Assessment of Antidepressant-like, Anxiolytic Effects and Impact on Memory of Pimpinella anisum L. Total Extract on Swiss Albino Mice
Abstract
1. Introduction
2. Results
2.1. Identification of the Major Constituents of the Seed Extract
2.2. Evaluation of the Antidepressant Activity
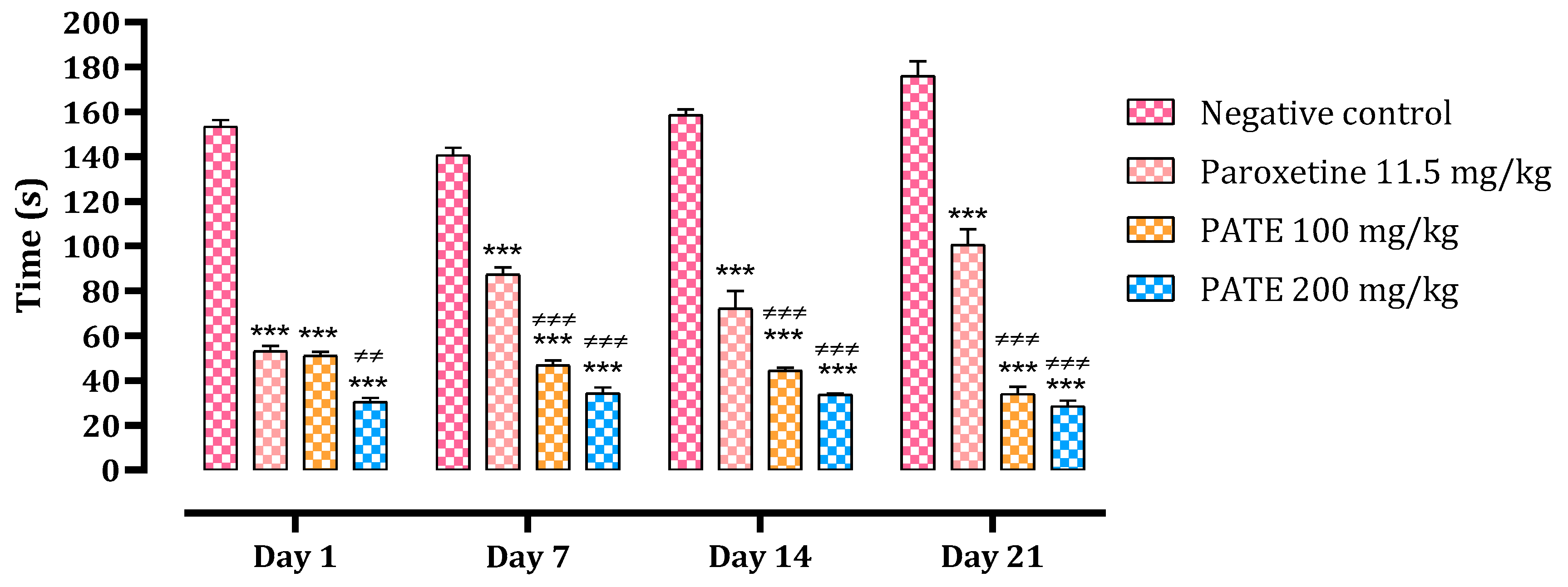
2.2.1. Forced Swimming Test
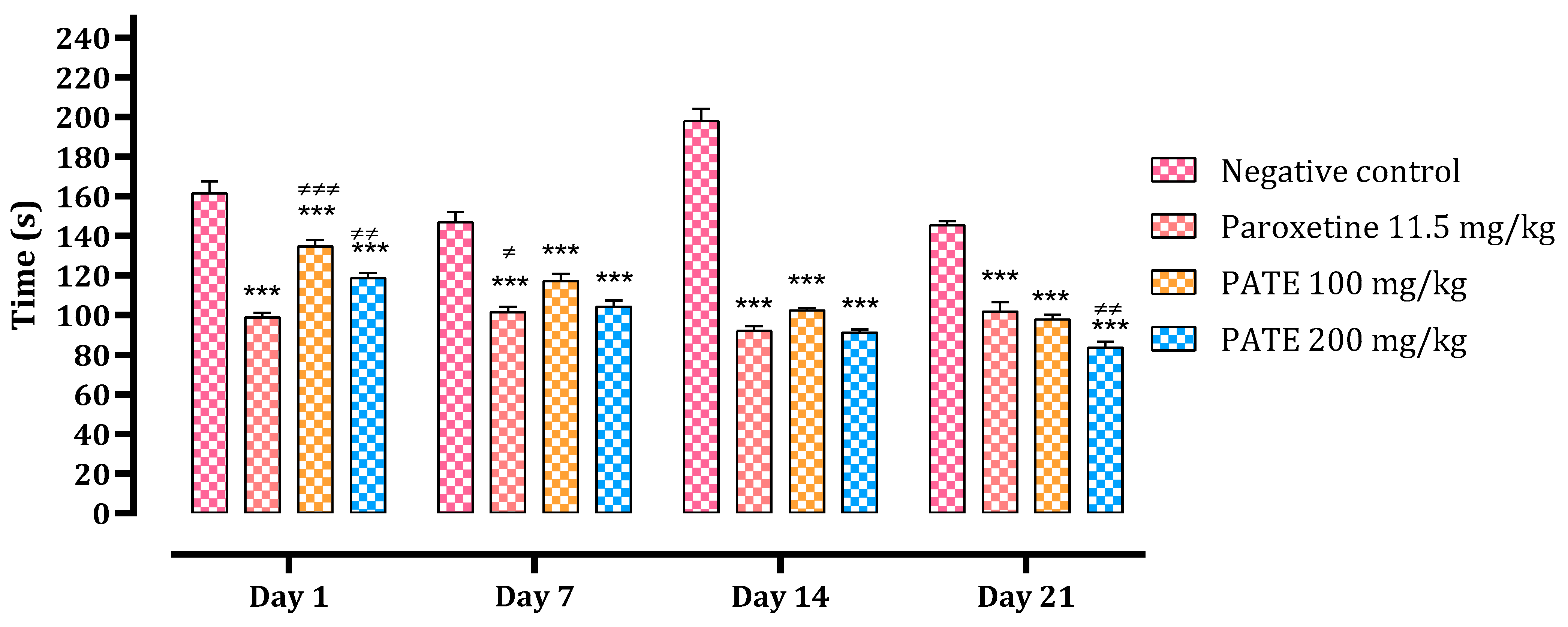
2.2.2. Tail Suspension Test
2.3. Evaluation of the Anxiolytic Activity
