Fluoride Metabolism in Pregnant Women: A Narrative Review of the Literature
Abstract
:1. Introduction
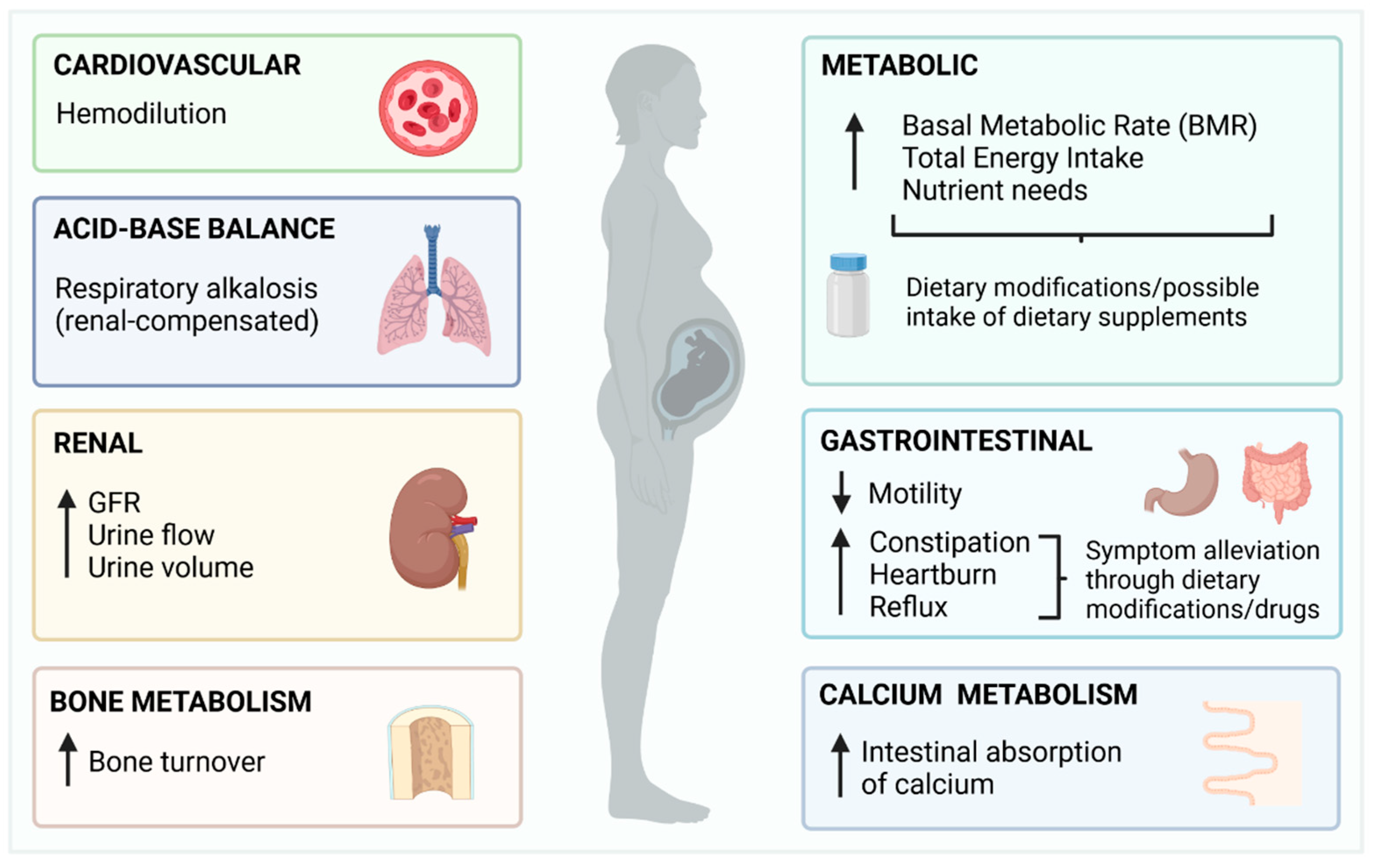
2. Overview of Pregnancy and Summary of Changes with the Potential to Impact Fluoride Metabolism
3. Available Evidence on the Absorption, Distribution, and Excretion of Fluoride in Pregnant Women
3.1. Literature Search
3.2. Fluoride Absorption in Pregnant Women
3.3. Distribution of Fluoride in Pregnant Women
3.3.1. Maternal Blood
3.3.2. Placenta
3.3.3. Placental Passage of Fluoride
3.3.4. Amniotic Fluid
3.3.5. Fetus
3.4. Urinary and fecal excretion of fluoride in pregnant women
4. Conclusions
- Although pregnancy is a physiological state that affects all major systems (cardiovascular, renal, respiratory, gastrointestinal, bone metabolism and acid-base balance) with high potential to affect fluoride metabolism, the evidence on how these changes affect the intake, distribution, and excretion of fluoride, is limited in quantity and quality.
