5-ALA, DTA-6, and Nitrogen Mitigate NaCl Stress by Promoting Photosynthesis and Carbon Metabolism in Rice Seedlings
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
2.2. Test Methods
2.3. Measurement of Morphological Indices
Measurement of Aboveground Morphological Indicators
2.4. Measurement of Physiological and Biochemical Indicators
2.4.1. Determination of Gas Exchange Parameters
2.4.2. Histochemical Staining
2.4.3. Determination of Osmoregulatory Substances
2.4.4. Determination of Ascorbic Acid (AsA) and Glutathione (GSH) Levels
2.4.5. Measurement of Indicators Related to Carbon Metabolism in Leaf Blades
2.5. Data Analysis
3. Results and Discussion
3.1. Effects of Plant Growth Regulators (PGRs) and Nitrogen Application on Rice Seedling Morphology
3.2. Effects of 5-ALA, DTA-6, and Nitrogen Application on the Photosynthetic Characteristics of Rice Seedlings
3.3. Effects of 5-ALA, DTA-6, and Nitrogen Application on H2O2 and O2− Distribution in Rice Seedling Leaves
3.4. Effects of 5-ALA, DTA-6, and Nitrogen Treatment on Osmoregulatory Substances in Rice Seedlings
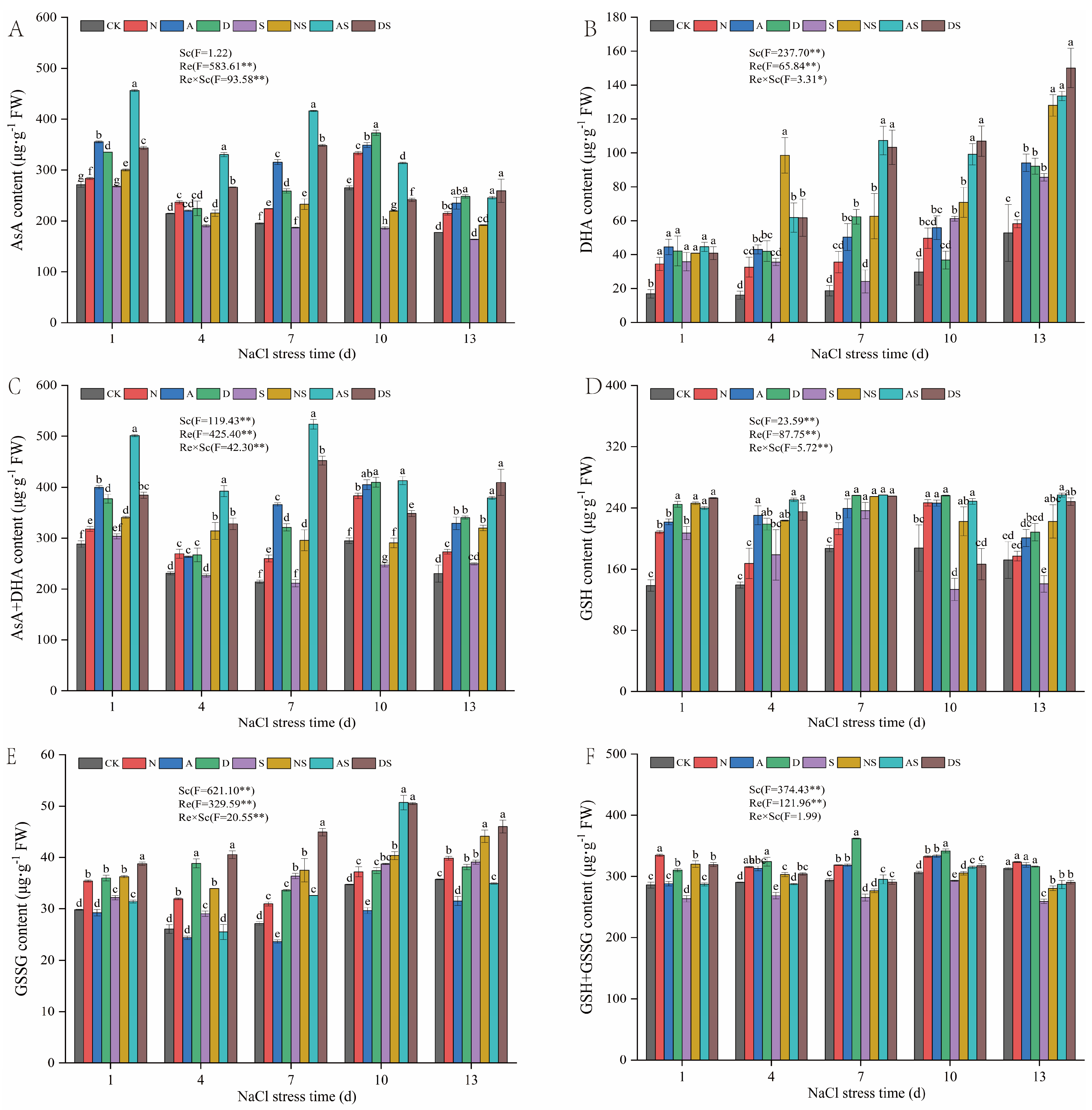
3.5. Effects of 5-ALA, DTA-6, and Nitrogen Application on Non-Enzymatic Antioxidants and Key Enzyme Activities in the AsA-GSH Cycle of Rice Seedlings
3.5.1. Effects of PGRs and Nitrogen Fertilizer on Non-Enzymatic Antioxidant Levels in AsA-GSH Cycle of Rice Seedlings
3.5.2. Effects of 5-ALA, DTA-6, and Nitrogen Fertilizer on the Activities of Key Enzymes in the AsA-GSH Cycle of Rice
3.6. Effects of 5-ALA, DTA-6, and Nitrogen Application on Carbon Metabolism in Rice
3.6.1. Effect of PGRs and Nitrogen Fertilizer on Major Carbohydrate Content of Rice Seedlings
3.6.2. Effects of 5-ALA, DTA-6, and Nitrogen Fertilizer on Enzyme Activities Related to Carbon Metabolism in Rice
4. Discussion
4.1. Effects of NaCl Stress on Rice Seedling Morphology and the Regulatory Effects of PGRs and Nitrogen Fertilizer
4.2. Effects of NaCl Stress on Photosynthesis and Osmoregulatory Substances in Rice and Regulatory Effects of PGRs and Nitrogen Fertilizer
4.3. Effects of NaCl Stress on Non-Enzymatic Antioxidants and Key Enzyme Activities in Rice and the Regulatory Effects of PGRs and Nitrogen Fertilizer
4.4. Effects of NaCl Stress on Carbon Metabolism in Rice and Regulatory Effects of PGRs and Nitrogen Fertilizer
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, C.; Mao, B.; Yuan, D.; Chu, C.; Duan, M. Salt tolerance in rice: Physiological responses and molecular mechanisms. Crop J. 2022, 10, 13–25. [Google Scholar] [CrossRef]
- Jamal, M.R.; Kristiansen, P.; Kabir, M.J.; Lobry de Bruyn, L.J.L. Challenges and Adaptations for Resilient Rice Production under Changing Environments in Bangladesh. Land 2023, 12, 1217. [Google Scholar] [CrossRef]
- Leng, G.; Leung, L.R.; Huang, M. Significant impacts of irrigation water sources and methods on modeling irrigation effects in the ACME L and Model. J. Adv. Model. Earth Syst. 2017, 9, 1665–1683. [Google Scholar] [CrossRef]
