The Effect of Cooking and Cooling Chickpea Pasta on Resistant Starch Content, Glycemic Response, and Glycemic Index in Healthy Adults
Abstract
1. Introduction
2. Materials and Methods
2.1. The Study Group
2.2. Study Protocol
2.3. Test Meals
- 1.
- Cooked in 100 g/100 mL of unsalted boiling water for 15 min, drained, and served directly to the subjects (FCP);
- 2.
- Cooked (in 100 g/100 mL of unsalted boiling water for 15 min), drained, cooled rapidly (using cold water), and stored at 4 °C for 24 h prior to the consumption by participants. To ensure blinding, before being served to the subjects, the cooked and cooled pasta was reheated in water (100 °C, 3 min) to the same temperature as the fresh pasta (CCP).
2.4. Analytical Methods
2.5. Glucose Measurements
2.6. Glycemic Index Determination
2.7. Sensory Assessment
2.8. Statistical Analysis
3. Results
3.1. Study Group
3.2. The Composition of Chickpea Pasta
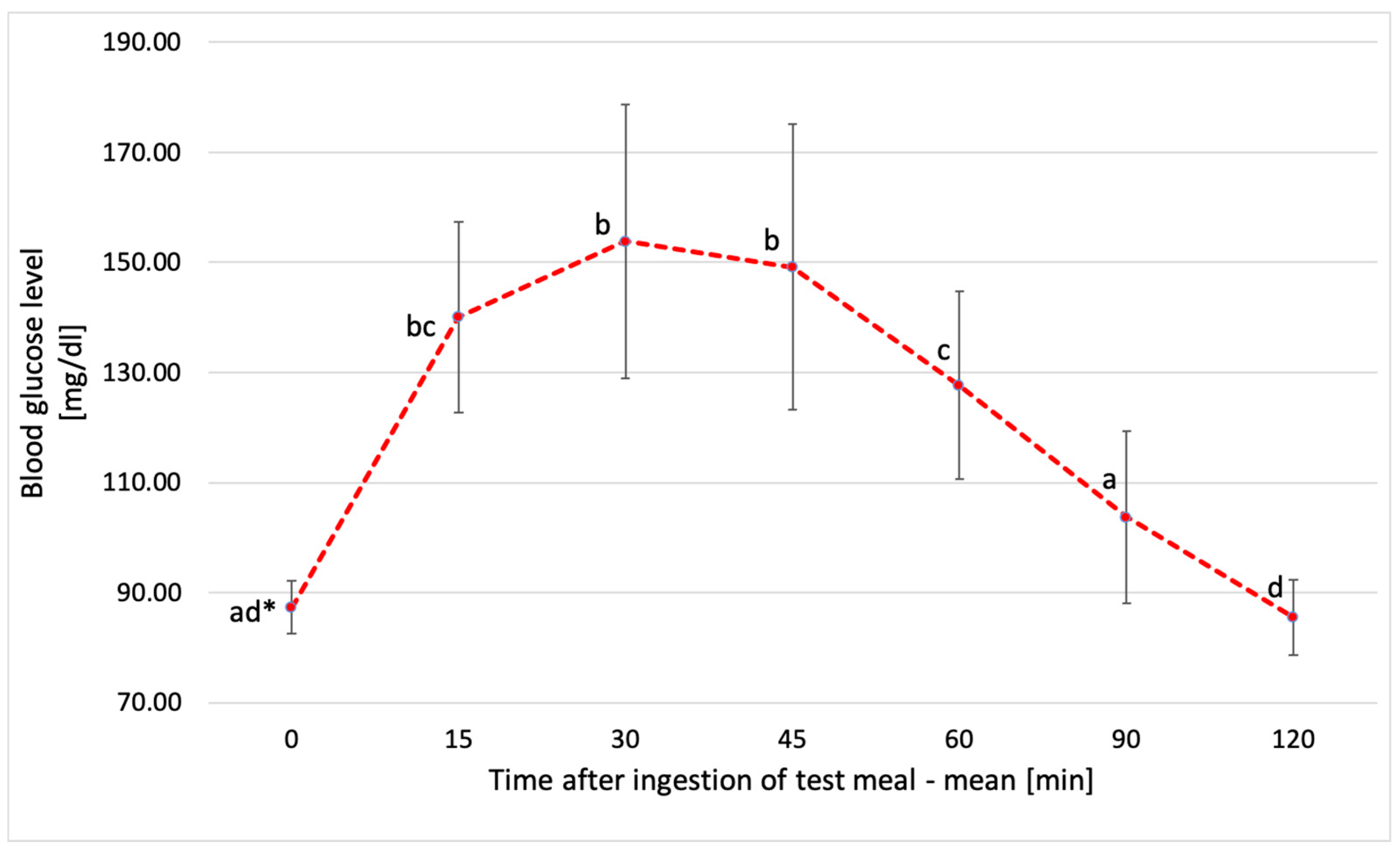
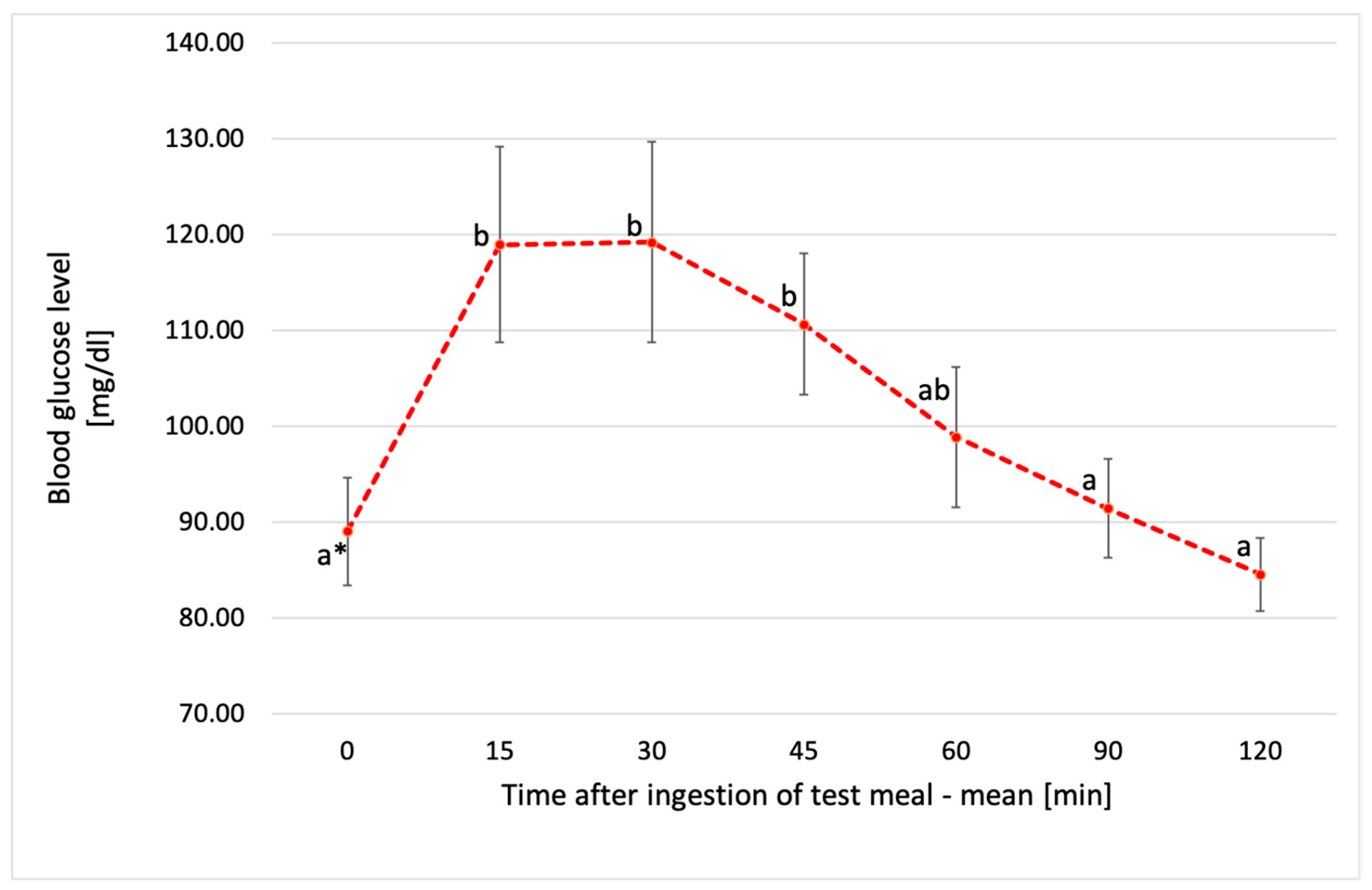
