Chemical Profiling and Biological Evaluation of Nepeta baytopii Extracts and Essential Oil: An Endemic Plant from Turkey
Abstract
:1. Introduction
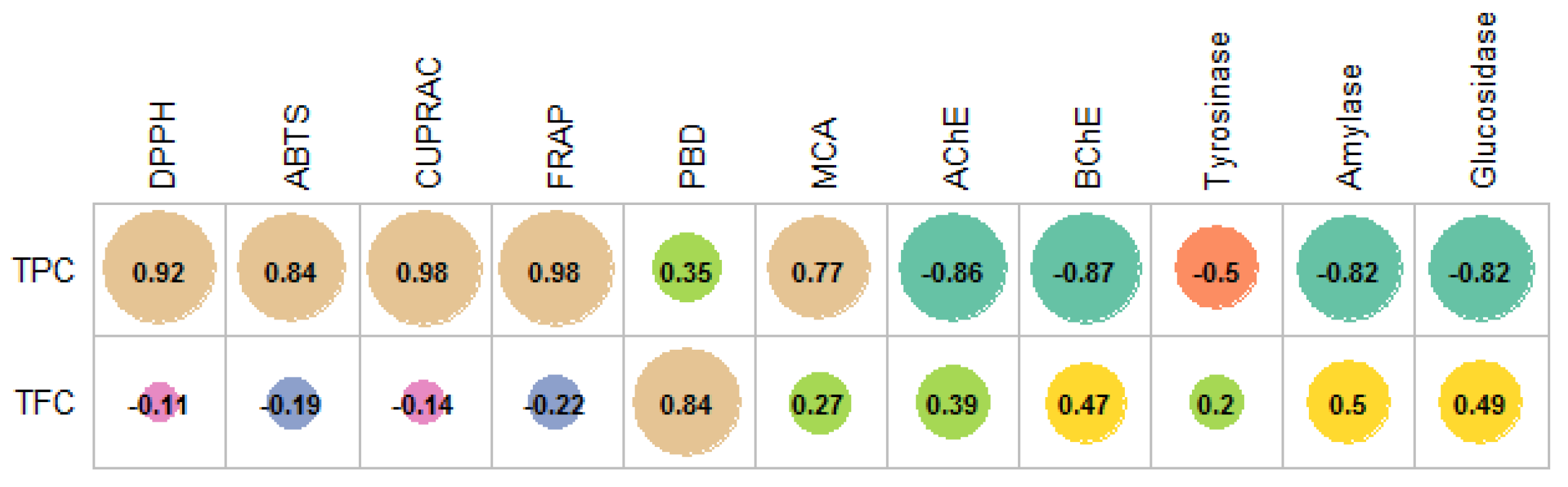
2. Results and Discussion
3. Materials and Methods
3.1. Plant Material
3.2. Extraction
3.3. UHPLC-MS Analysis
3.4. Essential Oil Components’ Analyses
3.5. Total Phenolic and Flavonoid Content
3.6. Determination of Antioxidant and Enzyme-Inhibitory Effects
3.7. Cell Culture
3.8. Determination of Cellular Viability and Selectivity
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumari, P.; Ujala; Bhargava, B. Phytochemicals from edible flowers: Opening a new arena for healthy lifestyle. J. Funct. Foods 2021, 78, 104375. [Google Scholar] [CrossRef]
- Dincer, Y.; Yuksel, S. Antiobesity effects of phytochemicals from an epigenetic perspective. Nutrition 2021, 84, 111119. [Google Scholar] [CrossRef]
- Zhang, L.; Virgous, C.; Si, H. Synergistic anti-inflammatory effects and mechanisms of combined phytochemicals. J. Nutr. Biochem. 2019, 69, 19–30. [Google Scholar] [CrossRef]
- Borrelli, F.; Borbone, N.; Capasso, R.; Montesano, D.; De Marino, S.; Aviello, G.; Aprea, G.; Masone, S.; Izzo, A.A. Potent relaxant effect of a Celastrus paniculatus extract in the rat and human ileum. J. Ethnopharmacol. 2009, 122, 434–438. [Google Scholar] [CrossRef]
- Borbone, N.; Borrelli, F.; Montesano, D.; Izzo, A.A.; De Marino, S.; Capasso, R.; Zollo, F. Identification of a New Sesquiterpene Polyol Ester from Celastrus paniculatus. Planta Med. 2007, 73, 792–794. [Google Scholar] [CrossRef]
- Montesano, D.; Rocchetti, G.; Putnik, P.; Lucini, L. Bioactive profile of pumpkin: An overview on terpenoids and their health-promoting properties. Curr. Opin. Food Sci. 2018, 22, 81–87. [Google Scholar] [CrossRef]
- Tamokou, J.; Mbaveng, A.; Kuete, V. Antimicrobial activities of African medicinal spices and vegetables. In Medicinal Spices and Vegetables from Africa; Elsevier: Amsterdam, The Netherlands, 2017; pp. 207–237. [Google Scholar]
- Tepe, B.; Daferera, D.; Tepe, A.-S.; Polissiou, M.; Sokmen, A. Antioxidant activity of the essential oil and various extracts of Nepeta flavida Hub.-Mor. from Turkey. Food Chem. 2007, 103, 1358–1364. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Ceylan, O.; Targan, S.; Ćavar Zeljković, S. Chemical composition and biological activities of the essential oils of two endemic Nepeta species. Ind. Crop. Prod. 2018, 125, 5–8. [Google Scholar] [CrossRef]
- Demirci, S.; Özhatay, N. An ethnobotanical study in Kahramanmaraş (Turkey); wild plants used for medicinal purpose in Andirin, Kahramanmaraş. Turk. J. Pharm. Sci. 2012, 9, 75–92. [Google Scholar]
- Mükemre, M.; Behçet, L.; Çakılcıoğlu, U. Ethnobotanical study on medicinal plants in villages of Çatak (Van-Turkey). J. Ethnopharmacol. 2015, 166, 361–374. [Google Scholar] [CrossRef]
- Everest, A.; Ozturk, E. Focusing on the ethnobotanical uses of plants in Mersin and Adana provinces (Turkey). J. Ethnobiol. Ethnomedicine 2005, 1, 6. [Google Scholar] [CrossRef] [Green Version]
- Mumcu, Ü.; Korkmaz, H. Ethnobotanical uses of alien and native plant species of Yeşilırmak Delta, Samsun, Turkey. Türk Biyol. Derg. 2018, 31, 102–113. [Google Scholar]
- Sharma, A.; Cooper, R.; Bhardwaj, G.; Cannoo, D.S. The genus Nepeta: Traditional uses, phytochemicals and pharmacological properties. J. Ethnopharmacol. 2021, 268, 25. [Google Scholar] [CrossRef]
- Salehi, B.; Valussi, M.; Jugran, A.K.; Martorell, M.; Ramírez-Alarcón, K.; Stojanović-Radić, Z.Z.; Antolak, H.; Kręgiel, D.; Mileski, K.S.; Sharifi-Rad, M.; et al. Nepeta species: From farm to food applications and phytotherapy. Trends Food Sci. Technol. 2018, 80, 104–122. [Google Scholar] [CrossRef]
- Kaska, A.; Çiçek, M.; Mammadov, R. Biological activities, phenolic constituents and mineral element analysis of two endemic medicinal plants from Turkey: Nepeta italica subsp. cadmea and Teucrium sandrasicum. S. Afr. J. Bot. 2019, 124, 63–70. [Google Scholar] [CrossRef]
