Abstract
Robust protocols for the regeneration of somatic embryos in vitro are essential for the efficient use of the most modern biotechnologies. Unfortunately, in perennial trees such as Citrus, plants regenerated from juvenile tissues usually exhibit strong, undesirable juvenile characters such as thorny habit and delayed flowering and fruit production. In this work, we tested whether the cell types (nucellar and stigma/style) used to regenerate Citrus plants through somatic embryogenesis affected the transition from the juvenile to mature phase. The results show that regenerants from nucellar cells presented persistent juvenile characters, whereas plants originating from stigma/style explants transited to the mature phase more rapidly. Our observations support the hypothesis that the totipotent cells originated from different cell types are not equivalent, possibly by maintaining memory of their previously differentiated state.
1. Introduction
The relevance of the Citrus industry and the continuous introduction of new improved genotypes encourage the use of biotechnologies based on somatic embryogenesis as an effective tool to rapidly regenerate genotypes of interest. One of the main problems that may limit the use of somatic embryogenesis is the occurrence of somaclonal variation. Plantlets derived from in vitro culture might develop altered characteristics and provide a wide range of culture-induced genetic variants [1] called somaclonal variations [2]. Several factors influence the onset of somaclonal variation, with the type and origin of explant being the most influencing elements [3]. Moreover, plantlets regenerated in vitro through somatic embryogenesis may display ploidy change that induces several anatomical and morphological changes in regenerants [4]. However, the detection of genetic instability in regenerants can be easily addressed through flow cytometric analysis and DNA-based techniques, such as RFLP, RAPD, ISSR, AFLP and microsatellites [5].
Improvement by conventional breeding for Citrus is problematic due to several factors such as sterility, nucellar embryony and long juvenile periods [6,7]. The long juvenile period is probably the major constraint for breeders. In Citrus, the mature and juvenile forms show distinct morphological characters such as leaf shape, branch habit, growth habit and degree of thorniness. The transition time from juvenile to mature forms varies from species to species [8] and it is also affected by environmental clues [9,10,11]. Early fruit production is a strongly desired character in Citrus and, as a consequence, attempts for shortening juvenile period is one of the greatest challenges. There are both historical work and ongoing efforts to use horticultural methods such as hybridization and clonal selection for shortening the juvenile period [12,13,14,15,16,17]. More recently, protocols for genetic transformation aimed to reduce juvenile period have been proposed [18,19,20,21,22].
Citrus genetic and sanitary improvement by conventional methods alone has many limitations that can be overcome using in vitro biotechnologies as somatic embryogenesis, which is reported as a key regeneration pathway in many experimental approaches to cultivar improvement [6]. The final success depends on several factors including the age of the explant. In Citrus, the best in vitro results, in terms of rapid proliferation rate, are normally obtained using stock material in the juvenile phase as explant source. Sim et al. [23] and Cervera et al. [24] have reported that explants collected from juvenile Citrus plants provide the best regeneration frequency in plant tissue culture as compared to explants collected from adult plants. The limited use of adult explants is due to the low morphogenetic potential of explants, and poor rooting of the shoots obtained [25,26]. For this reason, the regeneration of Citrus is usually achieved through the culture of nucellar tissues collected from immature, aborted and unfertilized ovules [27]. Unfortunately, plants regenerated from juvenile tissues usually exhibit strong and undesirable juvenile characters for several years. Other subsequent studies indicated the embryogenic potential of somatic tissues which are neither nucellar nor ovular in origin: anthers [28], juice vesicles [29] and stigmas/styles [30]. Among these three different types of explants, stigmas/styles showed the highest embryogenic potential in different Citrus species.
In this study, the effects of different cell types (nucellar and stigma/style) on the transition from the juvenile to the mature phase were evaluated on plants regenerated through somatic embryogenesis in four Citrus species. Flowering and morphological traits were assessed on plants, grafted onto sour orange, maintained in greenhouse and field conditions. Since DNA and ploidy variation may induce several morphological changes that could influence regenerant growth, the genetic fidelity of the regenerated plants was verified by flow cytometric analysis and DNA analysis (ISSR and RAPD).
2. Materials and Methods
2.1. Plant Regeneration
Somatic embryos were generated from stigma/style explants dissected from flowers before opening (Figure 1A,B) and undeveloped ovules were dissected from mature fruits (Figure 1C). Plant material of six cultivars belonging to four Citrus species (‘Femminello comune’ and ‘Lunario’ lemon (Citrus limon (L.) Burman F.), ‘Tardivo di Ciaculli’ mandarin (Citrus deliciosa Tenore), ‘AA CNR 31’ sour orange (Citrus aurantium L.), ‘Brasilian NL92’ and ‘Valencia late’ sweet orange (Citrus sinensis (L.) Osbeck)) was collected from plants growing in the Collesano field station (38° N, 14° E), Sicily. Flowers were surface sterilized by immersion for 5 min in 70% ethanol, 15 min in 2% sodium hypochlorite, followed by three 3 min rinses in sterile distilled water. Stigmas and styles were excised with a scalpel and vertically plated as single explants into medium-sized Petri dishes (100 × 15 mm) with the cut surface in contact with the medium. Mature fruits were harvested 6 months after anthesis. Each fruit was washed, the skin was peeled off and the fruits were surface-sterilized by immersion for 5 min in ethanol (70% v/v) and 30 min in 2% (w/v) sodium hypochlorite. Without rinsing, the fruits were cut open under sterile conditions, and the undeveloped ovules were dissected and transferred into medium-sized Petri dishes (100 × 15 mm). Ovule integuments were removed with the aid of a stereo microscope and plated. Explants were cultured on Murashige and Skoog (MS) medium [31] supplemented with 146 mM sucrose, 500 mg L–1 malt extract and 13.3 µM 6-benzylaminopurine. The pH of the media was adjusted to 5.7 ± 0.1 with 0.5 M of KOH before autoclaving. Explants and calluses were subcultured into fresh medium at 4–6-week intervals and maintained in a growth chamber at 25 ± 1 °C under a 16 h day length photoperiod. Germinated embryos were isolated and transferred into test tubes (1 embryo per 55 × 23 mm glass tube sealed with Parafilm M) containing 20 mL of the above-mentioned medium. Embryos were considered germinated when there was root extension and hypocotyl elongation. For acclimatization, plantlets (about 3 cm in length) were transplanted into autoclaved Jiffy peat pellets and maintained on a heating bench at 25 °C and at high relative humidity (95%). The conditions for the acclimatization of regenerated plants by grafting have been previously described in De Pasquale et al. [32].
