The Role of BDNF and TrkB in the Central Control of Energy and Glucose Balance: An Update
Abstract
1. Introduction
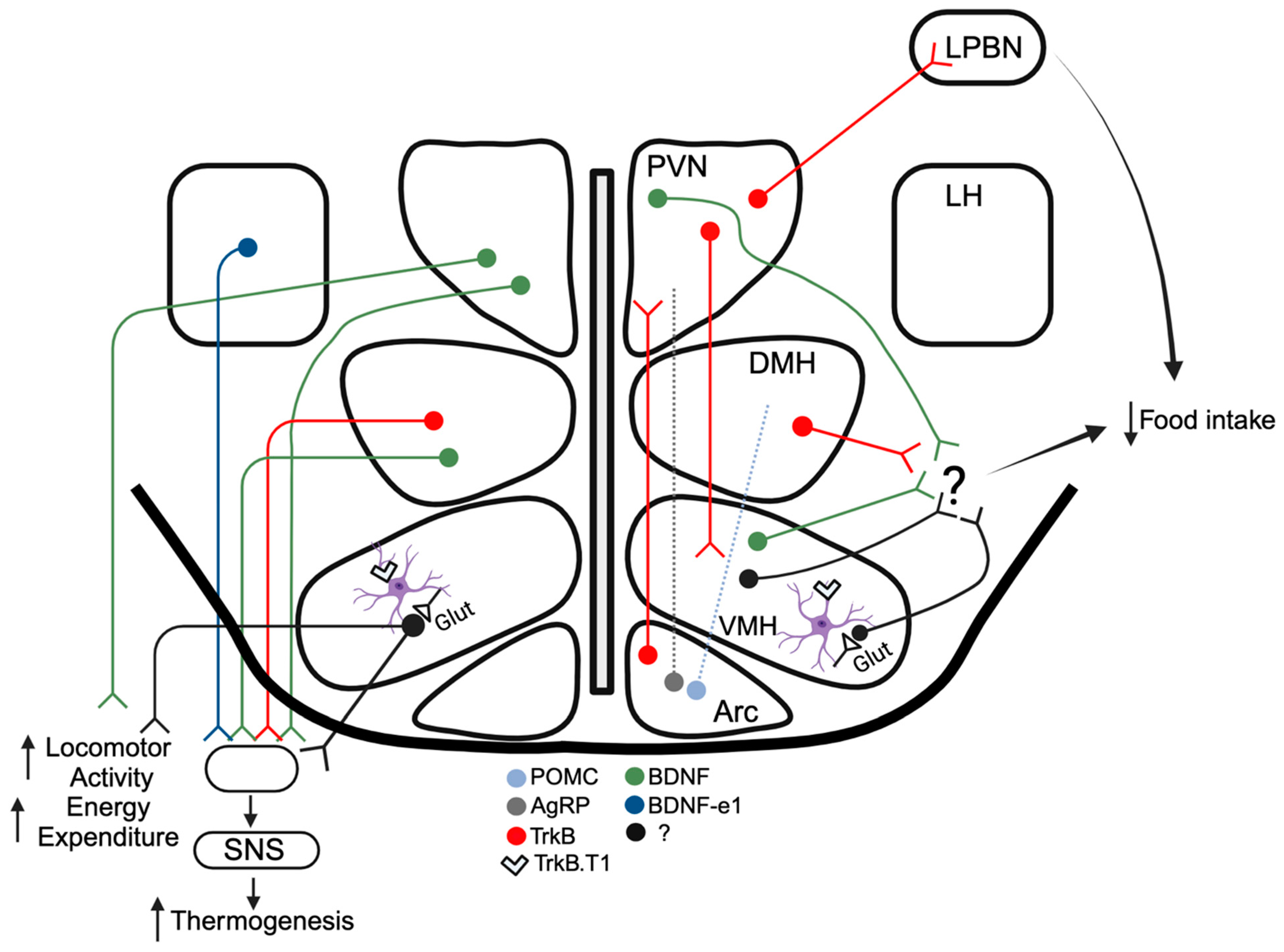
2. BDNF Signaling and Hypothalamic Control of Energy and Glucose Balance
2.1. Arcuate Nucleus
2.2. Paraventricular Nucleus
2.3. Ventromedial Hypothalamus
2.4. Dorsomedial Hypothalamus
2.5. Lateral Hypothalamus
2.6. Preoptic Area
3. Extrahypothalamic Actions of BDNF and TrkB Influencing Feeding and Body Weight
3.1. Dorsal Vagal Complex
3.2. Dopaminergic Reward Circuits
3.3. Amygdala
4. Summary
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Patapoutian, A.; Reichardt, L.F. Trk receptors: Mediators of neurotrophin action. Curr. Opin. Neurobiol. 2001, 11, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Middlemas, D.S.; Lindberg, R.A.; Hunter, T. trkB, a neural receptor protein-tyrosine kinase: Evidence for a full-length and two truncated receptors. Mol. Cell Biol. 1991, 11, 143–153. [Google Scholar] [PubMed]
- Ohira, K.; Kumanogoh, H.; Sahara, Y.; Homma, K.J.; Hirai, H.; Nakamura, S.; Hayashi, M. A truncated tropomyosin-related kinase B receptor, T1, regulates glial cell morphology via Rho GDP dissociation inhibitor 1. J. Neurosci. Off. J. Soc. Neurosci. 2005, 25, 1343–1353. [Google Scholar] [CrossRef]
- Lapchak, P.A.; Hefti, F. BDNF and NGF treatment in lesioned rats: Effects on cholinergic function and weight gain. Neuroreport 1992, 3, 405–408. [Google Scholar] [CrossRef] [PubMed]
- Martin-Iverson, M.T.; Todd, K.G.; Altar, C.A. Brain-derived neurotrophic factor and neurotrophin-3 activate striatal dopamine and serotonin metabolism and related behaviors: Interactions with amphetamine. J. Neurosci. Off. J. Soc. Neurosci. 1994, 14, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Pelleymounter, M.A.; Cullen, M.J.; Wellman, C.L. Characteristics of BDNF-induced weight loss. Exp. Neurol. 1995, 131, 229–238. [Google Scholar] [CrossRef]
- Lyons, W.E.; Mamounas, L.A.; Ricaurte, G.A.; Coppola, V.; Reid, S.W.; Bora, S.H.; Wihler, C.; Koliatsos, V.E.; Tessarollo, L. Brain-derived neurotrophic factor-deficient mice develop aggressiveness and hyperphagia in conjunction with brain serotonergic abnormalities. Proc. Natl. Acad. Sci. USA 1999, 96, 15239–15244. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Goulding, E.H.; Zang, K.; Cepoi, D.; Cone, R.D.; Jones, K.R.; Tecott, L.H.; Reichardt, L.F. Brain-derived neurotrophic factor regulates energy balance downstream of melanocortin-4 receptor. Nat. Neurosci. 2003, 6, 736–742. [Google Scholar] [CrossRef] [PubMed]
- Rios, M.; Fan, G.; Fekete, C.; Kelly, J.; Bates, B.; Kuehn, R.; Lechan, R.M.; Jaenisch, R. Conditional deletion of brain-derived neurotrophic factor in the postnatal brain leads to obesity and hyperactivity. Mol. Endocrinol. 2001, 15, 1748–1757. [Google Scholar] [CrossRef]
- Martinez-Ezquerro, J.D.; Rendon-Macias, M.E.; Zamora-Mendoza, G.; Serrano-Meneses, J.; Rosales-Rodriguez, B.; Escalante-Bautista, D.; Rodriguez-Cruz, M.; Sanchez-Gonzalez, R.; Arellano-Pineda, Y.; Lopez-Alarcon, M.; et al. Association Between the Brain-derived Neurotrophic Factor Val66Met Polymorphism and Overweight/Obesity in Pediatric Population. Arch. Med. Res. 2017, 48, 599–608. [Google Scholar] [CrossRef]
- Speliotes, E.K.; Willer, C.J.; Berndt, S.I.; Monda, K.L.; Thorleifsson, G.; Jackson, A.U.; Allen, H.L.; Lindgren, C.M.; Luan, J.; Magi, R.; et al. Association analyses of 249,796 individuals reveal 18 new loci associated with body mass index. Nat. Genet. 2010, 42, 937–948. [Google Scholar] [CrossRef] [PubMed]
- Vidovic, V.; Maksimovic, N.; Novakovic, I.; Damnjanovic, T.; Jekic, B.; Vidovic, S.; Majkic Singh, N.; Stamenkovic-Radak, M.; Nikolic, D.; Marisavljevic, D. Association of the Brain-derived Neurotrophic Factor Val66Met Polymorphism with Body Mass Index, Fasting Glucose Levels and Lipid Status in Adolescents. Balk. J. Med. Genet. 2020, 23, 77–82. [Google Scholar] [CrossRef]
- Han, J.C.; Liu, Q.R.; Jones, M.; Levinn, R.L.; Menzie, C.M.; Jefferson-George, K.S.; Adler-Wailes, D.C.; Sanford, E.L.; Lacbawan, F.L.; Uhl, G.R.; et al. Brain-derived neurotrophic factor and obesity in the WAGR syndrome. N. Engl. J. Med. 2008, 359, 918–927. [Google Scholar] [CrossRef]
