Contribution of Ascorbate and Glutathione in Endobacteria Bacillus subtilis-Mediated Drought Tolerance in Two Triticum aestivum L. Genotypes Contrasting in Drought Sensitivity
Abstract
:1. Introduction
2. Results
2.1. Growth Attributes
2.2. H2O2 and Lipid Peroxidation (MDA)
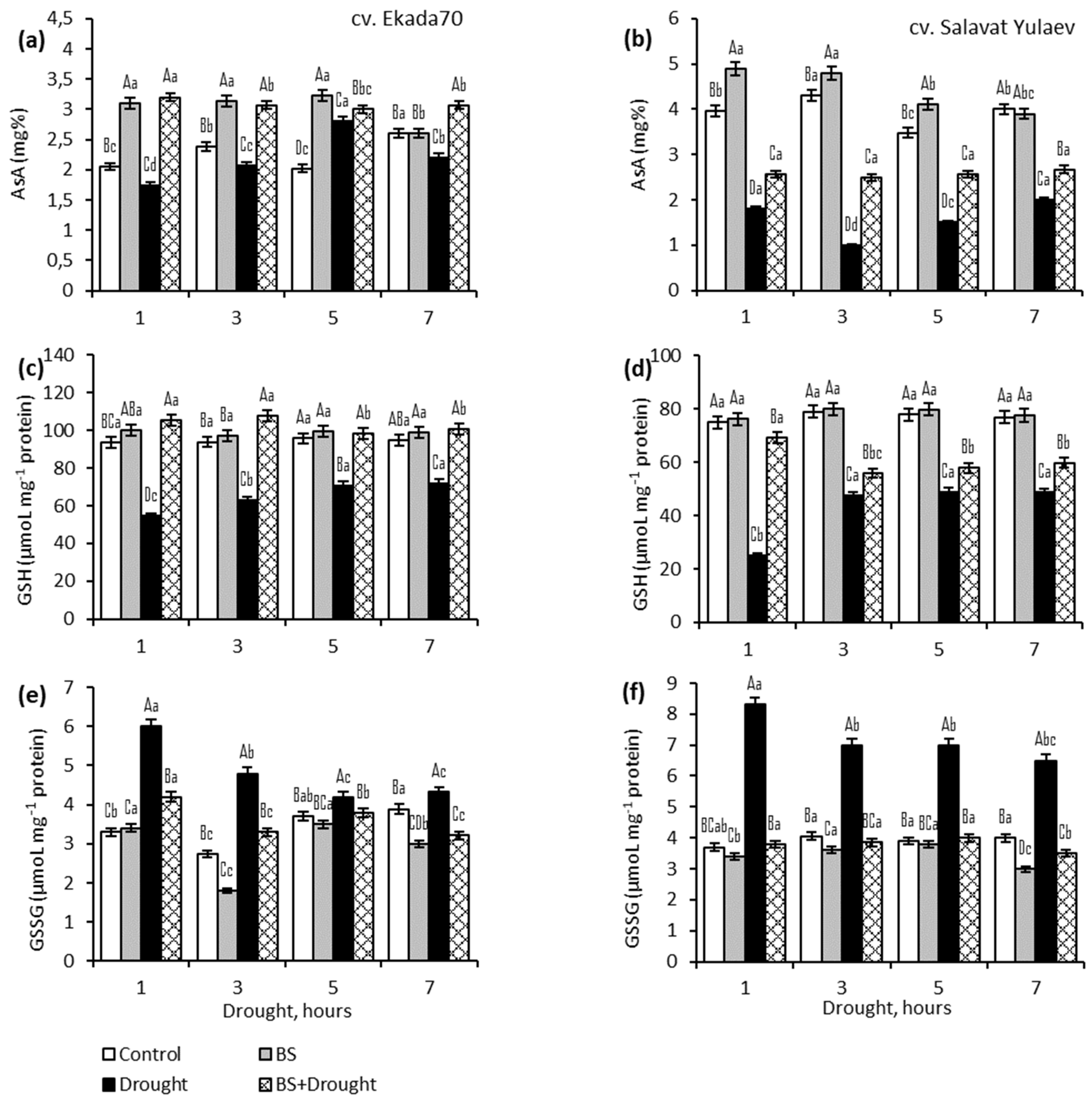
2.3. Ascorbic Acid (AsA) and Glutathione (GSH)
2.4. The Activity of Ascorbate Peroxidase (APX) and Glutathione Reductase (GR)
3. Discussion
4. Materials and Methods
4.1. Bacterial Strain and Inoculum Preparation
4.2. Plant Materials and Growth Conditions
4.3. Growth Parameters
4.4. Malondialdehyde (MDA) and Hydrogen Peroxide (H2O2) Contents
4.5. Measurement of Non-Enzymatic Antioxidants
4.5.1. Total of Reduced Glutathione (GSH) and Oxidized Glutathione (GSSG) Content
4.5.2. Ascorbate (AsA) content
4.6. Measurement of Enzymatic Antioxidants
4.6.1. Ascorbate Peroxidase (APX) Activity
4.6.2. Glutathione Reductase (GR) Activity
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APX | ascorbate peroxidase |
| AsA | ascorbate |
| DS | drought susceptible |
| DT | drought tolerant |
| GR | glutathione reductase |
| GSH | glutathione |
| GSSG | glutathione disulfide (oxidized glutathione) |
| H2O2 | hydrogen peroxide |
| MDA | malondialdehyde |
| PEG | polyethylene glycol |
| PGPB | plant growth-promoting bacteria |
| ROS | reactive oxygen species |
References
- Farooq, M.; Wahid, A.; Kobayashi, N.; Fujita, D.; Basra, S.M.A. Plant Drought Stress: Effects, Mechanisms and Management. Agron. Sustain. Dev. 2009, 29, 185–212. [Google Scholar] [CrossRef] [Green Version]
- FAO. The State of Food and Agriculture. Climate Change, Agriculture and Food Security; FAO: Rome, Italy, 2016; Available online: http://www.fao.org/3/a-i6030e.pdf (accessed on 21 October 2021).
- Asseng, S.; Martre, P.; Maiorano, A.; Rötter, R.P.; O’Leary, G.J.; Fitzgerald, G.J.; Girousse, C.; Motzo, R.; Giunta, F.; Ali Babar, M.; et al. Climate Change Impact and Adaptation for Wheat Protein. Glob. Chang. Biol. 2019, 25, 155–173. [Google Scholar] [CrossRef] [Green Version]
- FAO. Cereal Supply and Demand Brief. 2021. Available online: http://www.fao.org/worldfoodsituation/csdb/ru/ (accessed on 21 October 2021).
- Hasanuzzaman, M.; Nahar, K.; Gill, S.S.; Gill, R.; Fujita, M. Drought Stress Responses in Plants, Oxidative Stress, and Antioxidant Defense. In Climate Change and Plant Abiotic Stress Tolerance; Tuteja, N., Gill, S.S., Eds.; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2014; pp. 209–249. [Google Scholar]
