The Changes of Microbial Communities and Key Metabolites after Early Bursaphelenchus xylophilus Invasion of Pinus massoniana
Abstract
1. Introduction
2. Result
2.1. Microbial Richness and Diversity Indices
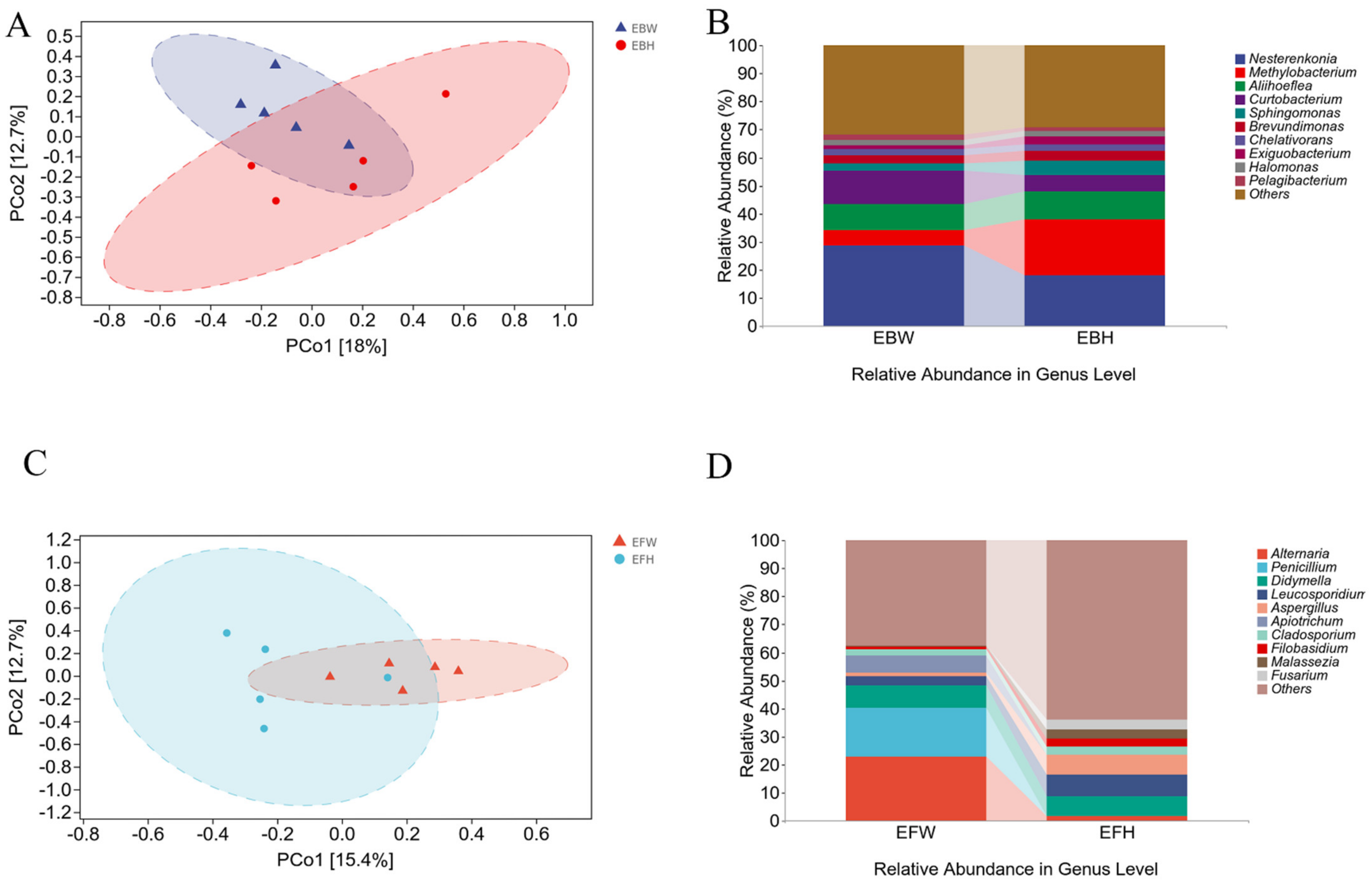
2.2. Diversity of Microbial Composition
2.3. Unique and Shared OTUs and the Key Marker OTU
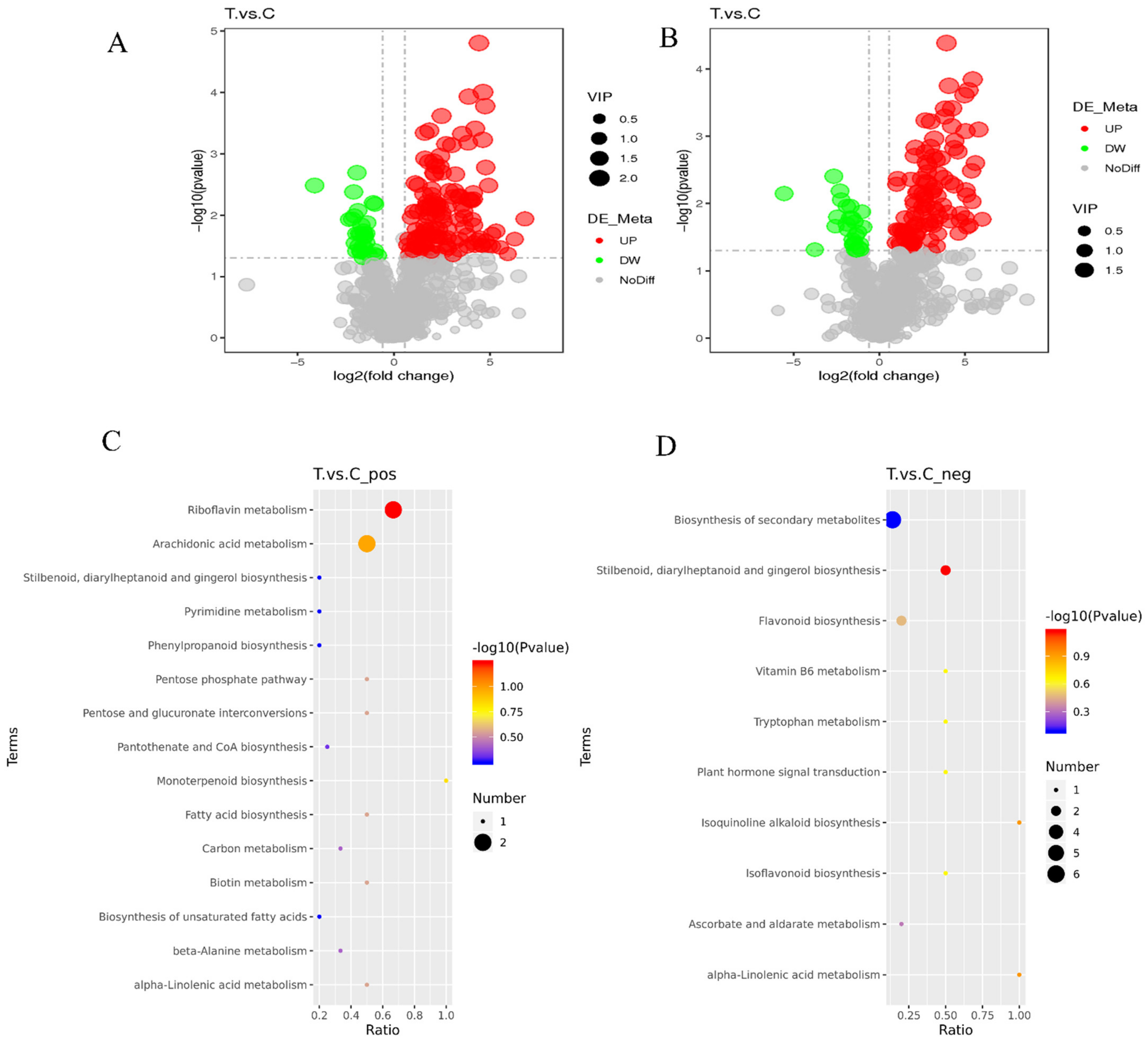
2.4. Difference Analysis between Metabolite Groups
2.5. Differential Metabolite Analysis and KEGG Metabolic Pathway Enrichment
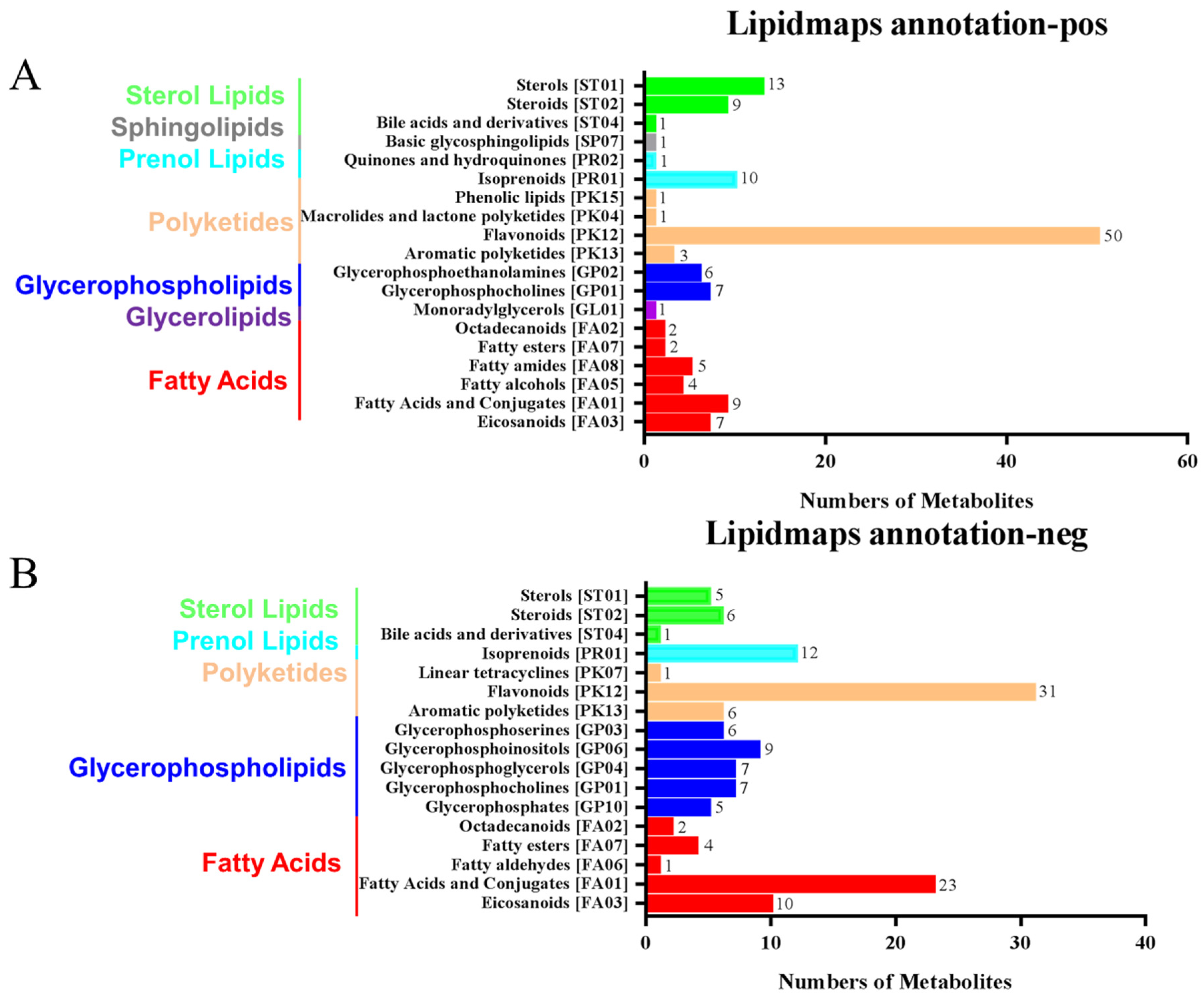
2.6. Annotation and Analysis of LIPID MAPS
3. Materials and Methods
3.1. Materials
3.2. Sample Handling
3.3. DNA Extraction and Amplicon Sequencing
3.4. Sequence Data Analysis
3.5. Bioinformatics and Statistical Analysis
3.6. Metabolites Extraction
3.6.1. Tissue Sample
3.6.2. UHPLC-MS/MS Analysis
3.6.3. Data Processing and Metabolite Identification
3.6.4. Data Analysis
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vicente, C.; Espada, M.; Vieira, P.; Mota, M. Pine Wilt Disease: A threat to European forestry. Eur. J. Plant Pathol. 2012, 133, 89–99. [Google Scholar] [CrossRef]
- Kojima, K.; Kamijyo, A.; Masumori, M.; Sasaki, S. Cellulase activities of pine-wood nematode isolates with different virulences. J. Jpn. For. Soc. 1994, 76, 258–262. [Google Scholar]
- Odani, K.; Sasaki, S.; Nishiyama, Y.; Yamamoto, N. Early Symptom Development of the Pine Wilt Disease by Hydrolytic Enzymes Produced by the Pine Wood Nematodes. Drug Saf. 2008, 38, 1032. [Google Scholar]
- Myers, R.F. Cambium destruction in conifers caused by pinewood nematodes. J. Nematol. 1986, 18, 398–402. [Google Scholar] [PubMed]
- Kuroda, K. The mechanism oftra—Cheid cavitation in trees infected with wilt diseases. Proc. IUFRO Work. Party 2002, 7, 17–23. [Google Scholar]
- Kawazu, K.; Zhang, H.; Kanzaki, H. Accumulation of benzoic acid in suspension cultured cells of Pinus thunbergii Parl. in response to phenylacetic acid administration. Biosci. Biotechnol. Biochem. 1996, 60, 1410–1412. [Google Scholar] [CrossRef]