- Çelik, G.; Kılıç, G.; Kanbolat, Ş.; Şener, S.Ö.; Karaköse, M.; Yaylı, N.; Karaoğlu, Ş.A. Biological activity, and volatile and phenolic compounds from five Lamiaceae species. Flavour Fragr. J. 2021, 36, 223–232. [Google Scholar] [CrossRef]
- Kaska, A.; Deniz, N.G.; Çiçek, M.; Mammadov, R. Evaluation of Antioxidant Properties, Phenolic Compounds, Anthelmintic, and Cytotoxic Activities of Various Extracts Isolated from Nepeta cadmea: An Endemic Plant for Turkey. J. Food Sci. 2018, 83, 1552–1559. [Google Scholar] [CrossRef] [PubMed]
- Yayli, B.; Tosun, G.; Karakse, M.; Renda, G.; Yayli, N. SPME/GC-MS Analysis of Volatile Organic Compounds from three Lamiaceae Species (Nepeta conferta Hedge & Lamond, Origanum onites L. and Satureja cuneifolia Ten.) Growing in Turkey. Asian J. Chem. 2014, 26, 2541–2544. [Google Scholar] [CrossRef]
- Dirmenci, T.; Yildiz, B.; Tümen, G. Threatened categories of four Nepeta L. (Lamiaceae) species endemic to the East Anatolia. Turk. J. Bot. 2004, 28, 221–226. [Google Scholar]
- Kilic, O.; Behcet, L.; Bagci, E. Essential Oil Compounds of Three Nepeta L. Taxa from Turkey and Their Chemotaxonomy. Asian J. Chem. 2013, 25, 8181–8183. [Google Scholar] [CrossRef]
- Dienaitė, L.; Pukalskienė, M.; Matias, A.A.; Pereira, C.V.; Pukalskas, A.; Venskutonis, P.R. Valorization of six Nepeta species by assessing the antioxidant potential, phytochemical composition and bioactivity of their extracts in cell cultures. J. Funct. Foods 2018, 45, 512–522. [Google Scholar] [CrossRef]
- Gökbulut, A.; Yilmaz, G. Nepeta humilis Bentham: First evaluation of phenolic profile and radical scavenging potential. J. Res. Pharm. 2020, 24, 901–907. [Google Scholar] [CrossRef]
- Teber, I.; Bursal, E. Phenolic Compounds and Antioxidant Activity of Nepeta nuda subsp. Albiflora. Int. Lett. Nat. Sci. 2020, 79, 1–8. [Google Scholar] [CrossRef]
- Işcan, G.; Göger, F.; Demirci, B.; Köse, Y.B. Chemical composition and biological activity of Nepeta cilicica. Bangladesh J. Pharmacol. 2017, 12, 204–209. [Google Scholar] [CrossRef] [Green Version]
- Tundis, R.; Nadjafi, F.; Menichini, F. Angiotensin-Converting Enzyme Inhibitory Activity and Antioxidant Properties of Nepeta crassifolia Boiss & Buhse and Nepeta binaludensis Jamzad. Phytother. Res. 2013, 27, 572–580. [Google Scholar] [CrossRef]
- Guvenalp, Z.; Ozbek, H.; Kuruuzum-Uz, A.; Kazaz, C.; Demirezer, L.O. Secondary metabolites from Nepeta heliotropifolia. Turk. J. Chem. 2009, 33, 667–675. [Google Scholar]
- Russo, E.B.; Marcu, J. Chapter Three—Cannabis Pharmacology: The Usual Suspects and a Few Promising Leads. In Advances in Pharmacology; Kendall, D., Alexander, S.P.H., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 80, pp. 67–134. [Google Scholar]
- Kaya, A.; Demirci, B.; Baser, K.H.C. Micromorphology of glandular trichomes of Nepeta congesta Fisch. & Mey. var. congesta (Lamiaceae) and chemical analysis of the essential oils. S. Afr. J. Bot. 2007, 73, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Bhardwaj, G.; Cannoo, D.S. Antioxidant potential, GC/MS and headspace GC/MS analysis of essential oils isolated from the roots, stems and aerial parts of Nepeta leucophylla. Biocatal. Agric. Biotechnol. 2021, 32, 101950. [Google Scholar] [CrossRef]
- Yang, S.; Bai, M.; Yang, J.; Yuan, Y.; Zhang, Y.; Qin, J.; Kuang, Y.; Sampietro, D.A. Chemical composition and larvicidal activity of essential oils from Peganum harmala, Nepeta cataria and Phellodendron amurense against Aedes aegypti (Diptera: Culicidae). Saudi Pharm. J. 2020, 28, 560–564. [Google Scholar] [CrossRef]
- Ali, L.; Khan, S.; Nazir, M.; Raiz, N.; Naz, S.; Zengin, G.; Mukhtar, M.; Parveen, S.; Shazmeen, N.; Saleem, M.; et al. Chemical profiling, in vitro biological activities and Pearson correlation between phenolic contents and antioxidant activities of Caragana brachyantha Rech.f. S. Afr. J. Bot. 2021, 140, 189–193. [Google Scholar] [CrossRef]
