and amoxicillin ineffective [3,12]. However, there is currently a significant increase in the prevalence of carbapenem-resistant K. pneumoniae (CRKP) isolates, and infections caused by these strains are a global public health concern [13].
Given this situation, it is imperative to explore alternative therapies involving secondary metabolites with antibacterial potential, such as flavonoids. Flavonoids constitute a diverse group of polyphenolic secondary metabolites, characterized by their pigmentary properties, and are abundant in various foods, including coffee, fruits, and vegetables [14,15]. The term “flavonoids” derives from the Latin word “flavus”, meaning “yellow”. In plants, flavonoids play a crucial role in protecting against bacterial, fungal, and viral infections, oxidative damage, influencing fertility, regulating growth, and contributing to visual characteristics through their pigmentation [16,17,18,19,20]. The anti-inflammatory, antioxidant, and antimicrobial properties of flavonoids have been investigated in numerous experimental and clinical studies, demonstrating promising outcomes [14,21,22,23,24,25]. These characteristics underscore their value for further research, particularly in understanding their potential against bacterial, fungal, and viral infections.
These compounds are chemically characterized by two benzene rings, referred to as the A and B rings, linked by an oxygen-containing heterocyclic ring, known as the C ring. They can be categorized into various subclasses based on specific characteristics, including the type of linkage between the B and C rings; the molecular structure of the B ring; the distribution of hydroxyl and glycosyl groups across the three rings; and factors such as the level of saturation, oxidation, and hydroxylation in the C ring pattern. Consequently, these compounds can be classified into different subgroups, such as flavonols, flavones, flavanones, flavanols, isoflavones, chalcones, and anthocyanins [26]. This structural diversity contributes to a wide range of biological properties and potential health benefits associated with flavonoids, including anti-inflammatory, antioxidant, and antimicrobial activities [27,28].
Although flavonoids possess various pharmacological properties, their therapeutic application is hindered by several limitations, including photosensitivity, rapid metabolization, low bioavailability, and a short biological half-life. The low solubility in water is due to the presence of aromatic rings and hydroxyl groups (-OH), which form preferential interactions with each other, reducing interaction with water molecules. Photosensitivity results from the conjugated structure of flavonoids, which, upon absorbing light, can generate instability, free radicals, and photooxidation. Additionally, the rigid and planar structure, combined with multiple hydroxyl groups, hinders penetration through cellular membranes [29,30,31]. These characteristics have been identified as significant obstacles to their use as therapeutic agents. However, advances in nanotechnology offer the potential to overcome these challenges using nanocarriers for flavonoid delivery [32,33].
Nanosystems consist of particles or structures with nanometric dimensions, designed to achieve emergent properties at this scale, such as increased specific surface area and distinct optical, magnetic, and electronic characteristics. These systems are often synthesized to interact precisely and in a controlled manner with biological systems [34,35]. The size of nanoparticles is optimized according to the therapeutic target to maximize interaction with target cells and influence absorption mechanisms. Nanoparticles with extremely small dimensions may not be recommended in certain contexts due to their ability to traverse capillaries and reach unintended tissues or organs, which can result in adverse effects. In contrast, larger nanoparticles may have limitations in efficiently penetrating target cells, potentially reducing treatment efficacy. However, larger nanoparticles can be advantageous for specific applications, such as treatments targeting the intestinal tract [34,36,37,38].
2. Medicinal Plants: Prevalence, Synthesis, and Action of Flavonoids
Medicinal plants have played a significant role in human development and disease treatment. Historically, traditional communities have utilized plants to prevent and treat infections and various illnesses [39]. In this context, ethnopharmacology stands out, an area that studies the properties of bioactive natural compounds traditionally used by the population, thus being a source of discoveries of new safe and cheap medicines [40,41,42].
In this scenario, these natural compounds can be produced by a living being from its primary metabolism, used for growth and reproduction, or a secondary being, used as strategies for survival in stressful situations, for defense against pathogens and predators, and in resistance to climatic adversities [43]. There are studies showing the presence of flavonoids in some species of algae; however, the total amount of these compounds in these organisms is low. The presence of genes associated with the flavonoid biosynthetic pathway have also been studied in fungi. Despite this, outside the Plant Kingdom, the abundance of flavonoids in other living organisms is very low [44,45].
As products of secondary metabolism, flavonoids are part of the phytochemical profile of plants in great abundance and variety, depending on the taxonomic groups, since plants of the same family usually have a similar composition of metabolites, and on environmental conditions, such as the climatic conditions of the region, genotypes, and abundance of resources [46].
Flavonoids are primarily produced in plants through the phenylpropanoid pathway, which involves several enzymatic sub-pathways, including biosynthesis from phenylalanine. One of the flavonoids synthesized via this pathway is quercetin. The biosynthesis of quercetin begins with the conversion of phenylalanine into cinnamic acid by the enzyme phenylalanine ammonia-lyase (PAL). This is followed by the conversion of cinnamic acid into p-coumaric acid and subsequently into p-coumaryl-CoA. Chalcone synthase (CHS) then converts p-coumaryl-CoA into naringenin, which is hydroxylated to dihydroquercetin by flavanone 3-hydroxylase (F3H) and finally converted into quercetin by flavonol synthase (FLS) [24,47]. In 2020, Franco et al. [48] evaluated the phytochemical profile of Bauhinia forficata Link., an angiosperm of the Fabaceae family native to the Atlantic Forest and widely used in folk medicine, especially in the treatment of diabetes [49], and found 11 flavonoids including kaempferitrin, a flavonoid that mimics insulin. Another species whose phytochemical profile was analyzed is Allium sativum L., a member of the Amaryllidaceae family, known for its bulb used in both culinary and medicinal applications. The analysis revealed rutin as the predominant flavonoid, a molecule known for its various biological properties, including antimicrobial and anti-inflammatory activities [50,51,52]. Hamad et al. [51] extracted and purified rutin from A. sativum L., determining its immunomodulatory and anti-Schistosoma activity. Their findings indicated a reduction in hepatosplenomegaly and control of oxidative stress induced by the parasite. Abidullah et al. [53] demonstrated antibacterial activity against strains of S. aureus, E. coli, and K. pneumoniae, with inhibition zones measuring 28 mm, 27 mm, and 22 mm, respectively, for 10 μL quantities. Additionally, Wang et al. [54] demonstrated inhibition of biofilm formation and reduction in the expression of genes related to quorum sensing and bacterial virulence.
Another species whose phytochemical profile was analyzed is Ginkgo biloba L., the sole member of the Ginkgoaceae family of gymnosperms. Widely used in traditional Chinese medicine for treating bacterial and fungal infections, Ginkgo biloba is known for its high production of kaempferol, quercetin, and isorhamnetin, flavonoids responsible for its biological properties [55]. In this context, Chassagne et al. [56] demonstrated the plant’s efficacy in inhibiting bacterial growth and biofilm formation against Streptococcus pyogenes, Staphylococcus aureus, and Cutibacterium acnes, pathogens significant in “skin and soft tissue infections (SSTIs)”.
These compounds act as antioxidants and modulate polar auxin transport (PAT), thereby mitigating the effects of free radicals and enhancing plant robustness under adverse biotic and abiotic stress conditions [19,57,58,59]. Additionally, flavonoids contribute to the coloration and aroma of petals, promoting pollinator attraction [60,61]. In humans, flavonoids exhibit a broad spectrum of pharmacological properties, including antiviral, antiparasitic, antibacterial, glucose regulation, immune system modulation, neuroprotection, cardiovascular protection, rejuvenation, anticancer, and anti-inflammatory activities [23,60,62,63].
In addition to the antimicrobial activity exhibited by several families of medicinal plants due to their high flavonoid content, it has been found that these compounds, when used in conjunction with commercial antibiotics, can exhibit synergism and potentiate the drug’s effect. This synergy allows for dose reduction and decreased toxicity of conventional treatments, as well as aids in the combat of antimicrobial resistance (AMR) [52]. Among the flavonoids used in antibacterial combination therapy, quercetin is particularly noteworthy. Quercetin, a polyphenolic flavonoid, is a major component in various plant groups such as Allium cepa (onion), Hypericum perforatum, Ginkgo biloba, and Camellia sinensis [64].
Lin et al. [54] studied the effect of quercetin in combination with colistin to evaluate the reversal of colistin resistance in resistant E. coli and K. pneumoniae strains. The authors observed synergistic activity with fractional inhibitory concentration index (FICI), which consists of a mathematical method to assess whether the interaction between drugs is synergistic, additive, or antagonistic, with values less than or equal to 0.5 indicative of a synergistic effect. The obtained values ranged from 0.141 to 0.375, and 90% of the expression of the resistance gene mcr-1 in E. coli and 95% of mgr-B in K. pneumoniae were inhibited. Pal and Tripathi [65] demonstrated the bactericidal and synergistic activity of quercetin in combination with meropenem against carbapenem-resistant K. pneumoniae and E. coli. They found synergistic activity with FICI values between 0.093 and 0.500 for K. pneumoniae strains, alongside altered expression of the blaVIM and ompC genes and changes in bacterial cell morphology. These studies underscore the potential of flavonoids as antimicrobial agents against clinically significant strains, highlighting their role in protecting plants against phytopathogens [27,66]. Several antibacterial mechanisms have been reported for these compounds, presenting activity through intracellular and extracellular mechanisms, including inhibition of nucleic acid synthesis, inhibition of bacterial motility, inhibition of the electron transport chain and ATP synthesis, inhibition of toxin production, inhibition of biofilm formation, inhibition of bacterial enzyme-dependent virulence mechanisms, membrane disruption, inhibition of efflux pumps, and prevention of quorum sensing (Figure 1). These compounds, therefore, not only interfere with the energy metabolism of bacteria but also inhibit the synthesis of nucleic acids, which are essential for bacterial replication and gene expression, effectively interrupting the life cycle of these microorganisms [67,68]. Thus, the use of plant-derived flavonoids represents a promising strategy for the development of new technologies in public health to combat antimicrobial resistance [21,69].
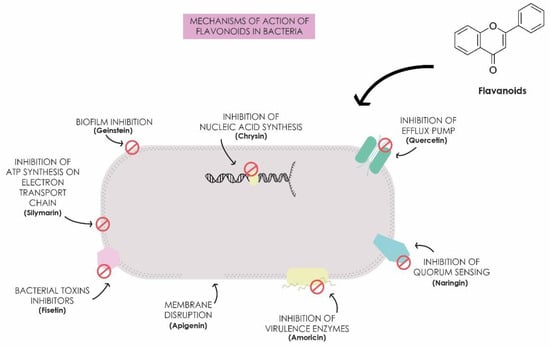
Figure 1.
Mechanisms of action of flavonoids in bacteria.