- Yeo, G.S.; Connie Hung, C.C.; Rochford, J.; Keogh, J.; Gray, J.; Sivaramakrishnan, S.; O’Rahilly, S.; Farooqi, I.S. A de novo mutation affecting human TrkB associated with severe obesity and developmental delay. Nat. Neurosci. 2004, 7, 1187–1189. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.; Yeo, G.; Hung, C.; Keogh, J.; Clayton, P.; Banerjee, K.; McAulay, A.; O’Rahilly, S.; Farooqi, I.S. Functional characterization of human NTRK2 mutations identified in patients with severe early-onset obesity. Int. J. Obes. 2007, 31, 359–364. [Google Scholar] [CrossRef]
- Bochukova, E.G.; Lawler, K.; Croizier, S.; Keogh, J.M.; Patel, N.; Strohbehn, G.; Lo, K.K.; Humphrey, J.; Hokken-Koelega, A.; Damen, L.; et al. A Transcriptomic Signature of the Hypothalamic Response to Fasting and BDNF Deficiency in Prader-Willi Syndrome. Cell Rep. 2018, 22, 3401–3408. [Google Scholar] [CrossRef]
- Dietrich, M.O.; Horvath, T.L. Feeding signals and brain circuitry. Eur. J. Neurosci. 2009, 30, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Waterson, M.J.; Horvath, T.L. Neuronal Regulation of Energy Homeostasis: Beyond the Hypothalamus and Feeding. Cell Metab. 2015, 22, 962–970. [Google Scholar] [CrossRef]
- Liao, G.Y.; Kinney, C.E.; An, J.J.; Xu, B. TrkB-expressing neurons in the dorsomedial hypothalamus are necessary and sufficient to suppress homeostatic feeding. Proc. Natl. Acad. Sci. USA 2019, 116, 3256–3261. [Google Scholar] [CrossRef]
- Liao, G.Y.; Li, Y.; Xu, B. Ablation of TrkB expression in RGS9-2 cells leads to hyperphagic obesity. Mol. Metab. 2013, 2, 491–497. [Google Scholar] [CrossRef]
- Unger, T.J.; Calderon, G.A.; Bradley, L.C.; Sena-Esteves, M.; Rios, M. Selective deletion of Bdnf in the ventromedial and dorsomedial hypothalamus of adult mice results in hyperphagic behavior and obesity. J. Neurosci. 2007, 27, 14265–14274. [Google Scholar] [CrossRef] [PubMed]
- Timmusk, T.; Palm, K.; Metsis, M.; Reintam, T.; Paalme, V.; Saarma, M.; Persson, H. Multiple promoters direct tissue-specific expression of the rat BDNF gene. Neuron 1993, 10, 475–489. [Google Scholar] [CrossRef] [PubMed]
- An, J.J.; Gharami, K.; Liao, G.Y.; Woo, N.H.; Lau, A.G.; Vanevski, F.; Torre, E.R.; Jones, K.R.; Feng, Y.; Lu, B.; et al. Distinct role of long 3’ UTR BDNF mRNA in spine morphology and synaptic plasticity in hippocampal neurons. Cell 2008, 134, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.Y.; An, J.J.; Gharami, K.; Waterhouse, E.G.; Vanevski, F.; Jones, K.R.; Xu, B. Dendritically targeted Bdnf mRNA is essential for energy balance and response to leptin. Nat. Med. 2012, 18, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Cone, R.D.; Cowley, M.A.; Butler, A.A.; Fan, W.; Marks, D.L.; Low, M.J. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int. J. Obes. Relat. Metab. Disord. J. Int. Assoc. Study Obes. 2001, 25 (Suppl. S5), S63–S67. [Google Scholar] [CrossRef] [PubMed]
- Aponte, Y.; Atasoy, D.; Sternson, S.M. AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat. Neurosci. 2011, 14, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Atasoy, D.; Betley, J.N.; Su, H.H.; Sternson, S.M. Deconstruction of a neural circuit for hunger. Nature 2012, 488, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Bewick, G.A.; Dhillo, W.S.; Darch, S.J.; Murphy, K.G.; Gardiner, J.V.; Jethwa, P.H.; Kong, W.M.; Ghatei, M.A.; Bloom, S.R. Hypothalamic cocaine- and amphetamine-regulated transcript (CART) and agouti-related protein (AgRP) neurons coexpress the NOP1 receptor and nociceptin alters CART and AgRP release. Endocrinology 2005, 146, 3526–3534. [Google Scholar] [CrossRef] [PubMed]
- Gropp, E.; Shanabrough, M.; Borok, E.; Xu, A.W.; Janoschek, R.; Buch, T.; Plum, L.; Balthasar, N.; Hampel, B.; Waisman, A.; et al. Agouti-related peptide-expressing neurons are mandatory for feeding. Nat. Neurosci. 2005, 8, 1289–1291. [Google Scholar] [CrossRef]
- Krashes, M.J.; Koda, S.; Ye, C.; Rogan, S.C.; Adams, A.C.; Cusher, D.S.; Maratos-Flier, E.; Roth, B.L.; Lowell, B.B. Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J. Clin. Investig. 2011, 121, 1424–1428. [Google Scholar] [CrossRef]
- Luquet, S.; Perez, F.A.; Hnasko, T.S.; Palmiter, R.D. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 2005, 310, 683–685. [Google Scholar] [CrossRef] [PubMed]