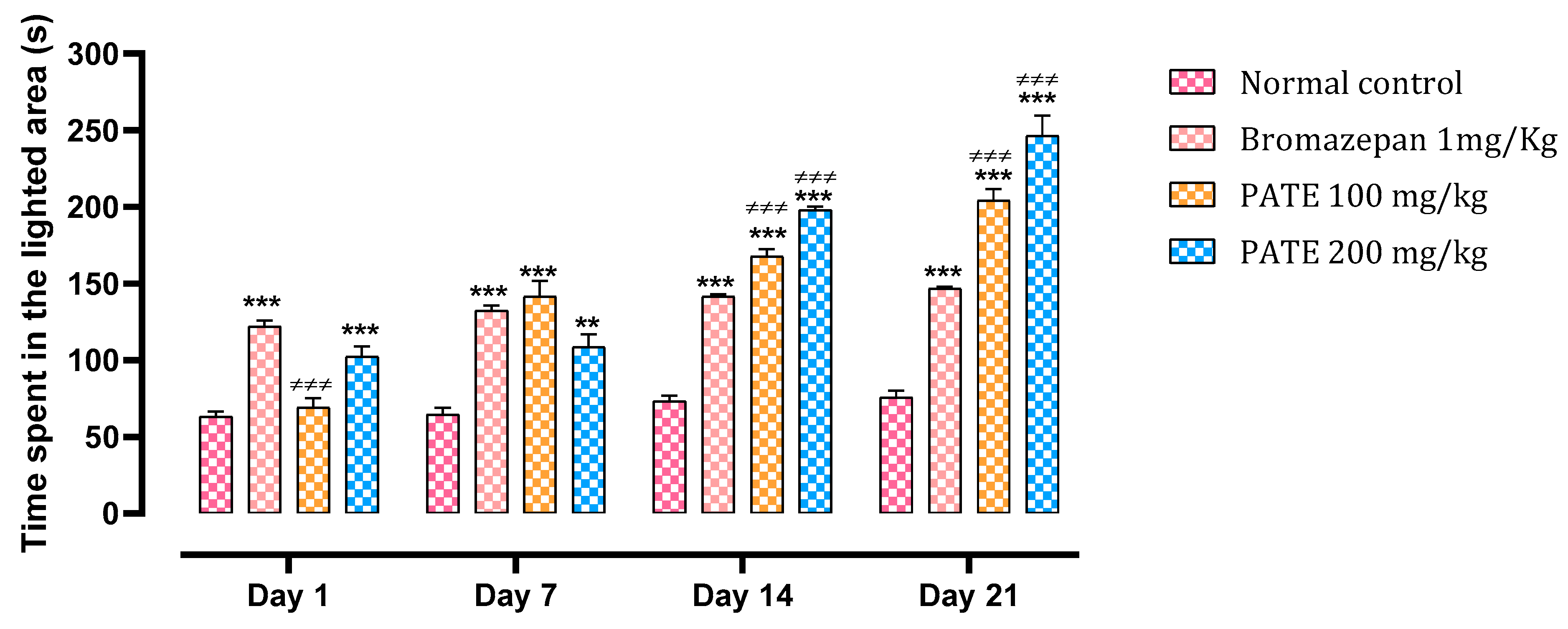
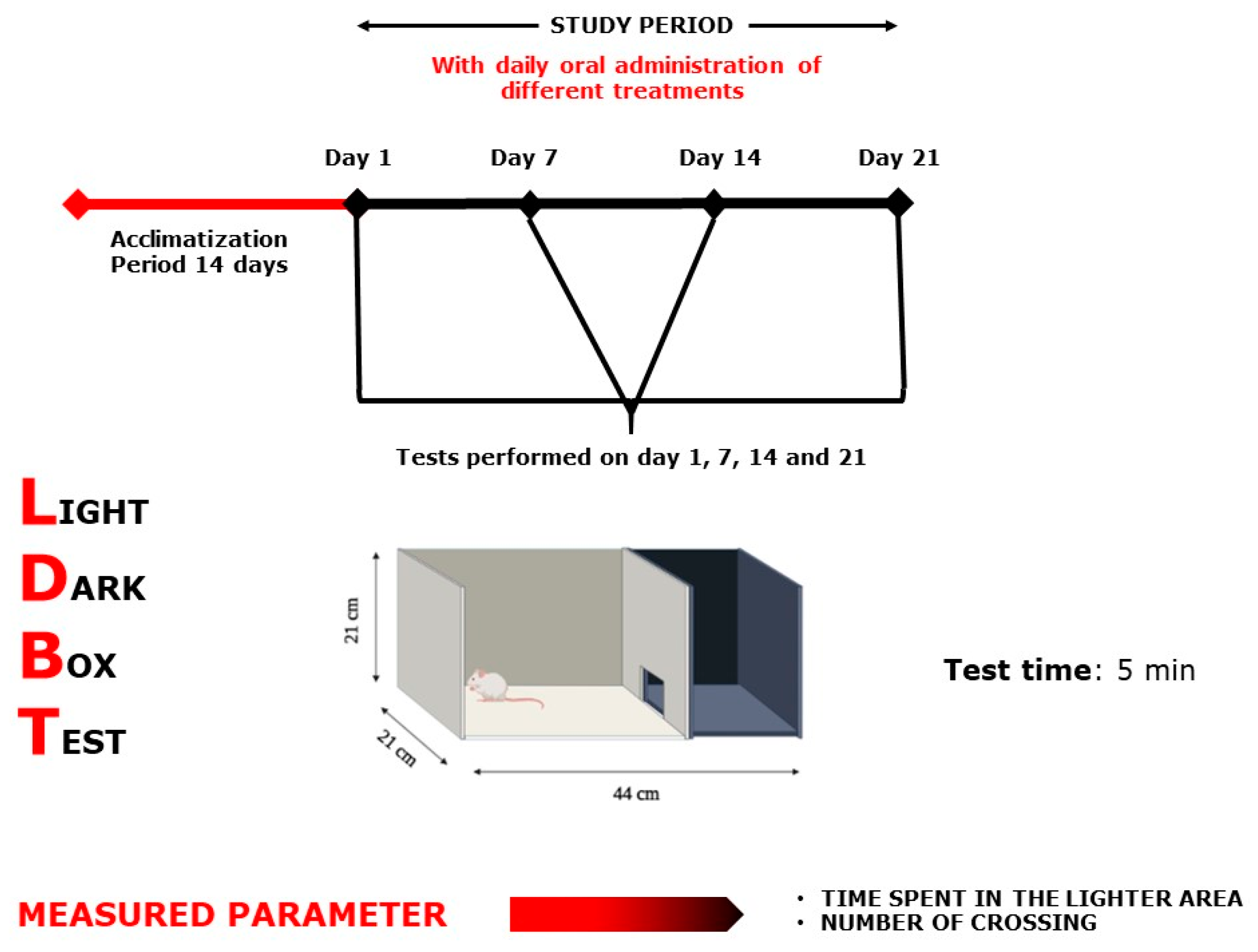
2.3.1. Light Dark Box Test
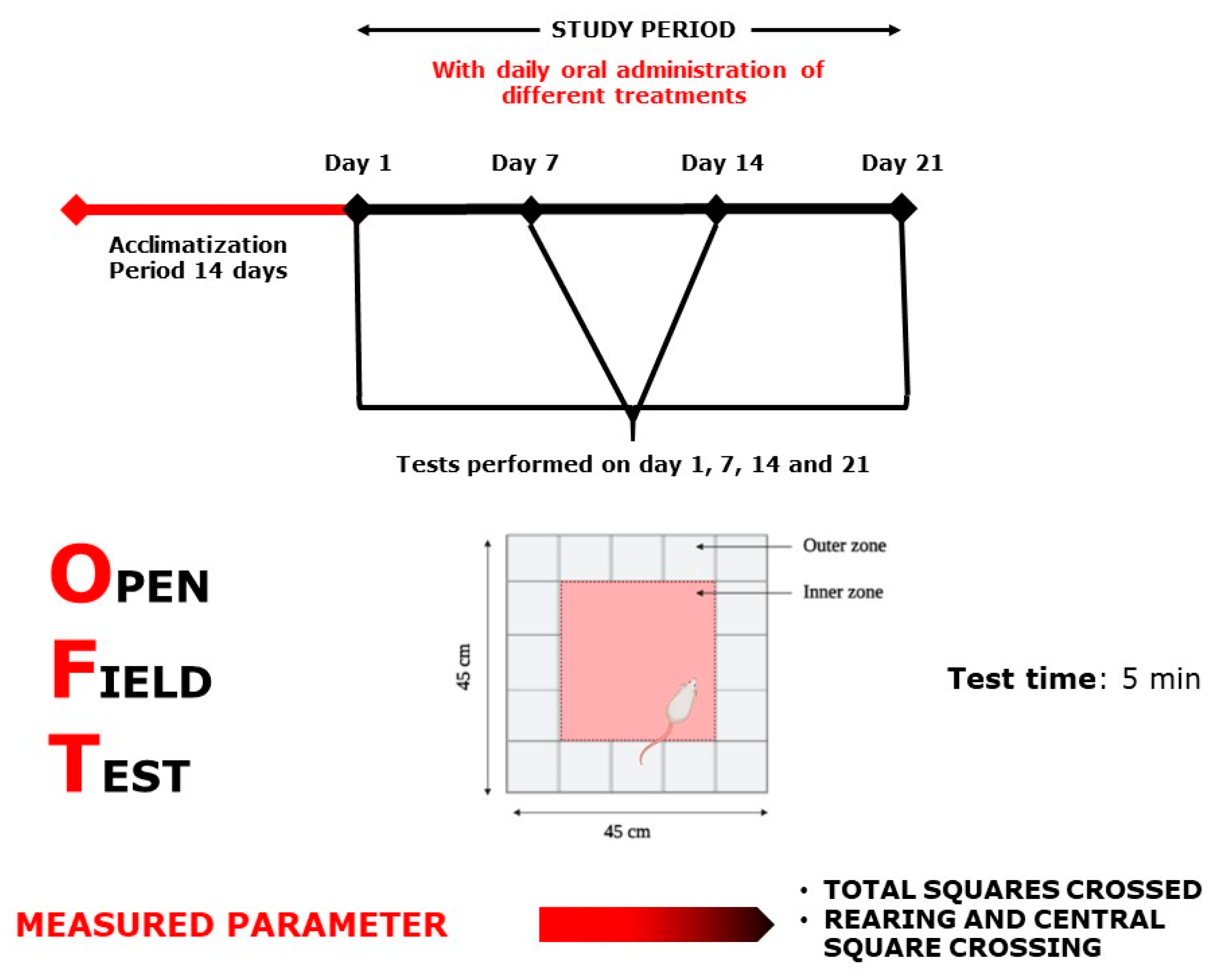
2.3.2. Open Field Test
2.4. Memory Impact Evaluation of PATE
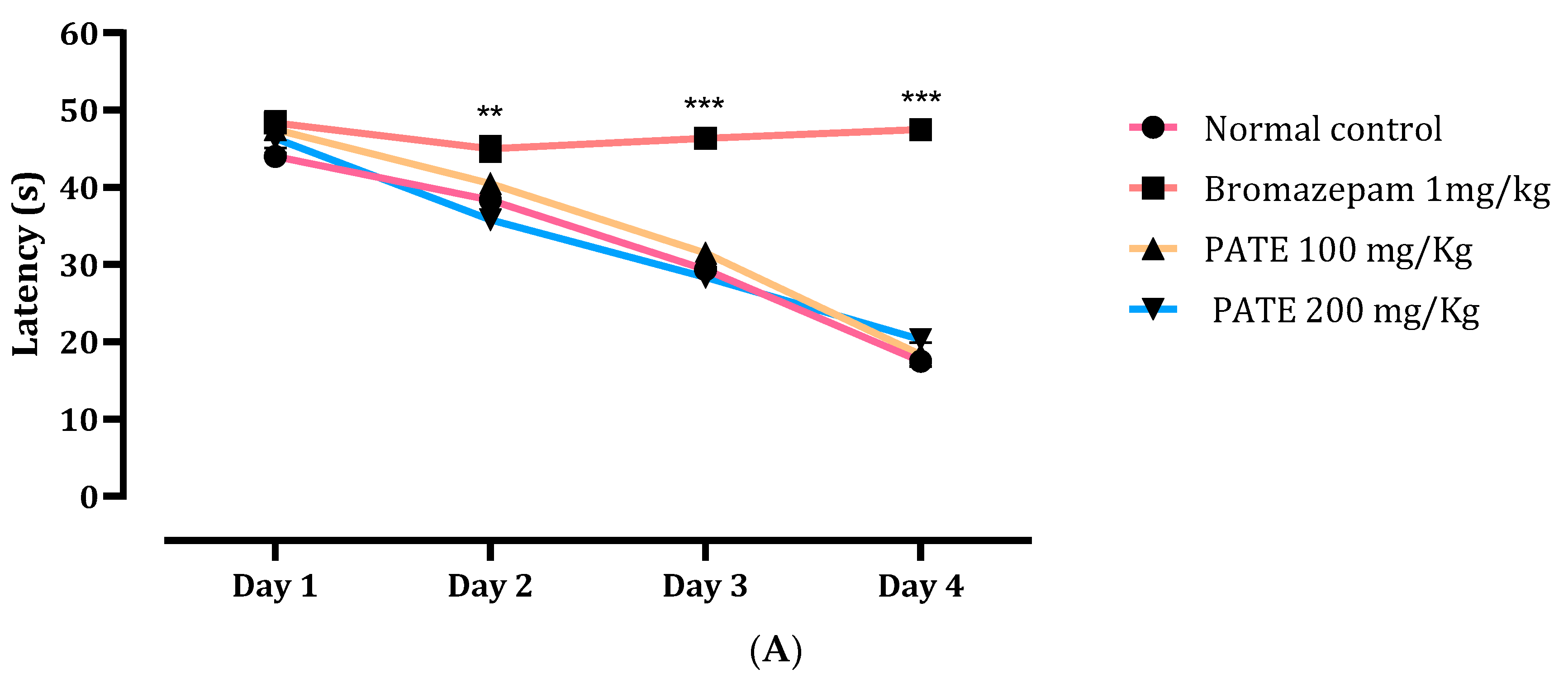
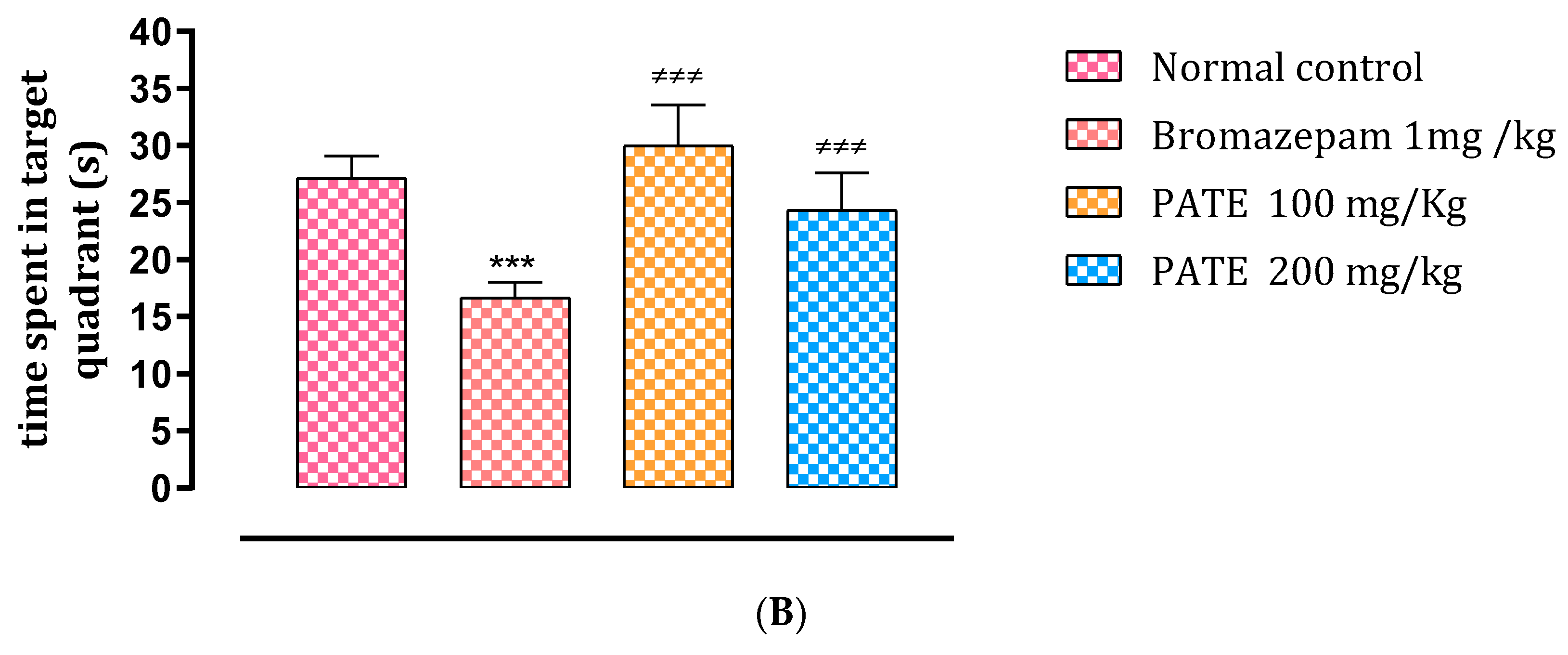
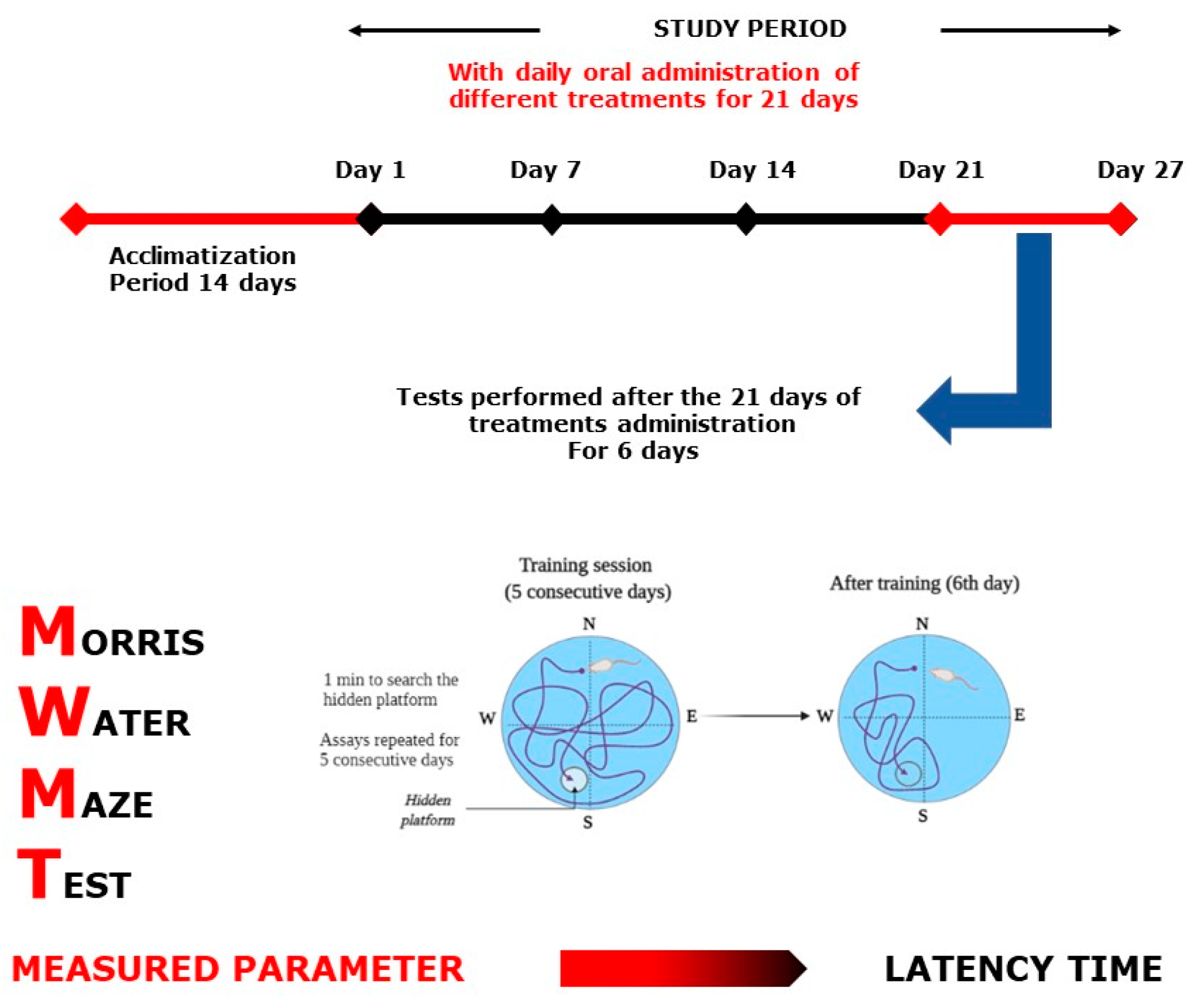
2.4.1. Morris Water Maze Test
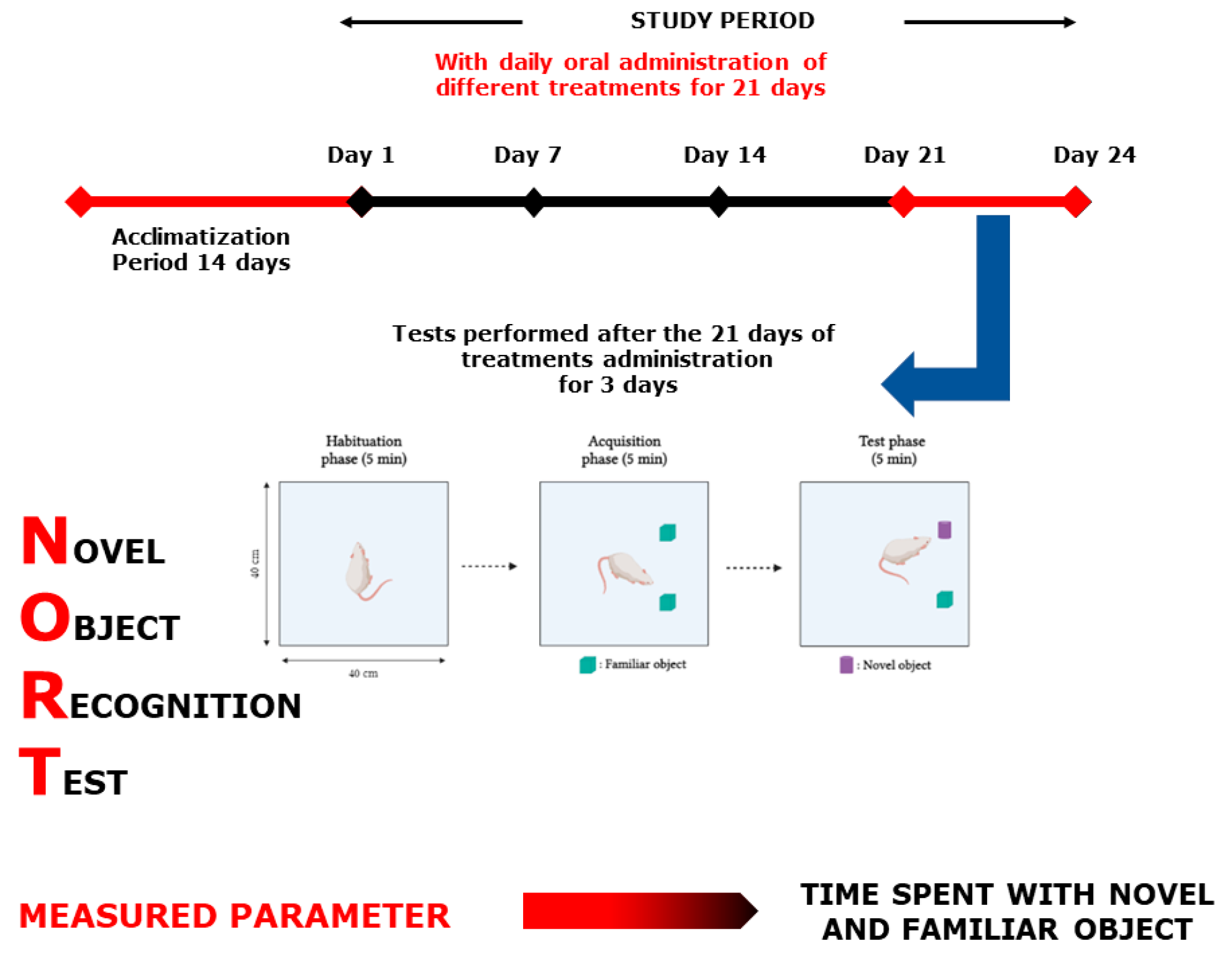
2.4.2. Novel Object Recognition Test