- Oku, H. Phytotoxins in pine (Pinus spp.) wilt disease Bursaphelenchus xyophilus. Nippon. Nogeik Kaishi 1990, 7, 1254–1257. [Google Scholar] [CrossRef][Green Version]
- Oku, H. Role ofphytotoxins in pine wilt disease. J. Nematol. 1988, 2, 245–251. [Google Scholar]
- Wang, J.; Han, S.; Li, Y.; Deng, X.; Zhang, X. Cloning of TLP-1 Gene and Prediction of TLP-1 Protein Structure of Bursaphelenchus xylophilus. J. Sichuan Agric. Univ. 2014, 32, 305–310. [Google Scholar]
- Dang, Q.L.; Son, S.W.; Cheon, H.-M.; Choi, G.J.; Choi, Y.H.; Jang, K.S.; Lim, C.H.; Kim, J.-C. Pyochelin isolated from Burkholderia arboris KRICT1 carried by pine wood nematodes exhibits phytotoxicity in pine callus. Nematology 2011, 13, 521–528. [Google Scholar] [CrossRef]
- Oku, H.; Shiraishi, T.; Ouchi, S.; Kurozumi, S.; Ohta, H. Pine Wilt Toxin, the Metabolite of a Bacterium Associated with a Nematode. Naturwissenschaften 1980, 67, 198–199. [Google Scholar] [CrossRef]
- Nascimento, F.X.; Espada, M.; Barbosa, P.; Rossi, M.J.; Vicente, C.S.L.; Mota, M. Non-specific transient mutualism between the plant parasitic nematode, Bursaphelenchus xylophilus, and the opportunistic bacterium Serratia quinivorans BXF1, a plant-growth promoting pine endophyte with antagonistic effects. Environ. Microbiol. 2016, 18, 5265–5276. [Google Scholar] [CrossRef]
- Zhao, B.; Liu, Y.; Lin, F. Mutual influences in growth and reproduction between pine wood nematode Bursaphelenchus xylophilus and bacteria it carries. Front. For. China 2006, 1, 324–327. [Google Scholar] [CrossRef]
- Pires, D.; Vicente, C.S.L.; Inacio, M.L.; Mota, M. The Potential of Esteya spp. for the Biocontrol of the Pinewood Nematode, Bursaphelenchus xylophilus. Microorganisms 2022, 10, 168. [Google Scholar] [CrossRef]
- Marques-Pereira, C.; Proenca, D.N.; Morais, P.V. The Role of Serratomolide-like Amino Lipids Produced by Bacteria of Genus Serratia in Nematicidal Activity. Pathogens 2022, 11, 198. [Google Scholar] [CrossRef]