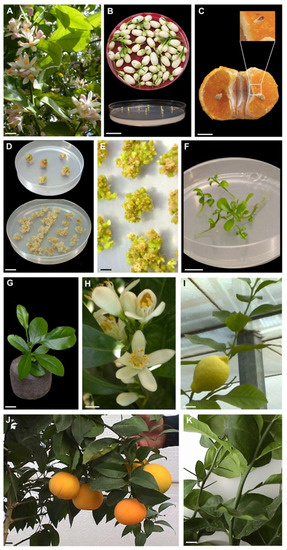
Figure 1.
Somatic embryogenesis and plant regeneration in Citrus. (A) Representative blooming Citrus (lemon) (bar = 2 cm); (B) Stigma/style explants dissected from orange flowers collected before opening (bar = 2 cm); (C) Undeveloped ovule in open pollinated fruit of mandarin harvested 6 months after anthesis (bar = 1 cm); (D) Creamy-white callus from the stigma/style and undeveloped ovule explants (bar = 2 cm); (E) Somatic embryos generated after 3–5 months of culture initiation at the surface of stigma/style explant-derived callus (bar = 3 mm); (F) Germinated somatic embryos growing on MS medium (bar = 1 cm); (G) Somatic embryo-derived plant of sweet orange transferred to Jiffy peat pellet (bar = 1 cm); (H) Sweet orange stigma/stile regenerants flowering under greenhouse condition (bar = 1 cm); (I,J) Fruits of ‘Femminello comune’ lemon and ‘Brasiliano NL 92’ sweet orange produced by three years old stigma/style regenerated plants growing in greenhouse (bar = 2 cm); (K) Thorny and thornless sour orange shoots from three years old stigma/style regenerants (bar = 2 cm).
2.2. Assessment of Ploidy by Flow Cytometric Analysis
Flow cytometry (FCM) was used to analyse the relative nuclear DNA content of the leaf cells collected either from regenerants and from the relative mother plants used as internal diploid standard (STD 2C). The analysis was carried out with the Partec PAS flow cytometer (Sysmex Partec, Görlitz, Germany, https://www.sysmex-partec.com/; accessed on 18 January 2021), equipped with a mercury lamp. Fully expanded leaves were chopped, using a sharp razor blade, in 400 μL nuclei extraction buffer (solution A of the ‘High Resolution Kit’ for PlantDNA, Sysmex Partec, Germany) for 30–60 s. After filtration through a 30 μm Cell-Trics disposable filter Cell-Trics Sysmex Partec, Germany, 1.6 mL staining solution containing the dye 4,6-diamidino-2-phenylindole (DAPI; solution B of the kit) was added. Routinely, 4000–5000 nuclei were measured per sample and histograms of DNA content were generated using the Partec FlowMax software package.
2.3. Assessment of Genetic Stability in Regenerants by ISSR and RAPD Markers
Leaves collected from regenerants and mother plants for each cultivar were harvested, washed, frozen in liquid nitrogen and stored at −80 °C until analyses. Genomic DNA was isolated from the samples as described by [33] and was quantified by measuring OD260 as described by [34]. The isolated genomic DNA was used for ISSR and RAPD analyses in order to assess genetic fidelity as described by Carra et al. [35].
A total of 6 ISSR primers [36] were used to amplify the DNA (Table S1). The primers were purchased from Life Technologies, Gaithersburg, Md. Each 25 μL amplification reaction consisted of 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 2 mM MgCl2, 800 μM dNTP, 0.5 μM of each primer, 1 U of Platinum Taq polymerase and 30 ng of template DNA. The amplification was performed under the following cycle program: initial denaturation step for 4 min at 94 °C, followed by 36 cycles at 94 °C for 30 s (denaturation), 48.5–52.0 °C (see Table S1) for 45 s (annealing) and 72 °C for 120 s (extension), followed by a final extension step at 72 °C for 7 min. A total of 25 µL of each PCR-reaction products were electrophoresed on a 1.5% (w/v) agarose gel containing 1 × TBE (45 mM Tris-borate, 1 mM EDTA) and 0.5 μg/mL aqueous solution of ethidium bromide. The gel was run for 4 h at 100 V and visualized under UV light lamp. Only those bands showing consistent amplification were considered; smeared and weak bands were excluded from the analysis. Polymorphic ISSR markers were scored for the presence or absence of bands.
RAPD analysis of the grapevine genotypes was performed using six decamer primers [37] (Table S2). DNA amplification reactions were carried out in a volume of 25 μL with 20 mM Tris-HCl (pH 8.4), 50 mM KCl, 3 mM MgCl2, 800 μM dNTP, 0.4 μM of each primer, 1.5 U of Platinum Taq polymerase and 25 ng of template DNA. The amplification was performed in a MJ Research thermocycler (Genenco) equipped with a Hot Bonnet under the following cycle program: initial denaturation step for 90 s at 94 °C, followed by 36 cycles at 94 °C for 1 min (denaturation), 36 °C for 60 s (annealing) and 72 °C for 2 min (extension), followed by a final extension step at 72 °C for 10 min. Reaction products were visualized and analyzed as cited for ISSR analysis.
2.4. Grafting Conditions
In May, scions generated from different embryogenic events were grafted (T-budding) on three-year-old sour orange rootstocks in greenhouse and field trials, 30 cm above the soil line as described in De Pasquale et al. [32]. As control, to represent the ‘true adult’ state, mature scions collected from mother plants were grafted onto sour orange rootstocks. After the successful graft implant, the upper part of the rootstock was removed.