- Hasanuzzaman, M.; Bhuyan, M.H.M.B.; Zulfiqar, F.; Raza, A.; Mohsin, S.M.; Mahmud, J.A.; Fujita, M.; Fotopoulos, V. Reactive Oxygen Species and Antioxidant Defense in Plants under Abiotic Stress: Revisiting the Crucial Role of a Universal Defense Regulator. Antioxidants 2020, 9, 681. [Google Scholar] [CrossRef]
- Lastochkina, O.; Aliniaeifard, S.; Kalhor, M.S.; Yuldashev, R.; Pusenkova, L.; Garipova, S. Plant Growth Promoting Bacteria Biotic Strategy to Cope with Abiotic Stresses in Wheat. In Wheat Production in Changing Environments: Management, Adaptation and Tolerance; Hasanuzzaman, M., Nahar, K., Hossain, A., Eds.; Springer: Singapore, 2019; pp. 579–614. [Google Scholar]
- Sood, G.; Kaushal, R.; Sharma, M. Significance of Inoculation with Bacillus subtilis to Alleviate Drought Stress in Wheat (Triticum aestivum L.). Vegetos 2020, 33, 782–792. [Google Scholar] [CrossRef]
- Goswami, M.; Deka, S. Plant Growth-Promoting Rhizobacteria—Alleviators of Abiotic Stresses in Soil: A Review. Pedosphere 2020, 30, 40–61. [Google Scholar] [CrossRef]
- Rashid, U.; Yasmin, H.; Hassan, M.N.; Naz, R.; Nosheen, A.; Sajjad, M.; Ilyas, N.; Keyani, R.; Jabeen, Z.; Mumtaz, S.; et al. Drought-tolerant Bacillus megaterium Isolated from Semi-Arid Conditions Induces Systemic Tolerance of Wheat Under Drought Conditions. Plant Cell Rep. 2021, 1–21. [Google Scholar] [CrossRef]
- Bokhari, A.; Essack, M.; Lafi, F.F.; Andres-Barrao, C.; Jalal, R.; Alamoudi, S.; Razali, R.; Alzubaidy, H.; Shah, K.H.; Siddique, S.; et al. Bioprospecting Desert Plant Bacillus Endophytic Strains for Their Potential to Enhance Plant Stress Tolerance. Sci. Rep. 2019, 9, 18154. [Google Scholar] [CrossRef]
- Bukhat, S.; Imran, A.; Javaid, S.; Shahid, M.; Majeed, A.; Naqqash, T. Communication of Plants with Microbial World: Exploring the Regulatory Networks for PGPR Mediated Defense Signaling. Microbiol. Res. 2020, 238, 126486. [Google Scholar] [CrossRef]
- Blake, C.; Christensen, M.N.; Kovács, Á. Molecular Aspects of Plant Growth Promotion and Protection by Bacillus subtilis. Mol. Plant-Microbe Interact. 2021, 34, 15–25. [Google Scholar] [CrossRef]
- Lastochkina, O.; Garshina, D.; Ivanov, S.; Yuldashev, R.; Khafizova, R.; Allagulova, C.; Fedorova, K.; Avalbaev, A.; Maslennikova, D.; Bosacchi, M. Seed Priming with Endophytic Bacillus subtilis Modulates Physiological Responses of Two Different Triticum aestivum L. Cultivars Under Drought Stress. Plants 2020, 9, 1810. [Google Scholar] [CrossRef]