- Maehara, N. Reduction of Bursaphelenchus xylophilus (Nematoda: Parasitaphelenchidae) population by inoculating Trichoderma spp. into pine wilt-killed trees. Biol. Control 2008, 44, 61–66. [Google Scholar] [CrossRef]
- Ni, Z.; Zhou, P.; Xu, M.; Xu, L.-A. Development and characterization of chloroplast microsatellite markers for Pinus massoniana and their application in Pinus (Pinaceae) species. J. Genet. 2018, 97, 53–59. [Google Scholar] [CrossRef]
- Liu, Q.; Wei, Y.; Xu, L.; Hao, Y.; Chen, X.; Zhou, Z. Transcriptomic Profiling Reveals Differentially Expressed Genes Associated with Pine Wood Nematode Resistance in Masson Pine (Pinus massoniana Lamb.). Sci. Rep. 2017, 7, 4693. [Google Scholar] [CrossRef]
- Meng, F.; Li, Y.; Liu, Z.; Wang, X.; Feng, Y.; Zhang, W.; Zhang, X. Potential Molecular Mimicry Proteins Responsive to α-pinene in Bursaphelenchus xylophilus. Int. J. Mol. Sci. 2020, 21, 982. [Google Scholar] [CrossRef]
- Gershenzon, J. Metabolic costs of terpenoid accumulation in higher plants. J. Chem. Ecol. 1994, 20, 1281–1328. [Google Scholar] [CrossRef]
- Turlings, T.C.; Loughrin, J.H.; McCall, P.J.; Rose, U.S.; Lewis, W.J.; Tumlinson, J.H. How caterpillar-damaged plants protect themselves by attracting parasitic wasps. Proc. Natl. Acad. Sci. USA 1995, 92, 4169–4174. [Google Scholar] [CrossRef] [PubMed]
- Chao, A. Nonparametric-Estimation of the Number of Classes in a Population. Scand. J. Stat. 1984, 11, 265–270. [Google Scholar]
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Simpson, E.H. Measurement of Diversity. Nature 1949, 163, 688. [Google Scholar] [CrossRef]
- Pielou, E.C. The measurement of diversity in different types of biological collections. J. Theor. Biol. 1966, 13, 131–144. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Katoh, K.; Misawa, K.; Kuma, K.; Miyata, T. MAFFT: A novel method for rapid multiple sequence alignment based on fast Fourier transform. Nucleic Acids Res. 2002, 30, 3059–3066. [Google Scholar] [CrossRef]
- Bokulich, N.A.; Kaehler, B.D.; Rideout, J.R.; Dillon, M.; Bolyen, E.; Knight, R.; Huttley, G.A.; Caporaso, J.G. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome 2018, 6, 90. [Google Scholar] [CrossRef]