- Maternal plasma and urinary fluoride levels depend on fluoride exposure and vary across gestation, but there is not enough quality evidence to determine the direction (increase/decrease) of such variation.
- There is no doubt that fluoride from maternal blood crosses the placenta and is absorbed and excreted by the fetus. The biological mechanisms behind this transfer, are however, unknown.
Author Contributions
Funding
Conflicts of Interest
References
- García, M.G.; Borgnino, L. Fluoride in the Context of the Environment. In Fluorine: Chemistry, Analysis, Function and Effects; Food and Nutritional Components in Focus; The Royal Society of Chemistry: London, UK, 2015; pp. 3–21. [Google Scholar]
- Smolin, L.A.; Grosvenor, M.B. The Trace Elements. In Nutrition: Science and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 486–534. [Google Scholar]
- Hayes, D.P. Nutritional hormesis. Eur. J. Clin. Nutr. 2007, 61, 147–159. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, M.S.; Whiting, P.F.; Wilson, P.M.; Sutton, A.J.; Chestnutt, I.; Cooper, J.; Misso, K.; Bradley, M.; Treasure, E.; Kleijnen, J. Systematic review of water fluoridation. BMJ 2000, 321, 855–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Künzel, W. Systemic use of fluoride--other methods: Salt, sugar, milk, etc. Caries Res. 1993, 27, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Iheozor-Ejiofor, Z.; Worthington, H.V.; Walsh, T.; O’Malley, L.; Clarkson, J.E.; Macey, R.; Alam, R.; Tugwell, P.; Welch, V.; Glenny, A.M. Water fluoridation for the prevention of dental caries. Cochrane Database Syst. Rev. 2015, 2015, CD010856. [Google Scholar] [CrossRef]
- Everett, E.T. Fluoride’s effects on the formation of teeth and bones, and the influence of genetics. J. Dent. Res. 2011, 90, 552–560. [Google Scholar] [CrossRef] [Green Version]
- Grandjean, P. Developmental fluoride neurotoxicity: An updated review. Environ. Health 2019, 18, 110. [Google Scholar] [CrossRef] [Green Version]
- Farmus, L.; Till, C.; Green, R.; Hornung, R.; Martinez Mier, E.A.; Ayotte, P.; Muckle, G.; Lanphear, B.P.; Flora, D.B. Critical windows of fluoride neurotoxicity in Canadian children. Environ. Res. 2021, 200, 111315. [Google Scholar] [CrossRef]
- Whitford, G.M. The Metabolism and Toxicity of Fluoride; Karger Publishers: Basel, Switzerland, 1996. [Google Scholar]
- Buzalaf, M.A.R.; Whitford, G.M. Fluoride metabolism. Monogr. Oral. Sci. 2011, 22, 20–36. [Google Scholar] [CrossRef]
- Duffy, T.P. Hematologic aspects of pregnancy. In Medical Complications During Pregnancy; Elsevier: Amsterdam, The Netherlands, 2004; pp. 69–86. [Google Scholar]
- Monga, M.; Mastrobattista, J. Maternal cardiovascular, respiratory, and renal adaptation to pregnancy. In Craesy and Resnik’s Maternal Fetal Medicine: Principles and Practice; Elsevier: Amsterdam, The Netherlands, 2009; pp. 101–109. [Google Scholar]
- Cornelis, T.; Odutayo, A.; Keunen, J.; Hladunewich, M. The kidney in normal pregnancy and preeclampsia. Semin. Nephrol. 2011, 31, 4–14. [Google Scholar] [CrossRef]