- Hou, J.; Liang, L.; Su, M.; Yang, T.; Mao, X.; Wang, Y. Variations in phenolic acids and antioxidant activity of navel orange at different growth stages. Food Chem. 2021, 360, 129980. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Li, W.; You, B.; Yang, S.; Xian, W.; Deng, Y.; Huang, W.; Yang, R. Phenolic profiles, bioaccessibility and antioxidant activity of plum (Prunus Salicina Lindl). Food Res. Int. 2021, 143, 110300. [Google Scholar] [CrossRef] [PubMed]
- Bibi Sadeer, N.; Montesano, D.; Albrizio, S.; Zengin, G.; Mahomoodally, M.F. The Versatility of Antioxidant Assays in Food Science and Safety—Chemistry, Applications, Strengths, and Limitations. Antioxidants 2020, 9, 709. [Google Scholar] [CrossRef] [PubMed]
- Lim, Y.P.; Pang, S.F.; Yusoff, M.M.; Abdul Mudalip, S.K.; Gimbun, J. Correlation between the extraction yield of mangiferin to the antioxidant activity, total phenolic and total flavonoid content of Phaleria macrocarpa fruits. J. Appl. Res. Med. Aromat. Plants 2019, 14, 100224. [Google Scholar] [CrossRef]
- Dong, X.; Hu, Y.; Li, Y.; Zhou, Z. The maturity degree, phenolic compounds and antioxidant activity of Eureka lemon [Citrus limon (L.) Burm. f.]: A negative correlation between total phenolic content, antioxidant capacity and soluble solid content. Sci. Hortic. 2019, 243, 281–289. [Google Scholar] [CrossRef]
- Hadi, N.; Sefidkon, F.; Shojaeiyan, A.; Šiler, B.; Jafari, A.-A.; Aničić, N.; Mišić, D. Phenolics’ composition in four endemic Nepeta species from Iran cultivated under experimental field conditions: The possibility of the exploitation of Nepeta germplasm. Ind. Crop. Prod. 2017, 95, 475–484. [Google Scholar] [CrossRef]
- Žugić, A.; Đorđević, S.; Arsić, I.; Marković, G.; Živković, J.; Jovanović, S.; Tadić, V. Antioxidant activity and phenolic compounds in 10 selected herbs from Vrujci Spa, Serbia. Ind. Crop. Prod. 2014, 52, 519–527. [Google Scholar] [CrossRef]
- Mohammed, A.B.A.; Yagi, S.; Tzanova, T.; Schohn, H.; Abdelgadir, H.; Stefanucci, A.; Mollica, A.; Mahomoodally, M.F.; Adlan, T.A.; Zengin, G. Chemical profile, antiproliferative, antioxidant and enzyme inhibition activities of Ocimum basilicum L. and Pulicaria undulata (L.) C.A. Mey. grown in Sudan. S. Afr. J. Bot. 2020, 132, 403–409. [Google Scholar] [CrossRef]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Dragićević, M.; Stupar, A.; Uysal, A.; Şenkardes, I.; Sinan, K.I.; Picot-Allain, M.C.N.; Ak, G.; et al. UHPLC-LTQ OrbiTrap MS analysis and biological properties of Origanum vulgare subsp. viridulum obtained by different extraction methods. Ind. Crop. Prod. 2020, 154, 112747. [Google Scholar] [CrossRef]
- Zengin, G.; Atasagun, B.; Zakariyyah Aumeeruddy, M.; Saleem, H.; Mollica, A.; Babak Bahadori, M.; Mahomoodally, M.F. Phenolic profiling and in vitro biological properties of two Lamiaceae species (Salvia modesta and Thymus argaeus): A comprehensive evaluation. Ind. Crop. Prod. 2019, 128, 308–314. [Google Scholar] [CrossRef]
- Toublet, F.-X.; Lalut, J.; Hatat, B.; Lecoutey, C.; Davis, A.; Since, M.; Corvaisier, S.; Freret, T.; Sopková-de Oliveira Santos, J.; Claeysen, S.; et al. Pleiotropic prodrugs: Design of a dual butyrylcholinesterase inhibitor and 5-HT6 receptor antagonist with therapeutic interest in Alzheimer’s disease. Eur. J. Med. Chem. 2021, 210, 113059. [Google Scholar] [CrossRef]
- Cespedes, C.L.; Balbontin, C.; Avila, J.G.; Dominguez, M.; Alarcon, J.; Paz, C.; Burgos, V.; Ortiz, L.; Peñaloza-Castro, I.; Seigler, D.S.; et al. Inhibition on cholinesterase and tyrosinase by alkaloids and phenolics from Aristotelia chilensis leaves. Food Chem. Toxicol. 2017, 109, 984–995. [Google Scholar] [CrossRef]
- Sun, L.; Wang, Y.; Miao, M. Inhibition of α-amylase by polyphenolic compounds: Substrate digestion, binding interactions and nutritional intervention. Trends Food Sci. Technol. 2020, 104, 190–207. [Google Scholar] [CrossRef]
- Nasr Bouzaiene, N.; Chaabane, F.; Sassi, A.; Chekir-Ghedira, L.; Ghedira, K. Effect of apigenin-7-glucoside, genkwanin and naringenin on tyrosinase activity and melanin synthesis in B16F10 melanoma cells. Life Sci. 2016, 144, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Fan, M.; Ding, H.; Zhang, G.; Hu, X.; Gong, D. Relationships of dietary flavonoid structure with its tyrosinase inhibitory activity and affinity. LWT 2019, 107, 25–34. [Google Scholar] [CrossRef]