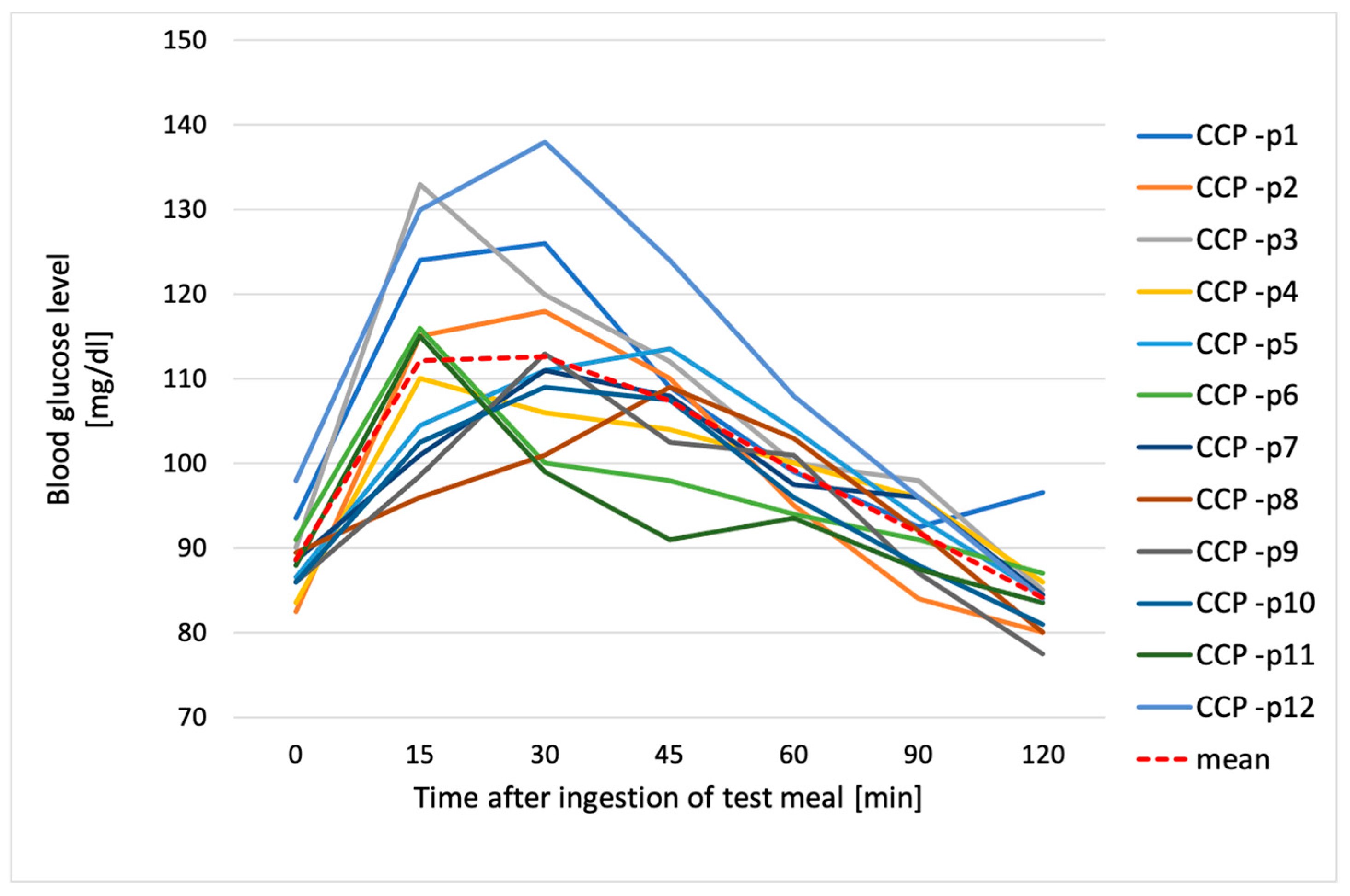
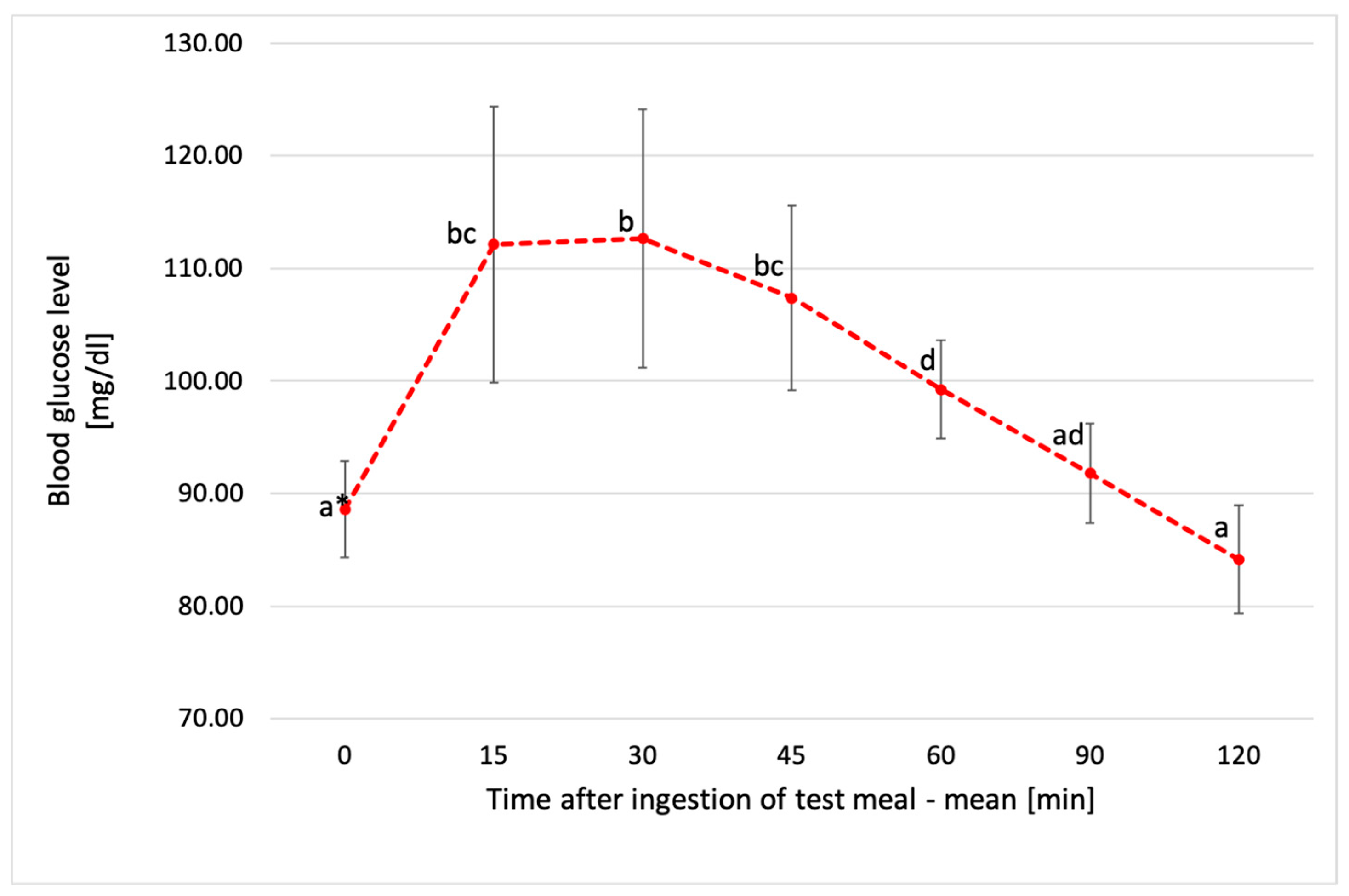
3.3. Glucose Response to Tested Chickpea Pasta
3.4. Sensory Profile of Tested Food
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Peña-Jorquera, H.; Cid-Jofré, V.; Landaeta-Díaz, L.; Petermann-Rocha, F.; Martorell, M.; Zbinden-Foncea, H.; Ferrari, G.; Jorquera-Aguilera, C.; Cristi-Montero, C. Plant-Based Nutrition: Exploring Health Benefits for Atherosclerosis, Chronic Diseases, and Metabolic Syndrome—A Comprehensive Review. Nutrients 2023, 15, 3244. [Google Scholar] [CrossRef] [PubMed]
- The Eat-Lancet Commission. Healthy Diets From Planet. Lancet 2019, 32. [Google Scholar]
- Klapp, A.-L.; Feil, N.; Risius, A. A Global Analysis of National Dietary Guidelines on Plant-Based Diets and Substitutions for Animal-Based Foods. Curr. Dev. Nutr. 2022, 6, nzac144. [Google Scholar] [CrossRef] [PubMed]
- Sustainable Healthy Diets; FAO and WHO: Rome, Italy, 2019; ISBN 978-92-5-131875-1.