- Lof, M.; Olausson, H.; Bostrom, K.; Janerot-Sjöberg, B.; Sohlstrom, A.; Forsum, E. Changes in basal metabolic rate during pregnancy in relation to changes in body weight and composition, cardiac output, insulin-like growth factor I, and thyroid hormones and in relation to fetal growth. Am. J. Clin. Nutr. 2005, 81, 678–685. [Google Scholar] [CrossRef]
- Blackburn, S.T. Maternal, Fetal, & Neonatal Physiology: A Clinical Perspective, 4th ed.; Elsevier Saunders: Maryland Heights, MO, USA, 2013. [Google Scholar]
- Forbes, L.E.; Graham, J.E.; Berglund, C.; Bell, R.C. Dietary Change during Pregnancy and Women’s Reasons for Change. Nutrients 2018, 10, 1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Body, C.; Christie, J.A. Gastrointestinal Diseases in Pregnancy: Nausea, Vomiting, Hyperemesis Gravidarum, Gastroesophageal Reflux Disease, Constipation, and Diarrhea. Gastroenterol. Clin. North Am. 2016, 45, 267–283. [Google Scholar] [CrossRef] [PubMed]
- Castiblanco-Rubio, G.A.; Muñoz-Rocha, T.V.; Cantoral, A.; Téllez-Rojo, M.M.; Ettinger, A.S.; Mercado-García, A.; Peterson, K.E.; Hu, H.; Martínez-Mier, E.A. Dietary fluoride intake over the course of pregnancy in Mexican women. Public Health Nutr. 2021, 24, 2388–2396. [Google Scholar] [CrossRef] [PubMed]
- Jensen, D.; Wolfe, L.A.; Slatkovska, L.; Webb, K.A.; Davies, G.A.; O’Donnell, D.E. Effects of human pregnancy on the ventilatory chemoreflex response to carbon dioxide. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2005, 288, R1369–R1375. [Google Scholar] [CrossRef] [Green Version]
- Bobrowski, R.A. Pulmonary physiology in pregnancy. Clin. Obstet. Gynecol. 2010, 53, 285–300. [Google Scholar] [CrossRef]
- Myers, V.C.; Muntwyler, E.; Bill, A.H. The acid-base balance disturbance of pregnancy. J. Biol. Chem. 1932, 98, 253–260. [Google Scholar] [CrossRef]
- Naylor, K.; Iqbal, P.; Fledelius, C.; Fraser, R.; Eastell, R. The effect of pregnancy on bone density and bone turnover. J. Bone Miner. Res. 2000, 15, 129–137. [Google Scholar] [CrossRef]
- Trautner, K.; Einwag, J. Influence of milk and food on fluoride bioavailability from NaF and Na2FPO3 in man. J. Dent. Res. 1989, 68, 72–77. [Google Scholar] [CrossRef]
- Whitford, G.M.; Pashley, D.H. Fluoride absorption: The influence of gastric acidity. Calcif. Tissue Int. 1984, 36, 302–307. [Google Scholar] [CrossRef]
- Nopakun, J.; Messer, H.H. Mechanism of fluoride absorption from the rat small intestine. Nutr. Res. 1990, 10, 771–779. [Google Scholar] [CrossRef]
- Hanhijarvi, H.; Kanto, J.; Ruponen, S. Human free ionized plasma fluoride concentrations during pregnancy, toxemia, and lactation. Fluoride 1974, 7, 143. [Google Scholar]
- Hanhijarvi, H. Maternal ionic plasma fluoride concentrations during pregnancy and after delivery. Fluoride 1981, 14, 4–9. [Google Scholar]
- Opydo-Szymaczek, J.; Borysewicz-Lewicka, M. Variations in concentration of fluoride in blood plasma of pregnant women and their possible consequences for amelogenesis in a fetus. Homo 2006, 57, 295–307. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.B.; Basu, N.; Martinez-Mier, E.A.; Sanchez, B.N.; Zhang, Z.; Liu, Y.; Parajuli, R.P.; Peterson, K.; Mercado-Garcia, A.; Bashash, M.; et al. Urinary and plasma fluoride levels in pregnant women from Mexico City. Environ. Res. 2016, 150, 489–495. [Google Scholar] [CrossRef] [Green Version]