- Orhan, I.E.; Jedrejek, D.; Senol, F.S.; Salmas, R.E.; Durdagi, S.; Kowalska, I.; Pecio, L.; Oleszek, W. Molecular modeling and in vitro approaches towards cholinesterase inhibitory effect of some natural xanthohumol, naringenin, and acyl phloroglucinol derivatives. Phytomedicine 2018, 42, 25–33. [Google Scholar] [CrossRef]
- Amin, I.; Majid, S.; Farooq, A.; Wani, H.A.; Noor, F.; Khan, R.; Shakeel, S.; Bhat, S.A.; Ahmad, A.; Madkhali, H.; et al. Chapter 8—Naringenin (4,5,7-trihydroxyflavanone) as a potent neuroprotective agent: From chemistry to medicine. In Studies in Natural Products Chemistry; Atta Ur, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 65, pp. 271–300. [Google Scholar]
- Kwon, Y. Luteolin as a potential preventive and therapeutic candidate for Alzheimer’s disease. Exp. Gerontol. 2017, 95, 39–43. [Google Scholar] [CrossRef]
- Martinez-Gonzalez, A.I.; Díaz-Sánchez, Á.G.; de la Rosa, L.A.; Bustos-Jaimes, I.; Alvarez-Parrilla, E. Inhibition of α-amylase by flavonoids: Structure activity relationship (SAR). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 206, 437–447. [Google Scholar] [CrossRef]
- Zheng, Y.; Yang, W.; Sun, W.; Chen, S.; Liu, D.; Kong, X.; Tian, J.; Ye, X. Inhibition of porcine pancreatic α-amylase activity by chlorogenic acid. J. Funct. Foods 2020, 64, 103587. [Google Scholar] [CrossRef]
- Agunloye, O.M.; Oboh, G.; Ademiluyi, A.O.; Ademosun, A.O.; Akindahunsi, A.A.; Oyagbemi, A.A.; Omobowale, T.O.; Ajibade, T.O.; Adedapo, A.A. Cardio-protective and antioxidant properties of caffeic acid and chlorogenic acid: Mechanistic role of angiotensin converting enzyme, cholinesterase and arginase activities in cyclosporine induced hypertensive rats. Biomed. Pharmacother. 2019, 109, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Delerue, T.; Fátima Barroso, M.; Dias-Teixeira, M.; Figueiredo-González, M.; Delerue-Matos, C.; Grosso, C. Interactions between Ginkgo biloba L. and Scutellaria baicalensis Georgi in multicomponent mixtures towards cholinesterase inhibition and ROS scavenging. Food Res. Int. 2021, 140, 109857. [Google Scholar] [CrossRef]
- Sarikurkcu, C.; Eskici, M.; Karanfil, A.; Tepe, B. Phenolic profile, enzyme inhibitory and antioxidant activities of two endemic Nepeta species: Nepeta nuda subsp. glandulifera and N. cadmea. S. Afr. J. Bot. 2019, 120, 298–301. [Google Scholar] [CrossRef]
- Sitarek, P.; Merecz-Sadowska, A.; Śliwiński, T.; Zajdel, R.; Kowalczyk, T. An In Vitro Evaluation of the Molecular Mechanisms of Action of Medical Plants from the Lamiaceae Family as Effective Sources of Active Compounds against Human Cancer Cell Lines. Cancers 2020, 12, 2957. [Google Scholar] [CrossRef]
- Mouhid, L.; Gómez de Cedrón, M.; Vargas, T.; García-Carrascosa, E.; Herranz, N.; García-Risco, M.; Reglero, G.; Fornari, T.; Ramírez de Molina, A. Identification of antitumoral agents against human pancreatic cancer cells from Asteraceae and Lamiaceae plant extracts. BMC Complementary Altern. Med. 2018, 18, 254. [Google Scholar] [CrossRef]
- Srancikova, A.; Horváthová, E.; Kozics, K. Biological effects of four frequently used medicinal plants of Lamiaceae. Neoplasma 2013, 60, 585–597. [Google Scholar] [CrossRef] [Green Version]
- Yao, M.; Menda, Y.; Bayouth, J.E. Chapter 13—Melanoma. In PET-CT in Radiotherapy Treatment Planning; Paulino, A.C., Teh, B.S., Eds.; Elsevier: Philadelphia, PA, USA, 2008; pp. 204–215. [Google Scholar]
- Ashrafi, B.; Rashidipour, M.; Gholami, E.; Sattari, E.; Marzban, A.; Kheirandish, F.; Khaksarian, M.; Taherikalani, M.; Soroush, S. Investigation of the phytochemicals and bioactivity potential of essential oil from Nepeta curvidens Boiss. & Balansa. S. Afr. J. Bot. 2020, 135, 109–116. [Google Scholar] [CrossRef]