- Koljalg, U.; Nilsson, R.H.; Abarenkov, K.; Tedersoo, L.; Taylor, A.F.S.; Bahram, M.; Bates, S.T.; Bruns, T.D.; Bengtsson-Palme, J.; Callaghan, T.M.; et al. Towards a unified paradigm for sequence-based identification of fungi. Mol. Ecol. 2013, 22, 5271–5277. [Google Scholar] [CrossRef]
- Jaccard, P. Nouvelles recherches sur la distribution florale. Bull. Soc. Vaud. Des Sci. Nat. 1908, 44, 223–270. [Google Scholar]
- Bray, J.R.; Curtis, J.T. An ordination of the upland forest communities of southern wisconsin. Ecol. Monogr. 1957, 27, 326–349. [Google Scholar] [CrossRef]
- Lozupone, C.; Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 2005, 71, 8228–8235. [Google Scholar] [CrossRef]
- Lozupone, C.A.; Hamady, M.; Kelley, S.T.; Knight, R. Quantitative and qualitative beta diversity measures lead to different insights into factors that structure microbial communities. Appl. Environ. Microbiol. 2007, 73, 1576–1585. [Google Scholar] [CrossRef]
- Ramette, A. Multivariate analyses in microbial ecology. Fems Microbiol. Ecol. 2007, 62, 142–160. [Google Scholar] [CrossRef]
- Zaura, E.; Keijser, B.J.F.; Huse, S.M.; Crielaard, W. Defining the healthy “core microbiome” of oral microbial communities. BMC Microbiol. 2009, 9, 259. [Google Scholar] [CrossRef]
- Breiman, L. Random forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M.C. Classification and Regression by randomForest. R News 2002, 2, 18–22. [Google Scholar]
- Want, E.J.; Masson, P.; Michopoulos, F.; Wilson, I.D.; Theodoridis, G.; Plumb, R.S.; Shockcor, J.; Loftus, N.; Holmes, E.; Nicholson, J.K. Global metabolic profiling of animal and human tissues via UPLC-MS. Nat. Protoc. 2013, 8, 17–32. [Google Scholar] [CrossRef]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. Metax: A flexible and comprehensive software for processing metabolomics data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef] [PubMed]