- Maslennikova, D.R.; Plotnikov, A.A.; Shakirova, F.M. Comparative Analysis of the Physiological Effect of Nitric Oxide and 6-Benzylaminopurine on the Components of the Glutathione Complex in the Roots of Wheat Seedlings. Agrokhimiya 2019, 3, 37. [Google Scholar]
- Li, Y.; Shi, H.; Zhang, H.; Chen, S. Amelioration of Drought Effects in Wheat and Cucumber by the Combined Application of Super Absorbent Polymer and Potential Biofertilizer. PeerJ 2019, 7, e6073. [Google Scholar] [CrossRef] [PubMed]
- Shukla, N.; Awasthi, R.P.; Rawat, L.; Kumar, J. Biochemical and physiological responses of rice (Oryza sativa L.) as influenced by Trichoderma harzianum under drought stress. Plant Physiol. Biochem. 2012, 54, 78–88. [Google Scholar] [CrossRef]
- Lastochkina, O.; Pusenkova, L.; Yuldashev, R.; Babaev, M.; Garipova, S.; Blagova, D.; Khairullin, R.; Aliniaeifard, S. Effects of Bacillus subtilis on Some Physiological and Biochemical Parameters of Triticum aestivum L. (Wheat) Under Salinity. Plant Physiol. Biochem. 2017, 121, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Puthiyottil, P.; Akkara, Y. Pre treatment with Bacillus subtilis mitigates drought induced photo-oxidative damages in okra by modulating antioxidant system and photochemical activity. Physiol. Mol. Biol. Plants 2021, 27, 945–957. [Google Scholar] [CrossRef]
- Cabane, M.; Afif, D.; Hawkins, S. Lignins and abiotic stresses. In Lignins Biosynthesis, Biodegradation and Bioengineering; Jouanin, L., Lapierre, C., Eds.; Academic Press, Advances in Botanical Research: London, UK, 2012; Volume 61, pp. 219–262. [Google Scholar]
- Lastochkina, O.; Aliniaeifard, S.; Garshina, D.; Garipova, S.; Pusenkova, L.; Allagulova, C.; Fedorova, K.; Baymiev, A.; Koryakov, I.; Sobhani, M. Seed priming with endophytic Bacillus subtilis strain-specifically improves growth of Phaseolus vulgaris plants under normal and salinity conditions and exerts anti-stress effect through induced lignin deposition in roots and decreased oxidative and osmotic damages. J. Plant Physiol. 2021, 263, 153462. [Google Scholar] [CrossRef]
- Kaushal, M. Portraying Rhizobacterial Mechanisms in Drought Tolerance: A Way Forward Toward Sustainable Agriculture. In PGPR Amelioration in Sustainable Agriculture; Singh, A.K., Rumar, A., Singh, P.K., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2019; pp. 195–216. [Google Scholar] [CrossRef]