- Jaradat, N.; Al-Maharik, N.; Abdallah, S.; Shawahna, R.; Mousa, A.; Qtishat, A. Nepeta curviflora essential oil: Phytochemical composition, antioxidant, anti-proliferative and anti-migratory efficacy against cervical cancer cells, and α-glucosidase, α-amylase and porcine pancreatic lipase inhibitory activities. Ind. Crop. Prod. 2020, 158, 112946. [Google Scholar] [CrossRef]
- Zengin, G.; Uysal, A.; Diuzheva, A.; Gunes, E.; Jekő, J.; Cziáky, Z.; Picot-Allain, C.M.N.; Mahomoodally, M.F. Characterization of phytochemical components of Ferula halophila extracts using HPLC-MS/MS and their pharmacological potentials: A multi-functional insight. J. Pharm. Biomed. Anal. 2018, 160, 374–382. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Mollica, A.; Stefanucci, A.; Aumeeruddy, M.Z.; Poorneeka, R.; Zengin, G. Volatile components, pharmacological profile, and computational studies of essential oil from Aegle marmelos (Bael) leaves: A functional approach. Ind. Crop. Prod. 2018, 126, 13–21. [Google Scholar] [CrossRef]
- Zengin, G.; Sarıkürkçü, C.; Aktümsek, A.; Ceylan, R. Antioxidant Potential and Inhibition of Key Enzymes Linked to Alzheimer’s Diseases and Diabetes Mellitus by Monoterpene-Rich Essential Oil from Sideritis galatica Bornm. Endemic to Turkey. Rec. Nat. Prod. 2016, 10, 195–206. [Google Scholar]
- Slinkard, K.; Singleton, V.L. Total phenol analysis: Automation and comparison with manual methods. Am. J. Enol. Vitic. 1977, 28, 49–55. [Google Scholar]
- Zengin, G.; Nithiyanantham, S.; Locatelli, M.; Ceylan, R.; Uysal, S.; Aktumsek, A.; Selvi, P.K.; Maskovic, P. Screening of in vitro antioxidant and enzyme inhibitory activities of different extracts from two uninvestigated wild plants: Centranthus longiflorus subsp. longiflorus and Cerinthe minor subsp. auriculata. Eur. J. Integr. Med. 2016, 8, 286–292. [Google Scholar] [CrossRef]
- Uysal, S.; Zengin, G.; Locatelli, M.; Bahadori, M.B.; Mocan, A.; Bellagamba, G.; De Luca, E.; Mollica, A.; Aktumsek, A. Cytotoxic and Enzyme Inhibitory Potential of Two Potentilla species (P. speciosa L. and P. reptans Willd.) and Their Chemical Composition. Front. Pharmacol. 2017, 8, 290. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.J.; Neves, V.; Martins, A.; Rauter, A.P.; Neng, N.R.; Nogueira, J.M.; Varela, J.; Barreira, L.; Custódio, L. In vitro antioxidant and anti-inflammatory properties of Limonium algarvense flowers’ infusions and decoctions: A comparison with green tea (Camellia sinensis). Food Chem. 2016, 200, 322–329. [Google Scholar] [CrossRef]
| Extracts | TPC (mg GAE/g) | TFC (mg RE/g) |
|---|---|---|
| n-Hexane | 13.23 ± 0.21 * e | 7.77 ± 0.07 e |
| Ethyl acetate | 19.57 ± 0.24 d | 27.02 ± 0.60 a |
| Methanol | 33.81 ± 0.22 c | 23.78 ± 0.87 b |
| Water/methanol | 41.25 ± 0.18 b | 10.61 ± 0.54 d |
| Water | 50.30 ± 0.13 a | 13.48 ± 0.18 c |
| Essential oil | nt | nt |
| No. | Name | Formula | Rt | [M + H]+ | [M − H]− | Fragment 1 | Fragment 2 | Fragment 3 | Fragment 4 | Fragment 5 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Quinic acid | C7H12O6 | 1.23 | 191.06 | 173.04 | 171.03 | 127.04 | 93.03 | 85.03 | |
| 2 | Pantothenic acid | C9H17NO5 | 6.13 | 220.12 | 202.11 | 184.10 | 174.11 | 116.03 | 90.06 | |
| 3 | Caftaric acid (2-O-Caffeoyltartaric acid) | C13H12O9 | 8.54 | 311.04 | 179.03 | 149.01 | 135.04 | 87.01 | ||
| 4 | Neochlorogenic acid (5-O-Caffeoylquinic acid) | C16H18O9 | 10.11 | 355.10 | 163.04 | 145.03 | 135.04 | 117.03 | 89.04 | |
| 5 | Unidentified iridoid | C16H24O9 | 13.14 | 405.14 | 359.14 | 197.08 | 179.07 | 153.05 | 71.01 | |
| 6 | Salicylic acid-O-hexoside | C13H16O8 | 13.50 | 299.08 | 137.02 | 113.02 | 93.03 | 85.03 | 71.01 | |
| 7 | Mussaenosidic acid or isomer | C16H24O10 | 13.57 | 375.13 | 213.08 | 169.09 | 151.08 | 125.06 | 107.05 | |
| 8 | Kynurenic acid | C10H7NO3 | 13.80 | 190.05 | 162.06 | 144.04 | 116.05 | 89.04 | ||
| 9 1 | Chlorogenic acid (3-O-Caffeoylquinic acid) | C16H18O9 | 14.84 | 355.10 | 163.04 | 145.03 | 135.04 | 117.03 | 89.04 | |
| 10 | Fertaric acid (2-O-Feruloyltartaric acid) | C14H14O9 | 14.85 | 325.06 | 193.05 | 178.03 | 149.06 | 134.04 | 87.01 | |
| 11 | Caffeic acid | C9H8O4 | 15.15 | 179.03 | 135.04 | 107.05 | ||||