- Xie, H.; Li, J.; Zhang, Y.; Xu, X.; Wang, L.; Ouyang, Z. Evaluation of coastal farming under salinization and optimized fertilization strategies in China. Sci. Total Environ. 2021, 797, 149038. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.Q.; Gao, Q. Comparative physiological and biochemical mechanisms of salt tolerance in five contrasting highland quinoa cultivars. BMC Plant Biol. 2020, 1, 70. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, T.B.; Ribas, A.F.; de Souza, S.G.H.; Budzinski, I.G.F.; Domingues, D.S. Physiological Responses to Drought, Salinity, and Heat Stress in Plants: A Review. Stresses 2022, 2, 113–135. [Google Scholar] [CrossRef]
- Radanielson, A.M.; Angeles, O.; Li, T.; Ismail, A.M.; Gaydon, D.S. Describing the physiological responses of different rice genotypes to salt stress using sigmoid and piecewise linear functions. Field Crops Res. 2018, 220, 46–56. [Google Scholar] [CrossRef]
- Liu, Z.; Mela, A.; Argenziano, M.G.; Banu, M.A.; Furnari, J.; Kotidis, C.; Sperring, C.P.; Humala, N.; Mahajan, A.; Bruce, J.N. Single-cell analysis of 5-aminolevulinic acid intraoperative labeling specificity for glioblastoma. J. Neurosurg. 2023, 1, 1–11. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; He, H.; Lin, Z.; Sun, J.; Zhang, F.; Zhou, L.; Wang, Z.; Zhang, Z.; Zou, H. Effects of 5-aminolevulinic acid (5-ALA) on physicochemical characteristics and growth of pomegranate (Punica granatum L.). Horticulturae 2023, 9, 860. [Google Scholar] [CrossRef]
- Tsai, Y.C.; Chen, K.C.; Cheng, T.S.; Lee, C.; Lin, S.H.; Tung, C.W.J.B.P.B. Chlorophyll fluorescence analysis in diverse rice varieties reveals the positive correlation between the seedlings salt tolerance and photosynthetic efficiency. BMC Plant Biol. 2019, 19, 403. [Google Scholar] [CrossRef]
- Elsheery, N.I.; Helaly, M.N.; El-Hefnawy, S.F.; Elhamahmy, M.M.; Abdelrazik, E.M.; Sardarov, Y.B.; Ahmad, P.; Zivcak, M.; Brestic, M.; Allakhverdiev, S.I. 5-Aminolevulinic acid (ALA) reduces arsenic toxicity stress in wheat (Triticum aestivum L.). J. Plant Growth Regul. 2023, 42, 3303–3322. [Google Scholar] [CrossRef]
- Fu, J.; Sun, Y.; Chu, X.; Xu, Y.; Hu, T. Exogenous 5-aminolevulenic acid promotes seed germination in Elymus nutans against oxidative damage induced by cold stress. PLoS ONE 2014, 9, e107152. [Google Scholar] [CrossRef]
- Li, X.; Li, J.; Islam, F.; Najeeb, U.; Pan, J.; Hou, Z.; Shou, J.; Qin, Y.; Xu, L. 5-Aminolevulinic acid could enhance the salinity tolerance by alleviating oxidative damages in Salvia miltiorrhiza. Food Sci. Technol. 2022, 42, e103121. [Google Scholar] [CrossRef]
- Qi, D.; Feng, N.; Zheng, D.; Liang, X. Effects of different compound seedling-strengthening agents on root morphology and resistance physiology of machine transplanted rice seedlings. J. South. Agric. 2019, 50, 974–981. [Google Scholar]
- Zhang, C.; He, P.; Li, Y.; Li, Y.; Yao, H.; Duan, J.; Hu, S.; Zhou, H.; Li, S. Exogenous Diethyl Aminoethyl Hexanoate, a Plant Growth Regulator, Highly Improved the Salinity Tolerance of Important Medicinal Plant Cassia obtusifolia L. J. Plant Growth Regul. 2015, 35, 330–344. [Google Scholar] [CrossRef]