- Cone, R.D. Anatomy and regulation of the central melanocortin system. Nat. Neurosci. 2005, 8, 571–578. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Atasoy, D.; Su, H.H.; Sternson, S.M. Hunger states switch a flip-flop memory circuit via a synaptic AMPK-dependent positive feedback loop. Cell 2011, 146, 992–1003. [Google Scholar] [CrossRef] [PubMed]
- Broberger, C.; Johansen, J.; Johansson, C.; Schalling, M.; Hokfelt, T. The neuropeptide Y/agouti gene-related protein (AGRP) brain circuitry in normal, anorectic, and monosodium glutamate-treated mice. Proc. Natl. Acad. Sci. USA 1998, 95, 15043–15048. [Google Scholar] [CrossRef] [PubMed]
- Liao, G.Y.; Bouyer, K.; Kamitakahara, A.; Sahibzada, N.; Wang, C.H.; Rutlin, M.; Simerly, R.B.; Xu, B. Brain-derived neurotrophic factor is required for axonal growth of selective groups of neurons in the arcuate nucleus. Mol. Metab. 2015, 4, 471–482. [Google Scholar] [CrossRef] [PubMed]
- An, J.J.; Liao, G.Y.; Kinney, C.E.; Sahibzada, N.; Xu, B. Discrete BDNF Neurons in the Paraventricular Hypothalamus Control Feeding and Energy Expenditure. Cell Metab. 2015, 22, 175–188. [Google Scholar] [CrossRef] [PubMed]
- Garfield, A.S.; Li, C.; Madara, J.C.; Shah, B.P.; Webber, E.; Steger, J.S.; Campbell, J.N.; Gavrilova, O.; Lee, C.E.; Olson, D.P.; et al. A neural basis for melanocortin-4 receptor-regulated appetite. Nat. Neurosci. 2015, 18, 863–871. [Google Scholar] [CrossRef]
- Shah, B.P.; Vong, L.; Olson, D.P.; Koda, S.; Krashes, M.J.; Ye, C.; Yang, Z.; Fuller, P.M.; Elmquist, J.K.; Lowell, B.B. MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. Proc. Natl. Acad. Sci. USA 2014, 111, 13193–13198. [Google Scholar] [CrossRef]
- Geerling, J.C.; Shin, J.W.; Chimenti, P.C.; Loewy, A.D. Paraventricular hypothalamic nucleus: Axonal projections to the brainstem. J. Comp. Neurol. 2010, 518, 1460–1499. [Google Scholar] [CrossRef]
- Wang, D.; He, X.; Zhao, Z.; Feng, Q.; Lin, R.; Sun, Y.; Ding, T.; Xu, F.; Luo, M.; Zhan, C. Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front. Neuroanat. 2015, 9, 40. [Google Scholar] [CrossRef]
- Stanley, S.; Pinto, S.; Segal, J.; Perez, C.A.; Viale, A.; DeFalco, J.; Cai, X.; Heisler, L.K.; Friedman, J.M. Identification of neuronal subpopulations that project from hypothalamus to both liver and adipose tissue polysynaptically. Proc. Natl. Acad. Sci. USA 2010, 107, 7024–7029. [Google Scholar] [CrossRef] [PubMed]
- Toriya, M.; Maekawa, F.; Maejima, Y.; Onaka, T.; Fujiwara, K.; Nakagawa, T.; Nakata, M.; Yada, T. Long-term infusion of brain-derived neurotrophic factor reduces food intake and body weight via a corticotrophin-releasing hormone pathway in the paraventricular nucleus of the hypothalamus. J. Neuroendocrinol. 2010, 22, 987–995. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Bomberg, E.; Billington, C.; Levine, A.; Kotz, C.M. Brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus reduces energy intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1003–R1012. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Godar, R.J.; Billington, C.J.; Kotz, C.M. Chronic administration of brain-derived neurotrophic factor in the hypothalamic paraventricular nucleus reverses obesity induced by high-fat diet. Am. J. Physiology. Regul. Integr. Comp. Physiol. 2010, 298, R1320–R1332. [Google Scholar] [CrossRef]
- Balthasar, N.; Dalgaard, L.T.; Lee, C.E.; Yu, J.; Funahashi, H.; Williams, T.; Ferreira, M.; Tang, V.; McGovern, R.A.; Kenny, C.D.; et al. Divergence of melanocortin pathways in the control of food intake and energy expenditure. Cell 2005, 123, 493–505. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Loh, K.H.; Wu, M.; Morgan, D.A.; Schneeberger, M.; Yu, X.; Chi, J.; Kosse, C.; Kim, D.; Rahmouni, K.; et al. A leptin-BDNF pathway regulating sympathetic innervation of adipose tissue. Nature 2020, 583, 839–844. [Google Scholar] [CrossRef] [PubMed]
- Luo, F.; Mu, Y.; Gao, C.; Xiao, Y.; Zhou, Q.; Yang, Y.; Ni, X.; Shen, W.L.; Yang, J. Whole-brain patterns of the presynaptic inputs and axonal projections of BDNF neurons in the paraventricular nucleus. J. Genet. Genom. 2019, 46, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.W.; Xu, B. Rapid and Lasting Effects of Activating BDNF-Expressing PVH Neurons on Energy Balance. eNeuro 2022, 9, ENEURO.0009-22.2022. [Google Scholar] [CrossRef]