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. PATE Preparation
4.3. HPLC Analysis
4.4. Animals
4.5. Antidepression-Like Tests
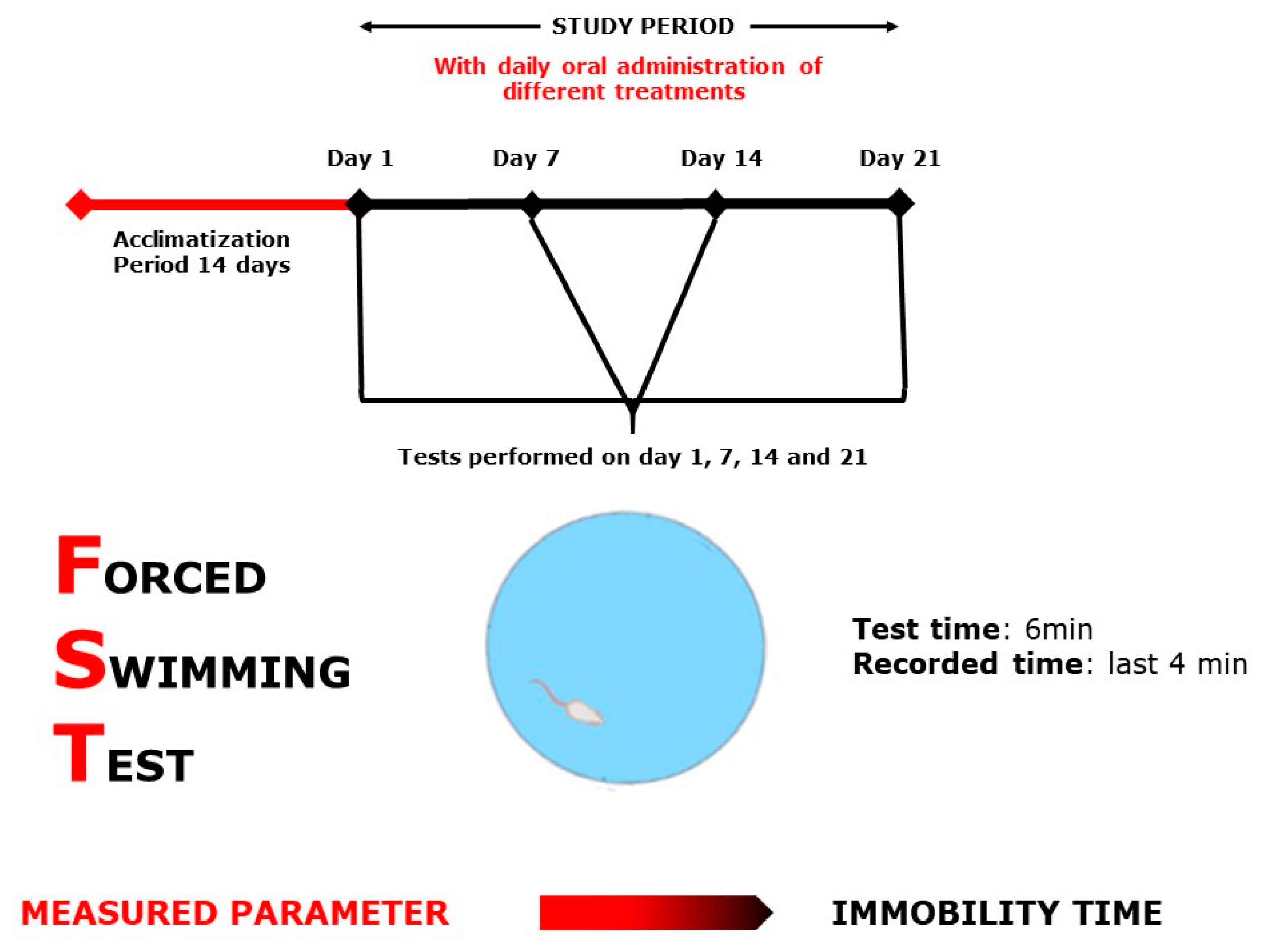
4.5.1. Forced Swimming Test
4.5.2. Tail Suspension Test
4.6. Anxiolytic Assessment Tests
4.6.1. Open Field Test
4.6.2. Light–Dark Box Test
4.7. Memory Assessment Tests
4.7.1. Morris Water Maze Test
4.7.2. Novel Object Recognition Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Friedrich, M.J. Depression is the leading cause of disability around the world. JAMA 2017, 317, 1517. [Google Scholar] [CrossRef] [PubMed]
- Gururajan, A.; Reif, A.; Cryan, J.F.; Slattery, D.A. The Future of Rodent Models in Depression Research. Nat. Rev. Neurosci. 2019, 20, 686–701. [Google Scholar] [CrossRef]
- Belzung, C.; Griebel, G. Measuring normal and pathological anxiety-like behaviour in mice: A review. Behav. Brain Res. 2001, 125, 141–149. [Google Scholar] [CrossRef]
- Griffin, C.E.; Kaye, A.M.; Bueno, F.R.; Kaye, A.D. Benzodiazepine pharmacology and central nervous system-mediated effects. Ochsner J. 2013, 13, 214–223. [Google Scholar]
- World Health Organization. Depression and Other Common Mental Disorders. Global Health Estimates. Available online: https://apps.who.int/iris/bitstream/handle/10665/254610/WHO-MSD-MER-2017.2-eng.pdf (accessed on 22 May 2019).