- Li, S.S.; Kendall, C.W.C.; de Souza, R.J.; Jayalath, V.H.; Cozma, A.I.; Ha, V.; Mirrahimi, A.; Chiavaroli, L.; Augustin, L.S.A.; Blanco Mejia, S.; et al. Dietary Pulses, Satiety and Food Intake: A Systematic Review and Meta-analysis of Acute Feeding Trials. Obesity 2014, 22, 1773–1780. [Google Scholar] [CrossRef]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the Management of Dyslipidaemias: Lipid Modification to Reduce Cardiovascular Risk. Eur. Heart J. 2020, 41, 111–188. [Google Scholar] [CrossRef]
- Trautwein, E.A.; McKay, S. The Role of Specific Components of a Plant-Based Diet in Management of Dyslipidemia and the Impact on Cardiovascular Risk. Nutrients 2020, 12, 2671. [Google Scholar] [CrossRef]
- Mann, J.; Cummings, J.H.; Englyst, H.N.; Key, T.; Liu, S.; Riccardi, G.; Summerbell, C.; Uauy, R.; van Dam, R.M.; Venn, B.; et al. FAO/WHO Scientific Update on Carbohydrates in Human Nutrition: Conclusions. Eur. J. Clin. Nutr. 2007, 61, 132–137. [Google Scholar] [CrossRef]
- 26642:2010(E); ISO International Standard Food Products—Determination of the Glycaemic Index (GI) and Recommendation for Food Classification. ISO: Geneva, Switzerland, 2010.
- Ni, C.; Jia, Q.; Ding, G.; Wu, X.; Yang, M. Low-Glycemic Index Diets as an Intervention in Metabolic Diseases: A Systematic Review and Meta-Analysis. Nutrients 2022, 14, 307. [Google Scholar] [CrossRef]
- Sharma, A.; Yadav, B.S. Ritika Resistant Starch: Physiological Roles and Food Applications. Food Rev. Int. 2008, 24, 193–234. [Google Scholar] [CrossRef]
- Bojarczuk, A.; Skąpska, S.; Mousavi Khaneghah, A.; Marszałek, K. Health Benefits of Resistant Starch: A Review of the Literature. J. Funct. Foods 2022, 93, 105094. [Google Scholar] [CrossRef]
- Sonia, S.; Witjaksono, F.; Ridwan, R. Effect of Cooling of Cooked White Rice on Resistant Starch Content and Glycemic Response. Asia Pac. J. Clin. Nutr. 2015, 24, 620–625. [Google Scholar] [CrossRef] [PubMed]
- Morales-Sánchez, E.; Cabrera-Ramírez, A.H.; Gaytán-Martínez, M.; Mendoza-Zuvillaga, A.L.; Velázquez, G.; Méndez-Montealvo, M.G.; Rodríguez-García, M.E. Heating-Cooling Extrusion Cycles as a Method to Improve the Physicochemical Properties of Extruded Corn Starch. Int. J. Biol. Macromol. 2021, 188, 620–627. [Google Scholar] [CrossRef] [PubMed]
- Robertson, M.D.; Bickerton, A.S.; Dennis, A.L.; Vidal, H.; Frayn, K.N. Insulin-Sensitizing Effects of Dietary Resistant Starch and Effects on Skeletal Muscle and Adipose Tissue Metabolism. Am. J. Clin. Nutr. 2005, 82, 559–567. [Google Scholar] [CrossRef] [PubMed]
- Johnston, K.L.; Thomas, E.L.; Bell, J.D.; Frost, G.S.; Robertson, M.D. Resistant Starch Improves Insulin Sensitivity in Metabolic Syndrome. Diabet. Med. 2010, 27, 391–397. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Obesity: Preventing and Managing the Global Epidemic. In Report of a WHO Consultation; Technical Report Series No 894; WHO: Geneva, Switzerland, 2000. [Google Scholar]
- Ruiz, R.P. Gravimetric Determination of Water by Drying and Weighing. In Current Protocols in Food Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar] [CrossRef]
- EN ISO 2171:2010; Cereals, Pulses, and by-Products—Determination of Ash Yield by Incineration. International Organization for Standardization: Geneva, Switzerland, 2010.