3. Nanocarriers Containing Flavonoids against Klebsiella pneumoniae
Flavonoids present an antibacterial therapeutic option, particularly for infections caused by antimicrobial-resistant strains of Klebsiella pneumoniae. However, their administration faces limitations, including low water solubility, which hampers absorption and bioavailability [70]. Furthermore, the chemical stability and pharmacokinetics of flavonoids can be compromised by metabolic processes, such as hepatic metabolism, intestinal degradation, and interactions with the intestinal microbiota, directly affecting their clinical efficacy. These factors constitute significant barriers to their application [71]. Consequently, loading these molecules into nanostructures emerges as a promising therapeutic approach for combating bacterial infections [15,72].
These nanostructures enhance the bioavailability of flavonoids, improving their absorption and distribution within the body. Additionally, they offer protection against metabolic degradation, enable controlled release, and reduce side and adverse effects (Figure 2). Incorporation into nanocarriers also facilitates the penetration of flavonoids into target cells, optimizing their biological effects, particularly their antimicrobial action due to the targeted delivery of the encapsulated compound; the surface of the nanostructures can be functionalized with specific ligands or by altering charge or size, making the nanosystem have better affinity with bacterial cells and less impact on human cells, thereby reducing toxicity levels [73,74]. Consequently, numerous studies have evaluated the antimicrobial potential of nanotechnology-based formulations for delivering flavonoids against K. pneumoniae (Table 1).
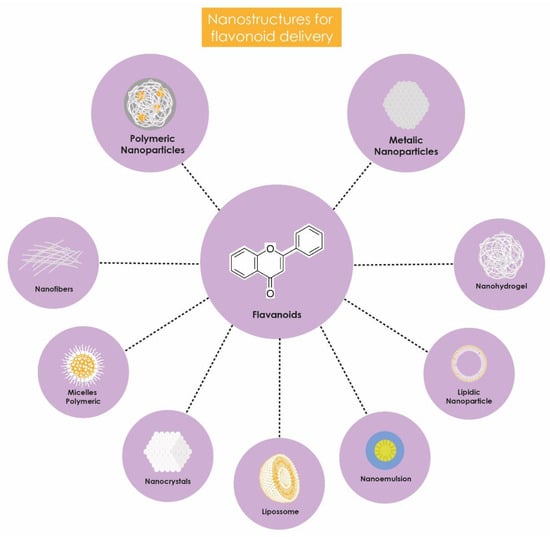
Figure 2.
Benefits of flavonoid encapsulation.

Table 1.
Physicochemical characteristics of nanostructures for flavonoid delivery.
3.1. Lipid Nanostructure
Lipid nanostructures are effective in encapsulating and releasing bioactive lipophilic compounds, utilizing lipid matrices to enhance solubility, stability, and bioavailability. Examples of such structures include nanoemulsions, nanostructured lipid carriers (NLC), and liposomes [58]. Nanoemulsions are colloidal systems composed of a mixture of two immiscible liquids, such as water and oil, stabilized by emulsifiers. These nanostructures are particularly suitable for topical administration due to their capability to penetrate the deeper layers of the epidermis [92].
Kaur et al. [75] developed nanoemulsions of catechins from Camellia sinensis to inhibit biofilm formation by resistant clinical isolates of K. pneumoniae collected from various health centers in Mumbai. The nanoemulsion was prepared using homogenization and ultrasonication techniques to combine bioactive compounds such as cranberry, curcumin, and Polyphenon 60. The antimicrobial activity of these nanoemulsions was evaluated using the microdilution method according to CLSI standards, and the antibiofilm activity was analyzed using the crystal violet method. The results indicated that growth was inhibited in 1:2 dilutions, containing 5.5 mg/mL of P60 and 15 mg/mL of cranberry (NE I), and 8 mg/mL of P60 and 2 mg/mL of curcumin (NE II). Additionally, there was significant efficacy in inhibiting biofilm growth, with an average inhibition of around 84% for the nanoemulsions, compared to approximately 64% for the free molecule.
In addition to nanoemulsions, nanostructured lipid carriers (NLCs) are utilized to deliver flavonoids. NLCs are drug delivery systems composed of a mixture of solid and liquid lipids that form a solid matrix. By increasing the available surface area, NLCs enhance the interaction of lipid fractions with epithelial membranes, facilitating adhesion to the walls of the gastrointestinal tract. Consequently, the formulation remains in the digestive system for a longer duration, thereby enhancing the absorption of the compounds [93,94,95].
Extracts rich in flavonoids are also effectively transported by liposomes, as demonstrated by Rubaka et al. [76]. They developed a liposomal nanocarrier loaded with Carissa spinarum polyphenols and coated with chitosan (Lip-CsP-chitosan) to enhance its antimicrobial action against K. pneumoniae ATCC. The antimicrobial activity was evaluated using the agar diffusion method. Lip-CsP-chitosan exhibited a relative inhibition zone diameter of 84.33 ± 2.51% compared to the free extract of C. spinarum and reduced the viability of K. pneumoniae by 57.45 ± 3.76% after 24 h, with a minimum inhibitory concentration of 31.25 mg/mL.
These studies demonstrate that the use of lipid nanostructures, such as nanoemulsions, NLCs, and liposomes, significantly enhances the delivery and efficacy of flavonoids against Klebsiella pneumoniae. These technologies are emerging as promising strategies to optimize the treatment of bacterial infections, providing an innovative approach to overcoming the limitations associated with the bioavailability and efficacy of antimicrobial agents.
3.2. Polymeric Nanostructures
Polymeric nanostructures are characterized by systems composed of polymers, which create nanostructured matrices for the controlled delivery of bioactive substances. These include nanogels, nanofibers, micelles, and polymeric nanoparticles. Nanogels, defined as polymeric matrices with dimensions in the nanometric scale, possess attributes that make them excellent carriers, including compatibility with biological systems, high encapsulation efficiency, precise release kinetics, and protection against degradation [96,97]. Based on these premises, Abbaszadeh et al. [77] developed a quercetin nanohydrogel using chitosan (ChiNH/Q). The antibacterial activity was demonstrated using the broth microdilution method, indicating the MIC against a resistant strain of Klebsiella pneumoniae PTCC 1290. This characteristic suggests its potential as an alternative antibiotic agent, as the nanohydrogel was able to reduce the MIC values for K. pneumoniae by 50%.
Chitosan is an abundant biopolymer widely used in nanotechnology for drug delivery systems due to its solubility, permeability enhancement, and mucoadhesive properties. It consists of a linear polysaccharide composed of D-glucosamine and N-acetyl-D-glucosamine units, linked by β-(1–4) glycosidic bonds, which allows for the formation of covalent bonds through reactions such as esterification, reductive amination, and etherification [98,99,100,101].
Abozahra et al. [83] investigated the antimicrobial activity of nanostructured lipid carriers (NLCs) containing rosemary (Baccharis dracunculifolia) and ginger (Zingiber officinale) essential oils, as well as the pharmaceutical form of chitosan nanoparticles against clinical isolates of K. pneumoniae obtained from patients at the Alexandria Fever Hospital [83]. Using the broth microdilution method, the minimum inhibitory concentration (MIC) of the essential oils was determined to be 1250 μg/mL. When encapsulated in NLCs, the MIC was reduced to 625 μg/mL. The lowest MIC was achieved with chitosan nanoparticles loaded with essential oils, enhancing the antibacterial efficacy of the essential oils against K. pneumoniae isolates by 8-fold. This positive effect may be attributed to the synergy between the essential oils and chitosan.
Nanofibers composed of biocompatible materials have frequently been utilized in therapeutic applications, proving to be excellent carriers for flavonoids. These structures are versatile, significantly enhancing patient adherence to therapy and yielding optimal results, particularly in wound healing applications [102,103,104]. In a study by Kannan et al. [78], electrospun nanofibers were synthesized containing flavonoid glycosides extracted from Glinus oppositifolius. These glycosides were previously isolated and subjected to a rigorous purification process using high-performance liquid chromatography methods and separation columns. The nanofibers demonstrated efficiency in antimicrobial tests, using the disk diffusion method against the resistant K. pneumoniae strain (MTCC 7028). The incorporation of flavonoid glycosides resulted in inhibition zones of 24 mm and 25 mm, exhibiting strong antibacterial activity
Polymeric micelles are spherical structures formed by surfactant polymers that self-organize in aqueous solutions. These micelles are excellent nanocarriers for antimicrobials, including flavonoids, due to their affinity with biological membranes and their ability to be incorporated into various pharmaceutical forms [105]. In a study by Miao et al. [79], methoxy poly(ethylene glycol)-poly(lactide) micelles loaded with luteolin (luteolin/MPEG-PLA) were formulated, demonstrating bactericidal effects against K. pneumoniae. The formulation not only increased the bioavailability of luteolin but also played a significant role in reducing inflammatory infiltrate in lung tissue in the murine models of lung infection induced by K. pneumoniae. These in vivo studies showed a significant therapeutic effect of luteolin/MPEG-PLA compared to free luteolin.
The encapsulation of flavonoids in polymeric nanoparticles, such as nanospheres and nanocapsules, enhances their controlled release and biological properties. Nanospheres are monolithic systems where the drug is dispersed or solubilized within a polymeric matrix, whereas nanocapsules consist of a polymeric membrane enclosing a core that can be solid or liquid. These nanocarriers improve the controlled delivery and efficacy of flavonoids at the target site. In this context, Balakrishnan et al. [80] developed poly(lactic-co-glycolic acid) (PLGA) nanoparticles loaded with hesperidin (HES-PLGA NPs). Their study, utilizing the broth microdilution method, demonstrated that HES-PLGA NPs at a concentration of 100 µg/mL exhibited significant antibacterial activity against multidrug-resistant clinical isolates of K. pneumoniae. Additionally, HES-PLGA NPs showed superior antioxidant activity compared to the reference standard ascorbic acid.
Alotaibi et al. [81] conducted a study involving the synthesis of PLGA nanocapsules loaded with epigallocatechin gallate (EGCG PLGA NCs). The antibacterial efficacy of free EGCG against the resistant strain K. pneumoniae ATCC 13883 was assessed using the well plate method, revealing an MIC of 128 µg/mL. In contrast, the MIC for EGCG encapsulated within PLGA nanocapsules was significantly lower at 16 µg/mL. Additionally, these nanocapsules demonstrated potential in mitigating kidney damage induced by cisplatin in murine models. This protective effect was evidenced by a notable reduction in renal biomarkers, including serum creatinine, KIM-1, and NGAL, alongside improvements in histopathological alterations associated with cisplatin-induced nephrotoxicity [106].
PLGA is widely utilized as a biopolymer for the synthesis of nanostructures due to its high performance and advantageous characteristics for drug delivery, including its ability to facilitate intracellular hydrolysis [95]. The encapsulation of isolated compounds and plant essential oils in PLGA has garnered significant scientific interest, particularly because these substances often contain high levels of flavonoids, which are critical to their therapeutic efficacy [107].