- Gillman, P.K. Tricyclic antidepressant pharmacology and therapeutic drug interactions updated. Br. J. Pharmacol. 2007, 151, 737–748. [Google Scholar] [CrossRef] [PubMed]
- Sanabria, E.; Cuenca, R.E.; Esteso, M.Á.; Maldonado, M. Benzodiazepines: Their Use either as Essential Medicines or as Toxics Substances. Toxics 2021, 9, 25. [Google Scholar] [CrossRef] [PubMed]
- Vgontzas, A.N.; Kales, A.; Bixler, E.O. Benzodiazepine side effects: Role of pharmacokinetics and pharmacodynamics. Pharmacology 1995, 51, 205–223. [Google Scholar] [CrossRef] [PubMed]
- Elizalde, N.; Gil-Bea, F.J.; Ramírez, M.J.; Aisa, B.; Lasheras, B.; Del Rio, J.; Tordera, R.M. Long-lasting behavioral effects and recognition memory deficit induced by chronic mild stress in mice: Effect of antidepressant treatment. Psychopharmacology 2008, 199, 1–14. [Google Scholar] [CrossRef]
- Amaghnouje, A.; Slighoua, M.; Es-Safi, I.; El Jaoudi, R.; Elyoubi, A.; Bousta, D.; Grafov, A. Ethnobotanical Survey of Medicinal Plants Used in the Traditional Treatment of Depression and Anxiety in Fez-Meknes Region. Phytothérapie 2020, 18, 220–229. [Google Scholar] [CrossRef]
- Es-Safi, I.; Mechchate, H.; Amaghnouje, A.; Calarco, A.; Boukhira, S.; Noman, O.M.; Mothana, R.A.; Nasr, F.A.; Bekkari, H.; Bousta, D. Defatted Hydroethanolic Extract of Ammodaucus leucotrichus Cosson and Durieu Seeds: Antidiabetic and Anti-Inflammatory Activities. Appl. Sci. 2020, 10, 9147. [Google Scholar] [CrossRef]
- Mechchate, H.; Es-safi, I.; Mohamed Al kamaly, O.; Bousta, D. Insight into Gentisic Acid Antidiabetic Potential Using In Vitro and In Silico Approaches. Molecules 2021, 26, 1932. [Google Scholar] [CrossRef] [PubMed]
- Shahamat, Z.; Abbasi-Maleki, S.; Mohammadi Motamed, S. Evaluation of antidepressant-like effects of aqueous and ethanolic extracts of Pimpinella anisum fruit in mice. Avicenna J. Phytomed. 2016, 6, 322–328. [Google Scholar] [PubMed]
- Mosavat, S.H.; Jaberi, A.R.; Sobhani, Z.; Mosaffa-Jahromi, M.; Iraji, A.; Moayedfard, A. Efficacy of Anise (Pimpinella anisum L.) oil for migraine headache: A pilot randomized placebo-controlled clinical trial. J. Ethnopharmacol. 2019, 236, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Pontes, V.C.B.; Rodrigues, D.P.; Caetano, A.; Gamberini, M.T. Preclinical investigation of the cardiovascular actions induced by aqueous extract of Pimpinella anisum L. seeds in rats. J. Ethnopharmacol. 2019, 237, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Shojaii, A.; Abdollahi Fard, M. Review of pharmacological properties and chemical constituents of Pimpinella anisum. Int. Sch. Res. Not. 2012, 2012, 510795. [Google Scholar] [CrossRef]
- Patel, V. Where There is no Psychiatrist: A Mental Health Care Manual; RCPsych Publications: London, UK, 2003; ISBN 1-901242-75-7. [Google Scholar]
- Drapeau, A.; Marchand, A.; Beaulieu-Prévost, D. Epidemiology of psychological distress. Ment. Illnesses-Underst. Predict. Control 2012, 69, 105–134. [Google Scholar]
- Mirowsky, J.; Ross, C.E. Measurement for a human science. J. Health Soc. Behav. 2002, 152–170. [Google Scholar] [CrossRef]
- Auerbach, R.P.; Mortier, P.; Bruffaerts, R.; Alonso, J.; Benjet, C.; Cuijpers, P.; Collaborators, W.W.-I. Mental disorder risk profiles in the World Health Organization World Mental Health Surveys International College Student Project. Int. J. Methods Psychiatr. Res. 2019, 28, e1752. [Google Scholar] [CrossRef]
- Es-Safi, I.; Mechchate, H.; Amaghnouje, A.; El Moussaoui, A.; Cerruti, P.; Avella, M.; Grafov, A.; Bousta, D. Marketing and legal status of phytomedicines and food supplements in Morocco. J. Complement. Integr. Med. 2021, 18, 279–285. [Google Scholar] [CrossRef]
- Amaghnouje, A.; Mechchate, H.; Es-safi, I.; Alotaibi, A.A.; Noman, O.M.; Nasr, F.A.; Al-zharani, M.; Cerruti, P.; Calarco, A.; EL Fatemi, H.; et al. Anxiolytic, Antidepressant-Like Proprieties and Impact on the Memory of the Hydro-Ethanolic Extract of Origanum majorana L. on Mice. Appl. Sci. 2020, 10, 8420. [Google Scholar] [CrossRef]
- Bouayed, J. Polyphenols: A Potential New Strategy for the Prevention and Treatment of Anxiety and Depression. Curr. Nutr. Food Sci. 2010, 6, 13–18. [Google Scholar] [CrossRef]
- Fajemiroye, J.O.; da Silva, D.M.; de Oliveira, D.R.; Costa, E.A. Treatment of anxiety and depression: Medicinal plants in retrospect. Fundam. Clin. Pharmacol. 2016, 30, 198–215. [Google Scholar] [CrossRef]
- Lee, B.; Shim, I.; Lee, H.; Hahm, D.-H. Oleuropein reduces anxiety-like responses by activating of serotonergic and neuropeptide Y (NPY)-ergic systems in a rat model of post-traumatic stress disorder. Anim. Cells Syst. 2018, 22, 109–117. [Google Scholar] [CrossRef]
- Es-safi, I.; Mechchate, H.; Amaghnouje, A.; Fati, J.; Kamaly, O.; Imtara, H.; Grafov, A.; Bari, A.; Bousta, D. An Insight into the Anxiolytic and Antidepressant-Like Proprieties of Carum Carvi L. and Their Association with Its Antioxidant Activity. Life 2021, 11, 207. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, P.; Bisht, S. Anti-anxiety activity of Coriandrum sativum assessed using different experimental anxiety models. Indian J. Pharmacol. 2011, 43, 574–577. [Google Scholar] [CrossRef]