- Javed, S.; Chang, Y.T.; Cho, Y.; Lee, Y.J.; Chang, H.C.; Haque, M.; Lin, Y.C.; Huang, W.H. Smith-Magenis syndrome protein RAI1 regulates body weight homeostasis through hypothalamic BDNF-producing neurons and neurotrophin downstream signalling. Elife 2023, 12, RP90333. [Google Scholar] [CrossRef]
- Alaimo, J.T.; Barton, L.V.; Mullegama, S.V.; Wills, R.D.; Foster, R.H.; Elsea, S.H. Individuals with Smith-Magenis syndrome display profound neurodevelopmental behavioral deficiencies and exhibit food-related behaviors equivalent to Prader-Willi syndrome. Res. Dev. Disabil. 2015, 47, 27–38. [Google Scholar] [CrossRef]
- Burns, B.; Schmidt, K.; Williams, S.R.; Kim, S.; Girirajan, S.; Elsea, S.H. Rai1 haploinsufficiency causes reduced Bdnf expression resulting in hyperphagia, obesity and altered fat distribution in mice and humans with no evidence of metabolic syndrome. Human Mol. Genet. 2010, 19, 4026–4042. [Google Scholar] [CrossRef]
- An, J.J.; Kinney, C.E.; Tan, J.W.; Liao, G.Y.; Kremer, E.J.; Xu, B. TrkB-expressing paraventricular hypothalamic neurons suppress appetite through multiple neurocircuits. Nat. Commun. 2020, 11, 1729. [Google Scholar] [CrossRef] [PubMed]
- Komori, T.; Morikawa, Y.; Nanjo, K.; Senba, E. Induction of brain-derived neurotrophic factor by leptin in the ventromedial hypothalamus. Neuroscience 2006, 139, 1107–1115. [Google Scholar] [CrossRef]
- Cheung, C.C.; Kurrasch, D.M.; Liang, J.K.; Ingraham, H.A. Genetic labeling of steroidogenic factor-1 (SF-1) neurons in mice reveals ventromedial nucleus of the hypothalamus (VMH) circuitry beginning at neurogenesis and development of a separate non-SF-1 neuronal cluster in the ventrolateral VMH. J. Comp. Neurol. 2013, 521, 1268–1288. [Google Scholar] [CrossRef]
- Coutinho, E.A.; Okamoto, S.; Ishikawa, A.W.; Yokota, S.; Wada, N.; Hirabayashi, T.; Saito, K.; Sato, T.; Takagi, K.; Wang, C.C.; et al. Activation of SF1 Neurons in the Ventromedial Hypothalamus by DREADD Technology Increases Insulin Sensitivity in Peripheral Tissues. Diabetes 2017, 66, 2372–2386. [Google Scholar] [CrossRef] [PubMed]
- Resch, J.M.; Maunze, B.; Gerhardt, A.K.; Magnuson, S.K.; Phillips, K.A.; Choi, S. Intrahypothalamic pituitary adenylate cyclase-activating polypeptide regulates energy balance via site-specific actions on feeding and metabolism. Am. J. Physiol. Endocrinol. Metab. 2013, 305, E1452–E1463. [Google Scholar] [CrossRef] [PubMed]
- Viskaitis, P.; Irvine, E.E.; Smith, M.A.; Choudhury, A.I.; Alvarez-Curto, E.; Glegola, J.A.; Hardy, D.G.; Pedroni, S.M.A.; Paiva Pessoa, M.R.; Fernando, A.B.P.; et al. Modulation of SF1 Neuron Activity Coordinately Regulates Both Feeding Behavior and Associated Emotional States. Cell Rep. 2017, 21, 3559–3572. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.V.; Akana, S.F.; Malkovska, I.; Dallman, M.F.; Parada, L.F.; Ingraham, H.A. Diminished hypothalamic bdnf expression and impaired VMH function are associated with reduced SF-1 gene dosage. J. Comp. Neurol. 2006, 498, 637–648. [Google Scholar] [CrossRef]
- Wang, C.; Bomberg, E.; Billington, C.J.; Levine, A.S.; Kotz, C.M. Brain-derived neurotrophic factor (BDNF) in the hypothalamic ventromedial nucleus increases energy expenditure. Brain Res. 2010, 1336, 66–77. [Google Scholar] [CrossRef]
- Wang, C.; Bomberg, E.; Levine, A.; Billington, C.; Kotz, C.M. Brain-derived neurotrophic factor in the ventromedial nucleus of the hypothalamus reduces energy intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007, 293, R1037–R1045. [Google Scholar] [CrossRef]
- Kamitakahara, A.; Xu, B.; Simerly, R. Ventromedial hypothalamic expression of Bdnf is required to establish normal patterns of afferent GABAergic connectivity and responses to hypoglycemia. Mol. Metab. 2016, 5, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Tran, P.V.; Lee, M.B.; Marin, O.; Xu, B.; Jones, K.R.; Reichardt, L.F.; Rubenstein, J.R.; Ingraham, H.A. Requirement of the orphan nuclear receptor SF-1 in terminal differentiation of ventromedial hypothalamic neurons. Mol. Cell Neurosci. 2003, 22, 441–453. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.; Guo, W.; You, H.; Lu, B. Regulation of Satiety by Bdnf-e2-Expressing Neurons through TrkB Activation in Ventromedial Hypothalamus. Biomolecules 2023, 13, 822. [Google Scholar] [CrossRef] [PubMed]