Zhang et al. [70] investigated the antimicrobial properties of essential oils extracted from guava leaves (Psidium guajava) when incorporated into chitosan nanoparticles targeting an environmental strain of K. pneumoniae. The study employed the ionic gelation technique, where the essential oil was loaded onto the surface of chitosan nanoparticles, with visual evidence of a bulge within the chitosan matrix, surrounded by discrete particles, as detailed by Hadidi et al. [108]. The guava leaf extract exhibited an inhibition zone of 12 mm against the multidrug-resistant K. pneumoniae strain. When loaded onto chitosan nanoparticles, the essential oil produced an inhibition zone of 22 mm at a concentration of 70 µg/mL.
In another study, Qanash et al. [82] investigated the encapsulation of Artemisia judaica extract, known for its high flavonoid content, into chitosan nanoparticles (CNPsLE) and assessed its antibacterial efficacy against clinical isolates of K. pneumoniae sourced from the Egyptian University Hospital using the broth microdilution method. High-performance liquid chromatography (HPLC) analysis of the Artemisia judaica (aerial parts) revealed the presence of 18 known compounds, with kaempferol, a natural flavonol, identified as the most predominant. Additionally, the extract contained other flavonoids such as luteolin, apigenin, rutin, and quercetin. Notably, the MIC for K. pneumoniae decreased approximately 7.62-fold when the extract was encapsulated in CNPsLE, with the MIC value of the free extract being 31.25 µg/mL compared to 4.1 µg/mL for the encapsulated form.
3.3. Metallic Nanostructure
Metallic nanostructures are used in various applications including, dentistry, molecular biology, cancer therapy and antimicrobial treatment [109,110,111]. The metallic nanostructures can be categorized in four types depending on the metal used in the synthesis process and on the physicochemical properties analyzed in the characterization method that those particles are based on: carbon, inorganic structures, organic structures, and composites [112].
Nanocarriers for flavonoid delivery have been extensively studied, with significant research focusing on their effectiveness, particularly when combined with gold nanocomposites, porous CuO nanobastons, magnetic iron oxide nanoparticles, and silver nanoparticles. Alhadrami et al. [84] developed gold nanocomposites (GNPs) coated with flavonoids, including quercetin, kaempferol, and chrysin, using glutathione (GSH) as a ligand to facilitate the interaction between the gold nanoparticles and the flavonoids. The antibacterial activity of these nanoconjugates was assessed using the agar diffusion method against the resistant strain K. pneumoniae (ATCC BAA-1705). The findings revealed that GNP-quercetin demonstrated the highest antimicrobial activity, with an MIC of 60 μg/mL. The authors proposed that these nanoconjugates may exert their antibacterial effects through two mechanisms: enhancing the rigidity of the bacterial membrane and inhibiting the B subunit of DNA gyrase (Gyr-B). Both in silico and in vitro analyses supported quercetin’s efficacy in binding to GNPs, reinforcing membrane rigidity, and inhibiting Gyr-B.
In addition to gold nanocomposites, porous CuO nanobastons are another class of nanometric structures, characterized by their rod-like shape and porous surface [113]. In this context, Mansi et al. [114] investigated the fabrication of microwave-induced porous CuO nanobastons loaded with quercetin and rutin. Metal nanoparticles, including CuO, have been extensively studied due to their antimicrobial properties and their diverse physical, biological, and chemical effects [115,116].
Several studies have highlighted the antimicrobial efficacy of silver nanoparticles (AgNPs) when combined with flavonoids such as quercetin. Hooda et al. [69] synthesized quercetin-impregnated silver nanoparticles, which exhibited enhanced antibacterial activity against K. pneumoniae ATCC 790603 as assessed by the agar well diffusion method. This increased activity can be attributed to the cell lysis mechanism inherent to silver nanoparticles.
Additionally, the therapeutic potential of gold nanoparticles (AuNPs) has been well-documented, owing to their remarkable properties and straightforward production methods [8]. Khosravi et al. [80] investigated AuNPs synthesized via green methods and coated with Anthemis atropatana extract against the resistant strain K. pneumoniae ATCC 13883. These AuNPs demonstrated antibacterial activity through mechanisms including increased intracellular oxidative stress mediated by reactive oxygen species (ROS), damage to the bacterial cell membrane resulting in altered permeability, and enhanced internalization of AuNPs. Furthermore, AuNPs exhibited efflux pump inhibitory activity, reduced exopolysaccharide production, urease inhibition, and low cytotoxicity. They also significantly decreased the expression of the mrkA, wzm, and acrB genes [117,118]. This reduction may be attributed to the inhibition of bacterial gene transcription by ROS and/or direct interaction of AuNPs with transcription factors involved in the regulation of these gene expressions.
In the study by Ali et al. [77], magnetic iron oxide nanoparticles were used to encapsulate epigallocatechin gallate (EGCG). The authors concluded that the observed antibacterial activity against resistant clinical isolates of K. pneumoniae, as evaluated by the microdilution method, and the anti-biofilm efficacy, assessed using the crystal violet method, were attributable to the ability of EGCG-MINPs to facilitate the penetration of EGCG through the bacterial cell wall and biofilms. This effect was further enhanced by the release of Fe3+ ions from the biomolecules, suggesting a potential interference with quorum sensing. Additionally, in silico molecular docking analyses demonstrated that EGCG-MINPs or Fe-EGCG complexes exhibited significantly higher binding affinity for human serum albumin (HSA) compared to free EGCG and Fe3+. These findings suggest that EGCG-MINPs could be promising nanosystems for the delivery of biocompatible nanodrugs in in vivo drug delivery applications.
3.4. Other Nanostructures
Carbon nanostructures have emerged as a significant innovation within the scientific community, attributed to their capacity to integrate unique properties with the inherent versatility of nanostructures, thereby unlocking novel avenues for advanced applications [41,102]. In this context, extensive research has been conducted on the delivery of quercetin, exemplified by the work of Shabana et al. [66], who encapsulated this flavonoid within mesoporous silica nanoparticles (QMSN) and subsequently assessed its antimicrobial efficacy via the disk diffusion method against multidrug-resistant clinical isolates of Klebsiella pneumoniae. The study demonstrated that QMSN at a concentration of 100 μg/mL exhibited a significant antibacterial effect, evidenced by an inhibition zone measuring 10.53 mm, which was notably larger than that observed for free quercetin at the same concentration, where the inhibition zone measured 7.63 mm, as reported in a prior study by Kumar et al. [21]. Furthermore, QMSN has been validated as an effective delivery nanosystem, characterized by its safety in application within living cells or organisms.
In a subsequent investigation, Shameem et al. [67] expanded on these findings by evaluating the impact of QMSNs on growth rates, feed utilization efficiency, health status indicators through biochemical indices, and pathogenic bacterial loads in Oreochromis niloticus fry. This study highlighted a notable enhancement in host immunity and resistance to infection by clinical isolates of Klebsiella pneumoniae, as assessed through pathogenicity tests, wherein 100 µL of bacterial suspension was injected intraperitoneally into the fish, with daily monitoring over a 15-day period to record mortality rates. This evidence underscores the potential of utilizing natural products, such as encapsulated flavonoids, as viable therapeutic alternatives [72,119].
Another nanotechnological strategy for flavonoid delivery is the employment of nanocrystals—organic nanostructures composed of crystalline particles at the nanometric scale—which serve to provide a protective barrier against degradation, enhance the solubility of bioactive compounds, and facilitate controlled release of therapeutic agents [120]. Memar et al. [74] synthesized rutin-containing nanocrystals (NRs) and evaluated their antibacterial activity using the broth microdilution method, revealing that while free rutin did not demonstrate significant antibacterial activity against environmental strains of Klebsiella pneumoniae, NRs exhibited markedly enhanced antibacterial properties.
Further advancements in the field were achieved by Seetharaman et al. [73], who developed nanocomposites of reduced graphene oxide conjugated with gold nanoparticles and hesperetin-coated flavonoids (Hes-Au/rGONCs), assessing their antimicrobial activity via the disk diffusion method. Their results indicated that Hes-Au/rGONCs significantly increased the bioavailability of hesperetin, induced cell death through the generation of reactive oxygen species (ROS), and demonstrated high antibacterial activity against Klebsiella pneumoniae MTCC-530. Collectively, these studies illustrate that the use of nanocarriers for flavonoid encapsulation represents a promising strategy to enhance their therapeutic efficacy. By improving stability, solubility, and the ability to traverse biological barriers, nanocarriers facilitate the application of these compounds in treating bacterial infections caused by Klebsiella pneumoniae.
4. Efficacy and Toxicity of Nanostructures
The nanosystems discussed in this review exhibit a wide range of characteristics and applications, each offering distinct advantages depending on the therapeutic need. Thus, the selection of the appropriate nanocarrier should consider the desired route of administration, the need for controlled release, and the specific properties required for the particular application.
Nanoemulsions are effective for both oral and topical administration, improving the solubility and bioavailability of compounds. In contrast, liposomes, which are ideal for intravenous or topical administration due to their ability to encapsulate lipophilic drugs, are ineffective for oral use due to degradation by gastrointestinal lipases [121,122,123]. Nanohydrogels offer remarkable flexibility in controlled drug release, with bacterial cellulose hydrogels standing out as advanced systems for drug delivery and biomedical applications [96]. Nanofibers, in turn, are widely used in tissue engineering and sustained release systems due to their high surface area and ability to mimic the extracellular matrix [124,125].
Polymeric micelles and nanocrystals are efficient in solubilizing poorly soluble drugs. Unlike liposomes, micelles, with their hydrophobic core and hydrophilic shell, offer greater stability and protection for drugs in aqueous environments and adverse gastrointestinal conditions [126,127,128]. PLGA nanoparticles and nanospheres are widely recognized for their biocompatibility and effectiveness in controlling drug release, making them suitable for various therapeutic applications [129,130].
Chitosan nanoparticles are valued for their biodegradability and potential for oral, intranasal, and topical administration, while mesoporous silica nanoparticles (MSNs) offer high loading capacity and controlled release, making them ideal for targeted therapies [131,132,133]. Gold compounds and silver nanoparticles have antimicrobial properties, used in topical treatments and disinfection, with gold compounds also showing potential in cancer therapies [64,109]. Magnetic iron oxide is used in imaging and targeted therapies due to its magnetic properties, and reduced graphene oxide nanocomposites with gold nanoparticles combine electronic and magnetic properties, showing promising applications in sensors and therapies [134].
The synergy between nanocarriers and therapeutic agents is a crucial aspect of advancing nanotechnology-based therapies, particularly in enhancing antimicrobial activity. A notable example of this synergy is the combination of silver nanoparticles, which have well-established antimicrobial properties, with bioactive compounds such as flavonoids. Similarly, chitosan, a natural polymer with antimicrobial properties, can create release systems that combine its antimicrobial actions when used as a carrier. This synergistic effect between nanocarriers and flavonoids results in a more effective therapeutic approach, amplifying antimicrobial activity [83,109,135].