- Abduweli Uyghurturk, D.; Goin, D.E.; Martinez-Mier, E.A.; Woodruff, T.J.; DenBesten, P.K. Maternal and fetal exposures to fluoride during mid-gestation among pregnant women in northern California. Environ. Health Glob. Access Sci. Source 2020, 19, 38. [Google Scholar] [CrossRef] [Green Version]
- Griffiths, S.K.; Campbell, J.P. Placental structure, function and drug transfer. Contin. Educ. Anaesth. Crit. Care Pain 2015, 15, 84–89. [Google Scholar] [CrossRef] [Green Version]
- Zipkin, I.; Babeaux, W.L. Maternal transfer of fluoride. J. Oral Ther. Pharmacol. 1965, 1, 652–665. [Google Scholar]
- Feltman, R.; Kosel, G. Prenatal Ingestion of Fluorides and Their Transfer to the Fetus. Science 1955, 122, 560. [Google Scholar] [CrossRef]
- Gardner, D.E.; Smith, F.A.; Hodge, H.C.; Overton, D.E.; Feltman, R. The Fluoride Content of Placental Tissue as Related to the Fluoride Content of Drinking Water. Science 1952, 115, 208–209. [Google Scholar] [CrossRef]
- Gedalia, I.; Brzezinski, A.; Zukerman, H.; Mayersdorf, A. Placental transfer of fluoride in the human fetus at low and high F-intake. J. Dent. Res. 1964, 43, 669–671. [Google Scholar] [CrossRef]
- Ericsson, Y.; Malmnäs, C. Placental Transfer of Fluorine Investigated with F18 in Man and Rabbit. Acta Obstet. Gynecol. Scand. 1962, 41, 144–158. [Google Scholar] [CrossRef]
- Armstrong, W.; Singer, L.; Makowski, E.L. Placental transfer of fluoride and calcium. Am. J. Obstet. Gynecol. 1970, 107, 432–434. [Google Scholar] [CrossRef]
- Shen, Y.W.; Taves, D.R. Fluoride concentrations in the human placenta and maternal and cord blood. Am. J. Obstet. Gynecol. 1974, 119, 205–207. [Google Scholar] [CrossRef]
- Fry, B.W.; Taves, D.R. Maternal and fetal fluorometabolite concentrations after exposure to methoxyflurane. Am. J. Obstet. Gynecol. 1974, 119, 199–204. [Google Scholar] [CrossRef]
- Palahniuk, R.J.; Cumming, M. Plasma fluoride levels following obstetrical use of methoxyflurane. Can. Anaesth. Soc. J. 1975, 22, 291–297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weiss, V.; de Carlini, C. Placental transfer of fluoride during methoxyflurane anaesthesia for cesarean section. Experientia 1975, 31, 339–341. [Google Scholar] [CrossRef]
- Louw, A.J.; Van Wyk, P.J. Placental transfer of fluoride. J. Dent. Assoc. South Afr. 1984, 39, 61–62. [Google Scholar]
- Ron, M.; Singer, L.; Menczel, J.; Kidroni, G. Fluoride concentration in amniotic fluid and fetal cord and maternal plasma. Eur. J. Obstet. Gynecol. Reprod. Biol. 1986, 21, 213–218. [Google Scholar] [CrossRef]
- Caldera, R.; Chavinie, J.; Fermanian, J.; Tortrat, D.; Laurent, A.M. Maternal-fetal transfer of fluoride in pregnant women. Biol. Neonate 1988, 54, 263–269. [Google Scholar] [CrossRef]
- Gupta, A.; Tangade, P.S.; Sunil, M.K.; Sahwney, H. Investigating relationship between fluoride ion concentration in mother and cord blood serum. Med. -Leg. Update 2012, 12, 94–97. [Google Scholar]
- Malhotra, A.; Tewari, A.; Chawla, H.S.; Gauba, K.; Dhall, K. Placental transfer of fluoride in pregnant women consuming optimum fluoride in drinking water. J. Indian Soc. Pedod. Prev. Dent. 1993, 11, 1–3. [Google Scholar] [PubMed]