- PN-A-04018:1975/Az3:2002; Agricultural and Food Products—Determination of Nitrogen by Kjeldahl Method and Conversion to Protein. Polish Committee for Standardization: Warsaw, Poland, 2002.
- PN-A-79011-4:1998; Food Concentrates—Test Methods—Determination of Fat Content. Polish Committee for Standardization: Warsaw, Poland, 1998.
- Association of Official Analytical Chemists (AOAC). Official Methods of Analysis of AOAC International, 16th ed.; Method 991.43; AOAC International: Gaithersburg, MD, USA, 1995. [Google Scholar]
- American Association of Cereal Chemists (AACC). Approved Methods of the American Association of Cereal Chemists, 10th ed.; Method 32-07; AACC: Arnold, MD, USA, 2000. [Google Scholar]
- McCleary, B.V.; Monaghan, D.A. Measurement of Resistant Starch. J. AOAC Int. 2002, 85, 665–675. [Google Scholar] [CrossRef]
- Zhao, Y.H.; Manthey, F.A.; Chang, S.K.C.; Hou, H.; Yuan, S.H. Quality Characteristics of Spaghetti as Affected by Green and Yellow Pea, Lentil, and Chickpea Flours. J. Food Sci. 2005, 70, s371–s376. [Google Scholar] [CrossRef]
- Laleg, K.; Barron, C.; Santé-Lhoutellier, V.; Walrand, S.; Micard, V. Protein Enriched Pasta: Structure and Digestibility of Its Protein Network. Food Funct. 2016, 7, 1196–1207. [Google Scholar] [CrossRef]
- Osorio-Díaz, P.; Bello-Pérez, L.A.; Sáyago-Ayerdi, S.G.; del P. Benítez-Reyes, M.; Tovar, J.; Paredes-López, O. Effect of Processing and Storage Time on in Vitro Digestibility and Resistant Starch Content of Two Bean ( Phaseolus vulgaris L) Varieties. J. Sci. Food Agric. 2003, 83, 1283–1288. [Google Scholar] [CrossRef]
- Goñi, I.; Valentín-Gamazo, C. Chickpea Flour Ingredient Slows Glycemic Response to Pasta in Healthy Volunteers. Food Chem. 2003, 81, 511–515. [Google Scholar] [CrossRef]
- Hodges, C.; Archer, F.; Chowdhury, M.; Evans, B.L.; Ghelani, D.J.; Mortoglou, M.; Guppy, F.M. Method of Food Preparation Influences Blood Glucose Response to a High-Carbohydrate Meal: A Randomised Cross-over Trial. Foods 2019, 9, 23. [Google Scholar] [CrossRef]
- Saget, S.; Costa, M.; Barilli, E.; Wilton de Vasconcelos, M.; Santos, C.S.; Styles, D.; Williams, M. Substituting Wheat with Chickpea Flour in Pasta Production Delivers More Nutrition at a Lower Environmental Cost. Sustain. Prod. Consum. 2020, 24, 26–38. [Google Scholar] [CrossRef]
- Begum, N.; Khan, Q.U.; Liu, L.G.; Li, W.; Liu, D.; Haq, I.U. Nutritional Composition, Health Benefits and Bio-Active Compounds of Chickpea (Cicer arietinum L.). Front. Nutr. 2023, 10, 1218468. [Google Scholar] [CrossRef] [PubMed]
- Zafar, T.A.; Kabir, Y. Chickpeas Suppress Postprandial Blood Glucose Concentration, and Appetite and Reduce Energy Intake at the next Meal. J. Food Sci. Technol. 2017, 54, 987–994. [Google Scholar] [CrossRef]
- Jenkins, D.J.A.; Kendall, C.W.C.; Augustin, L.S.A.; Mitchell, S.; Sahye-Pudaruth, S.; Blanco Mejia, S.; Chiavaroli, L.; Mirrahimi, A.; Ireland, C.; Bashyam, B.; et al. Effect of Legumes as Part of a Low Glycemic Index Diet on Glycemic Control and Cardiovascular Risk Factors in Type 2 Diabetes Mellitus. Arch. Intern. Med. 2012, 172, 1653. [Google Scholar] [CrossRef]