- Amaghnouje, A.; Mechchate, H.; Es-Safi, I.; Boukhira, S.; Aliqahtani, A.S.; Noman, O.M.; Nasr, F.A.; Conte, R.; Calarco, A.; Bousta, D. Subacute Assessment of the Toxicity and Antidepressant-Like Effects of Origanum Majorana L. Polyphenols in Swiss Albino Mice. Molecules 2020, 25, 5653. [Google Scholar] [CrossRef]
- Es-safi, I.; Mechchate, H.; Amaghnouje, A.; Kamaly, O.M.A.; Jawhari, F.Z.; Imtara, H.; Grafov, A.; Bousta, D. The Potential of Parsley Polyphenols and Their Antioxidant Capacity to Help in the Treatment of Depression and Anxiety: An In Vivo Subacute Study. Molecules 2021, 26, 2009. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Mombereau, C.; Vassout, A. The tail suspension test as a model for assessing antidepressant activity: Review of pharmacological and genetic studies in mice. Neurosci. Biobehav. Rev. 2005, 29, 571–625. [Google Scholar] [CrossRef] [PubMed]
- Fink, G. Encyclopedia of Stress; Academic Press: San Diego, CA, USA, 2007; ISBN 978-0-12-373947-6. [Google Scholar]
- Castagné, V.; Moser, P.; Porsolt, R.D. Behavioral Assessment of Antidepressant Activity in Rodents. In Methods of Behavior Analysis in Neuroscience; Frontiers in Neuroscience; Buccafusco; Buccafusco, J.J., Ed.; CRC Press/Taylor & Francis: Boca Raton, FL, USA, 2009; ISBN 978-1-4200-5234-3. [Google Scholar]
- Telner, J.I.; Singhal, R.L. Psychiatric progress. The learned helplessness model of depression. J. Psychiatr. Res. 1984, 18, 207–215. [Google Scholar] [CrossRef]
- Vollmayr, B.; Henn, F.A. Learned helplessness in the rat: Improvements in validity and reliability. Brain Res. Protoc. 2001, 8, 1–7. [Google Scholar] [CrossRef]
- Petit-Demouliere, B.; Chenu, F.; Bourin, M. Forced swimming test in mice: A review of antidepressant activity. Psychopharmacology 2005, 177, 245–255. [Google Scholar] [CrossRef] [PubMed]
- Cryan, J.F.; Slattery, D.A. Animal models of mood disorders: Recent developments. Curr. Opin. Psychiatry 2007, 20, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Lezak, K.R.; Missig, G.; Carlezon, W.A., Jr. Behavioral methods to study anxiety in rodents. Dialogues Clin. Neurosci. 2017, 19, 181–191. [Google Scholar] [PubMed]
- Crawley, J.N. Neuropharmacologic specificity of a simple animal model for the behavioral actions of benzodiazepines. Pharmacol. Biochem. Behav. 1981, 15, 695–699. [Google Scholar] [CrossRef]
- Bourin, M.; Hascoët, M. The mouse light/dark box test. Eur. J. Pharmacol. 2003, 463, 55–65. [Google Scholar] [CrossRef]
- Costall, B.; Jones, B.J.; Kelly, M.E.; Naylor, R.J.; Tomkins, D.M. Exploration of mice in a black and white test box: Validation as a model of anxiety. Pharmacol. Biochem. Behav. 1989, 32, 777–785. [Google Scholar] [CrossRef]
- Brandeis, R.; Brandys, Y.; Yehuda, S. The use of the Morris water maze in the study of memory and learning. Int. J. Neurosci. 1989, 48, 29–69. [Google Scholar] [CrossRef]
- Leger, M.; Quiedeville, A.; Bouet, V.; Haelewyn, B.; Boulouard, M.; Schumann-Bard, P.; Freret, T. Object recognition test in mice. Nat. Protoc. 2013, 8, 2531–2537. [Google Scholar] [CrossRef]
- Lee, S.; Kim, H.-B.; Hwang, E.-S.; Kim, E.-S.; Kim, S.-S.; Jeon, T.-D.; Song, M.-C.; Lee, J.-S.; Chung, M.-C.; Maeng, S.; et al. Antidepressant-like Effects of p-Coumaric Acid on LPS-induced Depressive and Inflammatory Changes in Rats. Exp. Neurobiol. 2018, 27, 189–199. [Google Scholar] [CrossRef]
- Khan, K.; Najmi, A.K.; Akhtar, M. A Natural Phenolic Compound Quercetin Showed the Usefulness by Targeting Inflammatory, Oxidative Stress Markers and Augment 5-HT Levels in One of the Animal Models of Depression in Mice. Drug Res. 2019, 69, 392–400. [Google Scholar] [CrossRef]
- Lorigooini, Z.; Nasiri boroujeni, S.; Balali-Dehkordi, S.; Ebrahimi, L.; Bijad, E.; Rahimi-Madiseh, M.; Amini-Khoei, H. Possible involvement of NMDA receptor in the anxiolytic-like effect of caffeic acid in mice model of maternal separation stress. Heliyon 2020, 6, e04833. [Google Scholar] [CrossRef] [PubMed]
- Topaz, G.R.; March, A.; Epiter-Smith, V.; Stieglitz, K.A. Inhibition of Monoamine Oxidase (MAO) via Green Tea Extracts: Structural Insights of Catechins as Potential Inhibitors of MAO. Front. Nat. Prod. Chem. 2019, 5, 1–38. [Google Scholar]
- Scheepens, A.; Bisson, J.; Skinner, M. p-Coumaric acid activates the GABA-A receptor in vitro and is orally anxiolytic in vivo. Phytother. Res. 2014, 28, 207–211. [Google Scholar] [CrossRef]
- Mansouri, M.T.; Soltani, M.; Naghizadeh, B.; Farbood, Y.; Mashak, A.; Sarkaki, A. A possible mechanism for the anxiolytic-like effect of gallic acid in the rat elevated plus maze. Pharmacol. Biochem. Behav. 2014, 117, 40–46. [Google Scholar] [CrossRef]
- Heitman, E.; Ingram, D.K. Cognitive and neuroprotective effects of chlorogenic acid. Nutr. Neurosci. 2017, 20, 32–39. [Google Scholar] [CrossRef]
- Kotwal, S.; Upaganlawar, A.B.; Mahajan, M.; Upasani, C. Protective effects of ferulic acid in alcohol withdrawal induced anxiety and depression in mice. Malays. J. Med. Biol. Res. 2017, 4, 71–78. [Google Scholar] [CrossRef][Green Version]