| 12 | Benzofuranecarbaldehyde | C9H6O2 | 15.53 | 147.04 | 119.05 | 91.05 | 65.04 | |||
| 13 | Cryptochlorogenic acid (4-O-Caffeoylquinic acid) | C16H18O9 | 16.08 | 355.10 | 163.04 | 145.03 | 135.04 | 117.03 | 89.04 | |
| 14 | 5-O-(4-Coumaroyl)quinic acid | C16H18O8 | 17.40 | 337.09 | 191.06 | 173.04 | 163.04 | 119.05 | 93.03 | |
| 15 | 4-O-(4-Coumaroyl)quinic acid | C16H18O8 | 18.03 | 337.09 | 191.06 | 173.04 | 163.04 | 119.05 | 93.03 | |
| 16 | Phaselic acid (2-O-Caffeoylmalic acid) | C13H12O8 | 18.62 | 295.05 | 179.03 | 135.04 | 133.01 | 115.00 | 71.01 | |
| 17 | Loliolide | C11H16O3 | 20.01 | 197.12 | 179.11 | 161.10 | 135.12 | 133.10 | 107.09 | |
| 18 | Eriodictyol-O-hexoside | C21H22O11 | 20.76 | 449.11 | 287.06 | 151.00 | 135.04 | 107.01 | 83.01 | |
| 19 | 7-Deoxyloganic acid isomer | C16H24O9 | 20.95 | 359.13 | 197.08 | 153.09 | 135.08 | 109.06 | 89.02 | |
| 20 | Luteolin-O-hexosylglucuronide | C27H28O17 | 21.44 | 623.12 | 285.04 | 217.05 | 199.04 | 175.04 | 133.03 | |
| 21 | Luteolin-7-O-glucuronide | C21H18O12 | 22.75 | 461.07 | 285.04 | 217.05 | 199.04 | 175.04 | 133.03 | |
| 22 | Luteolin-7-O-glucoside (Cynaroside) | C21H20O11 | 22.86 | 447.09 | 327.05 | 285.04 | 284.03 | 256.04 | 151.00 | |
| 23 | Apigenin-O-hexosylglucuronide | C27H28O16 | 22.97 | 607.13 | 269.05 | 151.00 | 113.02 | |||
| 24 1 | Apigenin-7-O-glucuronide | C21H18O11 | 24.52 | 445.08 | 269.05 | 175.02 | 151.00 | 113.02 | ||
| 25 | Rosmarinic acid (Labiatenic acid) | C18H16O8 | 24.71 | 359.08 | 197.05 | 179.03 | 161.02 | 135.04 | 72.99 | |
| 26 1 | Eriodictyol (3′,4′,5,7-Tetrahydroxyflavanone) | C15H12O6 | 25.40 | 287.06 | 151.00 | 135.04 | 125.02 | 107.01 | 83.01 | |
| 27 | 3-O-Methylrosmarinic acid | C19H18O8 | 26.58 | 373.09 | 197.05 | 179.03 | 175.04 | 160.02 | 135.04 | |
| 28 1 | Naringenin (4′,5,7-Trihydroxyflavanone) | C15H12O5 | 27.72 | 271.06 | 227.07 | 177.02 | 151.00 | 119.05 | 107.01 | |
| 29 1 | Luteolin (3′,4′,5,7-Tetrahydroxyflavone) | C15H10O6 | 28.37 | 285.04 | 217.05 | 199.04 | 175.04 | 151.00 | 133.03 | |
| 30 1 | Apigenin (4′,5,7-Trihydroxyflavone) | C15H10O5 | 30.23 | 269.05 | 227.04 | 225.06 | 151.00 | 149.02 | 117.03 | |
| 31 | Dimethoxy-trihydroxy(iso)flavone | C17H14O7 | 30.38 | 329.07 | 314.04 | 313.04 | 299.02 | 271.03 | ||
| 32 | Dihydrololiolide | C11H18O3 | 30.51 | 199.13 | 181.12 | 163.11 | 135.12 | 111.04 | 107.09 | |
| 33 | Undecanedioic acid | C11H20O4 | 31.30 | 215.13 | 197.12 | 153.13 | 125.10 | 57.03 | ||
| 34 | Malyngic acid or isomer | C18H32O5 | 32.54 | 327.22 | 309.21 | 291.20 | 229.14 | 211.13 | 171.10 | |
| 35 | Nakhsmyrin or isomer | C14H12O4 | 32.67 | 245.08 | 227.07 | 217.09 | 203.07 | 175.04 | ||
| 36 | Nakhsmyrin or isomer | C14H12O4 | 33.29 | 245.08 | 227.07 | 217.09 | 203.07 | 175.04 | ||
| 37 | Dodecanedioic acid | C12H22O4 | 33.74 | 229.14 | 211.13 | 185.15 | 167.14 | |||
| 38 | Pinellic acid | C18H34O5 | 33.83 | 329.23 | 311.22 | 293.21 | 229.14 | 211.13 | 99.08 | |
| 39 | Caffeic acid phenethyl ester | C17H16O4 | 34.10 | 283.10 | 179.03 | 178.03 | 161.02 | 135.04 | 133.03 | |
| 40 | Salvigenin (5-Hydroxy-4′,6,7-trimethoxyflavone) | C18H16O6 | 35.33 | 329.10 | 314.08 | 313.07 | 296.07 | 285.08 | 268.07 | |
| 41 | Octadecenedioic acid | C18H32O4 | 37.99 | 311.22 | 293.21 | 235.17 | 223.17 | |||
| 42 | Stearidonic acid | C18H28O2 | 40.16 | 275.20 | 257.19 | 231.21 | 59.01 | |||
| 43 | Hydroxyoctadecatrienoic acid | C18H30O3 | 40.22 | 293.21 | 275.20 | 235.17 | 223.13 | 171.10 | 59.01 | |
| 44 | Stearidonic acid methyl ester | C19H30O2 | 42.15 | 291.23 | 259.21 | 241.20 | 217.20 | 107.09 | 93.07 | |
| 45 | Linoleamide | C18H33NO | 44.44 | 280.26 | 263.24 | 245.23 | 109.10 | 95.09 | 81.07 | |
| 46 | Oleamide | C18H35NO | 45.68 | 282.28 | 265.25 | 247.24 | 97.10 | 83.09 | 69.07 |
| No. | Name | Formula | Rt | [M + H]+ | [M − H]− | Fragment 1 | Fragment 2 | Fragment 3 | Fragment 4 | Fragment 5 |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Quinic acid | C7H12O6 | 1.22 | 191.06 | 173.04 | 171.03 | 127.04 | 93.03 | 85.03 | |
| 2 | Pantothenic acid | C9H17NO5 | 6.16 | 220.12 | 202.11 | 184.10 | 174.11 | 116.03 | 90.06 | |