- Crujeiras, A.B.; Parra, D.; Abete, I.; Martínez, J.A. A Hypocaloric Diet Enriched in Legumes Specifically Mitigates Lipid Peroxidation in Obese Subjects. Free Radic Res. 2007, 41, 498–506. [Google Scholar] [CrossRef]
- Rehm, C.D.; Goltz, S.R.; Katcher, J.A.; Guarneiri, L.L.; Dicklin, M.R.; Maki, K.C. Trends and Patterns of Chickpea Consumption among United States Adults: Analyses of National Health and Nutrition Examination Survey Data. J. Nutr. 2023, 153, 1567–1576. [Google Scholar] [CrossRef]
- Llavata, B.; Albors, A.; Martin-Esparza, M.E. High Fibre Gluten-Free Fresh Pasta with Tiger Nut, Chickpea and Fenugreek: Technofunctional, Sensory and Nutritional Properties. Foods 2019, 9, 11. [Google Scholar] [CrossRef]
- Wani, A.A.; Singh, P.; Shah, M.A.; Schweiggert-Weisz, U.; Gul, K.; Wani, I.A. Rice Starch Diversity: Effects on Structural, Morphological, Thermal, and Physicochemical Properties—A Review. Compr. Rev. Food Sci. Food Saf. 2012, 11, 417–436. [Google Scholar] [CrossRef]
- Yamaguchi, Y.; Okawa, Y.; Ninomiya, K.; Kumagai, H.; Kumagai, H. Evaluation and Suppression of Retrogradation of Gelatinized Rice Starch. J. Nutr. Sci. Vitaminol. 2019, 65, S134–S138. [Google Scholar] [CrossRef]
- Pandolfo, A.; Messina, B.; Russo, G. Evaluation of Glycemic Index of Six Different Samples of Commercial and Experimental Pasta Differing in Wheat Varieties and Production Processes. Foods 2021, 10, 2221. [Google Scholar] [CrossRef]
- Sajilata, M.G.; Singhal, R.S.; Kulkarni, P.R. Resistant Starch? A Review. Compr. Rev. Food Sci. Food Saf. 2006, 5, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Zambrano, N.L.; Pérez-Carrillo, E.; Serna-Saldívar, S.O.; Tejada-Ortigoza, V. Effect of Thermal, Nonthermal, and Combined Treatments on Functional and Nutritional Properties of Chickpeas. Crit. Rev. Food Sci. Nutr. 2023, 1–19. [Google Scholar] [CrossRef]
- Aguilera, Y.; Esteban, R.M.; Benítez, V.; Mollá, E.; Martín-Cabrejas, M.A. Starch, Functional Properties, and Microstructural Characteristics in Chickpea and Lentil As Affected by Thermal Processing. J. Agric. Food Chem. 2009, 57, 10682–10688. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.R.; Thavarajah, D.; Thavarajah, P.; Payne, S.; Moore, J.; Ohm, J.-B. Processing, Cooking, and Cooling Affect Prebiotic Concentrations in Lentil (Lens culinaris Medikus). J. Food Compos. Anal. 2015, 38, 106–111. [Google Scholar] [CrossRef]
- Nakamura, Y.; Takemoto, A.; Oyanagi, T.; Tsunemi, S.; Kubo, Y.; Nakagawa, T.; Nagai, Y.; Tanaka, Y.; Sone, M. Effects of Cooked Rice Containing High Resistant Starch on Postprandial Plasma Glucose, Insulin, and Incretin in Patients with Type 2 Diabetes. Asia Pac. J. Clin. Nutr. 2023, 32, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Dewi AP, I.M. Effects of Freshly Cooked White Rice and Yesterday (Retrograded) White Rice on Postprandial Blood Glucose Levels in Prediabetic Female Subjects. JNC 2013, 2, 411–418. [Google Scholar]
- Chiu YT, S.M. Effect of Variety and Cooking Method on Resistant Starch Content of White Rice and Subsequent Postprandial Glucose Response and Appetite in Humans. Asia Pac. J. Nutr. 2013, 22, 372–379. [Google Scholar]