- Li, Z.; Lin, W. Synergetic effects of DA-6/24-EBL and NTA on uptake, subcellular distribution and chemical form of Cd in Amaranthus hybridus L. Soil Sci. Plant Nutr. 2020, 66, 653–661. [Google Scholar] [CrossRef]
- He, S.; Wu, Q.; He, Z. Growth-Promoting Hormone DA-6 Assists Phytoextraction and Detoxification of Cd by Ryegrass. Int. J. Phytoremediat. 2015, 17, 597–603. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, H.; Feng, N.; Zheng, D.; Liang, X.; Qi, D.; Li, J.; Han, Y.; Huang, W. Effects of S3307 and DTA-6 on the photosynthetic characteristics and yield of kidney bean plants in the reproductive stage. Acta Prataculturae Sin. 2018, 27, 162–170. [Google Scholar]
- Kim, G.; Sung, J. Transcriptional Expression of Nitrogen Metabolism Genes and Primary Metabolic Variations in Rice Affected by Different Water Status. Plants 2023, 12, 1649. [Google Scholar] [CrossRef]
- Hawkesford, M.J. Reducing the reliance on nitrogen fertilizer for wheat production. J. Cereal Sci. 2014, 59, 276–283. [Google Scholar] [CrossRef]
- Qiao, J.; Yang, L.; Yan, T.; Xue, F.; Zhao, D. Nitrogen fertilizer reduction in rice production for two consecutive years in the Taihu Lake area. Agric. Ecosyst. Environ. 2012, 146, 103–112. [Google Scholar] [CrossRef]
- Ribeiro, A.D.A.; Lacerda, C.F.; Neves, A.L.R.; De Sousa, C.H.C.; Braz, R.D.S.; De Oliveira, A.C.; Pereira, J.M.G.; Ferreira, J.F.D.S. Uses and losses of nitrogen by maize and cotton plants under salt stress. Arch. Agron. Soil Sci. 2021, 67, 1119–1133. [Google Scholar] [CrossRef]
- Wen-Zhi, Z.; Chi, X.; Jing-Wei, W.; HUANG, J.-S.; Qiang, Z.; Mou-Song, W.J.P. Impacts of salinity and nitrogen on the photosynthetic rate and growth of sunflowers (Helianthus annuus L.). Pedosphere 2014, 24, 635–644. [Google Scholar]
- Waqas, M.; Hawkesford, M.J.; Geilfus, C.M. Feeding the world sustainably: Efficient nitrogen use. Trends Plant Sci. 2023, 28, 505–508. [Google Scholar] [CrossRef]
- Liu, H.; Sun, J.; Zou, J.; Li, B.; Jin, H. MeJA-mediated enhancement of salt-tolerance of Populus wutunensis by 5-aminolevulinic acid. BMC Plant Biol. 2023, 23, 185. [Google Scholar] [CrossRef]
- Li, Y.H.; Tian, P.; Li, C.Z.; Yu, X.Z. Elucidating comportment of the glutamate and ornithine pathway on proline accumulation in rice under different nitrogenous nutrition. Int. J. Environ. Sci. Technol. 2021, 19, 2993–3000. [Google Scholar] [CrossRef]
- Kang, W.; Lin-Chi, H.E.; Xiao-Yun, W.; Jian-Mei, X. The Effect of Shading Rate, Base Fertilizer Type, ALA Treatment and Cover Agents on the Growth of Zingiber Mioga Rosc under Different Protected Cultivation Conditions. J. Jinling Inst. Technol. 2014, 4, 69–72. [Google Scholar]
- Yang, K. Effects of Plant Growth Regulators on Growth, Development and Yield of Cereals. Master’s Thesis, Heilongjiang Bayi Agric. Univ., Daqing, China, September 2020. [Google Scholar]
- Rahman, S.; Matsumuro, T.; Miyake, H.; Takeoka, Y. Salinity-induced ultrastructural alterations in leaf cells of rice (Oryza sativa L.). Plant Prod. Sci. 2000, 3, 422–429. [Google Scholar] [CrossRef]