| 3 | Caftaric acid (2-O-Caffeoyltartaric acid) | C13H12O9 | 8.42 | 311.04 | 179.03 | 149.01 | 135.04 | 87.01 | ||
| 4 | Neochlorogenic acid (5-O-Caffeoylquinic acid) | C16H18O9 | 10.12 | 355.10 | 163.04 | 145.03 | 135.04 | 117.03 | 89.04 | |
| 5 | Unidentified iridoid | C16H24O9 | 13.10 | 405.14 | 359.14 | 197.08 | 179.07 | 153.05 | 71.01 | |
| 6 | Salicylic acid-O-hexoside | C13H16O8 | 13.48 | 299.08 | 137.02 | 113.02 | 93.03 | 85.03 | 71.01 | |
| 7 | Mussaenosidic acid or isomer | C16H24O10 | 13.54 | 375.13 | 213.08 | 169.09 | 151.08 | 125.06 | 107.05 | |
| 8 | Kynurenic acid | C10H7NO3 | 13.76 | 190.05 | 162.06 | 144.04 | 116.05 | 89.04 | ||
| 9 1 | Chlorogenic acid (3-O-Caffeoylquinic acid) | C16H18O9 | 14.79 | 355.10 | 163.04 | 145.03 | 135.04 | 117.03 | 89.04 | |
| 10 | Fertaric acid (2-O-Feruloyltartaric acid) | C14H14O9 | 14.81 | 325.06 | 193.05 | 178.03 | 149.06 | 134.04 | 87.01 | |
| 11 | Benzofuranecarbaldehyde | C9H6O2 | 15.47 | 147.04 | 119.05 | 91.05 | 65.04 | |||
| 12 | Cryptochlorogenic acid (4-O-Caffeoylquinic acid) | C16H18O9 | 16.06 | 355.10 | 163.04 | 145.03 | 135.04 | 117.03 | 89.04 | |
| 13 | 5-O-(4-Coumaroyl)quinic acid | C16H18O8 | 17.37 | 337.09 | 191.06 | 173.04 | 163.04 | 119.05 | 93.03 | |
| 14 | 4-O-(4-Coumaroyl)quinic acid | C16H18O8 | 18.00 | 337.09 | 191.06 | 173.04 | 163.04 | 119.05 | 93.03 | |
| 15 | Loliolide | C11H16O3 | 19.98 | 197.12 | 179.11 | 161.10 | 135.12 | 133.10 | 107.09 | |
| 16 | Eriodictyol-O-glucuronide | C21H20O12 | 20.69 | 463.09 | 287.06 | 175.02 | 151.00 | 135.04 | 113.02 | |
| 17 | Eriodictyol-O-hexoside | C21H22O11 | 20.75 | 449.11 | 287.06 | 151.00 | 135.04 | 107.01 | 83.01 | |
| 18 | 7-Deoxyloganic acid isomer | C16H24O9 | 20.94 | 359.13 | 197.08 | 153.09 | 135.08 | 109.06 | 89.02 | |
| 19 | Luteolin-O-hexosylglucuronide | C27H28O17 | 21.43 | 623.12 | 285.04 | 217.05 | 199.04 | 175.04 | 133.03 | |
| 20 | Luteolin-7-O-glucuronide | C21H18O12 | 22.74 | 461.07 | 285.04 | 217.05 | 199.04 | 175.04 | 133.03 | |
| 21 | Luteolin-7-O-glucoside (Cynaroside) | C21H20O11 | 22.85 | 447.09 | 327.05 | 285.04 | 284.03 | 256.04 | 151.00 | |
| 22 | Apigenin-O-hexosylglucuronide | C27H28O16 | 22.96 | 607.13 | 269.05 | 151.00 | 113.02 | |||
| 23 1 | Apigenin-7-O-glucuronide | C21H18O11 | 24.51 | 445.08 | 269.05 | 175.02 | 151.00 | 113.02 | ||
| 24 | Rosmarinic acid (Labiatenic acid) | C18H16O8 | 24.71 | 359.08 | 197.05 | 179.03 | 161.02 | 135.04 | 72.99 | |
| 25 | N-trans-Feruloyltyramine | C18H19NO4 | 25.12 | 314.14 | 194.08 | 177.05 | 149.06 | 145.03 | 121.07 | |
| 26 1 | Eriodictyol (3′,4′,5,7-Tetrahydroxyflavanone) | C15H12O6 | 25.40 | 287.06 | 151.00 | 135.04 | 125.02 | 107.01 | 83.01 | |
| 27 | 3-O-Methylrosmarinic acid | C19H18O8 | 26.59 | 373.09 | 197.05 | 179.03 | 175.04 | 160.02 | 135.04 | |
| 28 1 | Luteolin (3′,4′,5,7-Tetrahydroxyflavone) | C15H10O6 | 28.39 | 285.04 | 217.05 | 199.04 | 175.04 | 151.00 | 133.03 | |
| 29 1 | Apigenin (4′,5,7-Trihydroxyflavone) | C15H10O5 | 30.23 | 269.05 | 227.04 | 225.06 | 151.00 | 149.02 | 117.03 | |
| 30 | Dihydrololiolide | C11H18O3 | 30.52 | 199.13 | 181.12 | 163.11 | 135.12 | 111.04 | 107.09 | |
| 31 | Undecanedioic acid | C11H20O4 | 31.32 | 215.13 | 197.12 | 153.13 | 125.10 | 57.03 | ||
| 32 | Malyngic acid or isomer | C18H32O5 | 32.55 | 327.22 | 309.21 | 291.20 | 229.14 | 211.13 | 171.10 | |
| 33 | Nakhsmyrin or isomer | C14H12O4 | 32.69 | 245.08 | 227.07 | 217.09 | 203.07 | 175.04 | ||
| 34 | Nakhsmyrin or isomer | C14H12O4 | 33.30 | 245.08 | 227.07 | 217.09 | 203.07 | 175.04 | ||
| 35 | Dodecanedioic acid | C12H22O4 | 33.76 | 229.14 | 211.13 | 185.15 | 167.14 | |||
| 36 | Pinellic acid | C18H34O5 | 33.85 | 329.23 | 311.22 | 293.21 | 229.14 | 211.13 | 99.08 | |
| 37 | Salvigenin (5-Hydroxy-4′,6,7-trimethoxyflavone) | C18H16O6 | 35.35 | 329.10 | 314.08 | 313.07 | 296.07 | 285.08 | 268.07 | |
| 38 | Octadecenedioic acid | C18H32O4 | 38.00 | 311.22 | 293.21 | 235.17 | 223.17 | |||
| 39 | Stearidonic acid | C18H28O2 | 40.19 | 275.20 | 257.19 | 231.21 | 59.01 | |||