2.5. Growth and Flowering Assessment of Plants Maintained in Greenhouse
Grafted plants were maintained in the greenhouse (six plants regenerated in vitro for each explant/genotype combination and two mother plants for each genotype) in order to check growth, flowering and thorns production. As Citrus plants tend to bloom in the uppermost part of the canopy, the lateral branches of the grafted plants were removed, starting from the second year of growth, to induce the scion growth in height as a single leader.
2.6. Morphological Analyses on Plants Transplanted in the Field
Grafted plants were maintained in the field (20 plants for each explant/genotype combination and eight mother plants for each genotype) for evaluation of leaf area of the plant and morphological characters (thorn length, thorns/nodes ratio). The experiment used a randomized block design (4 blocks) with 5 replications containing regenerated and mother plants. Leaf area of the plant and morphological analyses were performed on plants growing in the field at the end of the vegetative season (November) for one and three years, respectively.
2.7. Estimation of Leaf Area Using Linear Leaf Measurements
Non-destructive methods for measuring the leaf area (LA) through linear measurements have been reported for Citrus by many authors [38]. The following criteria for the selection of a regression equation were used: coefficients of determination (r2), standard errors of estimates, F test of analysis of variance and significance of the regression coefficients (SPSS-X, Inc., Chicago, IL, USA). We found that the leaf area estimation equations had the relative advantage of simplicity of calculation and the lowest standard error of estimates (Table 1).

Table 1.
Leaf area estimation equations.
3. Results
3.1. Somatic Embryogenesis
Most of the explants produced a creamy-white callus after 1–2 weeks of incubation (Figure 1D). The different genotypes showed a different embryogenic potential from stigma/style and undeveloped ovule explants. About 3–5 months after culture initiation, all of the cultivars regenerated somatic embryos from stigma/style explants (Figure 1E). On the contrary, ‘AA-CNR-31’ sour orange, ‘Valencia late’ sweet orange, ‘Femminello comune’ and ‘Lunario’ lemon generated few somatic embryos from undeveloped ovule explants. Only ‘Tardivo di Ciaculli’ mandarin and ‘Brasiliano NL 92’ sweet orange regenerated a sufficient number of somatic embryos from undeveloped ovules from field and greenhouse trials. About 12 weeks after germination, somatic embryos developed into plantlets (Figure 1F) at a high frequency (40–66%). When plantlets were transferred ex vitro in Jiffy peat pellets, the percentage of acclimatized plants was about 70% (Figure 1G).
3.2. Genetic Fidelity Analysis of the Regenerated Plants
Flow cytometric analysis was used to determine the ploidy level of regenerants. All plants regenerated trough somatic embryogenesis of the four different species showed the same ploidy of the mother plants confirming the stability of the ploidy level of plants regenerated from stigma/style and undeveloped ovule explants. Histograms of the DNA content of isolated nuclear suspension of regenerated plants are shown in Figure S1.
Six ISSR and six RAPD primers were screened out and used to amplify 16 DNA samples of regenerants from each cultivar and comparing them to the respective mother plant. A total of 135, 144, 146 and 134 well-resolved band classes were obtained for lemon, mandarin, sour orange and sweet orange, respectively.
The six ISSR primers gave 65, 76, 72 and 66 well-resolved band classes in lemon, mandarin (Figure S2A), sour orange and sweet orange. The sizes of amplified fragments were among 250 bp to 3.1 Kb. The mean number of ISSR bands obtained for each primer varied from 7 [primer (TCC)5RY] to 15 [primer (AG)8YT].
The six RAPD primers produced 70, 68, 74 and 68 well-resolved band classes in lemon, mandarin, sour orange and sweet orange, respectively, ranging from 300 bp to 3.5 Kb in size. The mean number of bands for each primer varied from 8 in ‘Valencia’ with primer OPM 04 (Figure S2B) to 17 in sour orange with primer OPAT 14. A total of 14,076 bands (number of plantlets analyzed × number of band classes obtained with all the primers) were generated by the RAPD and ISSR techniques. All regenerated plantlets appeared to be completely identical to the respective mother plants.
3.3. Growth and Flowering Assessment of Plants Maintained in Greenhouse
Mother plants and regenerated plants, growing under greenhouse conditions, were inspected for the presence or absence of flowers. The presence of flowers in plants maintained in the greenhouse for three years after grafting is reported in Table 2. During the first year of vegetation, mature and regenerated plants did not flower. During the second year, most mother plants were flowering and only few plants of lemon and sour orange produced flowers and some fruits, while juvenile characters started to be lost on some shoots.

Table 2.
Observations on flowering plants in greenhouse.
The third year, all the mother plants produced flowers and some of the plants regenerated from stigma/style of lemon, mandarin, sour and sweet orange were flowering under greenhouse condition (Figure 1H). Sour orange (‘AA-CNR-31’) showed the higher percentage of flowering plants (66%), whereas a lower percentage (50%) was observed in lemons (‘Femminello comune’ and ‘Lunario’) and ‘Brasiliano NL 92’ sweet orange. The lowest percentage was detected in ‘Tardivo di Ciaculli’ mandarin and ‘Valencia late’ sweet orange (33%). The third year some of the plants of ‘Femminello comune’ lemon, ‘Brasiliano NL 92’ sweet orange, ‘Tardivo di Ciaculli’ mandarin and ‘AA-CNR-31’ sour orange regenerated by stigma/style produced fruits in greenhouse (Figure 1I,J). Fruits produced by the plants regenerated in vitro from stigma/style did not show differences to those produced by mother plants. Juvenile characters of all genotypes regenerated trough stigma/stile culture started to be lost in several shoots (Figure 1K).
In contrast, none of the plants regenerated from undeveloped ovules (‘Tardivo di Ciaculli’ mandarin and ‘Brasiliano NL 92’ sweet orange) produced flowers in the first three years of greenhouse cultivation and most of the shoots retained their juvenile characters (Table 2).