- Yu, Q.; Sun, W.; Han, Y.; Hao, J.; Qin, X.; Liu, C.; Fan, S. Exogenous spermidine improves the sucrose metabolism of lettuce to resist high-temperature stress. Plant Growth Regul. 2022, 96, 497–509. [Google Scholar] [CrossRef]
- Zahra, J.; Nazim, H.; Faiza, I.; Zeng, J.; Tahir, A.; Zhang, G. Physiological and antioxidant responses of cultivated and wild barley under salt stress. Plant Soil Environ. 2020, 66, 334–344. [Google Scholar] [CrossRef]
- Clarke, J.D. Phenotypic Analysis of Arabidopsis Mutants: Diaminobenzidine Stain for Hydrogen Peroxide. Cold Spring Harb. Protoc. 2009, 2009, pdb-prot4981. [Google Scholar] [CrossRef]
- Perveen, S.; Hussain, S.A.; Sciences, P. Methionine-induced changes in growth, glycinebetaine, ascorbic acid, total soluble proteins and anthocyanin contents of two Zea mays L. varieties under salt stress. JAPS J. Anim. Plant Sci. 2021, 31, 131–142. [Google Scholar]
- Hussain, S.J.; Masood, A.; Anjum, N.A.; Khan, N.A. Sulfur-mediated control of salinity impact on photosynthesis and growth in mungbean cultivars screened for salt tolerance involves glutathione and proline metabolism, and glucose sensitivity. Acta Physiol. Plant. 2019, 41, 129. [Google Scholar] [CrossRef]
- Kampfenkel, K.; Vanmontagu, M.; Inzé, D. Extraction and determination of ascorbate and dehydroascorbate from plant tissue. Anal. Biochem. 1995, 225, 165–167. [Google Scholar] [CrossRef]
- Costa, H.; Gallego, S.M.; Tomaro, M.L. Effect of UV-B radiation on antioxidant defense system in sunflower cotyledons. Plant Sci. 2002, 162, 939–945. [Google Scholar] [CrossRef]
- Kaya, C.; Ashraf, M.; Alyemeni, M.N.; Ahmad, P. The role of endogenous nitric oxide in salicylic acid-induced up-regulation of ascorbate-glutathione cycle involved in salinity tolerance of pepper (Capsicum annuum L.) plants. Plant Physiol. Biochem. 2020, 147, 10–20. [Google Scholar] [CrossRef]
- Tyburski, J.; Tretyn, A. Glutathione and glutathione disulfide affect adventitious root formation and growth in tomato seedling cuttings. Acta Physiol. Plant. 2010, 32, 411–417. [Google Scholar] [CrossRef]
- Rahman, M.; Rahman, K.; Sathi, K.S.; Alam, M.M.; Nahar, K.; Fujita, M.; Hasanuzzaman, M. Supplemental selenium and boron mitigate salt-induced oxidative damages in Glycine max L. Plants 2021, 10, 2224. [Google Scholar] [CrossRef]
- Zhu, Z.; Wei, G.; Li, J.; Qian, Q.; Yu, J. Silicon alleviates salt stress and increases antioxidant enzymes activity in leaves of salt-stressed cucumber (Cucumis sativus L.). Plant Sci. 2004, 167, 527–533. [Google Scholar] [CrossRef]
- Shan, C.; Liu, R. Exogenous hydrogen peroxide up-regulates the contents of ascorbate and glutathione in the leaves of Vigna radiata (Linn.) Wilczek. exposed to salt stress. Braz. J. Bot. 2017, 40, 583–589. [Google Scholar]
- Hasanuzzaman, M.; Hossain, M.A.; Fujita, M. Nitric oxide modulates antioxidant defense and the methylglyoxal detoxification system and reduces salinity-induced damage of wheat seedlings. Plant Biotechnol. Rep. 2011, 5, 353–365. [Google Scholar] [CrossRef]