| 40 | Hydroxyoctadecatrienoic acid | C18H30O3 | 40.23 | 293.21 | 275.20 | 235.17 | 223.13 | 171.10 | 59.01 | |
| 41 | Stearidonic acid methyl ester | C19H30O2 | 42.15 | 291.23 | 259.21 | 241.20 | 217.19 | 107.09 | 93.07 | |
| 42 | Linoleamide | C18H33NO | 44.45 | 280.26 | 263.24 | 245.23 | 109.10 | 95.09 | 81.07 | |
| 43 | Oleamide | C18H35NO | 45.71 | 282.28 | 265.25 | 247.24 | 97.10 | 83.09 | 69.07 |
| No | Compounds | RRI a | (%) |
|---|---|---|---|
| 1 | Dihyroedulan I | 1530 | 6.1 |
| 2 | β-Bourbonene | 1531 | 3.7 |
| 3 | Linalool | 1548 | 4.1 |
| 4 | cis-p-mentha-2,8-dien-1-ol | 1678 | 5.7 |
| 5 | Verbonene | 1732 | 2.1 |
| 6 | (E)-β-Damascenone | 1841 | 0.8 |
| 7 | Caryophyllene oxide | 2017 | 39.3 |
| 8 | Hexahydrofarnesyl acetone | 2134 | 2.7 |
| 9 | Spathulenol | 2147 | 15.6 |
| 10 | n-Hexadecanoic acid | 2912 | 11.0 |
| Total identified (%) | 91.1 |
| Extracts | PBD (mmol TE/g) | DPPH (mg TE/g) | ABTS (mg TE/g) | CUPRAC (mg TE/g) | FRAP (mg TE/g) | MCA (mg EDTAE/g) |
|---|---|---|---|---|---|---|
| n-Hexane | 1.28 ± 0.15 c | na | 12.04 ± 0.84 d | 44.08 ± 0.35 e | 22.38 ± 0.66 e | 0.34 ± 0.02 e |
| Ethyl acetate | 2.36 ± 0.20 a | na | 13.33 ± 0.58 d | 75.55 ± 0.60 d | 29.24 ± 0.27 d | 22.15 ± 2.16 b |
| Methanol | 2.45 ± 0.15 a | 90.88 ± 0.37 c | 92.43 ± 1.30 b | 165.54 ± 1.87 c | 88.78 ± 1.36 c | 15.61 ± 0.54 c |
| Water/methanol | 1.87 ± 0.06 b | 94.40 ± 0.09 a | 129.22 ± 0.78 a | 221.71 ± 2.59 b | 124.78 ± 1.40 b | 26.88 ± 2.10 a |
| Water | 2.09 ± 0.07 a,b | 93.16 ± 0.20 b | 86.56 ± 2.54 c | 229.37 ± 1.38 a | 129.55 ± 1.23 a | 27.14 ± 0.58 a |
| Essential oil | 2.22 ± 0.15 a,b | na | 12.10 ± 0.53 d | 21.42 ± 0.11 f | 10.95 ± 0.13 f | 6.52 ± 0.07 d |
| Sample/Cell line | HepG2 | B16 4A5 | S17 |
|---|---|---|---|
| DMSO 0.5% | 101 ± 7 | 88.2 ± 2.1 | 79.3 ± 4.9 |
| Methanol | 31.7 ± 0.5 | 76.7 ± 2.3 | 34.8 ± 0.9 |
| Water | 108.6 ± 12.6 | 70.2 ± 3.1 | 61.5 ± 5.6 |
| Extracts | AChE (mg GALAE/g)) | BChE (mg GALAE/g) | α-Amylase (mmol ACAE/g) | α-Glucosidase (mmol ACAE/g) | Tyrosinase (mg KAE/g) |
|---|---|---|---|---|---|
| n-Hexane | 3.97 ± 0.32 b | 6.93 ± 1.14 b | 0.66 ± 0.01 b | 7.87 ± 0.02 b | 77.84 ± 1.83 b |
| Ethyl acetate | 4.57 ± 0.06 a | 10.85 ± 0.73 a | 0.84 ± 0.02 a | 7.76 ± 0.01 b | 78.60 ± 1.58 b |
| Methanol | 3.65 ± 0.11 b | 2.98 ± 0.46 c | 0.67 ± 0.02 b | 8.15 ± 0.08 a | 96.06 ± 0.70 a |
| Water/methanol | 2.68 ± 0.07 c | na | 0.50 ± 0.01 c | 0.61 ± 0.04 e | 95.31 ± 1.77 a |
| Water | na | na | 0.10 ± 0.01 e | 1.06 ± 0.09 d | 6.15 ± 1.02 d |
| Essential oil | na | na | 0.24 ± 0.01 d | 1.64 ± 0.01 c | 21.41 ± 3.57 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zengin, G.; Mahomoodally, M.F.; Aktumsek, A.; Jekő, J.; Cziáky, Z.; Rodrigues, M.J.; Custodio, L.; Polat, R.; Cakilcioglu, U.; Ayna, A.; et al. Chemical Profiling and Biological Evaluation of Nepeta baytopii Extracts and Essential Oil: An Endemic Plant from Turkey. Plants 2021, 10, 1176. https://doi.org/10.3390/plants10061176
Zengin G, Mahomoodally MF, Aktumsek A, Jekő J, Cziáky Z, Rodrigues MJ, Custodio L, Polat R, Cakilcioglu U, Ayna A, et al. Chemical Profiling and Biological Evaluation of Nepeta baytopii Extracts and Essential Oil: An Endemic Plant from Turkey. Plants. 2021; 10(6):1176. https://doi.org/10.3390/plants10061176
Chicago/Turabian StyleZengin, Gokhan, Mohamad Fawzi Mahomoodally, Abdurrahman Aktumsek, József Jekő, Zoltán Cziáky, Maria João Rodrigues, Luisa Custodio, Rıdvan Polat, Ugur Cakilcioglu, Adnan Ayna, and et al. 2021. "Chemical Profiling and Biological Evaluation of Nepeta baytopii Extracts and Essential Oil: An Endemic Plant from Turkey" Plants 10, no. 6: 1176. https://doi.org/10.3390/plants10061176
APA StyleZengin, G., Mahomoodally, M. F., Aktumsek, A., Jekő, J., Cziáky, Z., Rodrigues, M. J., Custodio, L., Polat, R., Cakilcioglu, U., Ayna, A., Gallo, M., Montesano, D., & Picot-Allain, C. (2021). Chemical Profiling and Biological Evaluation of Nepeta baytopii Extracts and Essential Oil: An Endemic Plant from Turkey. Plants, 10(6), 1176. https://doi.org/10.3390/plants10061176