- Han, G.; Mannaa, M.; Kim, N.; Jeon, H.W.; Jung, H.; Lee, H.H.; Kim, J.; Park, J.; Park, A.R.; Kim, J.C.; et al. Response of Pine Rhizosphere Microbiota to Foliar Treatment with Resistance-Inducing Bacteria against Pine Wilt Disease. Microorganisms 2021, 9, 688. [Google Scholar] [CrossRef] [PubMed]
- Proença, D.N.; Francisco, R.; Kublik, S.; Schöler, A.; Vestergaard, G.; Schloter, M.; Morais, P.V. The Microbiome of Endophytic, Wood Colonizing Bacteria from Pine Trees as Affected by Pine Wilt Disease. Sci. Rep. 2017, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, X.; Li, Y.; Liu, Z.; Li, D.; Wen, X.; Feng, Y.; Zhang, X. Pinewood Nematode Alters the Endophytic and Rhizospheric Microbial Communities of Pinus massoniana. Microb. Ecol. 2020, 81, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Tan, J.; Chen, F. Bacillus pumilus strain LYMC-3 shows nematicidal activity against Bursaphelenchus xylophilus via the production of a guanidine compound. Biocontrol Sci. Technol. 2018, 1, 12. [Google Scholar] [CrossRef]
- Proença, D.N.; Santo, C.E.; Grass, G.; Morais, P.V. Draft Genome Sequence of Serratia sp. Strain M24T3, Isolated from Pinewood Disease Nematode Bursaphelenchus xylophilus. J. Bacteriol. 2012, 194, 3764–3764. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, Y.; Wang, C.; Wang, Y.; Sung, C. Esteya vermicola controls the pinewood nematode, Bursaphelenchus xylophilus, in pine seedlings. Econ. Res.-Ekon. Istraživanja 2017, 49, 86–91. [Google Scholar] [CrossRef]
- Alves, M.; Pereira, A.; Vicente, C.; Matos, P.; Henriques, J.; Lopes, H.; Nascimento, F.; Mota, M.; Correia, A.; Henriques, I. The role of bacteria in pine wilt disease: Insights from microbiome analysis. FEMS Microbiol. Ecol. 2018, 94, fiy077. [Google Scholar] [CrossRef]
- Ma, Y.; Qu, Z.L.; Liu, B.; Tan, J.J.; Asiegbu, F.O.; Sun, H. Bacterial Community Structure of Pinus thunbergii Naturally Infected by the Nematode Bursaphelenchus xylophilus. Microorganisms 2020, 8, 307. [Google Scholar] [CrossRef]