- Ameroso, D.; Meng, A.; Chen, S.; Felsted, J.; Dulla, C.G.; Rios, M. Astrocytic BDNF signaling within the ventromedial hypothalamus regulates energy homeostasis. Nat. Metab. 2022, 4, 627–643. [Google Scholar] [CrossRef] [PubMed]
- Araque, A.; Parpura, V.; Sanzgiri, R.P.; Haydon, P.G. Tripartite synapses: Glia, the unacknowledged partner. Trends Neurosci. 1999, 22, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Araque, A.; Sanzgiri, R.P.; Parpura, V.; Haydon, P.G. Astrocyte-induced modulation of synaptic transmission. Can. J. Physiol. Pharmacol. 1999, 77, 699–706. [Google Scholar] [CrossRef]
- Dallerac, G.; Chever, O.; Rouach, N. How do astrocytes shape synaptic transmission? Insights from electrophysiology. Front. Cell. Neurosci. 2013, 7, 159. [Google Scholar] [CrossRef] [PubMed]
- Danbolt, N.C. Glutamate uptake. Progress Neurobiol. 2001, 65, 1–105. [Google Scholar]
- Gegelashvili, G.; Dehnes, Y.; Danbolt, N.C.; Schousboe, A. The high-affinity glutamate transporters GLT1, GLAST, and EAAT4 are regulated via different signalling mechanisms. Neurochem. Int. 2000, 37, 163–170. [Google Scholar] [CrossRef]
- Bernardinelli, Y.; Muller, D.; Nikonenko, I. Astrocyte-synapse structural plasticity. Neural Plast. 2014, 2014, 232105. [Google Scholar] [CrossRef]
- Genoud, C.; Quairiaux, C.; Steiner, P.; Hirling, H.; Welker, E.; Knott, G.W. Plasticity of astrocytic coverage and glutamate transporter expression in adult mouse cortex. PLoS Biol. 2006, 4, e343. [Google Scholar] [CrossRef] [PubMed]
- Cordeira, J.W.; Felsted, J.A.; Teillon, S.; Daftary, S.; Panessiti, M.; Wirth, J.; Sena-Esteves, M.; Rios, M. Hypothalamic dysfunction of the thrombospondin receptor alpha2delta-1 underlies the overeating and obesity triggered by brain-derived neurotrophic factor deficiency. J. Neurosci. Off. J. Soc. Neurosci. 2014, 34, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Bauer, C.S.; Tran-Van-Minh, A.; Kadurin, I.; Dolphin, A.C. A new look at calcium channel alpha2delta subunits. Curr. Opin. Neurobiol. 2010, 20, 563–571. [Google Scholar] [CrossRef] [PubMed]
- Davies, A.; Hendrich, J.; Van Minh, A.T.; Wratten, J.; Douglas, L.; Dolphin, A.C. Functional biology of the alpha(2)delta subunits of voltage-gated calcium channels. Trends Pharmacol. Sci. 2007, 28, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Eroglu, C.; Allen, N.J.; Susman, M.W.; O’Rourke, N.A.; Park, C.Y.; Ozkan, E.; Chakraborty, C.; Mulinyawe, S.B.; Annis, D.S.; Huberman, A.D.; et al. Gabapentin receptor alpha2delta-1 is a neuronal thrombospondin receptor responsible for excitatory CNS synaptogenesis. Cell 2009, 139, 380–392. [Google Scholar] [CrossRef] [PubMed]
- DeToledo, J.C.; Toledo, C.; DeCerce, J.; Ramsay, R.E. Changes in body weight with chronic, high-dose gabapentin therapy. Ther. Drug Monit. 1997, 19, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Gee, N.S.; Brown, J.P.; Dissanayake, V.U.; Offord, J.; Thurlow, R.; Woodruff, G.N. The novel anticonvulsant drug, gabapentin (Neurontin), binds to the alpha2delta subunit of a calcium channel. J. Biol. Chem. 1996, 271, 5768–5776. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, C.; Rademacher, M.; Hoffmann, J.M.; Schmidt, D.; Elger, C.E. Bodyweight gain under pregabalin therapy in epilepsy: Mitigation by counseling patients? Seizure 2008, 17, 327–332. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fagan, M.P.; Ameroso, D.; Meng, A.; Rock, A.; Maguire, J.; Rios, M. Essential and sex-specific effects of mGluR5 in ventromedial hypothalamus regulating estrogen signaling and glucose balance. Proc. Natl. Acad. Sci. USA 2020, 117, 19566–19577. [Google Scholar] [CrossRef]
- van den Pol, A.N.; Romano, C.; Ghosh, P. Metabotropic glutamate receptor mGluR5 subcellular distribution and developmental expression in hypothalamus. J. Comp. Neurol. 1995, 362, 134–150. [Google Scholar] [CrossRef]
- Meng, A.; Ameroso, D.; Rios, M. mGluR5 in Astrocytes in the Ventromedial Hypothalamus Regulates Pituitary Adenylate Cyclase-Activating Polypeptide Neurons and Glucose Homeostasis. J. Neurosci. 2023, 43, 5918–5935. [Google Scholar] [CrossRef]