- Kim, K.; Jang, Y.-J.; Lee, S.-M.; Oh, B.-T.; Chae, J.-C.; Lee, K.-J. Alleviation of Salt Stress by Enterobacter sp. EJ01 in Tomato and Arabidopsis is Accompanied by Up-Regulation of Conserved Salinity Responsive Factors in Plants. Mol. Cell 2014, 37, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Ma, Z.; Zhu, L.; Xia, X.; Xie, Y.; Zhu, J.; Wang, J. Rhizobacterial Strain Bacillus megaterium BOFC15 Induces Cellular Polyamine Changes That Improve Plant Growth and Drought Resistance. Int. J. Mol. Sci. 2016, 17, 976. [Google Scholar] [CrossRef]
- Abd El-Daim, I.A.; Bejai, S.; Meijer, J. Bacillus velezensis 5113 Induced Metabolic and Molecular Reprogramming During Abiotic Stress Tolerance in Wheat. Sci. Rep. 2019, 9, 16282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasim, W.A.; Osman, M.E.; Omar, M.N.; Abd El-Daim, I.A.; Bejai, S.; Meijer, J. Control of Drought Stress in Wheat Using Plant-Growth-Promoting Bacteria. J. Plant Growth Regul. 2013, 32, 122–130. [Google Scholar] [CrossRef]
- Netrusov, A.I.; Egorova, M.A.; Zakharchuk, L.M. Praktikum po Mikrobiologii (A Practical Course in Microbiology); Izdat. Tsentr Akademiya: Moscow, Russia, 2005; 608p. [Google Scholar]
- Mokronosova, A.T. Small Workshop on Plant Physiology; Moscow State University: Moscow, Russia, 1994; 184p. [Google Scholar]
- Bindschedler, L.V.; Minibaeva, F.; Gardner, S.L.; Gerrish, C.; Davies, D.R.; Bolwell, G.P. Early Signalling Events in the Apoplastic Oxidative Burst in Suspension Cultured French Bean Cells Involve cAMP and Ca2+. New Phytol. 2001, 151, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Hissin, P.J.; Hilf, R.A. A Fluorometric Method for Determination of Oxidize and Reduced Glutathione in Tissues. Anal. Biochem. 1976, 74, 214–226. [Google Scholar] [CrossRef]
- Pleshkov, B.P. Workshop on Plant Biochemistry; Izdat. Colos: Moscow, Russia, 1976; 256p. [Google Scholar]
- Verma, S.; Dubey, R.S. Lead Toxicity Induces Lipid Peroxidation and Alters the Activities of Antioxidant Enzymes in Growing Rice Plants. Plant Sci. 2003, 164, 645–655. [Google Scholar] [CrossRef]
- Rao, M.V.; Paliyath, G.; Ormrod, D.P. Ultraviolet-B- and Ozone-induced Biochemical Changes in Antioxidant Enzymes of Arabidopsis Thaliana. Plant Physiol. 1996, 110, 125–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradford, M.M. A Rapid and Sensitive Methods for Quantitation of Microgram Quantities of Protein Utilizing the Principle of Protein Dye Binding. Anal. Biochem. 1976, 74, 248–254. [Google Scholar] [CrossRef]