- Brambilla, E.; Belluomo, G.; Malerba, A.; Buscaglia, M.; Strohmenger, L. Oral administration of fluoride in pregnant women, and the relation between concentration in maternal plasma and in amniotic fluid. Arch. Oral Biol. 1994, 39, 991–994. [Google Scholar] [CrossRef]
- Shimonovitz, S.; Patz, D.; Ever-Hadani, P.; Singer, L.; Zacut, D.; Kidroni, G.; Ron, M. Umbilical cord fluoride serum levels may not reflect fetal fluoride status. J. Perinat. Med. 1995, 23, 279–282. [Google Scholar] [CrossRef] [PubMed]
- Montherrat-Carret, L.; Perrat-Mabilon, B.; Barbey, E.; Bouloc, R.; Boivin, G.; Michelet, A.; Magloire, H. Chemical and X-ray analysis of fluoride, phosphorus, and calcium in human foetal blood and hard tissues. Arch. Oral Biol. 1996, 41, 1169–1178. [Google Scholar] [CrossRef]
- Opydo-Szymaczek, J.; Borysewicz-Lewicka, M. Transplacental passage of fluoride in pregnant Polish women assessed on the basis of fluoride concentrations in maternal and cord blood plasma. Fluoride 2007, 40, 46–50. [Google Scholar]
- Abboud, T.K.; Shnider, S.M.; Wright, R.G.; Rolbin, S.H.; Craft, J.B.; Henriksen, E.H.; Johnson, J.; Jones, M.J.; Hughes, S.C.; Levinson, G. Enflurane analgesia in obstetrics. Anesth. Analg. 1981, 60, 133–137. [Google Scholar] [CrossRef]
- Wickstrom, I.; Kjellmer, I.; Kristianson, B.; Magno, R. Anesthesia for cesarean section--VII. Early effects on neonatal renal function of enflurane anesthesia for cesarean section. Acta Anaesthesiol. Scand. 1980, 24, 190–194. [Google Scholar] [CrossRef]
- Dahlgren, B.E. Urinary fluoride concentration in mothers and neonates after methoxyflurane-nitrous oxide analgesia during labour. Acta Pharm. Suec. 1978, 15, 211–217. [Google Scholar]
- Abboud, T.K.; D’Onofrio, L.; Reyes, A.; Mosaad, P.; Zhu, J.; Mantilla, M.; Gangolly, J.; Crowell, D.; Cheung, M.; Afrasiabi, A.; et al. Isoflurane or halothane for cesarean section: Comparative maternal and neonatal effects. Acta Anaesthesiol. Scand. 1989, 33, 578–581. [Google Scholar] [CrossRef]
- Martin, D.J. The Evanston dental caries study; determination of fluorine in foods, bones, and teeth. J. Dent. Res. 1948, 27, 27–33. [Google Scholar] [CrossRef]
- Brzezinski, A.; Bercovici, B.; Gedalia, J. Fluorine in the human fetus. Obstet. Gynecol. 1960, 15, 329–331. [Google Scholar] [PubMed]
- Blayney, J.R.; Hill, I.N. Evanston dental caries study. XXIV. Prenatal Fluorides--Value of waterborne fluorides during pregnancy. J. Am. Dent. Assoc. 1964, 69, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Gedalia, I.; Brzezinski, A.; Portuguese, N.; Bercovici, B. The fluoride content of teeth and bones of human fetuses. Arch. Oral Biol. 1964, 9, 331–340. [Google Scholar] [CrossRef]
- Gedalia, I.; Zukerman, H.; Leventhal, H. Fluoride content of teeth and bones of human fetuses: In areas with about 1 ppm of fluoride in drinking water. J. Am. Dent. Assoc. 1965, 71, 1121–1123. [Google Scholar] [CrossRef]
- Gedalia, I.; Garti, A.; Lewin-Epstein, J. Ash and fluoride contents of the different human foetal teeth from areas of low and high fluoride concentrations in the drinking water. Arch. Oral. Biol. 1967, 12, 1485–1490. [Google Scholar] [CrossRef]