3.4. Observations of Juvenility Mature and Regenerated Plants Maintained in the Field
Plant leaf area and number of thorns per plant, was measured only the first year. Regenerants (stigma/style and undeveloped ovule) showed a higher vegetative growth as compared to the mother plants (Figure 2). In fact, all regenerants had a higher plant leaf area than the mother plants. The lowest plant leaf area was observed in mandarin mother plants (22.3 dm2), higher values were observed in stigma/style and undeveloped ovule regenerants: 32.2 and 38.7 dm2, respectively. Generally, the plant leaf area of undeveloped ovules regenerants is superior to stigma/style regenerants (Figure 2).
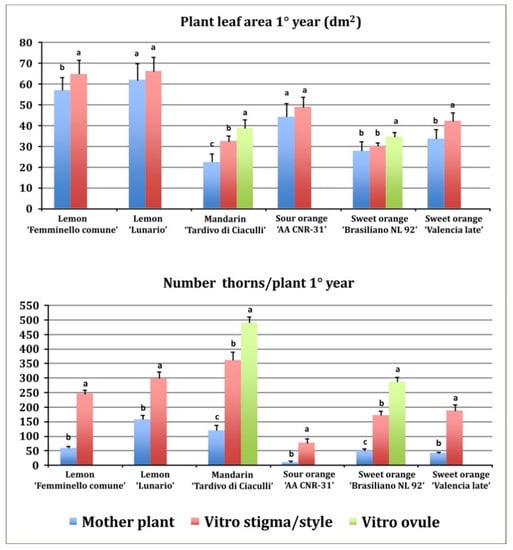
Figure 2.
Plant leaf area and number of thorns per plant in the first year of growth in the field. Different letters on bars indicate significantly different values at a particular genotype according to the t-test for ‘Femminello comune’, ‘Lunario’ lemon, ‘AA CNR 31’ sour orange and ‘Valencia late’ sweet orange and according to Tukey’s multiple comparison test for ‘Tardivo di Ciaculli’ mandarin and ‘Brasilian NL92’ sweet orange. Tests were performed at p < 0.05 significance level. Bars indicate standard error.
All regenerants exhibited a greater number of thorns as compared to mother plants (Figure 2). Mandarin regenerants from stigma/style and undeveloped ovule explants showed the higher number of thorns (361 and 490, respectively) in the first year, nevertheless also the mother plants showed a high number of thorns (119). In the first year, the lowest number of thorns was observed in sour orange mother plants (9). The different genotypes showed different levels of thorniness: both in regenerated plants, ranging from 75 in sour orange (stigma/style regenerants) to 490 in mandarin (undeveloped ovule regenerants) and in mother plants, ranging from 9 in sour orange to 157 in ‘Lunario’ lemon (Figure 2).
The four species showed great differences in thorn length between mother and regenerated plants (Figure 3).
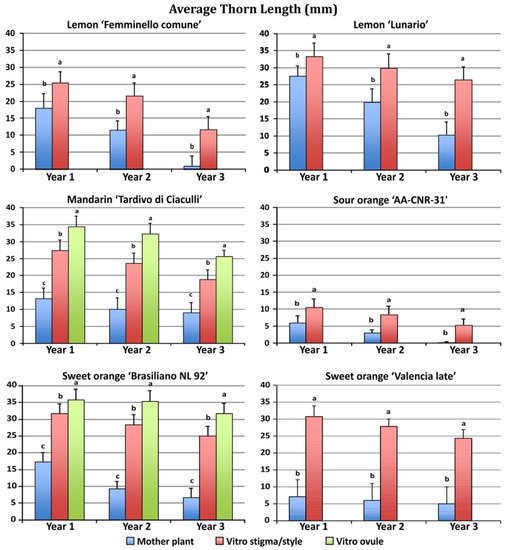
Figure 3.
Average thorn length in plants regenerated from different explants growing in the field during the first three years after grafting. Different letters on bars indicate significantly different values at a particular genotype according to the t-test for ‘Femminello comune’, ‘Lunario’ lemon, ‘AA CNR 31’ sour orange and ‘Valencia late’ sweet orange and according to Tukey’s multiple comparison test for ‘Tardivo di Ciaculli’ mandarin and ‘Brasilian NL92’ sweet orange. Tests were performed at p < 0.05 significance level within each year. Bars indicate standard error.
Regenerated plants after one year of growth in the field showed much longer thorns than mother plants. Moreover, the undeveloped ovule regenerants showed a longer thorn length when compared to the stigma/style regenerants. ‘Brasiliano NL 92’ sweet orange stigma/style and undeveloped ovule regenerants showed the highest thorn length (31.7 and 35.8 mm, respectively) in the first year, yet also the mother plants showed long thorns (17.3 mm).
A similar behavior was observed in mandarin, with stigma/style and undeveloped ovule regenerants showing the highest thorn length (27.4 and 34.5 mm, respectively) as compared to the mother plants (13.2 mm). The lowest thorn length was observed in sour orange, in which stigma/style regenerants had an average thorn length of 10.5 mm and the mother plants thorns were 6.0 mm long. During the second and third year of growth, the thorn length of the regenerated plants was reduced as compared to the first year, but thorns were still longer in regenerated compared with mother plants. The second and third years, plants regenerated from undeveloped ovules produced longer spines than plants regenerated from stigma/style.
Consistent differences were observed in thorn/node ratio between mature and regenerated plants during the three years of growth in the field (Figure 4).
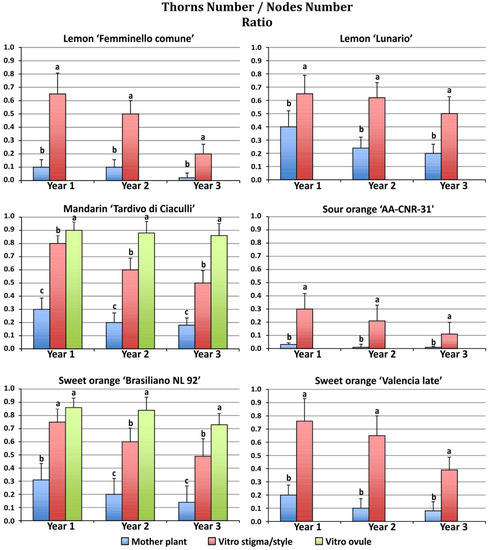
Figure 4.