- Xue, Q.; Xiang, Y.; Wu, X.-Q.; Li, M.-J. Bacterial Communities and Virulence Associated with Pine Wood Nematode Bursaphelenchus xylophilus from Different Pinus spp. Int. J. Mol. Sci. 2019, 20, 3342. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, L.; Zhou, J.; Yu, H.; Zhang, C.; Lv, Y.; Lin, Z.; Hu, S.; Zou, Z.; Sun, J. Enhancement of oxidative stress contributes to increased pathogenicity of the invasive pine wood nematode. Philos. Trans. R. Soc. B Biol. Sci. 2019, 374, 1767. [Google Scholar] [CrossRef]
- Han, L.-B.; Li, Y.-B.; Wang, F.-X.; Wang, W.-Y.; Liu, J.; Wu, J.-H.; Zhong, N.-Q.; Wu, S.-J.; Jiao, G.-L.; Wang, H.-Y.; et al. The Cotton Apoplastic Protein CRR1 Stabilizes Chitinase 28 to Facilitate Defense against the Fungal Pathogen Verticillium dahliae. Plant Cell 2019, 31, 520–536. [Google Scholar] [CrossRef]
- James, L.F.; Hartley, W.J.; Van Kampen, K.R. Syndromes of astragalus poisoning in livestock. J. Am. Vet. Med. Assoc. 1981, 178, 146–150. [Google Scholar]
- Braun, K.; Romero, J.; Liddell, C.; Creamer, R. Production of swainsonine by fungal endophytes of locoweed. Mycol. Res. 2003, 107, 980–988. [Google Scholar] [CrossRef]
- Grum, D.S.; Cook, D.; Baucom, D.; Mott, I.W.; Gardner, D.R.; Creamer, R.; Allen, J.G. Production of the Alkaloid Swainsonine by a Fungal Endophyte in the HostSwainsona canescens. J. Nat. Prod. 2013, 76, 1984–1988. [Google Scholar] [CrossRef]
- Ghallab, D.S.; Shawky, E.; Ibrahim, R.S.; Mohyeldin, M.M. Comprehensive metabolomics unveil the discriminatory metabolites of some Mediterranean Sea marine algae in relation to their cytotoxic activities. Sci. Rep. 2022, 12, 8094. [Google Scholar] [CrossRef]
- Salvatore, M.M.; Di Lelio, I.; DellaGreca, M.; Nicoletti, R.; Salvatore, F.; Russo, E.; Volpe, G.; Becchimanzi, A.; Mahamedi, A.E.; Berraf-Tebbal, A.; et al. Secondary Metabolites, including a New 5,6-Dihydropyran-2-One, Produced by the Fungus Diplodia corticola. Aphicidal Activity of the Main Metabolite, Sphaeropsidin A. Molecules 2022, 27, 2327. [Google Scholar] [CrossRef]