- Du, L. The effect of fluorine on the developing human brain. Chin. J. Pathol. 1992, 21, 218–220. [Google Scholar]
- He, H.; Cheng, Z.; Liu, W. Effects of fluorine on the human fetus. Chin. J. Control. Endem. Dis. 1989, 4, 136–138. [Google Scholar]
- Maheshwari, U.R.; King, J.; Brunetti, A.J.; Hodge, H.C.; Newbrun, E.; Margen, S. Fluoride balances in pregnant and nonpregnant women. J. Occup. Med. 1981, 23, 465–468. [Google Scholar] [CrossRef]
- Maheshwari, U.R.; King, J.C.; Leybin, L.; Newbrun, E.; Hodge, H.C. Fluoride balances during early and late pregnancy. J. Occup. Med. 1983, 25, 587–590. [Google Scholar]
- Gedalia, J.; Brzezinski, A.; Bercovici, B. Urinary fluorine levels in women during pregnancy and after delivery. J. Dent. Res. 1959, 38, 548–551. [Google Scholar] [CrossRef]
- Opydo-Szymaczek, J.; Borysewicz-Lewicka, M. Urinary fluoride levels for assessment of fluoride exposure of pregnant women in Poznan, Poland. Fluoride 2005, 38, 312–317. [Google Scholar]
- Till, C.; Green, R.; Grundy, J.G.; Hornung, R.; Neufeld, R.; Martinez-Mier, E.A.; Ayotte, P.; Muckle, G.; Lanphear, B. Community Water Fluoridation and Urinary Fluoride Concentrations in a National Sample of Pregnant Women in Canada. Environ. Health Perspect. 2018, 126, 107001. [Google Scholar] [CrossRef] [PubMed]
- Castiblanco-Rubio, G.A.; Muñoz-Rocha, T.V.; Téllez-Rojo, M.M.; Ettinger, A.S.; Mercado-García, A.; Peterson, K.E.; Hu, H.; Cantoral, A.; Martínez-Mier, E.A. Dietary Influences on Urinary Fluoride over the Course of Pregnancy and at One-Year Postpartum. Biol. Trace Elem. Res. 2021, 200, 1568–1579. [Google Scholar] [CrossRef]
- Hanhijarvi, H. Inorganic plasma fluoride concentrations and its renal excretion in certain physiological and pathological conditions in man. Fluoride 1975, 8, 198–207. [Google Scholar]

| Reference | Fluoride Source | Type of Sample | Analytical Method | n | Collection Time | Maternal F (mg/L) |
|---|---|---|---|---|---|---|
| Hanhijarvi [27], 1974 | 1 mg/L CWF | Plasma | ISE, microdiffusion | 48 | Not pregnant | 0.023 ± 0.001 |
| 149 | Labor | 0.018 ± 0.000 | ||||
| 67 | 0–10 months pregnant | 0.018 ± 0.001 | ||||
| Hanhijarvi [28], 1981 | 1.2 mg/L CWF | Plasma | ISE, microdiffusion | 12 | Not pregnant | 0.019 ± 0.006 |
| 11 | 10 weeks | 0.018 ± 0.000 | ||||
| 45 | 19 weeks | 0.017 ± 0.005 | ||||
| 10 | 36 weeks | 0.016 ± 0.003 | ||||
| 79 | 7 days after delivery | 0.018 ± 0.006 | ||||
| Opydo-Szymaczek et al. [29], 2006 | 0.8 mg/L CWF | Plasma | ISE, known addition | 31 | 28 weeks | 0.062 ± 0.021 |
| 33 weeks | 0.071 ± 0.022 | |||||
| Delivery | 0.070 ± 0.026 | |||||
| Thomas et al. [30], 2016 | 250mg/kg CSF | Plasma | ISE, microdiffusion | 220 | 13.5 ± 2.3 weeks | 0.023 ± 0.022 |
| 255 | 25.3 ± 2.4 weeks | 0.019 ± 0.017 | ||||
| 152 | 34.5 ± 2.1 weeks | 0.018 ± 0.017 | ||||
| Uyghurturk et al. [31], 2020 | <0.7 mg/L CWF | Serum | ISE, microdiffusion | 24 | 20.5 ± 2 weeks | 0.011 ± 0.009 |
| >0.7 mg/L CWF | Serum | ISE, microdiffusion | 24 | 20.5 ± 2 weeks | 0.021 ± 0.015 |