Average thorn/node ratio in plants regenerated from different explants growing in the field during the first three years after grafting. Different letters on bars indicate significantly different values at a particular genotype according to the t-test for ‘Femminello comune’, ‘Lunario’ lemon, ‘AA CNR 31’ sour orange and ‘Valencia late’ sweet orange and according to Tukey’s multiple comparison test for ‘Tardivo di Ciaculli’ mandarin and ‘Brasilian NL92’ sweet orange. Tests were performed at p < 0.05 significance level within each year. Bars indicate standard error.
All regenerants exhibited a higher thorn/node ratio as compared to mother plants. Among the plants originating from somatic embryogenesis the undeveloped ovule regenerants showed a higher thorn/node ratio when compared to the stigma/style regenerants. Mandarin regenerants from stigma/style and undeveloped ovule explants showed the highest thorn/node ratio (0.80 and 0.90, respectively) in the first year, while the mother plants showed a lower thorn/node ratio (0.30). In the first year, the lowest thorn/node ratio was observed in sour orange mother plants (0.03). The highest thorn/node ratio of the mother plants was observed in ‘Lunario’ lemon (0.4). During the second and third year of growth, the thorn/node ratio of the regenerated plants was reduced as compared to the first year, with the exception of the mandarin undeveloped ovule regenerants, which maintained similar values in the three years of observation (0.90 and 0.87 for the second and third year, respectively).
At any evaluation time, our observations showed that the thorniness of regenerated plants was higher than those of mother plants; however, some regenerants developed thornless apical shoots. If the thornless shoots were collected and regrafted on sour orange, they kept the acquired mature characters (data not reported). Most juvenile characters (thorniness, internode length, absence of flowers, etc.) were no longer present after mature shoots from plants regenerated in vitro were grafted again and the new vegetation displayed mature morphology nearly identical to mature plants. The only exception was observed in sweet orange and mandarin, which in some cases reverted to the juvenile form. However, in all species, it was possible to obtain plants regenerated from stigma/style explants that displayed mature morphology within three years after grafting. In contrast, undeveloped ovule regenerants retained their juvenile characteristics during the three years of observation after grafting.
4. Discussion
Since plants cannot escape adversity, they developed a high regeneration ability for survival to biotic or abiotic stresses. Compared to animals, plants generally have a high level of plasticity and exhibit a remarkable regenerative capacity, both in vivo and in vitro, that varies widely between species and tissue types. Growing evidence suggests that some forms of plant regeneration involve the reprogramming of differentiated somatic cells, while others are induced through the activation of relatively undifferentiated cells in somatic tissues [39]. An extreme example of this adaptation is the generation of adult plants via somatic embryogenesis without the need for fertilization [40]. The regenerative potential can be increased in vitro by exogenously supplied plant growth regulators, wherein the interaction between auxin and cytokinin influences the developmental fate of cells inducing shoot or root regeneration. A balanced concentration of auxin and cytokinin induces an unorganized growth of a cell mass known as ‘callus’ due to its resemblance to the wound-healing plant tissue [41].
Somatic embryogenesis is a powerful biotechnological tool for the propagation and genetic improvement of plants. In Citrus, the production of embryogenic callus lines was first reported by Rangan et al. [42] from excised nucelli culture, the regeneration of somatic embryos from stigma and style cultures was first reported by Carimi et al. [43] and, since then, somatic embryogenesis has been induced directly or indirectly via callus formation in several Citrus species to produce plants for mass propagation, breeding program [6] and virus-free plants [44,45,46,47,48,49]. Even if somatic embryogenesis is widely used, little information is available on the behavior of Citrus plants regenerated in vitro from stigma/style and nucellar culture when grafted on rootstock and transferred under greenhouse conditions.
Here, we tested the genetic fidelity and agronomical aspects of plants regenerated from stigma/style explants or nucellar tissue and grown in the field or greenhouse. Shifts in morphological characters in regenerated plants can be expressed in terms of loss of apical dominance, number and size of leaves and, most importantly, in the time of flowering.
In order to investigate the presence of somaclonal variations on the regenerated plants, we used two different PCR-based techniques, ISSR and RAPD, and flow cytometry to analyse the regenerants. No somaclonal variation compared to the mother plants was obtained, revealing the homogeneity of the produced plantlets. We also observed that fruits produced by the plants regenerated in vitro (from stigma and style cultures) were identical to those produced by mother plants, confirming that regenerated plants are genetically true-to-type. Moreover, this technology is also effective in the elimination of several viral infections [47]. This strategy can be considered as a possible in vitro process for the healthy plant regeneration of Citrus with a very low risk of generating somaclonal variants.
Somatic embryo regeneration from mature explants induced a partial reversion to a juvenile state, as widely demonstrated both for woody and herbaceous plants [50,51]. In fact, with the exception of sour orange, most of the regenerated plants showed strong juvenile characters in the first stages of growth, characterized by a high presence of thorns on stems and branches. During the first year of growth (post-grafting), mature and regenerated plants exhibited juvenile growth, characterized by the absence of flowers and high thorniness. As time passed, both mature and regenerated plants reduced these juvenile characters. Most of the lemons and sour oranges had completely thornless single shoots, whereas, in other shoots, thorns were still abundantly present. The loss of juvenility in the early stages of growth confirms previous observations that lemons and sour oranges produced fruits earlier than other Citrus species, with fruits present on thornless branches 3 years after the embryogenic event [46]. Similar results were reported for plantlets of Calamondin Citrus derived from somatic embryos grafted onto Japanese citron rootstock which, after one year in the field, produced flowers and fruits normally [52]. Propagated plants obtained by regrafting thornless budwood of stigma/style regenerants onto sour orange rootstock did not show any juvenile characters and they were flowering and fruiting regularly. This suggests that the protocol for plantlets regeneration by somatic embryogenesis from stigma/style explants maintains the genomic integrity of the chosen cultivar, reaching the mature stage in a relatively short time.