- Du, J.; Shu, S.; An, Y.; Zhou, H.; Guo, S.; Sun, J. Influence of exogenous spermidine on carbon–nitrogen metabolism under Ca (NO3)2 stress in cucumber root. Plant Growth Regul. 2017, 81, 103–115. [Google Scholar] [CrossRef]
- Baxter, C.J.; Foyer, C.H.; Turner, J.; Rolfe, S.A.; Quick, W.P. Elevated sucrose-phosphate synthase activity in transgenic tobacco sustains photosynthesis in older leaves and alters development. J. Exp. Bot. 2003, 54, 1813–1820. [Google Scholar] [CrossRef] [PubMed]
- Wongmetha, O.; Ke, L.S.; Liang, Y.S. The changes in physical, bio-chemical, physiological characteristics and enzyme activities of mango cv. Jinhwang during fruit growth and development. NJAS Wagening. J. Life Sci. 2015, 72, 7–12. [Google Scholar] [CrossRef]
- Zhu, Y.; Guo, J.; Feng, R.; Jia, J.; Han, W.; Gong, H. The regulatory role of silicon on carbohydrate metabolism in Cucumis sativus L. under salt stress. Plant Soil 2016, 406, 231–249. [Google Scholar] [CrossRef]
- Dai, H.; Fu, M.; Yang, X.; Chen, Q. Ethylene inhibited sprouting of potato tubers by influencing the carbohydrate metabolism pathway. J. Food Sci. Technol. 2016, 53, 3166–3174. [Google Scholar] [CrossRef]
- Singh, A.; Roychoudhury, A.; Samanta, S.; Banerjee, A. Fluoride stress-mediated regulation of tricarboxylic acid cycle and sugar metabolism in rice seedlings in absence and presence of exogenous calcium. J. Plant Growth Regul. 2021, 40, 1579–1593. [Google Scholar] [CrossRef]
- Farooq, M.; Khan, M.A.; Zhao, D.D.; Asif, S.; Kim, E.G.; Jang, Y.H.; Park, J.R.; Lee, I.J.; Kim, K.M. Extrinsic role of gibberellin mitigating salinity effect in different rice genotypes. Front. Plant Sci. 2022, 13, 1041181. [Google Scholar] [CrossRef]
- Hussain, S.; Zhong, C.; Bai, Z.; Cao, X.; Zhu, L.; Hussain, A.; Zhu, C.; Fahad, S.; James, A.B.; Zhang, J. Effects of 1-methylcyclopropene on rice growth characteristics and superior and inferior spikelet development under salt stress. J. Plant Growth Regul. 2018, 37, 1368–1384. [Google Scholar] [CrossRef]
- Hadia, E.; Slama, A.; Romdhane, L.; Cheikh M’Hamed, H.; Fahej, M.A.S.; Radhouane, L. Seed Priming of Bread Wheat Varieties with Growth Regulators and Nutrients Improves Salt Stress Tolerance Particularly for the Local Genotype. J. Plant Growth Regul. 2022, 42, 304–318. [Google Scholar] [CrossRef]
- Liu, J.; Wu, Y.; Dong, G.; Zhu, G.; Zhou, G. Progress of Research on the Physiology and Molecular Regulation of Sorghum Growth under Salt Stress by Gibberellin. Int. J. Mol. Sci. 2023, 24, 6777. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Yang, J. Nitrogen (N) transformation in paddy rice field: Its effect on N uptake and relation to improved N management. Crop Environ. 2022, 1, 7–14. [Google Scholar] [CrossRef]
- Liu, J.; Ouyang, X.; Shen, J.; Li, Y.; Sun, W.; Jiang, W.; Wu, J. Nitrogen and phosphorus runoff losses were influenced by chemical fertilization but not by pesticide application in a double rice-cropping system in the subtropical hilly region of China. Sci. Total Environ. 2020, 715, 136852. [Google Scholar] [CrossRef] [PubMed]