Although there are no in vivo studies on the use of flavonoid-containing nanoparticles for treating pulmonary infections caused by Klebsiella pneumoniae, there are studies that explore the applications of flavonoid-containing nanoparticles for intranasal administration. These findings suggest that such a nanotechnological approach may offer new opportunities for effective delivery and enhancement of treatment for infections caused by K. pneumoniae [136].
Encapsulating flavonoids in nanometric structures can enhance their stability, solubility, and ability to cross biological barriers. This approach often allows for the use of lower concentrations, which in turn reduces the toxicity of therapeutic regimens [98,137].
Riaz et al. [138] conducted a study to evaluate the antibacterial activity and in vivo hemolysis rate of conjugated flavonoids (CFs) and gold nanoparticles coated with flavonoids (FAuNPs). The results showed that both CF and FAuNPs exhibited antimicrobial activity against Gram-positive and Gram-negative bacteria. The encapsulation of flavonoids led to a significant reduction in the minimum inhibitory concentration (MIC), decreasing from 500 µg/mL to 25 µg/mL. Furthermore, in the hemolysis experiment, the nanoparticles demonstrated a significantly lower hemolysis rate compared to free flavonoids. At a concentration of 150 µg/mL, FAuNPs exhibited only half the hemolysis rate observed with free flavonoids, which had a hemolysis rate of approximately 7%. These findings highlight the potential of gold nanoparticles coated with flavonoids as a promising approach to reduce toxicity and enhance the therapeutic efficacy of these compounds.
In a study by Elkhateeb et al. [139], the antimicrobial activity of curcumin (CUR) and curcumin-loaded nanostructured lipid carriers (CURC-NLCs) was evaluated against Gram-negative and Gram-positive bacteria. The results revealed that CUR demonstrated antibacterial activity with an MIC ranging from 31.25 to 500 µg/mL. In contrast, CURC-NLCs exhibited lower MICs, ranging from 15.6 to 250 µg/mL, indicating reduced toxicity and increased safety of the compound when encapsulated.
The use of carbon nanotubes incorporated with flavonoids is also a well-known and elucidated nanosystem. When incorporating these compounds, assessing cytotoxicity is crucial. In a study, Espíndola et al. [140] evaluated single-walled and multi-walled carbon nanotubes as nanocarriers for the delivery of 7-hydroxyflavone (7-HF) and assessed their cytotoxic effects on several cell lines, including Vero E6 (normal monkey kidney epithelial cells). The nanotubes exhibited nearly negligible cytotoxicity, maintaining cellular viability, which demonstrates the safety and reliability of these nanosystems.
Shabana et al. [88] conducted a study on mesoporous silica nanoparticles loaded with quercetin against bacterial infections such as Klebsiella pneumoniae in Oreochromis niloticus species. In addition to antimicrobial evaluation, cytotoxicity was assessed in the tilapia gill cell line (TG). The results showed a cell viability rate above 90% across five different concentrations ranging from 20 to 100 µg/mL. These findings suggest that these nanoparticles have promising potential as therapeutic agents for treating bacterial infections in aquaculture. However, future in vivo studies and evaluation of these nanoparticles’ effects in pulmonary models are essential, considering that Klebsiella pneumoniae is a significant pathogen in pulmonary diseases. This would enhance understanding of their efficacy and safety in clinical contexts.
5. Material and Methods
The methodology employed in this narrative review involved a comprehensive screening of the literature to identify original studies that provided information on nanocarriers for the delivery of flavonoids against K. pneumoniae. Specifically, independent searches were conducted for articles published from 2019 to June 2024 in the PubMed, Science Direct, Google Scholar, and Scopus databases using the following search terms: “K. pneumoniae AND nanostructure AND delivery AND flavonoids”. Excluded from the review were reviews, systematic reviews, letters to the editor, and other non-original studies. Additionally, studies written in languages other than English, those without access to the full text, studies with aggregated data, and those involving animals were excluded. Studies that did not provide detailed information on the analysis of flavonoids in plant extracts were also excluded.
6. Conclusions
The synthesis of nanocarriers for flavonoid delivery has gained substantial recognition as a highly advantageous strategy, heralding a new therapeutic paradigm aimed at combating both sensitive and resistant strains of Klebsiella pneumoniae. The application of nanotechnology in this context optimizes the bioavailability of flavonoids, thereby significantly enhancing their delivery efficacy and amplifying their therapeutic effects against K. pneumoniae.
Among the most prevalent flavonoids isolated from plants, quercetin, rutin, catechins, kaempferol, and apigenin are particularly noteworthy. However, their clinical efficacy is often compromised by low water solubility and susceptibility to degradation. To address these limitations, flavonoids and their derivatives can be encapsulated and/or delivered using various nanocarriers, including mesoporous silica nanoparticles, nanohydrogels, silver nanoparticles, gold nanocomposites, CuO nanorods, poly(lactic-co-glycolic acid) nanoparticles, graphene oxide nanocomposites, nanocrystals, methoxy poly(ethylene glycol)-poly(lactide) micelles, magnetic iron oxide nanoparticles, nanoemulsions, polymeric nanoparticles, liposomes, and nanofibers. This encapsulation strategy is promising as it protects flavonoids from adverse environmental factors, enhances their solubility, and enables controlled release and targeted delivery, thereby expanding their therapeutic potential.
In this context, it is imperative to explore and optimize different controlled-release systems and to tailor nanocarriers to address the specific characteristics of infections and resistance profiles associated with Klebsiella pneumoniae. Such advancements are crucial, as the discussed nanocarriers have the potential to establish themselves as new therapeutic modalities based on nanotechnology. Additionally, it is essential to conduct further evaluations on the biocompatibility and toxicity of the employed nanomaterials, ensuring that their clinical use does not compromise patient safety. These studies are vital to ensure that the therapeutic benefits of nanocarriers outweigh any potential risks, thereby promoting safe and effective application in clinical practice.
The application of nanostructures for flavonoid delivery represents a significant advancement in the development of novel therapeutic regimens against infections caused by Klebsiella pneumoniae. Continued in vivo and clinical studies are therefore essential to develop innovative approaches for directing flavonoids to specific targets, maximizing their antibacterial potential, and overcoming bacterial resistance.
Author Contributions
Conceptualization, H.L.R.d.Q.M. and L.A.d.A.C.; methodology, H.L.R.d.Q.M.; resources, I.M.F.C.; writing—original draft preparation, H.L.R.d.Q.M., L.L.d.O. and D.N.d.O.; writing—review and editing, H.L.R.d.Q.M., K.F.A.L., I.M.F.C. and L.A.d.A.C.; visualization, I.M.F.C. and L.A.d.A.C.; supervision, I.M.F.C. and L.A.d.A.C.; project administration, H.L.R.d.Q.M., I.M.F.C. and L.A.d.A.C.; funding acquisition, I.M.F.C. All authors have read and agreed to the published version of the manuscript.
Funding
The current study was supported by the Research Productivity of CNPq (#312690/902023-1), CNPq/MCTI/CT-Health 52/2022–Actions in Science, Technology and Innovation to the Combat of Antimicrobial Resistance (RAM) (#408785/2022.5) and Foundation for the Support of Science and Technology of the State of Pernambuco (FACEPE) for the M.Sc. scholarship (IBPG-2253-2.12/22).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Zou, H.; Zhou, Z.; Berglund, B.; Zheng, B.; Meng, M.; Zhao, L.; Zhang, H.; Wang, Z.; Wu, T.; Li, Q.; et al. Persistent Transmission of Carbapenem-Resistant, Hypervirulent Klebsiella pneumoniae between a Hospital and Urban Aquatic Environments. Water Res. 2023, 242, 120263. [Google Scholar] [CrossRef]
- Adeolu, M.; Alnajar, S.; Naushad, S.; Gupta, R.S. Genome-Based Phylogeny and Taxonomy of the ‘Enterobacteriales’: Proposal for Enterobacterales Ord. Nov. Divided into the Families Enterobacteriaceae, Erwiniaceae Fam. Nov., Pectobacteriaceae Fam. Nov., Yersiniaceae Fam. Nov., Hafniaceae Fam. Nov., Morganellaceae Fam. Nov., and Budviciaceae Fam. Nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 5575–5599. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Yuan, X.-D.; Pang, T.; Duan, S.-H. The Risk Factors of Carbapenem-Resistant Klebsiella pneumoniae Infection: A Single-Center Chinese Retrospective Study. Infect. Drug Resist. 2022, 15, 1477–1485. [Google Scholar] [CrossRef] [PubMed]