| Reference | CWF (mg/L) | Type of Sample | Analytical Method | n | Collection Time | Maternal F (mg/L) | Fetal F− (mg/L) | Ratio Fetal/Maternal | Correlation |
|---|---|---|---|---|---|---|---|---|---|
| Gedalia et al. [36], 1964 | 0.06 | Not specified | Ashing and distillation | 39 | V | 0.150 ± 0.060 | 0.160 ± 0.070 | 1.07 | - |
| 0.60 | 30 | 0.230 ± 0.100 | 0.170 ± 0.050 | 0.74 | - | ||||
| Armstrong et al. [38], 1970 | - | Plasma (venous) | Diffusion of Hydrogen F | 16 | C | 0.100 ± 0.008 | 0.140 ± 0.013 | 1.40 | - |
| Plasma (arterial) | 16 | 0.110 ± 0.011 | 0.140 ± 0.010 | 1.27 | - | ||||
| Shen & Taves [39], 1973 | - | Serum | Morin-thorium reagent | 16 | V | 0.017 ± 0.002 | 0.013 ± 0.002 | 0.76 | 0.86 |
| Fry & Taves [40], 1973 | - | Plasma | ISE, microdiffusion | 7 | V | - | - | 0.25 | - |
| Palahniuk & Cumming [41], 1975 | - | Plasma | ISE, not specified | 50 | V | - | - | 0.81 | - |
| 41 | L + V | - | - | 0.63 | - | ||||
| 14 | C | - | - | 0.44 | - | ||||
| Weiss & Carlini [42], 1975 | - | Serum | ISE, not specified | 15 | C | - | - | 0.47 | 0.77 |
| Louw et al. [43], 1984 | - | Plasma | ISE, direct | 10 | C | 0.270 | 0.250 | 0.92 | 0.94 |
| Ron et al. [44], 1986 | 0.50 | Plasma | ISE, direct | 50 | V | 0.033 ± 0.003 | 0.028 ± 0.005 | 0.84 | - |
| Caldera et al. [45], 1988 | 0.50 | Plasma | Gas chromatography | 46 | V | 0.022 ± 0.015 | 0.028 ± 0.021 | 1.26 | - |
| Gupta et al. [46], 1993 | - | Plasma | ISE, not specified | 25 | V | - | - | 0.60 | - |
| Malhotra et al. [47], 1993 | 1.20 | Plasma | ISE, direct | 25 | V | 0.250 ± 0.080 | 0.230 ± 0.840 | 0.92 | 0.97 |
| Shimonovitz et al. [49], 1995 | 0.50 | Serum | ISE, known-addition | 22 | V | 0.018 ± 0.012 | 0.030 ± 0.015 | 1.66 | - |
| Montherrat-Carret et al. [50], 1996 | 0.10 | Serum | ISE, direct | 5 | 17–25 weeks | 0.034 ± 0.011 | 0.031 ± 0.011 | 0.91 | - |
| Opydo-Szymaczek et al. [51], 2007 | 0.80 | Plasma | ISE, known-addition | 30 | V | 0.067 ± 0.023 | 0.055 ± 0.008 | 0.82 | 0.45 |
| Reference | Fluoride Source | Type of Sample | Analytical Method | Collection time | Sample Size | F concentration (mg/L) | Dilution Adjustment | |
|---|---|---|---|---|---|---|---|---|
| Gedalia et al. [66], 1959 | 0.5–0.6 mg/L F CWF | Spot samples voided between 9:00 a.m. and 12:00 p.m. | Colorimetric after ashing and steam distillation | Pregnancy (months) | 4 | 117 | 0.53 | None |
| 5 | 80 | 0.43 | ||||||
| 6 | 91 | 0.34 | ||||||
| 7 | 89 | 0.28 | ||||||
| 8 | 81 | 0.22 | ||||||
| 0.5–0.6 mg/L F CWF | Spot samples voided between 9:00 a.m. and 12:00 p.m. | Colorimetric after ashing and steam distillation | Postpartum (months) | 9 | 88 | 0.29 | None | |
| 1 | 55 | 0.39 | ||||||
| 2 & 3 | 64 | 0.49 | ||||||
| 4 | 45 | 0.50 | ||||||
| 8 | 32 | 0.23 | ||||||
| Maheshwari et al. [64], 1981 | 0.41 mg F/day, controlled diet high in animal protein | Pool of 3 days of 24 h samples | ISE, microdiffusion | Pregnancy (weeks) | 20–40 | 6 | 0.62 ± 0.09 | Not required |
| 0.27 mg F/day, controlled diet high in vegetable protein | Pool of 3 days of 24 h samples | ISE, microdiffusion | Pregnancy (weeks) | 20–40 | 4 | 0.44 ± 0.07 | Not required | |