- Mi, W.; Gao, Q.; Xia, S.; Zhao, H.; Wu, L.; Mao, W.; Hu, Z.; Liu, Y. Medium-term effects of different types of N fertilizer on yield, apparent N recovery, and soil chemical properties of a double rice cropping system. Field Crops Res. 2019, 234, 87–94. [Google Scholar] [CrossRef]
- Feijuan, W.; Yiting, Z.; Qinxin, G.; Haifeng, T.; Jiahui, H.; Haoran, L.; Hewen, W.; Guangwei, X.; Cheng, Z. Effects of Exogenous 5-Aminolevulinic Acid and 24-Epibrassinolide on Cd Accumulation in Rice from Cd-Contaminated Soil. Rice Sci. 2018, 25, 320–329. [Google Scholar] [CrossRef]
- Fan, H.; Guo, S.; Jiao, Y.; Zhang, R.; Li, J. Effects of exogenous nitric oxide on growth, active oxygen species metabolism, and photosynthetic characteristics in cucumber seedlings under NaCl stress. Front. Agric. China 2007, 1, 308–314. [Google Scholar] [CrossRef]
- Hamani, A.K.M.; Wang, G.; Soothar, M.K.; Shen, X.; Gao, Y.; Qiu, R.; Mehmood, F. Responses of leaf gas exchange attributes, photosynthetic pigments and antioxidant enzymes in NaCl-stressed cotton (Gossypium hirsutum L.) seedlings to exogenous glycine betaine and salicylic acid. BMC Plant Biol. 2020, 20, 434. [Google Scholar] [CrossRef]
- Sil, P.; Das, P.; Biswas, S.; Mazumdar, A.; Biswas, A.K.J.E.S.; Research, P. Modulation of photosynthetic parameters, sugar metabolism, polyamine and ion contents by silicon amendments in wheat (Triticum aestivum L.) seedlings exposed to arsenic. Environ. Sci. Pollut. Res. 2019, 26, 13630–13648. [Google Scholar] [CrossRef]
- Zhu, X.G.; Long, S.P.; Ort, D.R. Improving photosynthetic efficiency for greater yield. Annu. Rev. Plant Biol. 2010, 61, 235–261. [Google Scholar] [CrossRef]
- Fang, X.; An, Y.; Zheng, J.; Shangguan, L.; Wang, L. Genome-wide identification and comparative analysis of GST gene family in apple (Malus domestica) and their expressions under ALA treatment. 3 Biotech 2020, 10, 307. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, S.; Cai, Q.; Wang, Z.; Cao, J.; Yu, T.; Xie, T. Exogenous diethyl aminoethyl hexanoate ameliorates low temperature stress by improving nitrogen metabolism in maize seedlings. PLoS ONE 2020, 15, e0232294. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Zhu, H.; Zhu, H.; Tao, Y.; Liu, C.; Liu, J.; Yang, F.; Li, M. Exogenous ABA Enhances the Antioxidant Defense System of Maize by Regulating the AsA-GSH Cycle under Drought Stress. Sustainability 2022, 14, 3071. [Google Scholar] [CrossRef]
- Kamrun, N.; Mirza, H.; Alam, M.M.; Fujita, M. Exogenous Spermidine Alleviates Low Temperature Injury in Mung Bean (Vigna radiata L.) Seedlings by Modulating Ascorbate-Glutathione and Glyoxalase Pathway. Int. J. Mol. Sci. 2015, 16, 30117–30132. [Google Scholar]
- Qin, S.; Liu, H.; Nie, Z.; Gao, W.; Li, C.; Lin, Y.; Zhao, P. AsA-GSH Cycle and Antioxidant Enzymes Play Important Roles in Cd Tolerance of Wheat. Bull. Environ. Contam. Toxicol. 2018, 101, 684–690. [Google Scholar] [CrossRef] [PubMed]
- Ren, M.; Liu, S.; Mao, G.; Tang, C.; Gai, P.; Guo, X.; Zheng, H.; Wang, W.; Tang, Q. Simultaneous Application of Red and Blue Light Regulate Carbon and Nitrogen Metabolism, Induces Antioxidant Defense System and Promote Growth in Rice Seedlings under Low Light Stress. Int. J. Mol. Sci. 2023, 24, 10706. [Google Scholar] [CrossRef]