- Garfield, A.S.; Shah, B.P.; Burgess, C.R.; Li, M.M.; Li, C.; Steger, J.S.; Madara, J.C.; Campbell, J.N.; Kroeger, D.; Scammell, T.E.; et al. Dynamic GABAergic afferent modulation of AgRP neurons. Nat. Neurosci. 2016, 19, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Luiten, P.G.; Room, P. Interrelations between lateral, dorsomedial and ventromedial hypothalamic nuclei in the rat. An HRP study. Brain Res. 1980, 190, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Rezai-Zadeh, K.; Yu, S.; Jiang, Y.; Laque, A.; Schwartzenburg, C.; Morrison, C.D.; Derbenev, A.V.; Zsombok, A.; Munzberg, H. Leptin receptor neurons in the dorsomedial hypothalamus are key regulators of energy expenditure and body weight, but not food intake. Mol. Metab. 2014, 3, 681–693. [Google Scholar] [CrossRef]
- Thompson, R.H.; Swanson, L.W. Organization of inputs to the dorsomedial nucleus of the hypothalamus: A reexamination with Fluorogold and PHAL in the rat. Brain Res. Brain Res. Rev. 1998, 27, 89–118. [Google Scholar] [CrossRef]
- Houtz, J.; Liao, G.Y.; An, J.J.; Xu, B. Discrete TrkB-expressing neurons of the dorsomedial hypothalamus regulate feeding and thermogenesis. Proc. Natl. Acad. Sci. USA 2021, 118, e2017218118. [Google Scholar] [CrossRef]
- Zhou, Q.; Liu, C.; Chen, T.; Liu, Y.; Cao, R.; Ni, X.; Yang, W.Z.; Shen, Q.; Sun, H.; Shen, W.L. Cooling-activated dorsomedial hypothalamic BDNF neurons control cold defense in mice. J. Neurochem. 2022, 163, 220–232. [Google Scholar] [CrossRef] [PubMed]
- Anand, B.K.; Brobeck, J.R. Localization of a “feeding center” in the hypothalamus of the rat. Proc. Soc. Exp. Biol. Medicine. Soc. Exp. Biol. Med. 1951, 77, 323–324. [Google Scholar] [CrossRef]
- Date, Y.; Ueta, Y.; Yamashita, H.; Yamaguchi, H.; Matsukura, S.; Kangawa, K.; Sakurai, T.; Yanagisawa, M.; Nakazato, M. Orexins, orexigenic hypothalamic peptides, interact with autonomic, neuroendocrine and neuroregulatory systems. Proc. Natl. Acad. Sci. USA 1999, 96, 748–753. [Google Scholar] [CrossRef]
- Zamir, N.; Skofitsch, G.; Jacobowitz, D.M. Distribution of immunoreactive melanin-concentrating hormone in the central nervous system of the rat. Brain Res. 1986, 373, 240–245. [Google Scholar] [CrossRef]
- You, H.; Chu, P.; Guo, W.; Lu, B. A subpopulation of Bdnf-e1-expressing glutamatergic neurons in the lateral hypothalamus critical for thermogenesis control. Mol. Metab. 2020, 31, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Boulant, J.A. Role of the preoptic-anterior hypothalamus in thermoregulation and fever. Clin. Infect. Dis. 2000, 31 (Suppl. S5), S157–S161. [Google Scholar] [CrossRef]
- Boulant, J.A.; Dean, J.B. Temperature receptors in the central nervous system. Annu. Rev. Physiol. 1986, 48, 639–654. [Google Scholar] [CrossRef] [PubMed]
- Katz, A.; Meiri, N. Brain-derived neurotrophic factor is critically involved in thermal-experience-dependent developmental plasticity. J. Neurosci. 2006, 26, 3899–3907. [Google Scholar] [CrossRef]
- Tan, C.L.; Cooke, E.K.; Leib, D.E.; Lin, Y.C.; Daly, G.E.; Zimmerman, C.A.; Knight, Z.A. Warm-Sensitive Neurons that Control Body Temperature. Cell 2016, 167, 47–59.e15. [Google Scholar] [CrossRef]
- Cheng, W.; Gordian, D.; Ludwig, M.Q.; Pers, T.H.; Seeley, R.J.; Myers, M.G., Jr. Hindbrain circuits in the control of eating behaviour and energy balance. Nat. Metab. 2022, 4, 826–835. [Google Scholar] [CrossRef] [PubMed]
- Grill, H.J.; Hayes, M.R. Hindbrain neurons as an essential hub in the neuroanatomically distributed control of energy balance. Cell Metab. 2012, 16, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Grill, H.J.; Kaplan, J.M. The neuroanatomical axis for control of energy balance. Front. Neuroendocrinol. 2002, 23, 2–40. [Google Scholar] [CrossRef]
- Conner, J.M.; Lauterborn, J.C.; Yan, Q.; Gall, C.M.; Varon, S. Distribution of brain-derived neurotrophic factor (BDNF) protein and mRNA in the normal adult rat CNS: Evidence for anterograde axonal transport. J. Neurosci. 1997, 17, 2295–2313. [Google Scholar] [CrossRef]