- Tohge, T.; Borghi, M.; Fernie, A.R. The natural variance of the Arabidopsis floral secondary metabolites. Sci. Data 2018, 5, 180051. [Google Scholar] [CrossRef]
- Mullin, M.; Klutsch, J.G.; Cale, J.A.; Hussain, A.; Zhao, S.; Whitehouse, C.; Erbilgin, N. Primary and Secondary Metabolite Profiles of Lodgepole Pine Trees Change with Elevation, but Not with Latitude. J. Chem. Ecol. 2021, 47, 280–293. [Google Scholar] [CrossRef]
- Castander-Olarieta, A.; Montalbán, I.A.; De Medeiros Oliveira, E.; Dell’Aversana, E.; D’Amelia, L.; Carillo, P.; Steiner, N.; Fraga, H.P.D.F.; Guerra, M.P.; Goicoa, T.; et al. Effect of Thermal Stress on Tissue Ultrastructure and Metabolite Profiles During Initiation of Radiata Pine Somatic Embryogenesis. Front. Plant Sci. 2019, 9, 2004. [Google Scholar] [CrossRef]
- Cale, J.A.; Klutsch, J.G.; Dykstra, C.B.; Peters, B.; Erbilgin, N. Pathophysiological responses of pine defensive metabolites largely lack differences between pine species but vary with eliciting ophiostomatoid fungal species. Tree Physiol. 2019, 39, 1121–1135. [Google Scholar] [CrossRef] [PubMed]
- Bentivenha, J.P.F.; Canassa, V.F.; Baldin, E.L.L.; Borguini, M.G.; Lima, G.P.P.; Lourencao, A.L. Role of the Rutin and Genistein Flavonoids in Soybean Resistance to Piezodorus guildinii (Hemiptera: Pentatomidae). Arthropod-Plant Interact. 2018, 12, 311–320. [Google Scholar] [CrossRef]
- Yao, Q.; Peng, Z.; Tong, H.; Yang, F.; Xing, G.; Wang, L.; Zheng, J.; Zhang, Y.; Su, Q. Tomato Plant Flavonoids Increase Whitefly Resistance and Reduce Spread of Tomato yellow leaf curl virus. J. Econ. Entomol. 2019, 112, 2790–2796. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Chen, Q.; Bu, Y.; Luo, R.; Hao, S.; Zhang, J.; Tian, J.; Yao, Y. Flavonoid Accumulation Plays an Important Role in the Rust Resistance of Malus Plant Leaves. Front. Plant Sci. 2017, 8, 1286. [Google Scholar] [CrossRef]