- Assoni, L.; Couto, A.J.M.; Vieira, B.; Milani, B.; Lima, A.S.; Converso, T.R.; Darrieux, M. Animal Models of Klebsiella pneumoniae Mucosal Infections. Front. Microbiol. 2024, 15, 1367422. [Google Scholar] [CrossRef]
- Chen, I.-R.; Lin, S.-N.; Wu, X.-N.; Chou, S.-H.; Wang, F.-D.; Lin, Y.-T. Clinical and Microbiological Characteristics of Bacteremic Pneumonia Caused by Klebsiella pneumoniae. Front. Cell. Infect. Microbiol. 2022, 12, 903682. [Google Scholar] [CrossRef] [PubMed]
- Stojowska-Swędrzyńska, K.; Łupkowska, A.; Kuczyńska-Wiśnik, D.; Laskowska, E. Antibiotic Heteroresistance in Klebsiella pneumoniae. Int. J. Mol. Sci. 2021, 23, 449. [Google Scholar] [CrossRef]
- Moutel, M.; Peju, E.; Belan, M.; Gavaud, A.; Mira, J.-P.; Charlier, C.; Canouï, E.; Gastli, N. Hypervirulent Klebsiella pneumoniae-Related Bacteremia in Intensive Care Unit: A Retrospective Cohort Study. Infect. Dis. Now 2024, 54, 104892. [Google Scholar] [CrossRef]
- Xu, Q.; Liu, C.; Wu, Z.; Zhang, S.; Chen, Z.; Shi, Y.; Gu, S. Demographics and Prognosis of Patients with Pyogenic Liver Abscess Due to Klebsiella pneumonia or Other Species. Heliyon 2024, 10, e29463. [Google Scholar] [CrossRef]
- Luo, K.; Tang, J.; Qu, Y.; Yang, X.; Zhang, L.; Chen, Z.; Kuang, L.; Su, M.; Mu, D. Nosocomial Infection by Klebsiella pneumoniae among Neonates: A Molecular Epidemiological Study. J. Hosp. Infect. 2021, 108, 174–180. [Google Scholar] [CrossRef]
- Sree, R.A.; Gupta, A.; Gupta, N.; Veturi, S.; Reddy, L.S.K.; Begum, M.; Shravani, E.; Challa, H.R.; Reddy, S.S.; Singamsetty, A.; et al. Ceftazidime-Avibactam Alone or in Combination with Aztreonam versus Polymyxins in the Management of Carbapenem-Resistant Klebsiella pneumoniae Nosocomial Infections (CAPRI Study): A Retrospective Cohort Study from South India. Infection 2024, 52, 429–437. [Google Scholar] [CrossRef]
- Pei, N.; Sun, W.; He, J.; Li, Y.; Chen, X.; Liang, T.; Kristiansen, K.; Liu, W.; Li, J. Genome-Wide Association Study of Klebsiella pneumoniae Identifies Variations Linked to Carbapenems Resistance. Front. Microbiol. 2022, 13, 997769. [Google Scholar] [CrossRef] [PubMed]
- De La Cadena, E.; Mojica, M.F.; García-Betancur, J.C.; Appel, T.M.; Porras, J.; Pallares, C.J.; Solano-Gutiérrez, J.S.; Rojas, L.J.; Villegas, M.V. Molecular Analysis of Polymyxin Resistance among Carbapenemase-Producing Klebsiella pneumoniae in Colombia. Antibiotics 2021, 10, 284. [Google Scholar] [CrossRef]
- Zhen, X.; Stålsby Lundborg, C.; Sun, X.; Gu, S.; Dong, H. Clinical and Economic Burden of Carbapenem-Resistant Infection or Colonization Caused by Klebsiella pneumoniae, Pseudomonas aeruginosa, Acinetobacter baumannii: A Multicenter Study in China. Antibiotics 2020, 9, 514. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.; Khan, A.; Ahmad, I.; Alghamdi, S.; Rajab, B.S.; Babalghith, A.O.; Alshahrani, M.Y.; Islam, S.; Islam, M.d.R. Flavonoids a Bioactive Compound from Medicinal Plants and Its Therapeutic Applications. BioMed Res. Int. 2022, 2022, 5445291. [Google Scholar] [CrossRef] [PubMed]
- Wen, K.; Fang, X.; Yang, J.; Yao, Y.; Nandakumar, K.S.; Salem, M.L.; Cheng, K. Recent Research on Flavonoids and Their Biomedical Applications. Curr. Med. Chem. 2021, 28, 1042–1066. [Google Scholar] [CrossRef] [PubMed]
- Bag, S.; Mondal, A.; Majumder, A.; Mondal, S.K.; Banik, A. Flavonoid Mediated Selective Cross-Talk between Plants and Beneficial Soil Microbiome. Phytochem. Rev. 2022, 21, 1739–1760. [Google Scholar] [CrossRef]
- Bhakta, S.; Negi, S.; Tak, H.; Singh, S.; Ganapathi, T.R. MusaATAF2-like Protein Regulates Shoot Development and Multiplication by Inducing Cytokinin Hypersensitivity and Flavonoid Accumulation in Banana Plants. Plant Cell Rep. 2022, 41, 1197–1208. [Google Scholar] [CrossRef]
- Marone, D.; Mastrangelo, A.M.; Borrelli, G.M.; Mores, A.; Laidò, G.; Russo, M.A.; Ficco, D.B.M. Specialized Metabolites: Physiological and Biochemical Role in Stress Resistance, Strategies to Improve Their Accumulation, and New Applications in Crop Breeding and Management. Plant Physiol. Biochem. 2022, 172, 48–55. [Google Scholar] [CrossRef]
- Marhava, P. Recent Developments in the Understanding of PIN Polarity. New Phytol. 2022, 233, 624–630. [Google Scholar] [CrossRef]
- Shah, A.; Smith, D.L. Flavonoids in Agriculture: Chemistry and Roles in, Biotic and Abiotic Stress Responses, and Microbial Associations. Agronomy 2020, 10, 1209. [Google Scholar] [CrossRef]
- Huang, W.; Wang, Y.; Tian, W.; Cui, X.; Tu, P.; Li, J.; Shi, S.; Liu, X. Biosynthesis Investigations of Terpenoid, Alkaloid, and Flavonoid Antimicrobial Agents Derived from Medicinal Plants. Antibiotics 2022, 11, 1380. [Google Scholar] [CrossRef] [PubMed]
- Mozaffarian, D. Dietary and Policy Priorities to Reduce the Global Crises of Obesity and Diabetes. Nat. Food 2020, 1, 38–50. [Google Scholar] [CrossRef]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-Inflammatory Effects of Flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Veiko, A.G.; Olchowik-Grabarek, E.; Sekowski, S.; Roszkowska, A.; Lapshina, E.A.; Dobrzynska, I.; Zamaraeva, M.; Zavodnik, I.B. Antimicrobial Activity of Quercetin, Naringenin and Catechin: Flavonoids Inhibit Staphylococcus aureus-Induced Hemolysis and Modify Membranes of Bacteria and Erythrocytes. Molecules 2023, 28, 1252. [Google Scholar] [CrossRef]
- Weng, Z.; Zeng, F.; Wang, M.; Guo, S.; Tang, Z.; Itagaki, K.; Lin, Y.; Shen, X.; Cao, Y.; Duan, J.; et al. Antimicrobial Activities of Lavandulylated Flavonoids in Sophora Flavences against Methicillin-Resistant Staphylococcus aureus via Membrane Disruption. J. Adv. Res. 2024, 57, 197–212. [Google Scholar] [CrossRef]
- Kumar, S.; Pandey, A.K. Chemistry and Biological Activities of Flavonoids: An Overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef]
- Biharee, A.; Sharma, A.; Kumar, A.; Jaitak, V. Antimicrobial Flavonoids as a Potential Substitute for Overcoming Antimicrobial Resistance. Fitoterapia 2020, 146, 104720. [Google Scholar] [CrossRef]
- Chagas, M.d.S.S.; Behrens, M.D.; Moragas-Tellis, C.J.; Penedo, G.X.M.; Silva, A.R.; Gonçalves-de-Albuquerque, C.F. Flavonols and Flavones as Potential Anti-Inflammatory, Antioxidant, and Antibacterial Compounds. Oxidative Med. Cell. Longev. 2022, 2022, 9966750. [Google Scholar] [CrossRef]
- Dong, X.; Li, X.; Ruan, X.; Kong, L.; Wang, N.; Gao, W.; Wang, R.; Sun, Y.; Jin, M. A Deep Insight into the Structure-Solubility Relationship and Molecular Interaction Mechanism of Diverse Flavonoids in Molecular Solvents, Ionic Liquids, and Molecular Solvent/Ionic Liquid Mixtures. J. Mol. Liq. 2023, 385, 122359. [Google Scholar] [CrossRef]
- Yuann, J.-M.P.; Lee, S.-Y.; Yang, M.-J.; Huang, S.-T.; Cheng, C.-W.; Liang, J.-Y. A Study of Catechin Photostability Using Photolytic Processing. Processes 2021, 9, 293. [Google Scholar] [CrossRef]
- Kaushal, N.; Singh, M.; Singh Sangwan, R. Flavonoids: Food Associations, Therapeutic Mechanisms, Metabolism and Nanoformulations. Food Res. Int. 2022, 157, 111442. [Google Scholar] [CrossRef]
- Grgić, J.; Šelo, G.; Planinić, M.; Tišma, M.; Bucić-Kojić, A. Role of the Encapsulation in Bioavailability of Phenolic Compounds. Antioxidants 2020, 9, 923. [Google Scholar] [CrossRef]
- Buosi, F.S.; Alaimo, A.; Di Santo, M.C.; Elías, F.; García Liñares, G.; Acebedo, S.L.; Castañeda Cataña, M.A.; Spagnuolo, C.C.; Lizarraga, L.; Martínez, K.D.; et al. Resveratrol Encapsulation in High Molecular Weight Chitosan-Based Nanogels for Applications in Ocular Treatments: Impact on Human ARPE-19 Culture Cells. Int. J. Biol. Macromol. 2020, 165, 804–821. [Google Scholar] [CrossRef]
- Saka, R.; Chella, N. Nanotechnology for Delivery of Natural Therapeutic Substances: A Review. Environ. Chem. Lett. 2021, 19, 1097–1106. [Google Scholar] [CrossRef]
- Seaberg, J.; Montazerian, H.; Hossen, M.N.; Bhattacharya, R.; Khademhosseini, A.; Mukherjee, P. Hybrid Nanosystems for Biomedical Applications. ACS Nano 2021, 15, 2099–2142. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Qi, Y.; Liu, G.; Song, Y.; Jiang, X.; Du, B. Size-Dependent In Vivo Transport of Nanoparticles: Implications for Delivery, Targeting, and Clearance. ACS Nano 2023, 17, 20825–20849. [Google Scholar] [CrossRef]
- Hu, W.; Wang, C.; Gao, D.; Liang, Q. Toxicity of Transition Metal Nanoparticles: A Review of Different Experimental Models in the Gastrointestinal Tract. J. Appl. Toxicol. 2023, 43, 32–46. [Google Scholar] [CrossRef]