| Growth Parameters | 0% PEG (Non-Stressed) | 12% PEG (Stressed) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Control (Non-Inoculated) | BS | Control (Non-Inoculated) | BS | ||||||
| DT | DS | DT | DS | DT | DS | DT | DS | ||
| Seed germination (%) | 97 ± 1 | 96 ± 2 | 99 ± 1 | 97 ± 1 | 57 ± 2 | 39 ± 3 | 74 ± 2 * | 42 ± 2 | |
| Lenght (cm) | Roots | 2.40 ± 0.12 | 3.42 ± 0.09 | 2.59 ± 0.10 * | 3.54 ± 0.14 * | 2.16 ± 0.10 | 3.25 ± 0.09 | 2.41 ± 0.09 * | 3.51 ± 0.09 * |
| Shoots | 3.05 ± 0.05 | 3.60 ± 0.07 | 3.26 ± 0.05 * | 3.65 ± 0.06 | 2.44 ± 0.05 | 3.29 ± 0.07 | 3.24 ± 0.05 * | 3.57 ± 0.06 * | |
| FW (g) | Roots | 0.60 ± 0.01 | 0.99 ± 0.02 | 0.65 ± 0.03 | 0.85 ± 0.09 | 0.53 ± 0.01 | 0.76 ± 0.02 | 0.56 ± 0.01 | 0.71 ± 0.06 |
| Shoots | 0.66 ± 0.02 | 0.94 ± 0.05 | 0.63 ± 0.02 | 1.03 ± 0.02 * | 0.38 ± 0.01 | 0.43 ± 0.04 | 0.41 ± 0.06 | 0.43 ± 0.008 | |
| DW (g) | Roots | 0.065 ± 0.001 | 0.08 ± 0.009 | 0.059 ± 0.007 | 0.07 ± 0.01 | 0.06 ± 0.003 | 0.17 ± 0.03 | 0.062 ± 0.02 | 0.16 ± 0.008 |
| Shoots | 0.050 ± 0.002 | 0.08 ± 0.007 | 0.057 ± 0.003 | 0.09 ± 0.008 | 0.04 ± 0.007 | 0.09 ± 0.01 | 0.05 ± 0.006 | 0.09 ± 0.005 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maslennikova, D.; Lastochkina, O. Contribution of Ascorbate and Glutathione in Endobacteria Bacillus subtilis-Mediated Drought Tolerance in Two Triticum aestivum L. Genotypes Contrasting in Drought Sensitivity. Plants 2021, 10, 2557. https://doi.org/10.3390/plants10122557
Maslennikova D, Lastochkina O. Contribution of Ascorbate and Glutathione in Endobacteria Bacillus subtilis-Mediated Drought Tolerance in Two Triticum aestivum L. Genotypes Contrasting in Drought Sensitivity. Plants. 2021; 10(12):2557. https://doi.org/10.3390/plants10122557
Chicago/Turabian StyleMaslennikova, Dilara, and Oksana Lastochkina. 2021. "Contribution of Ascorbate and Glutathione in Endobacteria Bacillus subtilis-Mediated Drought Tolerance in Two Triticum aestivum L. Genotypes Contrasting in Drought Sensitivity" Plants 10, no. 12: 2557. https://doi.org/10.3390/plants10122557
APA StyleMaslennikova, D., & Lastochkina, O. (2021). Contribution of Ascorbate and Glutathione in Endobacteria Bacillus subtilis-Mediated Drought Tolerance in Two Triticum aestivum L. Genotypes Contrasting in Drought Sensitivity. Plants, 10(12), 2557. https://doi.org/10.3390/plants10122557