| 0.41 mg F/day, controlled diet high in animal protein | Pool of 3 days of 24 h samples | ISE, microdiffusion | Not pregnant | - | 6 | 0.44 ± 0.04 | Not required | |
| 0.27 mg F/day, controlled diet high in vegetable protein | Pool of 3 days of 24 h samples | ISE, microdiffusion | Not pregnant | - | 6 | 0.36 ± 0.05 | Not required | |
| Maheshwari et al. [65], 1983 | 1.35 mg F/day controlled diet | Pool of 3 days of 24 h samples | ISE, microdiffusion | Pregnancy (weeks) | 10–20 | 6 | 0.95 ± 0.11 | Not required |
| 1.40 mg F/day controlled diet | Pool of 3 days of 24 h samples | ISE, microdiffusion | Pregnancy (weeks) | 30–40 | 5 | 1.03 ± 0.14 | Not required | |
| 1.28 mg F/day controlled diet | Pool of 3 days of 24 h samples | ISE, microdiffusion | Not pregnant | - | 7 | 1.15 ± 0.24 | Not required | |
| Opydo-Szymackzek et al. [67], 2005 | 0.4–0.8 mg/L CWF | Spot morning urine (fasting) | ISE, direct | Pregnancy (weeks) | 28–33 | 31 | 0.65 ± 0.316 | None |
| 0.84 ± 0.352 | ||||||||
| Not pregnant | - | 30 | 1.30 ± 0.301 | |||||
| Thomas et al. [30], 2016 | 250 mg/kg in CSF | Spot morning urine (nonfasting) | ISE, microdiffusion | Pregnancy (weeks) | 13.5 ± 2.3 | 436 | 0.92 ± 0.46 | CRE |
| 25.3 ± 2.4 | 199 | 0.95 ± 0.47 | ||||||
| 34.5 ± 2.1 | 246 | 0.87 ± 0.48 | ||||||
| Till et al. [68], 2020 | <0.3 mg/L CWF | Spot urine (collection time not specified) | ISE, microdiffusion | Pregnancy (weeks) | 11.6 ± 1.6 | 541 | 0.31 ± 0.39 | SG |
| 19.1 ± 2.4 | 507 | 0.39 ± 0.32 | ||||||
| 33.1 ± 1.5 | 475 | 0.48 ± 0.32 | ||||||
| 0.6–0.8 mg/L CWF | Spot urine (collection time not specified) | ISE, microdiffusion | Pregnancy (weeks) | 11.6 ± 1.6 | 762 | 0.52 ± 0.46 | SG | |
| 19.1 ± 2.4 | 728 | 0.71 ± 0.47 | ||||||
| 33.1 ± 1.5 | 711 | 0.88 ± 0.55 | ||||||
| Uyghurturk et al. [31], 2020 | <0.7 mg/L CWF | Spot urine (collection time not specified) | ISE, microdiffusion | Pregnancy (weeks) | 20.5 ± 2.1 | 24 | 0.57 ± 0.35 | SG |
| >0.7 mg/L CWF | 24 | 0.69 ± 0.34 | ||||||
| Castiblanco et al. [69], 2021 | 250 mg/kg CSF | Spot urine (collection time not specified) | ISE, microdiffusion | Pregnancy (weeks) | 13.1 ± 2.1 | 135 | 0.82 ± 0.47 | SG |
| 25.3 ± 2.1 | 101 | 0.83 ± 0.39 | ||||||
| 33.9 ± 2.6 | 71 | 0.86 ± 0.51 | ||||||
| ISE, microdiffusion | Postpartum | 12.2 ± 0.8 | 421 | 0.83 ± 0.37 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castiblanco-Rubio, G.A.; Martinez-Mier, E.A. Fluoride Metabolism in Pregnant Women: A Narrative Review of the Literature. Metabolites 2022, 12, 324. https://doi.org/10.3390/metabo12040324
Castiblanco-Rubio GA, Martinez-Mier EA. Fluoride Metabolism in Pregnant Women: A Narrative Review of the Literature. Metabolites. 2022; 12(4):324. https://doi.org/10.3390/metabo12040324
Chicago/Turabian StyleCastiblanco-Rubio, Gina A., and E. Angeles Martinez-Mier. 2022. "Fluoride Metabolism in Pregnant Women: A Narrative Review of the Literature" Metabolites 12, no. 4: 324. https://doi.org/10.3390/metabo12040324
APA StyleCastiblanco-Rubio, G. A., & Martinez-Mier, E. A. (2022). Fluoride Metabolism in Pregnant Women: A Narrative Review of the Literature. Metabolites, 12(4), 324. https://doi.org/10.3390/metabo12040324