- Loewe, A.; Einig, W.; Shi, L.; Dizengremel, P.; Hampp, R. Mycorrhiza formation and elevated CO2 both increase the capacity for sucrose synthesis in source leaves of spruce and aspen. New Phytol. 2000, 145, 565–574. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, C.; Hussain, S.; Huang, J.; Liang, Q.; Zhu, L.; Cao, X.; Kong, Y.; Li, Y.; Wang, J. Effects of nitric oxide on nitrogen metabolism and the salt resistance of rice (Oryza sativa L.) seedlings with different salt tolerances. Plant Physiol. Biochem. 2020, 155, 374–383. [Google Scholar] [CrossRef]
- Zhao, M.; Meng, Y.; Wang, Y.; Sun, G.; Liu, X.; Li, J.; Wei, S.; Gu, W. Exogenous Hemin alleviates cadmium stress in maize by enhancing sucrose and nitrogen metabolism and regulating endogenous hormones. Int. J. Phytoremediat. 2023, 25, 368–380. [Google Scholar] [CrossRef]








| Treatment | DTA-6 (mg·L−1) | 5-ALA (mg·L−1) | NaCl (%) (w/w) | N (g/pot) |
|---|---|---|---|---|
| CK | 0 | 0 | 0 | 0 |
| N | 0 | 0 | 0 | 0.05 |
| A | 0 | 40 | 0 | 0 |
| D | 30 | 0 | 0 | 0 |
| S | 0 | 0 | 0.3 | 0 |
| NS | 0 | 0 | 0.3 | 0.05 |
| AS | 0 | 40 | 0.3 | 0 |
| DS | 30 | 0 | 0.3 | 0 |
| Morphological Indicators | Treatment | Day after NaCl Treatment (d) | ||||
|---|---|---|---|---|---|---|
| 1 | 4 | 7 | 10 | 13 | ||
| Shoot length (cm) | CK | 29.07 ± 0.18 bc | 28.03 ± 0.03 d | 32.07 ± 0.07 d | 34.07 ± 0.15 e | 37.63 ± 0.07 d |
| N | 30.07 ± 0.15 a | 29.53 ± 0.09 c | 36.43 ± 0.19 c | 37.67 ± 0.18 bc | 39.07 ± 0.55 c | |
| A | 28.90 ± 0.06 bc | 34.40 ± 0.21 ab | 38.47 ± 0.09 a | 38.43 ± 0.28 b | 40.00 ± 0.21 b | |
| D | 30.13 ± 0.37 a | 34.10 ± 0.32 b | 38.60 ± 0.10 a | 39.90 ± 0.20 a | 42.50 ± 0.06 a | |
| S | 29.03 ± 0.23 bc | 27.30 ± 0.17 e | 29.53 ± 0.39 e | 33.30 ± 0.12 e | 36.03 ± 0.03 e | |
| NS | 29.60 ± 0.31 ab | 29.17 ± 0.12 c | 35.67 ± 0.58 c | 36.67 ± 0.24 d | 37.20 ± 0.15 d | |
| AS | 28.67 ± 0.33 c | 34.83 ± 0.17 a | 37.43 ± 0.15 b | 37.50 ± 0.50 c | 42.70 ± 0.15 a | |
| DS | 29.97 ± 0.19 a | 34.53 ± 0.03 ab | 37.63 ± 0.07 b | 39.63 ± 0.27 a | 39.93 ± 0.07 b | |
| F-value | Sc (F = 21.50 **) Re (F = 281.69 **) Sc × Re (F = 4.4 *) | |||||
| Leaf area (cm2) | CK | 935.23 ± 15.30 bc | 1075.83 ± 9.22 bc | 1154.77 ± 6.20 bc | 1251.67 ± 21.95 b | 1368.53 ± 18.73 d |
| N | 1080.40 ± 25.03 a | 1106.97 ± 14.11 ab | 1266.23 ± 25.20 a | 1430.83 ± 48.39 a | 1518.20 ± 7.37 c | |
| A | 830.17 ± 12.86 d | 1116.10 ± 26.68 ab | 1204.6 ± 31.22 abc | 1393.97 ± 54.58 a | 1676.30 ± 28.02 a | |
| D | 960.67 ± 8.04 b | 1174.23 ± 21.18 a | 1254.03 ± 57.62 ab | 1454.23 ± 29.72 a | 1599.03 ± 11.71 b | |
| S | 827.03 ± 8.783 d | 857.93 ± 11.07 d | 946.53 ± 32.59 d | 1044.90 ± 7.97 c | 1147.50 ± 25.67 f | |
| NS | 928.70 ± 61.55 bc | 1045.03 ± 14.50 bc | 1108.20 ± 50.34 c | 1163.00 ± 11.09 bc | 1250.47 ± 35.81 e | |
| AS | 885.20 ± 30.04 bcd | 1029.20 ± 13.02 c | 1106.43 ± 10.42 c | 1155.17 ± 54.18 bc | 1310.83 ± 26.74 de | |