The loss of juvenile characters proceeded more quickly in mother plants than in regenerated plants. The most obvious explanation is that since the mother plants scions were derived from adult plants, the rejuvenation process was limited to the first year after grafting. Conversely, regenerants by somatic embryogenesis retained the full juvenile potential for a longer period. However, when comparing scions originated by in vitro somatic embryogenesis to scions taken directly from mother plants in the fields, we always need to keep in mind that the somatic embryogenesis process removes most of the viral and endophytic communities of the parent tissue [53]. Therefore, it is possible that the differences in growth and juvenile characters were (also) a result of the interaction of the microbial community in mother plants, versus the “clean” scions derived from somatic embryos.
Most interestingly, we observed a different behavior in plants regenerated from stigma/style versus ovule explants. Regenerants from ovules showed a greater number and density of thorns and they never flowered within the three years of observation. Conversely, regenerants from stigma/style reduced juvenile characters 12–18 months after grafting in the terminal portion of some shoots, and many showed flowers and fruits in the third year. Since both types of regenerants were derived from somatic embryogenesis in vitro, they were originally both free of the viral and endophytic communities. Therefore, the observed differences probably depended on the source of genetic material. Regenerants derived from nucellus, which is a non-vascularized tissue of maternal origin, can be regarded as a pocket of juvenile tissue in an otherwise adult plant [54]. Our results support the hypothesis that the totipotent cells originated from different cell types are not equivalent, possibly by maintaining memory of their previously differentiated state, possibly by epigenetic modifications. Our future research will address the molecular mechanisms underlying the process of juvenility in regenerants.
Supplementary Materials
The following are available online at: https://www.mdpi.com/article/10.3390/plants11141811/s1, Figure S1: Flow cytometry and ploidy evaluation of regenerants: Citrus limon ‘Lunario’ (A), Citrus deliciosa ‘Tardivo di Ciaculli’ (B), Citrus sinensis ‘Valencia late’ (C), Citrus aurantium ‘AA CNR 31’ (D). Leaf nuclei suspensions were stained with DAPI. Each sample was analysed using the leaf nuclei of the relative mother plant as internal diploid standard (STD 2C). Nuclei DNA fluorescence intensity values and nuclei counts are shown on X and Y axes, respectively, Figure S2: Representative images of molecular markers: (A) Patterns of mother plant (M) and 16 regenerated plantlets (R1-16) of Citrus deliciosa ‘Tardivo di Ciaculli’ obtained with the ISSR primer UBC-835. L, 100 bp ladder; (B) Patterns of mother plant (M) and 16 regenerated plantlets (R1-16) of Citrus sinensis ‘Valencia late’ obtained with the RAPD primer OPM04. L, 100 bp ladder, Table S1: Primer sequences and annealing temperatures used for ISSR analysis, Table S2: Primer sequences used for RAPD analysis.
Author Contributions
Conceptualization, L.A., F.C. and A.C.; methodology, C.C., A.M., F.C. and A.C.; formal analysis, C.C., L.A., F.C. and A.C.; investigation, C.C., L.A., A.M., A.M.D., F.C. and A.C.; resources, A.M.; data curation, R.D.M. and A.C.; writing—original draft preparation, C.C., L.A., S.F.D.B., F.C., R.D.M., F.M., A.M.D. and A.C.; writing—review and editing, C.C., L.A., S.F.D.B., A.M., F.C., R.D.M., F.M., A.M.D. and A.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are openly available in Supplementary Materials here.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bednarek, P.T.; Orłowska, R. Plant tissue culture environment as a switch-key of (epi) genetic changes. Plant Cell Tissue Organ Cult. 2020, 140, 245–257. [Google Scholar] [CrossRef]
- Larkin, P.J.; Scowcroft, W.R. Somaclonal variation—A novel source of variability from cell cultures for plant improvement. Appl. Genet. 1981, 60, 197–214. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.; Gill, M.I.S.; Gosal, S.S. Somatic embryogenesis, in vitro selection and plantlet regeneration for citrus improvement. In Biotechnologies of Crop Improvement; Springer: Cham, Switzerland, 2018; Volume 1, pp. 373–406. [Google Scholar]
- Catalano, C.; Abbate, L.; Motisi, A.; Crucitti, D.; Cangelosi, V.; Pisciotta, A.; Di Lorenzo, R.; Carimi, F.; Carra, A. Autotetraploid Emergence via Somatic Embryogenesis in Vitis vinifera Induces Marked Morphological Changes in Shoots, Mature Leaves, and Stomata. Cells 2021, 10, 1336. [Google Scholar] [CrossRef]
- Bairu, M.W.; Aremu, A.O.; Van Staden, J. Somaclonal variation in plants: Causes and detection methods. Plant Growth Regul. 2011, 63, 147–173. [Google Scholar] [CrossRef]
- Omar, A.A.; Dutt, M.; Gmitter, F.G.; Grosser, J.W. Somatic embryogenesis: Still a relevant technique in citrus improvement. In In Vitro Embryogenesis in Higher Plants; Humana Press: New York, NY, USA, 2016; pp. 289–327. [Google Scholar]
- Zhang, S.; Liang, M.; Wang, N.; Xu, Q.; Deng, X.; Chai, L. Reproduction in woody perennial Citrus: An update on nucellar embryony and self-incompatibility. Plant Reprod. 2018, 31, 43–57. [Google Scholar] [CrossRef]