- Yan, Q.; Radeke, M.J.; Matheson, C.R.; Talvenheimo, J.; Welcher, A.A.; Feinstein, S.C. Immunocytochemical localization of TrkB in the central nervous system of the adult rat. J. Comp. Neurol. 1997, 378, 135–157. [Google Scholar] [CrossRef]
- Bariohay, B.; Lebrun, B.; Moyse, E.; Jean, A. Brain-derived neurotrophic factor plays a role as an anorexigenic factor in the dorsal vagal complex. Endocrinology 2005, 146, 5612–5620. [Google Scholar] [CrossRef] [PubMed]
- Bariohay, B.; Roux, J.; Tardivel, C.; Trouslard, J.; Jean, A.; Lebrun, B. Brain-derived neurotrophic factor/tropomyosin-related kinase receptor type B signaling is a downstream effector of the brainstem melanocortin system in food intake control. Endocrinology 2009, 150, 2646–2653. [Google Scholar] [CrossRef]
- Spaeth, A.M.; Kanoski, S.E.; Hayes, M.R.; Grill, H.J. TrkB receptor signaling in the nucleus tractus solitarius mediates the food intake-suppressive effects of hindbrain BDNF and leptin. Am. J. Physiology. Endocrinol. Metab. 2012, 302, E1252–E1260. [Google Scholar] [CrossRef]
- Ozek, C.; Zimmer, D.J.; De Jonghe, B.C.; Kalb, R.G.; Bence, K.K. Ablation of intact hypothalamic and/or hindbrain TrkB signaling leads to perturbations in energy balance. Mol. Metab. 2015, 4, 867–880. [Google Scholar] [CrossRef]
- Bassareo, V.; De Luca, M.A.; Di Chiara, G. Differential Expression of Motivational Stimulus Properties by Dopamine in Nucleus Accumbens Shell versus Core and Prefrontal Cortex. J. Neurosci. 2002, 22, 4709–4719. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, L.; Hoebel, B.G. Food reward and cocaine increase extracellular dopamine in the nucleus accumbens as measured by microdialysis. Life Sci. 1988, 42, 1705–1712. [Google Scholar] [CrossRef]
- Rada, P.; Avena, N.M.; Hoebel, B.G. Daily bingeing on sugar repeatedly releases dopamine in the accumbens shell. Neuroscience 2005, 134, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Numan, S.; Lane-Ladd, S.B.; Zhang, L.; Lundgren, K.H.; Russell, D.S.; Seroogy, K.B.; Nestler, E.J. Differential regulation of neurotrophin and trk receptor mRNAs in catecholaminergic nuclei during chronic opiate treatment and withdrawal. J. Neurosci. 1998, 18, 10700–10708. [Google Scholar] [CrossRef] [PubMed]
- Numan, S.; Seroogy, K.B. Expression of trkB and trkC mRNAs by adult midbrain dopamine neurons: A double-label in situ hybridization study. J. Comp. Neurol. 1999, 403, 295–308. [Google Scholar] [CrossRef]
- Freeman, A.Y.; Soghomonian, J.J.; Pierce, R.C. Tyrosine kinase B and C receptors in the neostriatum and nucleus accumbens are co-localized in enkephalin-positive and enkephalin-negative neuronal profiles and their expression is influenced by cocaine. Neuroscience 2003, 117, 147–156. [Google Scholar] [CrossRef]
- Cordeira, J.W.; Frank, L.; Sena-Esteves, M.; Pothos, E.N.; Rios, M. Brain-derived neurotrophic factor regulates hedonic feeding by acting on the mesolimbic dopamine system. J. Neurosci. Off. J. Soc. Neurosci. 2010, 30, 2533–2541. [Google Scholar] [CrossRef] [PubMed]
- Pu, L.; Liu, Q.S.; Poo, M.M. BDNF-dependent synaptic sensitization in midbrain dopamine neurons after cocaine withdrawal. Nat. Neurosci. 2006, 9, 605–607. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.H.; Zhang, J.Y.; Holmes, A.; Pan, B.X. Amygdala Circuit Substrates for Stress Adaptation and Adversity. Biol. Psychiatry 2021, 89, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Krause, S.; Schindowski, K.; Zechel, S.; von Bohlen und Halbach, O. Expression of trkB and trkC receptors and their ligands brain-derived neurotrophic factor and neurotrophin-3 in the murine amygdala. J. Neurosci. Res. 2008, 86, 411–421. [Google Scholar] [CrossRef] [PubMed]
- Mottarlini, F.; Rizzi, B.; Targa, G.; Fumagalli, F.; Caffino, L. Long-lasting BDNF signaling alterations in the amygdala of adolescent female rats exposed to the activity-based anorexia model. Front. Behav. Neurosci. 2022, 16, 1087075. [Google Scholar] [CrossRef] [PubMed]
- Klenotich, S.J.; Dulawa, S.C. The activity-based anorexia mouse model. Methods Mol. Biol. 2012, 829, 377–393. [Google Scholar] [PubMed]
- Xie, X.; Yang, H.; An, J.J.; Houtz, J.; Tan, J.W.; Xu, H.; Liao, G.Y.; Xu, Z.X.; Xu, B. Activation of Anxiogenic Circuits Instigates Resistance to Diet-Induced Obesity via Increased Energy Expenditure. Cell Metab. 2019, 29, 917–931.e914. [Google Scholar] [CrossRef] [PubMed]