- Yang, B.; Liu, C.; Pan, X.; Fu, W.; Fan, Z.; Jin, Y.; Bai, F.; Cheng, Z.; Wu, W. Identification of Novel phoP-phoQ Regulated Genes That Contribute to Polymyxin B Tolerance in Pseudomonas aeruginosa. Microorganisms 2021, 9, 344. [Google Scholar] [CrossRef]
- Rahman, I.U.; Afzal, A.; Iqbal, Z.; Ijaz, F.; Ali, N.; Shah, M.; Ullah, S.; Bussmann, R.W. Historical Perspectives of Ethnobotany. Clin. Dermatol. 2019, 37, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Agidew, M.G. Phytochemical Analysis of Some Selected Traditional Medicinal Plants in Ethiopia. Bull. Natl. Res. Cent. 2022, 46, 87. [Google Scholar] [CrossRef]
- Caballero-Serrano, V.; McLaren, B.; Carrasco, J.C.; Alday, J.G.; Fiallos, L.; Amigo, J.; Onaindia, M. Traditional Ecological Knowledge and Medicinal Plant Diversity in Ecuadorian Amazon Home Gardens. Glob. Ecol. Conserv. 2019, 17, e00524. [Google Scholar] [CrossRef]
- Süntar, I. Importance of Ethnopharmacological Studies in Drug Discovery: Role of Medicinal Plants. Phytochem. Rev. 2020, 19, 1199–1209. [Google Scholar] [CrossRef]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Davies, K.M.; Jibran, R.; Zhou, Y.; Albert, N.W.; Brummell, D.A.; Jordan, B.R.; Bowman, J.L.; Schwinn, K.E. The Evolution of Flavonoid Biosynthesis: A Bryophyte Perspective. Front. Plant Sci. 2020, 11, 7. [Google Scholar] [CrossRef]
- Mohanta, T.K. Fungi Contain Genes Associated with Flavonoid Biosynthesis Pathway. J. Funct. Foods 2020, 68, 103910. [Google Scholar] [CrossRef]
- Sabaragamuwa, R.; Perera, C.O. Total Triterpenes, Polyphenols, Flavonoids, and Antioxidant Activity of Bioactive Phytochemicals of Centella Asiatica by Different Extraction Techniques. Foods 2023, 12, 3972. [Google Scholar] [CrossRef] [PubMed]
- Nabavi, S.M.; Šamec, D.; Tomczyk, M.; Milella, L.; Russo, D.; Habtemariam, S.; Suntar, I.; Rastrelli, L.; Daglia, M.; Xiao, J.; et al. Flavonoid Biosynthetic Pathways in Plants: Versatile Targets for Metabolic Engineering. Biotechnol. Adv. 2020, 38, 107316. [Google Scholar] [CrossRef] [PubMed]
- Franco, R.R.; Mota Alves, V.H.; Ribeiro Zabisky, L.F.; Justino, A.B.; Martins, M.M.; Saraiva, A.L.; Goulart, L.R.; Espindola, F.S. Antidiabetic Potential of Bauhinia forficata Link Leaves: A Non-Cytotoxic Source of Lipase and Glycoside Hydrolases Inhibitors and Molecules with Antioxidant and Antiglycation Properties. Biomed. Pharmacother. 2020, 123, 109798. [Google Scholar] [CrossRef] [PubMed]
- Chávez-Bustos, E.A.; Morales-González, A.; Anguiano-Robledo, L.; Madrigal-Santillán, E.O.; Valadez-Vega, C.; Lugo-Magaña, O.; Mendoza-Pérez, J.A.; Fregoso-Aguilar, T.A. Bauhinia forficata Link, Antioxidant, Genoprotective, and Hypoglycemic Activity in a Murine Model. Plants 2022, 11, 3052. [Google Scholar] [CrossRef]
- Zugaro, S.; Benedetti, E.; Caioni, G. Garlic (Allium sativum L.) as an Ally in the Treatment of Inflammatory Bowel Diseases. Curr. Issues Mol. Biol. 2023, 45, 685–698. [Google Scholar] [CrossRef]
- Hamad, R.S. Rutin, a Flavonoid Compound Derived from Garlic, as a Potential Immunomodulatory and Anti-Inflammatory Agent against Murine Schistosomiasis mansoni. Nutrients 2023, 15, 1206. [Google Scholar] [CrossRef]
- Semwal, R.; Joshi, S.K.; Semwal, R.B.; Semwal, D.K. Health Benefits and Limitations of Rutin—A Natural Flavonoid with High Nutraceutical Value. Phytochem. Lett. 2021, 46, 119–128. [Google Scholar] [CrossRef]
- Abidullah, M.; Jadhav, P.; Sujan, S.S.; Shrimanikandan, A.G.; Reddy, C.R.; Wasan, R.K. Potential Antibacterial Efficacy of Garlic Extract on Staphylococcus aureus, Escherichia coli, and Klebsiella pneumoniae: An In Vitro Study. J. Pharm. Bioallied Sci. 2021, 13, S590–S594. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Y.; Nie, G. Multifunctional Biomolecule Nanostructures for Cancer Therapy. Nat. Rev. Mater. 2021, 6, 766–783. [Google Scholar] [CrossRef] [PubMed]
- Ražná, K.; Sawinska, Z.; Ivanišová, E.; Vukovic, N.; Terentjeva, M.; Stričík, M.; Kowalczewski, P.Ł.; Hlavačková, L.; Rovná, K.; Žiarovská, J.; et al. Properties of Ginkgo biloba L.: Antioxidant Characterization, Antimicrobial Activities, and Genomic MicroRNA Based Marker Fingerprints. Int. J. Mol. Sci. 2020, 21, 3087. [Google Scholar] [CrossRef]
- Chassagne, F.; Huang, X.; Lyles, J.T.; Quave, C.L. Validation of a 16th Century Traditional Chinese Medicine Use of Ginkgo biloba as a Topical Antimicrobial. Front. Microbiol. 2019, 10, 775. [Google Scholar] [CrossRef]
- Pang, X.; Suo, J.; Liu, S.; Xu, J.; Yang, T.; Xiang, N.; Wu, Y.; Lu, B.; Qin, R.; Liu, H.; et al. Combined Transcriptomic and Metabolomic Analysis Reveals the Potential Mechanism of Seed Germination and Young Seedling Growth in Tamarix hispida. BMC Genom. 2022, 23, 109. [Google Scholar] [CrossRef]
- Peng, Z.; Wang, Y.; Zuo, W.-T.; Gao, Y.-R.; Li, R.-Z.; Yu, C.-X.; Liu, Z.-Y.; Zheng, Y.; Shen, Y.-Y.; Duan, L.-S. Integration of Metabolome and Transcriptome Studies Reveals Flavonoids, Abscisic Acid, and Nitric Oxide Comodulating the Freezing Tolerance in Liriope spicata. Front. Plant Sci. 2022, 12, 764625. [Google Scholar] [CrossRef]
- Yu, Y.; Guo, D.-D.; Min, D.-H.; Cao, T.; Ning, L.; Jiang, Q.-Y.; Sun, X.-J.; Zhang, H.; Tang, W.; Gao, S.-Q.; et al. Foxtail Millet MYB-like Transcription Factor SiMYB16 Confers Salt Tolerance in Transgenic Rice by Regulating Phenylpropane Pathway. Plant Physiol. Biochem. 2023, 195, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Jucá, M.M.; Cysne Filho, F.M.S.; de Almeida, J.C.; Mesquita, D.d.S.; Barriga, J.R.d.M.; Dias, K.C.F.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.K.A.M.; Ribeiro, J.E.; et al. Flavonoids: Biological Activities and Therapeutic Potential. Nat. Prod. Res. 2020, 34, 692–705. [Google Scholar] [CrossRef]
- Vidana Gamage, G.C.; Lim, Y.Y.; Choo, W.S. Sources and Relative Stabilities of Acylated and Nonacylated Anthocyanins in Beverage Systems. J. Food Sci. Technol. 2022, 59, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The Effects of Polyphenols and Other Bioactives on Human Health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Coria, H.; Arrieta-Cruz, I.; Gutiérrez-Juárez, R.; López-Valdés, H.E. Anti-Inflammatory Effects of Flavonoids in Common Neurological Disorders Associated with Aging. Int. J. Mol. Sci. 2023, 24, 4297. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.L.A.; Bhattacharya, D. Antimicrobial Activity of Quercetin: An Approach to Its Mechanistic Principle. Molecules 2022, 27, 2494. [Google Scholar] [CrossRef]
- Pal, A.; Tripathi, A. Demonstration of Bactericidal and Synergistic Activity of Quercetin with Meropenem among Pathogenic Carbapenem Resistant Escherichia coli and Klebsiella pneumoniae. Microb. Pathog. 2020, 143, 104120. [Google Scholar] [CrossRef]
- Wen, W.; Alseekh, S.; Fernie, A.R. Conservation and Diversification of Flavonoid Metabolism in the Plant Kingdom. Curr. Opin. Plant Biol. 2020, 55, 100–108. [Google Scholar] [CrossRef]
- Zhou, H.; Chen, L.; Ouyang, K.; Zhang, Q.; Wang, W. Antibacterial Activity and Mechanism of Flavonoids from Chimonanthus salicifolius S. Y. Hu. and Its Transcriptome Analysis against Staphylococcus aureus. Front. Microbiol. 2023, 13, 1103476. [Google Scholar] [CrossRef]
- Dey, J.; Mahapatra, S.R.; Raj, T.K.; Misra, N.; Suar, M. Identification of Potential Flavonoid Compounds as Antibacterial Therapeutics against Klebsiella pneumoniae Infection Using Structure-Based Virtual Screening and Molecular Dynamics Simulation. Mol. Divers. 2023, 1–18. [Google Scholar] [CrossRef]
- El-Naggar, N.E.-A. Streptomyces-Based Cell Factories for Production of Biomolecules and Bioactive Metabolites. In Microbial Cell Factories Engineering for Production of Biomolecules; Elsevier: Amsterdam, The Netherlands, 2021; pp. 183–234. [Google Scholar]
- Zhang, Y.; Guan, R.; Huang, H. Anti-Allergic Effects of Quercetin and Quercetin Liposomes in RBL-2H3 Cells. Endocr. Metab. Immune Disord.—Drug Targets 2023, 23, 692–701. [Google Scholar] [CrossRef]
- Sajid, M.; Channakesavula, C.N.; Stone, S.R.; Kaur, P. Synthetic Biology towards Improved Flavonoid Pharmacokinetics. Biomolecules 2021, 11, 754. [Google Scholar] [CrossRef]
- Silva, A.R.D.A.; Braga, J.C.C.M.; Vieira, V.V.R.; Jesus, G.D.S.D.; Bezerra, D.C.; Coimbra, V.C.S.; Teles, A.M.; Bezerra, N.P.C. Antimicrobial Resistance Profile of Aeromonas Spp. Isolated from Tambaqui Fish (Colossoma macropomum). Rev. Ambiente Água 2024, 18, e2936. [Google Scholar] [CrossRef]
- Imperlini, E.; Massaro, F.; Buonocore, F. Antimicrobial Peptides against Bacterial Pathogens: Innovative Delivery Nanosystems for Pharmaceutical Applications. Antibiotics 2023, 12, 184. [Google Scholar] [CrossRef]
- Coksu, I.; Dokuz, S.; Akgul, B.; Ozbek, T.; Abamor, E.S.; Duranoglu, D.; Acar, S. Enhancing the Treatment of Staphylococcus aureus Infections: A Nanosystem with Including Dual Antimicrobial Peptide. J. Drug Deliv. Sci. Technol. 2024, 97, 105830. [Google Scholar] [CrossRef]