| DS | 855.20 ± 37.22 cd | 1095.1 ± 49.50 bc | 1148.67 ± 18.23 bc | 1193.70 ± 64.38 b | 1291.67 ± 9.00 e | |
| F-value | Sc (F = 109.69 **) Re (F = 15.34 **) Sc × Re(F = 0.35) | |||||
| Shoot fresh weight (×10−2 g) | CK | 21.77 ± 0.12 bc | 27.47 ± 0.09 d | 34.43 ± 0.39 d | 48.13 ± 0.45 c | 56.87 ± 1.02 c |
| N | 25.00 ± 0.31 a | 29.20 ± 0.20 cd | 43.23 ± 1.28 b | 60.07 ± 0.82 a | 70.60 ± 2.03 a | |
| A | 22.53 ± 0.18 b | 34.80 ± 0.67 b | 47.97 ± 0.15 a | 53.77 ± 0.37 b | 62.20 ± 0.75 b | |
| D | 22.70 ± 0.17 b | 39.60 ± 0.32 a | 48.33 ± 1.12 a | 59.83 ± 1.13 a | 71.47 ± 1.98 a | |
| S | 19.70 ± 0.64 d | 21.20 ± 0.49 e | 32.77 ± 0.49 d | 44.10 ± 1.35 d | 52.50 ± 0.89 d | |
| NS | 24.70 ± 0.38 a | 27.27 ± 0.12 d | 39.13 ± 0.42 c | 53.07 ± 1.56 b | 54.23 ± 0.71 cd | |
| AS | 20.77 ± 0.24 d | 31.00 ± 0.70 c | 44.97 ± 1.78 b | 48.13 ± 0.57 c | 55.13 ± 0.32 cd | |
| DS | 20.37 ± 0.69 d | 31.13 ± 1.63 c | 39.90 ± 0.25 c | 46.53 ± 0.60 cd | 53.03 ± 0.19 d | |
| F-value | Sc (F = 178.32 **) Re (F = 57.96 **) Sc × Re (F = 10.68 **) | |||||
| Shoot dry weight (×10−2 g) | CK | 3.40 ± 0.12 ab | 4.67 ± 0.12 e | 7.07 ± 0.09 d | 9.17 ± 0.09 bc | 12.40 ± 0.15 bc |
| N | 3.63 ± 0.32 a | 5.27 ± 0.176 cd | 8.93 ± 0.09 b | 11.40 ± 0.17 a | 13.37 ± 0.52 b | |
| A | 3.27 ± 0.09 ab | 6.37 ± 0.09 a | 9.97 ± 0.09 a | 11.80 ± 0.15 a | 14.60 ± 0.55 a | |
| D | 3.70 ± 0.06 a | 6.50 ± 0.06 a | 9.73 ± 0.38 a | 11.60 ± 0.06 a | 14.37 ± 0.09 a | |
| S | 3.07 ± 0.03 b | 3.8 ± 0.06 f | 6.03 ± 0.20 e | 8.00 ± 0.25 d | 10.07 ± 0.18 d | |
| NS | 3.63 ± 0.07 a | 4.97 ± 0.09 de | 7.4 ± 0.12 d | 8.77 ± 0.29 c | 11.43 ± 0.26 c | |
| AS | 3.17 ± 0.07 b | 5.73 ± 0.23 b | 8.27 ± 0.17 c | 9.40 ± 0.06 b | 12.00 ± 0.42 c | |
| DS | 3.10 ± 0.10 b | 5.53 ± 0.22 bc | 7.30 ± 0.17 d | 9.17 ± 0.12 bc | 11.60 ± 0.06 c | |
| F-value | Sc (F = 149.76 **) Re (F = 41.27 **) Sc × Re (F = 1.65) | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Tan, C.; Li, Y.; Meng, F.; Du, Y.; Zhang, S.; Jiang, W.; Feng, N.; Zhao, L.; Zheng, D. 5-ALA, DTA-6, and Nitrogen Mitigate NaCl Stress by Promoting Photosynthesis and Carbon Metabolism in Rice Seedlings. Metabolites 2024, 14, 142. https://doi.org/10.3390/metabo14030142
Wang Y, Tan C, Li Y, Meng F, Du Y, Zhang S, Jiang W, Feng N, Zhao L, Zheng D. 5-ALA, DTA-6, and Nitrogen Mitigate NaCl Stress by Promoting Photosynthesis and Carbon Metabolism in Rice Seedlings. Metabolites. 2024; 14(3):142. https://doi.org/10.3390/metabo14030142
Chicago/Turabian StyleWang, Yaxin, Chaolu Tan, Yinghao Li, Fengyan Meng, Youwei Du, Shuyu Zhang, Wenxin Jiang, Naijie Feng, Liming Zhao, and Dianfeng Zheng. 2024. "5-ALA, DTA-6, and Nitrogen Mitigate NaCl Stress by Promoting Photosynthesis and Carbon Metabolism in Rice Seedlings" Metabolites 14, no. 3: 142. https://doi.org/10.3390/metabo14030142
APA StyleWang, Y., Tan, C., Li, Y., Meng, F., Du, Y., Zhang, S., Jiang, W., Feng, N., Zhao, L., & Zheng, D. (2024). 5-ALA, DTA-6, and Nitrogen Mitigate NaCl Stress by Promoting Photosynthesis and Carbon Metabolism in Rice Seedlings. Metabolites, 14(3), 142. https://doi.org/10.3390/metabo14030142