- Korte, M.; Carroll, P.; Wolf, E.; Brem, G.; Thoenen, H.; Bonhoeffer, T. Hippocampal long-term potentiation is impaired in mice lacking brain-derived neurotrophic factor. Proc. Natl. Acad. Sci. USA 1995, 92, 8856–8860. [Google Scholar] [CrossRef] [PubMed]
- McAllister, A.K.; Katz, L.C.; Lo, D.C. Opposing roles for endogenous BDNF and NT-3 in regulating cortical dendritic growth. Neuron 1997, 18, 767–778. [Google Scholar] [CrossRef]
- Liao, L.; Pilotte, J.; Xu, T.; Wong, C.C.; Edelman, G.M.; Vanderklish, P.; Yates, J.R., 3rd. BDNF induces widespread changes in synaptic protein content and up-regulates components of the translation machinery: An analysis using high-throughput proteomics. J. Proteome Res. 2007, 6, 1059–1071. [Google Scholar] [CrossRef]
- Shimizu, E.; Hashimoto, K.; Iyo, M. Ethnic difference of the BDNF 196G/A (val66met) polymorphism frequencies: The possibility to explain ethnic mental traits. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2004, 126, 122–123. [Google Scholar] [CrossRef] [PubMed]
| Brain Region | Food Intake | Thermogenesis | Locomotor Activity | Energy Expenditure (EE) | Glucose Balance |
|---|---|---|---|---|---|
| Arcuate nucleus (Arc) | ↑ Activation of TrkB+ neurons during refeeding following fasting period [35] | ||||
| Pariventricular nucleus (PVN) | Depletion of BDNF or TrkB ↑ food intake [36,52] Activation of BDNF+ or TrkB+ neurons ↓ food intake [48,52] | Depletion of BDNF ↓ thermogenesis [36] Activation of BDNF+ neurons ↑ thermogenesis [48] | Depletion of BDNF or TrkB ↓ locomotor activity [36,52] Activation of BDNF+ or TrkB+ neurons ↑ locomotor activity [48,52] | Depletion of BDNF ↓ EE [36] Activation of BDNF+ neurons ↑ EE in females [48] | Depletion of BDNF results in glucose intolerance and hyperinsulinemia [36] |
| Ventromedial hypothalamus (VMH) | Depletion of BDNF or Bdnf-e2 ↑ food intake [21,63] Depletion of Bdnf-e1 ↑ food intake only when socially isolated or on HFD [63] Depletion of astrocytic TrkB.T1 ↑ food intake [64] | Depletion of astrocytic TrkB.T1 ↓ thermogenesis [64] | Depletion of astrocytic TrkB.T1 ↓ locomotor activity [64] | BDNF depletion induces hyperglycemia and hyperinsulinemia [21] Depletion of astrocytic TrkB.T1 results in glucose intolerance [64] | |
| Dorsomedial hypothalamus (DMH) | Activation of TrkB+ neurons ↓ food intake, whereas inhibition of TrkB+ neurons ↑ food intake [19] | Activation of BDNF+ neurons in response to cold temperatures ↑ thermogenesis [87] Activation of TrkB+ neurons ↑ adaptive thermogenesis [86] | Activation of BDNF+ neurons ↑ locomotor activity [87] Activation of TrkB+ neurons ↑ locomotor activity [86] | Activation of BDNF+ neurons ↑ EE [87] Activation of TrkB+ neurons ↑ EE [86] | Depletion of TrkB induces glucose intolerance [19] |
| Lateral hypothalamus (LH) | Depletion of Bdnf-e1 ↓ adaptive thermogenesis [91] TrkB agonist ↑ thermogenesis [91] | ||||
| Preoptic area (POA) | Activation of BDNF/PACAP+ neurons by environmental warmth ↓ thermogenesis [95] | ||||
| Dorsal vagal complex (DVC) | Infusion of BDNF into DVC ↓ food intake [103] Depletion of TrkB+ ↑ food intake [104] | ||||
| Ventral tegmental area (VTA) | Knock down of BDNF ↑ HFD but not chow intake [111] | ||||
| Amygdala | Depletion of BDNF elicits resistance to DIO [117] | Depletion of BDNF ↑ thermogenesis [117] | Depletion of BDNF ↑EE [117] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harvey, T.; Rios, M. The Role of BDNF and TrkB in the Central Control of Energy and Glucose Balance: An Update. Biomolecules 2024, 14, 424. https://doi.org/10.3390/biom14040424
Harvey T, Rios M. The Role of BDNF and TrkB in the Central Control of Energy and Glucose Balance: An Update. Biomolecules. 2024; 14(4):424. https://doi.org/10.3390/biom14040424
Chicago/Turabian StyleHarvey, Theresa, and Maribel Rios. 2024. "The Role of BDNF and TrkB in the Central Control of Energy and Glucose Balance: An Update" Biomolecules 14, no. 4: 424. https://doi.org/10.3390/biom14040424
APA StyleHarvey, T., & Rios, M. (2024). The Role of BDNF and TrkB in the Central Control of Energy and Glucose Balance: An Update. Biomolecules, 14(4), 424. https://doi.org/10.3390/biom14040424