- Yadav, B.S.; Sharma, A.; Yadav, R.B. Studies on Effect of Multiple Heating/Cooling Cycles on the Resistant Starch Formation in Cereals, Legumes and Tubers. Int. J. Food Sci. Nutr. 2009, 60, 258–272. [Google Scholar] [CrossRef]
- Carreira, M.C.; Lajolo, F.M.; Menezes, E.W. de Glycemic Index: Effect of Food Storage under Low Temperature. Braz. Arch. Biol. Technol. 2004, 47, 569–574. [Google Scholar] [CrossRef]
- Hoover, R.; Zhou, Y. In Vitro and in Vivo Hydrolysis of Legume Starches by α-Amylase and Resistant Starch Formation in Legumes—A Review. Carbohydr. Polym. 2003, 54, 401–417. [Google Scholar] [CrossRef]
- Strozyk, S.; Rogowicz-Frontczak, A.; Pilacinski, S.; LeThanh-Blicharz, J.; Koperska, A.; Zozulinska-Ziolkiewicz, D. Influence of Resistant Starch Resulting from the Cooling of Rice on Postprandial Glycemia in Type 1 Diabetes. Nutr. Diabetes 2022, 12, 21. [Google Scholar] [CrossRef]
- Lu, L.; Venn, B.; Lu, J.; Monro, J.; Rush, E. Effect of Cold Storage and Reheating of Parboiled Rice on Postprandial Glycaemic Response, Satiety, Palatability and Chewed Particle Size Distribution. Nutrients 2017, 9, 475. [Google Scholar] [CrossRef]




| Characteristic | Total (n = 12) | Women (n = 8) | Men (n = 4) |
|---|---|---|---|
| Age (years) | 25.3 ± 4.3 | 24.00 ± 4.0 | 28 ± 4.1 |
| Weight (kg) | 66 ± 10.0 | 61.38 ± 5.9 | 75.25 ± 12.1 |
| Height (m) | 1.7 ± 0.1 | 1.66 ± 0.1 | 1.81 ± 0.1 |
| BMI (kg/m2) | 22.5 ± 1.9 | 22.35 ± 2.2 | 22.91 ± 2.0 |
| Fasting blood glucose (mg/dL) | 87.33 ± 4.7 | 86.13 ± 3.5 | 89.75 ± 5.3 |
| Attribute | FCP | CCP | p * FCP vs. CCP | ||
|---|---|---|---|---|---|
| Mean ± SD | Minimum–Maximum | Mean ± SD | Minimum–Maximum | ||
| Visual appearance | 5.33 ± 0.9 | 3–6 | 5.58 ± 0.9 | 4–7 | 0.48 |
| Flavor | 4.5 ± 1.0 | 3–6 | 4.58 ± 1.4 | 1–6 | 0.36 |
| Smell | 4.25 ± 0.6 | 3–3 | 4.17 ± 0.6 | 3–5 | 0.72 |
| Texture | 4.08 ± 1.6 | 2–6 | 3.67 ± 1.4 | 1–6 | 0.21 |
| Overall acceptability | 4.83 ± 0.7 | 4–6 | 4.75 ± 1.2 | 2–6 | 1.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bojarczuk, A.; Kęszycka, P.; Marszałek, K.; Gajewska, D. The Effect of Cooking and Cooling Chickpea Pasta on Resistant Starch Content, Glycemic Response, and Glycemic Index in Healthy Adults. Metabolites 2024, 14, 585. https://doi.org/10.3390/metabo14110585
Bojarczuk A, Kęszycka P, Marszałek K, Gajewska D. The Effect of Cooking and Cooling Chickpea Pasta on Resistant Starch Content, Glycemic Response, and Glycemic Index in Healthy Adults. Metabolites. 2024; 14(11):585. https://doi.org/10.3390/metabo14110585
Chicago/Turabian StyleBojarczuk, Adrianna, Paulina Kęszycka, Krystian Marszałek, and Danuta Gajewska. 2024. "The Effect of Cooking and Cooling Chickpea Pasta on Resistant Starch Content, Glycemic Response, and Glycemic Index in Healthy Adults" Metabolites 14, no. 11: 585. https://doi.org/10.3390/metabo14110585
APA StyleBojarczuk, A., Kęszycka, P., Marszałek, K., & Gajewska, D. (2024). The Effect of Cooking and Cooling Chickpea Pasta on Resistant Starch Content, Glycemic Response, and Glycemic Index in Healthy Adults. Metabolites, 14(11), 585. https://doi.org/10.3390/metabo14110585