- Kaur, A.; Gabrani, R.; Dang, S. Nanoemulsions of Green Tea Catechins and Other Natural Compounds for the Treatment of Urinary Tract Infection: Antibacterial Analysis. Adv. Pharm. Bull. 2019, 9, 401–408. [Google Scholar] [CrossRef]
- Rubaka, C.; Gathirwa, J.W.; Malebo, H.M.; Swai, H.; Sibuyi, N.R.S.; Hilonga, A.; Dube, A. Chitosan-Coated Liposomes of Carrisa Spinarum Extract: Synthesis, Analysis and Anti-Pneumococcal Potency. Bioinspired Biomim. Nanobiomater. 2023, 12, 12–23. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Rashidipour, M.; Khosravi, P.; Shahryarhesami, S.; Ashrafi, B.; Kaviani, M.; Moradi Sarabi, M. Biocompatibility, Cytotoxicity, Antimicrobial and Epigenetic Effects of Novel Chitosan-Based Quercetin Nanohydrogel in Human Cancer Cells. Int. J. Nanomed. 2020, 15, 5963–5975. [Google Scholar] [CrossRef]
- Kannan, K.S.; Kandavel, D.; Balamurugan, S.; Rajalakshmi, P. Novel Electrospun Nanofibers Incorporated with Flavonoid Glycosides from Glinus oppositifolius (L.) Aug. DC. for Antibacterial Dressings. J. Integr. Sci. Technol. 2022, 10, 53–60. [Google Scholar]
- Miao, J.; Lin, F.; Huang, N.; Teng, Y. Improving Anti-Inflammatory Effect of Luteolin with Nano-Micelles in the Bacteria-Induced Lung Infection. J. Biomed. Nanotechnol. 2021, 17, 1229–1241. [Google Scholar] [CrossRef]
- Balakrishnan, K.; Casimeer, S.; Ghidan, A.Y.; Antary, T.; Singaravelu, A. Exploration of Antioxidant, Antibacterial Activities of Green Synthesized Hesperidin Loaded PLGA Nanoparticles. Biointerface Res. Appl. Chem. 2021, 11, 14520–14528. [Google Scholar] [CrossRef]
- Alotaibi, B.; El-Masry, T.A.; Elekhnawy, E.; El-Kadem, A.H.; Saleh, A.; Negm, W.A.; Abdelkader, D.H. Aqueous Core Epigallocatechin Gallate PLGA Nanocapsules: Characterization, Antibacterial Activity against Uropathogens, and In Vivo Reno-Protective Effect in Cisplatin Induced Nephrotoxicity. Drug Deliv. 2022, 29, 1848–1862. [Google Scholar] [CrossRef]
- Qanash, H.; Bazaid, A.; Aldarhami, A.; Alharbi, B.; Almashjary, M.; Hazzazi, M.; Felemban, H.; Abdelghany, T. Phytochemical Characterization and Efficacy of Artemisia judaica Extract Loaded Chitosan Nanoparticles as Inhibitors of Cancer Proliferation and Microbial Growth. Polymers 2023, 15, 391. [Google Scholar] [CrossRef] [PubMed]
- Abozahra, R.; Abdelhamid, S.M.; Wen, M.M.; Abdelwahab, I.; Baraka, K. A Nanoparticles Based Microbiological Study on the Effect of Rosemary and Ginger Essential Oils against Klebsiella pneumoniae. Open Microbiol. J. 2020, 14, 205–212. [Google Scholar] [CrossRef]
- Alhadrami, H.A.; Orfali, R.; Hamed, A.A.; Ghoneim, M.M.; Hassan, H.M.; Hassane, A.S.I.; Rateb, M.E.; Sayed, A.M.; Gamaleldin, N.M. Flavonoid-Coated Gold Nanoparticles as Efficient Antibiotics against Gram-Negative Bacteria—Evidence from In Silico-Supported In Vitro Studies. Antibiotics 2021, 10, 968. [Google Scholar] [CrossRef]
- Hooda, H.; Singh, P.; Bajpai, S. Effect of Quercitin Impregnated Silver Nanoparticle on Growth of Some Clinical Pathogens. Mater. Today Proc. 2020, 31, 625–630. [Google Scholar] [CrossRef]
- Khosravi, M.; Mirzaie, A.; Kashtali, A.B.; Noorbazargan, H. Antibacterial, Anti-Efflux, Anti-Biofilm, Anti-Slime (Exopolysaccharide) Production and Urease Inhibitory Efficacies of Novel Synthesized Gold Nanoparticles Coated Anthemis Atropatana Extract against Multidrug-Resistant Klebsiella pneumoniae Strains. Arch. Microbiol. 2020, 202, 2105–2115. [Google Scholar] [CrossRef] [PubMed]
- Ali, K.; Rakesh, S.; Khalid, S.; Khan, A.U. Moist Heat Synthesis of Magnetic EGCG-Cappedα-Fe2O3 Nanoparticles and Their In Vitro and In Silico Interactions with Pristine HSA- and NDM-1-Producing Bacteria. ACS Omega 2023, 8, 48775–48786. [Google Scholar] [CrossRef]
- Shabana, M.S.; Taju, G.; Majeed, A.; Karthika, M.; Ramasubramanian, V.; Sahul Hameed, A.S. Preparation and Evaluation of Mesoporous Silica Nanoparticles Loaded Quercetin against Bacterial Infections in Oreochromis niloticus. Aquac. Rep. 2021, 21, 100808. [Google Scholar] [CrossRef]
- Shameem, M.S.; Mayavan, K.; Venkatachalam, R. Influence of Quercetin Loaded Mesoporous Silica Nanoparticles (QMSNs) on Immunity and Diseases Resistance in Nile Tilapia (Oreochromis niloticus). J. Appl. Aquac. 2023, 35, 585–604. [Google Scholar] [CrossRef]
- Memar, M.Y.; Yekani, M.; Sharifi, S.; Dizaj, S. Antibacterial and Biofilm Inhibitory Effects of Rutin Nanocrystals. Biointerface Res. Appl. Chem. 2022, 13, 132. [Google Scholar] [CrossRef]
- Seetharaman, P.K.; Sivapunniyam, A.; Ramalingam, P.; Ramalingam, K.R.; Liu, B. Fabrication of a Citrus Flavonoid Hesperetin-Capped Gold Nanoparticles-Reduced Graphene Oxide Nanocomposites (Hes-Au/rGONCs) as a Potential Therapeutic Agent for Triple Negative Breast Cancer and Bacterial Infections. Surf. Interfaces 2023, 42, 103347. [Google Scholar] [CrossRef]
- Jadhav, S.T.; Salunkhe, V.R.; Bhinge, S.D. Nanoemulsion Drug Delivery System Loaded with Imiquimod: A QbD-Based Strategy for Augmenting Anti-Cancer Effects. Future J. Pharm. Sci. 2023, 9, 120. [Google Scholar] [CrossRef]
- Talegaonkar, S.; Bhattacharyya, A. Potential of Lipid Nanoparticles (SLNs and NLCs) in Enhancing Oral Bioavailability of Drugs with Poor Intestinal Permeability. AAPS PharmSciTech 2019, 20, 121. [Google Scholar] [CrossRef] [PubMed]
- Mitsutake, H.; Rodrigues da Silva, G.H.; de Paula, E.; Breitkreitz, M.C. When It Is Too Much: Identifying Butamben Excess on the Surface of Pharmaceutical Preformulation Samples by Raman Mapping. J. Pharm. Biomed. Anal. 2023, 235, 115644. [Google Scholar] [CrossRef]
- Elmowafy, M.; Al-Sanea, M.M. Nanostructured Lipid Carriers (NLCs) as Drug Delivery Platform: Advances in Formulation and Delivery Strategies. Saudi Pharm. J. 2021, 29, 999–1012. [Google Scholar] [CrossRef] [PubMed]
- Correia, A.A.V.; de Almeida Campos, L.A.; de Queiroz Macêdo, H.L.R.; de Lacerda Coriolano, D.; Agreles, M.A.A.; Xavier, D.E.; de Siqueira Ferraz-Carvalho, R.; de Andrade Aguiar, J.L.; Cavalcanti, I.M.F. Antibacterial and Antibiofilm Potential of Bacterial Cellulose Hydrogel Containing Vancomycin against Multidrug-Resistant Staphylococcus aureus and Staphylococcus epidermidis. Biology 2024, 13, 354. [Google Scholar] [CrossRef] [PubMed]
- Nordström, R.; Andrén, O.C.J.; Singh, S.; Malkoch, M.; Davoudi, M.; Schmidtchen, A.; Malmsten, M. Degradable Dendritic Nanogels as Carriers for Antimicrobial Peptides. J. Colloid Interface Sci. 2019, 554, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Detsi, A.; Kavetsou, E.; Kostopoulou, I.; Pitterou, I.; Pontillo, A.R.N.; Tzani, A.; Christodoulou, P.; Siliachli, A.; Zoumpoulakis, P. Nanosystems for the Encapsulation of Natural Products: The Case of Chitosan Biopolymer as a Matrix. Pharmaceutics 2020, 12, 669. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Abd-elmaksoud, G.A.; Abusaif, M.S.; Ammar, Y.A.; Al-Sharbasy, S.; Migahed, M.A. Construction, Characterization, DFT Computational Study, and Evaluation the Performance of Some New N-Amino Pyridinone Schiff Base Catalyzed with Ceric(IV) Ammonium Nitrate (CAN) as Corrosion Inhibitors in Some Petroleum Applications. Arab. J. Sci. Eng. 2023, 48, 16167–16185. [Google Scholar] [CrossRef]
- Wang, W.; Xue, C.; Mao, X. Chitosan: Structural Modification, Biological Activity and Application. Int. J. Biol. Macromol. 2020, 164, 4532–4546. [Google Scholar] [CrossRef]
- Alam, A.; Karmakar, R.; Rengan, A.K.; Khandelwal, M. Nanofiber-Based Systems for Stimuli-Responsive and Dual Drug Delivery: Present Scenario and the Way Forward. ACS Biomater. Sci. Eng. 2023, 9, 3160–3184. [Google Scholar] [CrossRef]
- Jiang, Z.; Zheng, Z.; Yu, S.; Gao, Y.; Ma, J.; Huang, L.; Yang, L. Nanofiber Scaffolds as Drug Delivery Systems Promoting Wound Healing. Pharmaceutics 2023, 15, 1829. [Google Scholar] [CrossRef]
- Singh, B.; Kim, J.; Shukla, N.; Lee, J.; Kim, K.; Park, M.-H. Smart Delivery Platform Using Core–Shell Nanofibers for Sequential Drug Release in Wound Healing. ACS Appl. Bio Mater. 2023, 6, 2314–2324. [Google Scholar] [CrossRef] [PubMed]
- Mustafai, A.; Zubair, M.; Hussain, A.; Ullah, A. Recent Progress in Proteins-Based Micelles as Drug Delivery Carriers. Polymers 2023, 15, 836. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.E.; Yang, G.; Park, Y.B.; Kang, H.C.; Cho, Y.-Y.; Lee, H.S.; Lee, J.Y. Epigallocatechin-3-Gallate Prevents Acute Gout by Suppressing NLRP3 Inflammasome Activation and Mitochondrial DNA Synthesis. Molecules 2019, 24, 2138. [Google Scholar] [CrossRef]
- Su, H.; Huang, C.; Liu, Y.; Kong, S.; Wang, J.; Huang, H.; Zhang, B. Preparation and Characterization of Cinnamomum Essential Oil–Chitosan Nanocomposites: Physical, Structural, and Antioxidant Activities. Processes 2020, 8, 834. [Google Scholar] [CrossRef]