- Furr, J.R.; Cooper, W.C.; Reece, P.C. An investigation on flower formation in adult and juvenile citrus trees. Am. J. Bot. 1947, 34, 1–8. [Google Scholar] [CrossRef]
- Hackett, W.P. Juvenility, maturation, and rejuvenation in woody plants. Hortic. Rev. 1985, 7, 109–155. [Google Scholar]
- Huijser, P.; Schmid, M. The control of developmental phase transitions in plants. Development 2011, 138, 4117–4129. [Google Scholar] [CrossRef]
- Wang, J.W.; Park, M.Y.; Wang, L.J.; Koo, Y.; Chen, X.Y.; Weigel, D.; Poethig, R.S. MiRNA control of vegetative phase change in trees. PLoS Genet. 2011, 7, e1002012. [Google Scholar] [CrossRef]
- Snowball, A.M.; Warrington, I.J.; Halligan, E.A.; Mullins, M.G. Phase change in citrus: The effects of main stem node number, branch habit and paclobutrazol application on flowering in citrus seedlings. J. Hortic. Sci. 1994, 69, 149–160. [Google Scholar] [CrossRef]
- Snowball, A.M.; Halligan, E.A.; Warrington, I.J.; Mullins, M.G. Phase change in citrus: Growth and flowering of citrus seedlings from thirteen genetically diverse seedling families. J. Hortic. Sci. 1994, 69, 141–148. [Google Scholar] [CrossRef]
- Krajewski, A.J.; Rabe, E. Citrus flowering: A critical evaluation. J. Hortic. Sci. 1995, 70, 357–374. [Google Scholar] [CrossRef]
- Mitani, N.; Matsumoto, R.; Yoshioka, T.; Kuniga, T. Citrus hybrid seedlings reduce initial time to flower when grafted onto shiikuwasha rootstock. Sci. Hortic. 2008, 116, 452–455. [Google Scholar] [CrossRef]
- Nishikawa, F. Regulation of floral induction in citrus. J. Jpn. Soc. Hortic. Sci. 2013, 82, 283–292. [Google Scholar] [CrossRef]
- Caruso, M.; Smith, M.W.; Froelicher, Y.; Russo, G.; Gmitter, F.G., Jr. Traditional breeding. In The Genus Citrus; Woodhead Publishing: Sawston, UK, 2020; pp. 129–148. [Google Scholar]
- Cervera, M.; Juárez, J.; Navarro, L.; Peña, L. Genetic transformation of mature Citrus plants. In Transgenic Plants: Methods and Protocols. Methods in Molecular Biology; Peña, L., Ed.; Humana Press: Totowa, NJ, USA, 2005; Volume 286. [Google Scholar]
- Endo, T.; Shimada, T.; Fujii, H.; Kobayashi, Y.; Araki, T.; Omura, M. Ectopic expression of an FT homolog from Citrus confers an early flowering phenotype on trifoliate orange (Poncirus trifoliata L. Raf.). Transgenic Res. 2005, 14, 703–712. [Google Scholar] [CrossRef]
- Pena, L.; Cervera, M.; Fagoaga, C.; Romero, J.; Juarez, J.; Pina, J.A.; Navarro, L. Citrus. In Biotechnology in Agriculture and Forestry, Transgenic Crops; Pua, E.C., Davey, M.R., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2007; Volume 60, pp. 35–50. [Google Scholar]
- Velázquez, K.; Agüero, J.; Vives, M.C.; Aleza, P.; Pina, J.A.; Moreno, P.; Navarro, L.; Guerri, J. Precocious flowering of juvenile citrus induced by a viral vector based on Citrus leaf blotch virus: A new tool for genetics and breeding. Plant Biotechnol. J. 2016, 14, 1976–1985. [Google Scholar] [CrossRef]
- Salonia, F.; Ciacciulli, A.; Poles, L.; Pappalardo, H.D.; La Malfa, S.; Licciardello, C. New Plant Breeding Techniques in Citrus for the Improvement of Important Agronomic Traits. A Review. Front. Plant Sci. 2020, 11, 1234. [Google Scholar] [CrossRef]
- Sim, G.E.; Goh, C.J.; Loh, C.S. Micropropagation of Citrus mitis Blanco- multiple bud formation from shoot and root explants in the presence of 6-benzylaminopurine. Plant Sci. 1989, 59, 203–210. [Google Scholar] [CrossRef]
- Cervera, M.; Juárez, J.; Navarro, A.; Pina, J.A.; Duran-Vila, N.; Navarro, L.; Peña, L. Genetic transformation and regeneration n of mature tissues of woody fruit plants bypassing the juvenile stage. Transgenic Res. 1998, 7, 51–59. [Google Scholar] [CrossRef]
- Bassan, M.M.; Mourão Filho, F.A.A.; Miyata, L.Y.; Mendes, B.M.J. In vitro organogenesis from internodal segments of adult sweet orange plants. Pesqui. Agropecuária Bras. 2011, 46, 672–674. [Google Scholar] [CrossRef][Green Version]
- Cardoso, J.C.; Curtolo, M.; Latado, R.R.; Martinelli, A.P. Somatic embryogenesis of a seedless sweet orange (Citrus sinensis (L.) Osbeck). Vitr. Cell. Dev. Biol. Plant 2017, 53, 619–623. [Google Scholar] [CrossRef]
- Carimi, F.; Tortorici, M.C.; De Pasquale, F.; Crescimanno, F.G. Somatic embryogenesis and plant regeneration from undeveloped ovules and stigma/style explants of sweet orange navel group [Citrus sinensis (L.) Osb.]. Plant Cell Tissue Organ Cult. 1998, 54, 183–189. [Google Scholar] [CrossRef]
- Hidaka, T.; Yamada, Y.; Shichijo, T. Plantlet formation from anthers of C. aurantium L. Proc. Intl. Soc. Citric. 1981, 1, 153–155. [Google Scholar]
- Nito, N.; Iwamasa, M. In vitro plantlet formation from juice vesicle callus of satsuma (Citrus unshiu Marc.). Plant Cell Tissue Organ Cult. 1990, 20, 137–140. [Google Scholar] [CrossRef]
- Carimi, F.; De Pasquale, F.; Crescimanno, F.G. Somatic embryogenesis in Citrus from styles culture. Plant Sci. 1995, 105, 81–86. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- De Pasquale, F.; Giuffrida, S.; Carimi, F. Minigrafting of shoots, roots, inverted roots, and somatic embryos for rescue of in vitro Citrus regenerants. J. Am. Soc. Hort. Sci. 1999, 124, 152–157. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid DNA isolation procedure from small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Sambrook, J.; Fritsch, E.F.; Maniatis, T. Molecular Cloning. A Laboratory Manual, 2nd ed.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1989. [Google Scholar]