- Lee, I.H.; Han, H.; Koh, Y.H.; Kim, I.S.; Lee, S.-W.; Shim, D. Comparative Transcriptome Analysis of Pinus densiflora Following Inoculation with Pathogenic (Bursaphelenchus xylophilus) or Non-pathogenic Nematodes (B. thailandae). Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, R.; Li, D.; Wang, F. Transcriptomic and Coexpression Network Analyses Revealed Pine Chalcone Synthase Genes Associated with Pine Wood Nematode Infection. Int. J. Mol. Sci. 2021, 22, 11195. [Google Scholar] [CrossRef]
- Abelleira, A.; Picoaga, A.; Mansilla, J.P.; Aguin, O. Detection of Bursaphelenchus xylophilus, Causal Agent of Pine Wilt Disease on Pinus pinaster in Northwestern Spain. Plant Dis. 2011, 95, 776. [Google Scholar] [CrossRef]




| Samples | Species Richness Indices | |
|---|---|---|
| Location | Host Status | OTUs Observed |
| Endophytic bacteria (EB) | Wilted pines | 160.40 ± 35.78 |
| Healthy pines | 199.00 ± 104.60 | |
| Endophytic fungi (EF) | Wilted pines | 49.80 ± 25.08 |
| Healthy pines | 72.20 ± 42.59 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, Y.; Li, Y.; Ma, L.; Li, D.; Zhang, W.; Feng, Y.; Liu, Z.; Wang, X.; Wen, X.; Zhang, X. The Changes of Microbial Communities and Key Metabolites after Early Bursaphelenchus xylophilus Invasion of Pinus massoniana. Plants 2022, 11, 2849. https://doi.org/10.3390/plants11212849
An Y, Li Y, Ma L, Li D, Zhang W, Feng Y, Liu Z, Wang X, Wen X, Zhang X. The Changes of Microbial Communities and Key Metabolites after Early Bursaphelenchus xylophilus Invasion of Pinus massoniana. Plants. 2022; 11(21):2849. https://doi.org/10.3390/plants11212849
Chicago/Turabian StyleAn, Yibo, Yongxia Li, Ling Ma, Dongzhen Li, Wei Zhang, Yuqian Feng, Zhenkai Liu, Xuan Wang, Xiaojian Wen, and Xingyao Zhang. 2022. "The Changes of Microbial Communities and Key Metabolites after Early Bursaphelenchus xylophilus Invasion of Pinus massoniana" Plants 11, no. 21: 2849. https://doi.org/10.3390/plants11212849
APA StyleAn, Y., Li, Y., Ma, L., Li, D., Zhang, W., Feng, Y., Liu, Z., Wang, X., Wen, X., & Zhang, X. (2022). The Changes of Microbial Communities and Key Metabolites after Early Bursaphelenchus xylophilus Invasion of Pinus massoniana. Plants, 11(21), 2849. https://doi.org/10.3390/plants11212849