- Hadidi, M.; Pouramin, S.; Adinepour, F.; Haghani, S.; Jafari, S.M. Chitosan Nanoparticles Loaded with Clove Essential Oil: Characterization, Antioxidant and Antibacterial Activities. Carbohydr. Polym. 2020, 236, 116075. [Google Scholar] [CrossRef] [PubMed]
- Agreles, M.A.A.; Cavalcanti, I.D.L.; Cavalcanti, I.M.F. Synergism between Metallic Nanoparticles and Antibiotics. Appl. Microbiol. Biotechnol. 2022, 106, 3973–3984. [Google Scholar] [CrossRef]
- Cavalcanti, I.M.F.; Pontes-Neto, J.G.; Kocerginsky, P.O.; Bezerra-Neto, A.M.; Lima, J.L.C.; Lira-Nogueira, M.C.B.; Maciel, M.A.V.; Neves, R.P.; Pimentel, M.F.; Santos-Magalhães, N.S. Antimicrobial Activity of β-Lapachone Encapsulated into Liposomes against Meticillin-Resistant Staphylococcus aureus and Cryptococcus neoformans Clinical Strains. J. Glob. Antimicrob. Resist. 2015, 3, 103–108. [Google Scholar] [CrossRef]
- Kisimba, K.; Krishnan, A.; Faya, M.; Byanga, K.; Kasumbwe, K.; Vijayakumar, K.; Prasad, R. Synthesis of Metallic Nanoparticles Based on Green Chemistry and Their Medical Biochemical Applications: Synthesis of Metallic Nanoparticles. J. Renew. Mater. 2023, 11, 2575–2591. [Google Scholar] [CrossRef]
- Skłodowski, K.; Chmielewska-Deptuła, S.J.; Piktel, E.; Wolak, P.; Wollny, T.; Bucki, R. Metallic Nanosystems in the Development of Antimicrobial Strategies with High Antimicrobial Activity and High Biocompatibility. Int. J. Mol. Sci. 2023, 24, 2104. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Banerjee, S.; Ali, S.A.; Karmakar, A.; Patra, A.K. Exploring the High Activity of Hierarchical Copper Oxide Nanomaterials: Unveiling the Influence of Reduced Size and Enhanced Surface Area. Catal. Commun. 2023, 184, 106784. [Google Scholar] [CrossRef]
- Mansi, K.; Kumar, R.; Narula, D.; Pandey, S.K.; Kumar, V.; Singh, K. Microwave-Induced CuO Nanorods: A Comparative Approach between Curcumin, Quercetin, and Rutin to Study Their Antioxidant, Antimicrobial, and Anticancer Effects against Normal Skin Cells and Human Breast Cancer Cell Lines MCF-7 and T-47D. ACS Appl. Bio Mater. 2022, 5, 5762–5778. [Google Scholar] [CrossRef]
- Adeniji, O.O.; Ojemaye, M.O.; Okoh, A.I. Antibacterial Activity of Metallic Nanoparticles against Multidrug-Resistant Pathogens Isolated from Environmental Samples: Nanoparticles/Antibiotic Combination Therapy and Cytotoxicity Study. ACS Appl. Bio Mater. 2022, 5, 4814–4826. [Google Scholar] [CrossRef] [PubMed]
- Pacheco Coello, F.J. Synthesis of Lithium and Silver Nanoparticles by Green Chemistry and Its Antimicrobial Effect on Staphylococcus aureus and Klebsiella pneumoniae. Rev. Bol. Quim. 2024, 41, 42–48. [Google Scholar] [CrossRef]
- Ibrahim, H.M.; Reda, M.M.; Klingner, A. Preparation and Characterization of Green Carboxymethylchitosan (CMCS)—Polyvinyl Alcohol (PVA) Electrospun Nanofibers Containing Gold Nanoparticles (AuNPs) and Its Potential Use as Biomaterials. Int. J. Biol. Macromol. 2020, 151, 821–829. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, X.; Jin, S.; Wang, H.; Yuan, L.; Brash, J.L. Rapid Antibacterial Effect of Sunlight-Exposed Silicon Nanowire Arrays Modified with Au/Ag Alloy Nanoparticles. J. Mater. Chem. B 2019, 7, 6202–6209. [Google Scholar] [CrossRef]
- Pathak, D.; Mazumder, A. Potential of Flavonoids as Promising Phytotherapeutic Agents to Combat Multidrug-Resistant Infections. Curr. Pharm. Biotechnol. 2024, 25, 1664–1692. [Google Scholar] [CrossRef]
- Rofeal, M.; El-Malek, F.A.; Qi, X. In Vitro Assessment of Green Polyhydroxybutyrate/Chitosan Blend Loaded with Kaempferol Nanocrystals as a Potential Dressing for Infected Wounds. Nanotechnology 2021, 32, 375102. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.-J. Challenges and Recent Progress in Oral Drug Delivery Systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef]
- Duong, V.-A.; Nguyen, T.-T.-L.; Maeng, H.-J. Recent Advances in Intranasal Liposomes for Drug, Gene, and Vaccine Delivery. Pharmaceutics 2023, 15, 207. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, A.M.; Zuccari, G. Nanotechnological Manipulation of Nutraceuticals and Phytochemicals for Healthy Purposes: Established Advantages vs. Still Undefined Risks. Polymers 2021, 13, 2262. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, M.; Alijani, S.; Montazeri, M.; Esmaeilizadeh, N.; Sadeghi-Soureh, S.; Pilehvar-Soltanahmadi, Y. Recent Advances in Electrospun Nanofiber-mediated drug Delivery Strategies for Localized Cancer Chemotherapy. J. Biomed. Mater. Res 2020, 108, 1444–1458. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Chen, Y.; Wang, X.; Xu, X.; Shen, Y.; Khan, A.U.R.; Aldalbahi, A.; Fetz, A.E.; Bowlin, G.L.; El-Newehy, M.; et al. Electrospinning Nanofiber Scaffolds for Soft and Hard Tissue Regeneration. J. Mater. Sci. Technol. 2020, 59, 243–261. [Google Scholar] [CrossRef]
- Hwang, D.; Ramsey, J.D.; Kabanov, A.V. Polymeric Micelles for the Delivery of Poorly Soluble Drugs: From Nanoformulation to Clinical Approval. Adv. Drug Deliv. Rev. 2020, 156, 80–118. [Google Scholar] [CrossRef]
- Fan, W.; Wei, Q.; Xiang, J.; Tang, Y.; Zhou, Q.; Geng, Y.; Liu, Y.; Sun, R.; Xu, L.; Wang, G.; et al. Mucus Penetrating and Cell-Binding Polyzwitterionic Micelles as Potent Oral Nanomedicine for Cancer Drug Delivery. Adv. Mater. 2022, 34, 2109189. [Google Scholar] [CrossRef] [PubMed]
- McGuckin, M.B.; Wang, J.; Ghanma, R.; Qin, N.; Palma, S.D.; Donnelly, R.F.; Paredes, A.J. Nanocrystals as a Master Key to Deliver Hydrophobic Drugs via Multiple Administration Routes. J. Control. Release 2022, 345, 334–353. [Google Scholar] [CrossRef]
- Abdel-Emam, R.A.; Ali, M.F.; Hassan, A.S.; Abd-Ellatief, R.B. Development and Evaluation of Dexamethasone-Loaded Bioadhesive Polymeric Nanocapsules for Mitigating Cardiac and Gastric Adverse Effects of Free Dexamethasone. J. Pharm. Investig. 2024. [Google Scholar] [CrossRef]
- Gebreel, R.M.; Abdellatif, M.M.; Attia, A. Lomefloxacin-Loaded PLGA Hollow Nanospheres for Healing MRSA-Infected Diabetic Wound; Optimization, in Vitro and in Vivo Studies. J. Drug Deliv. Sci. Technol. 2024, 100, 106050. [Google Scholar] [CrossRef]
- Chai, M.; Gao, Y.; Liu, J.; Deng, Y.; Hu, D.; Jin, Q.; Ji, J. Polymyxin B-Polysaccharide Polyion Nanocomplex with Improved Biocompatibility and Unaffected Antibacterial Activity for Acute Lung Infection Management. Adv. Healthc. Mater. 2020, 9, 1901542. [Google Scholar] [CrossRef]
- Dilnawaz, F.; Acharya, S.; Kanungo, A. A Clinical Perspective of Chitosan Nanoparticles for Infectious Disease Management. Polym. Bull. 2024, 81, 1071–1095. [Google Scholar] [CrossRef] [PubMed]
- Mudhakir, D.; Sadaqa, E.; Permana, Z.; Mumtazah, J.E.; Zefrina, N.F.; Xeliem, J.N.; Hanum, L.F.; Kurniati, N.F. Dual-Functionalized Mesoporous Silica Nanoparticles for Celecoxib Delivery: Amine Grafting and Imidazolyl PEI Gatekeepers for Enhanced Loading and Controlled Release with Reduced Toxicity. Molecules 2024, 29, 3546. [Google Scholar] [CrossRef] [PubMed]
- Ajinkya, N.; Yu, X.; Kaithal, P.; Luo, H.; Somani, P.; Ramakrishna, S. Magnetic Iron Oxide Nanoparticle (IONP) Synthesis to Applications: Present and Future. Materials 2020, 13, 4644. [Google Scholar] [CrossRef] [PubMed]
- Al-Zahrani, S.S.; Bora, R.S.; Al-Garni, S.M. Antimicrobial Activity of Chitosan Nanoparticles. Biotechnol. Biotechnol. Equip. 2021, 35, 1874–1880. [Google Scholar] [CrossRef]
- Dhas, N.; Mehta, T. Intranasal Delivery of Chitosan Decorated PLGA Core/Shell Nanoparticles Containing Flavonoid to Reduce Oxidative Stress in the Treatment of Alzheimer’s Disease. J. Drug Deliv. Sci. Technol. 2021, 61, 102242. [Google Scholar] [CrossRef]
- Ranjbar, S.; Emamjomeh, A.; Sharifi, F.; Zarepour, A.; Aghaabbasi, K.; Dehshahri, A.; Sepahvand, A.M.; Zarrabi, A.; Beyzaei, H.; Zahedi, M.M.; et al. Lipid-Based Delivery Systems for Flavonoids and Flavonolignans: Liposomes, Nanoemulsions, and Solid Lipid Nanoparticles. Pharmaceutics 2023, 15, 1944. [Google Scholar] [CrossRef]
- Riaz, S.; Fatima Rana, N.; Hussain, I.; Tanweer, T.; Nawaz, A.; Menaa, F.; Janjua, H.A.; Alam, T.; Batool, A.; Naeem, A.; et al. Effect of Flavonoid-Coated Gold Nanoparticles on Bacterial Colonization in Mice Organs. Nanomaterials 2020, 10, 1769. [Google Scholar] [CrossRef]
- Elkhateeb, O.; Badawy, M.E.I.; Tohamy, H.G.; Abou-Ahmed, H.; El-Kammar, M.; Elkhenany, H. Curcumin-Infused Nanostructured Lipid Carriers: A Promising Strategy for Enhancing Skin Regeneration and Combating Microbial Infection. BMC Vet. Res. 2023, 19, 206. [Google Scholar] [CrossRef]
- Espíndola, C.; Correa, A.J.; López-López, M.; López-Cornejo, P.; Bernal, E.; Lebrón, J.A.; Ostos, F.J.; Benhnia, M.R.-E.-I.; Moyá, M.L. Single -and Multi-Walled Carbon Nanotubes as Nanocarriers for the Delivery of 7-Hydroxyflavone. Pharmaceutics 2022, 14, 2806. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).