- Carra, A.; Sajeva, M.; Abbate, L.; Siragusa, M.; Pathirana, R.; Carimi, F. Factors affecting somatic embryogenesis in eight Italian grapevine cultivars and the genetic stability of embryo-derived regenerants as assessed by molecular markers. Sci. Hortic. 2016, 204, 123–127. [Google Scholar] [CrossRef]
- Fang, D.Q.; Roose, M.L. Identification of closely related citrus cultivars with inter-simple sequence repeat markers. Theor. Appl. Genet. 1997, 95, 408–417. [Google Scholar] [CrossRef]
- Coletta Filho, H.D.; Machado, M.A.; Targon, M.L.P.N.; Moreira, M.C.P.Q.D.G.; Pompeu, J., Jr. Analysis of the genetic diversity among mandarins (Citrus spp.) using RAPD markers. Euphytica 1998, 102, 133–139. [Google Scholar] [CrossRef]
- Ascenso, J.C.; Soost, R.K. Relationships between leaf surface area and linear dimension in seedling Citrus populations. J. Am. Soc. Hort. Sci. 1976, 101, 696–698. [Google Scholar]
- Ikeuchi, M.; Ogawa, Y.; Iwase, A.; Sugimoto, K. Plant regeneration: Cellular origins and molecular mechanisms. Development 2016, 143, 1442–1451. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.H.; Tang, L.P.; Zhao, X.Y.; Zhang, X.S. Plant cell totipotency: Insights into cellular reprogramming. J. Integr. Plant Biol. 2021, 63, 228–243. [Google Scholar] [CrossRef]
- Fehér, A. Callus, dedifferentiation, totipotency, somatic embryogenesis: What these terms mean in the era of molecular plant biology? Front. Plant Sci. 2019, 10, 536. [Google Scholar] [CrossRef]
- Rangan, S.; Murashige, T.; Bitters, W.P. In vitro initiation of nucellar embryons in monoembryonic citrus. HortScience 1968, 3, 226–227. [Google Scholar]
- Carimi, F.; De Pasquale, F.; Crescimanno, F.G. Somatic embryogenesis from styles of lemon (Citrus limon). Plant Cell Tissue Organ Cult. 1994, 37, 209–211. [Google Scholar] [CrossRef]
- D’Onghia, A.M.; De Pasquale, F.; Carimi, F.; Savino, V.; Crescimanno, F.G. Somatic embryogenesis from style culture as possible means for virus elimination in Citrus. J. Phytopath. 1997, 145, 77–79. [Google Scholar] [CrossRef]
- D’Onghia, A.M.; Carimi, F.; De Pasquale, F.; Djelouah, K.; Martelli, G.P. Somatic embryogenesis from style culture: A new technique for the sanitization, conservation and safe exchange of citrus germplasm. In Proceedings of the Int. Soc. Citric., ‘IXth International Citrus Congress’, Orlando, FL, USA, 3–7 December 2000; Volume 1, pp. 147–149. [Google Scholar]
- D’Onghia, A.M.; Carimi, F.; De Pasquale, F.; Djelouah, K.; Martelli, G.P. Elimination of Citrus psorosis virus by somatic embryogenesis from stigma and style cultures. Plant Pathol. 2001, 50, 266–269. [Google Scholar] [CrossRef]
- Meziane, M.; Frasheri, D.; Carra, A.; Boudjeniba, M.; D’Onghia, A.M.; Mercati, F.; Djelouah, K.; Carimi, F. Attempts to eradicate graft-transmissible infections through somatic embryogenesis in Citrus ssp. and analysis of genetic stability of regenerated plants. Eur. J. Plant Pathol. 2017, 148, 85–95. [Google Scholar] [CrossRef]
- El-Sawy, A.; Gomaa, A.; Abd-El-Zaher, M.H.; Reda, A.; Danial, N. Production of somatic embryogenesis via in vitro culture of stigma and style for elimination of Citrus psorosis virus (CPsV) from some citrus genotypes. J. Hort. Sci. Ornam. Plants 2013, 5, 110–117. [Google Scholar]
- Mahmoud, K.B.; Najar, A.; JedidI, E.; HamdI, I.; Jemmali, A. Detection of two viroids in the Tunisian sweet orange (Citrus sinensis L.) cv. Maltese and sanitation via somatic embryogenesis. J. Chem. Pharm. Res. 2017, 9, 154–159. [Google Scholar]
- Ballester, A.; Vieitez, A.M. Partial rejuvenation of mature hardwood trees through somatic embryogenesis: The example of pedunculate oak. In Proceedings of the IUFRO Working Party 2.09.02 Conference on “Integrating Vegetative Propagation, Biotechnologies and Genetic Improvement for Tree Production and Sustainable Forest Management”, Brno, Czech Republic, 25–28 June 2012. [Google Scholar]
- Heide, O.M. Juvenility, maturation and rejuvenation in plants: Adventitious bud formation as a novel rejuvenation process. J. Hortic. Sci. Biotechnol. 2019, 94, 2–11. [Google Scholar] [CrossRef]
- Devy, N.F.; Yenni, Y.; Hardiyanto, H. The growth performance of citrus derived from somatic embryogenesis plantlet and scion stock. Indones. J. Agric. Sci. 2014, 15, 71–78. [Google Scholar] [CrossRef]
- Carimi, F. Somatic embryogenesis and organogenesis in citrus for sanitation and in vitro conservation. In Improvement of the Citrus Sector by the Setting up of the Common Conservation Strategies for the Free Exchange of Healthy Citrus Genetic Resources; D’Onghia, A.M., Menini, U., Martelli, G.P., Eds.; Options Méditerranéennes: Série B; CIHEAM publications: Rome, Italy, 2001; Volume 33, pp. 115–128. [Google Scholar]
- Button, J.; Kochba, J. Tissue culture in the citrus industry. In Applied and Fundamental Aspects of Plant Cell, Tissue, and Organ Culture; Reinert, J., Bajaj, Y.P.S., Eds.; Springer: Berlin/Heidelberg, Germany, 1977; pp. 70–92. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).