- Priprem, A.; Watanatorn, J.; Sutthiparinyanont, S.; Phachonpai, W.; Muchimapura, S. Anxiety and cognitive effects of quercetin liposomes in rats. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 70–78. [Google Scholar] [CrossRef]
- Can, Ö.D.; Turan, N.; Demir Özkay, Ü.; Öztürk, Y. Antidepressant-like effect of gallic acid in mice: Dual involvement of serotonergic and catecholaminergic systems. Life Sci. 2017, 190, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Park, S.-H.; Sim, Y.-B.; Han, P.-L.; Lee, J.-K.; Suh, H.-W. Antidepressant-like effect of chlorogenic acid isolated from Artemisia capillaris Thunb. Anim. Cells Syst. 2010, 14, 253–259. [Google Scholar] [CrossRef]
- Zeni, A.L.B.; Zomkowski, A.D.E.; Maraschin, M.; Rodrigues, A.L.S.; Tasca, C.I. Ferulic acid exerts antidepressant-like effect in the tail suspension test in mice: Evidence for the involvement of the serotonergic system. Eur. J. Pharmacol. 2012, 679, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Anjaneyulu, M.; Chopra, K.; Kaur, I. Antidepressant Activity of Quercetin, a Bioflavonoid, in Streptozotocin-Induced Diabetic Mice. J. Med. Food 2003, 6, 391–395. [Google Scholar] [CrossRef]
- Diniz, L.R.L.; Souza, M.T.D.S.; Barboza, J.N.; Almeida, R.N.D.; Sousa, D.P.D. Antidepressant Potential of Cinnamic Acids: Mechanisms of Action and Perspectives in Drug Development. Molecules 2019, 24, 4469. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Guide for the Care and Use of Laboratory Animals, 8th ed.; The National Academies Collection: Reports Funded by National Institutes of Health; National Academies Press: Washington, DC, USA, 2011; ISBN 978-0-309-15400-0. [Google Scholar]
- Porsolt, R.D.; Anton, G.; Blavet, N.; Jalfre, M. Behavioural despair in rats: A new model sensitive to antidepressant treatments. Eur. J. Pharmacol. 1978, 47, 379–391. [Google Scholar] [CrossRef]
- Choleris, E.; Thomas, A.W.; Kavaliers, M.; Prato, F.S. A detailed ethological analysis of the mouse open field test: Effects of diazepam, chlordiazepoxide and an extremely low frequency pulsed magnetic field. Neurosci. Biobehav. Rev. 2001, 25, 235–260. [Google Scholar] [CrossRef]
- Maurmann, N.; Reolon, G.K.; Rech, S.B.; Fett-Neto, A.G.; Roesler, R. A valepotriate fraction of Valeriana glechomifolia shows sedative and anxiolytic properties and impairs recognition but not aversive memory in mice. Evid. Based Complement. Alternat. Med. 2011, 2011, 720853. [Google Scholar] [CrossRef] [PubMed]
- Linck, V.M.; da Silva, A.L.; Figueiro, M.; Caramao, E.B.; Moreno, P.R.H.; Elisabetsky, E. Effects of inhaled Linalool in anxiety, social interaction and aggressive behavior in mice. Phytomedicine 2010, 17, 679–683. [Google Scholar] [CrossRef]
- Doukkali, Z.; Taghzouti, K.; Kamal, R.; Jemeli, M.E.; Bouidida, E.H. Anti-anxiety effects of Mercurialis annua aqueous extract in the elevated plus maze test. J. Pharmacol. Rep. 2016, 1, 1–5. [Google Scholar]
- Walesiuk, A.; Braszko, J.J. Preventive action of Ginkgo biloba in stress-and corticosterone-induced impairment of spatial memory in rats. Phytomedicine 2009, 16, 40–46. [Google Scholar] [CrossRef]
- Ennaceur, A. Tests of unconditioned anxiety—Pitfalls and disappointments. Physiol. Behav. 2014, 135, 55–71. [Google Scholar] [CrossRef]
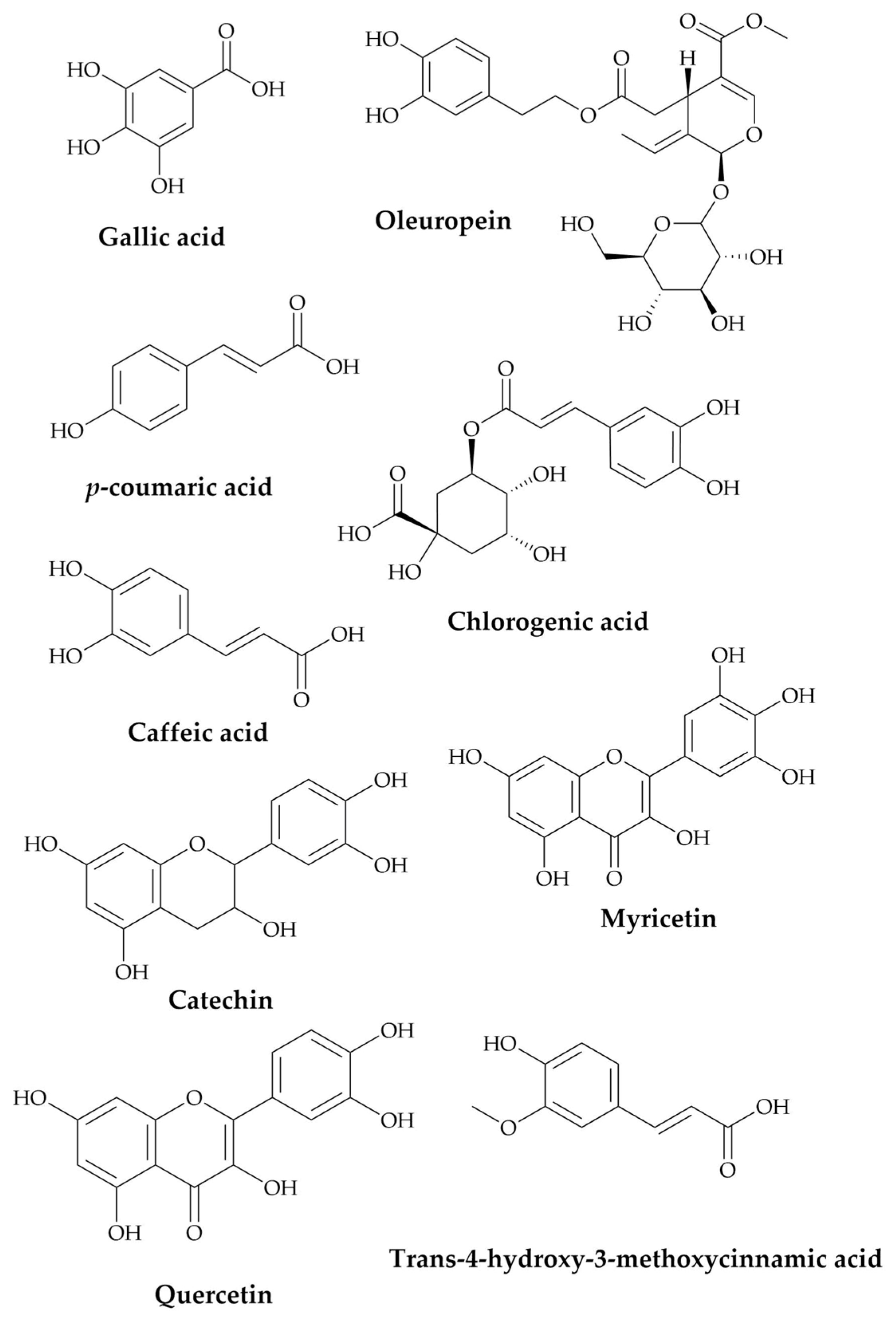


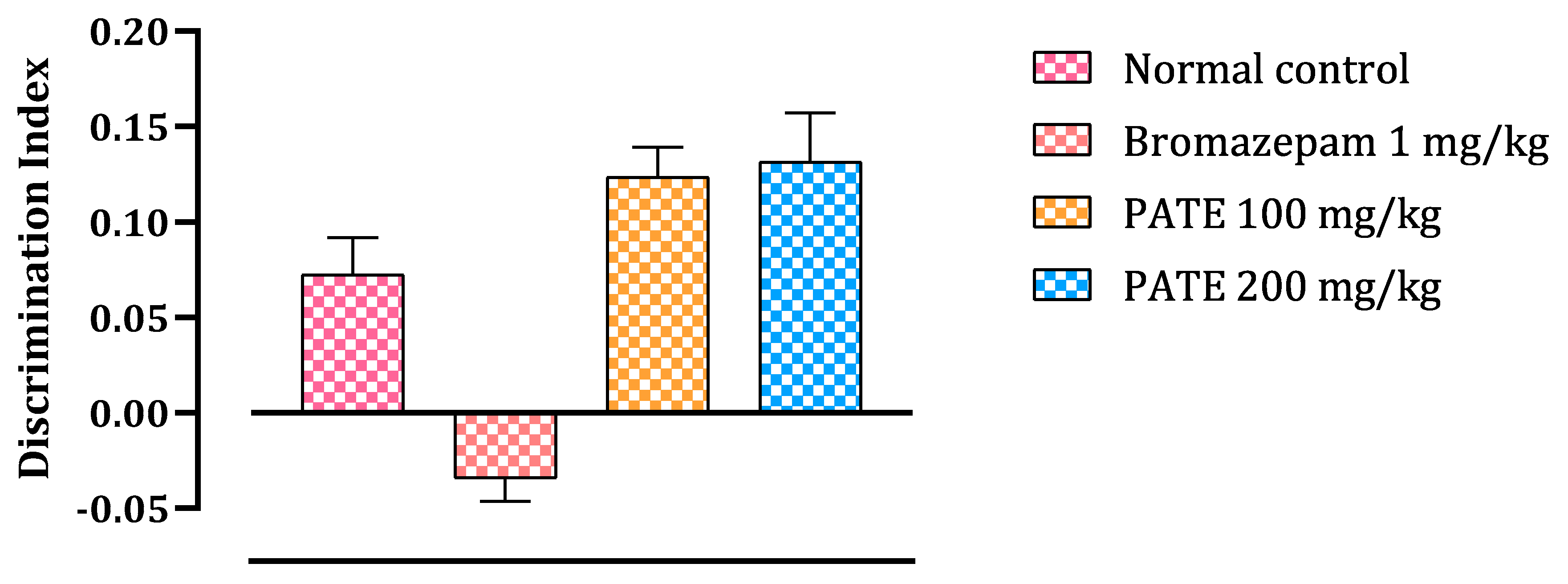

| Compounds | RT (Retention Time) | Concentration (μg/g) |
|---|---|---|
| Gallic acid | 6.652 | 4.17 |
| Catechin | 11.097 | 11.66 |
| Chlorogenic acid | 17.555 | 2.78 |
| Caffeic acid | 18.28 | 3.7 |
| Oleuropein | 21.03 | 8.6 |
| p-coumaric acid | 21.648 | 16.4 |
| Trans-4-hydroxy-3-methoxycinnamic acid | 22.097 | 0.8 |
| Myricetin | 22.381 | 0.06 |
| Quercetin | 23.224 | 0.35 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Es-safi, I.; Mechchate, H.; Amaghnouje, A.; Elbouzidi, A.; Bouhrim, M.; Bencheikh, N.; Hano, C.; Bousta, D. Assessment of Antidepressant-like, Anxiolytic Effects and Impact on Memory of Pimpinella anisum L. Total Extract on Swiss Albino Mice. Plants 2021, 10, 1573. https://doi.org/10.3390/plants10081573
Es-safi I, Mechchate H, Amaghnouje A, Elbouzidi A, Bouhrim M, Bencheikh N, Hano C, Bousta D. Assessment of Antidepressant-like, Anxiolytic Effects and Impact on Memory of Pimpinella anisum L. Total Extract on Swiss Albino Mice. Plants. 2021; 10(8):1573. https://doi.org/10.3390/plants10081573
Chicago/Turabian StyleEs-safi, Imane, Hamza Mechchate, Amal Amaghnouje, Amine Elbouzidi, Mohamed Bouhrim, Noureddine Bencheikh, Christophe Hano, and Dalila Bousta. 2021. "Assessment of Antidepressant-like, Anxiolytic Effects and Impact on Memory of Pimpinella anisum L. Total Extract on Swiss Albino Mice" Plants 10, no. 8: 1573. https://doi.org/10.3390/plants10081573
APA StyleEs-safi, I., Mechchate, H., Amaghnouje, A., Elbouzidi, A., Bouhrim, M., Bencheikh, N., Hano, C., & Bousta, D. (2021). Assessment of Antidepressant-like, Anxiolytic Effects and Impact on Memory of Pimpinella anisum L. Total Extract on Swiss Albino Mice. Plants, 10(8), 1573. https://doi.org/10.3390/plants10081573








