HPLC-DAD-MS Identification and Quantification of Phenolic Components in Japanese Knotweed and American Pokeweed Extracts and Their Phytotoxic Effect on Seed Germination
Abstract
:1. Introduction
2. Results and Discussion
2.1. Phytochemical Analysis of Japanese Knotweed and American Pokeweed Extract and Phytotoxic Activity of Their Extracts
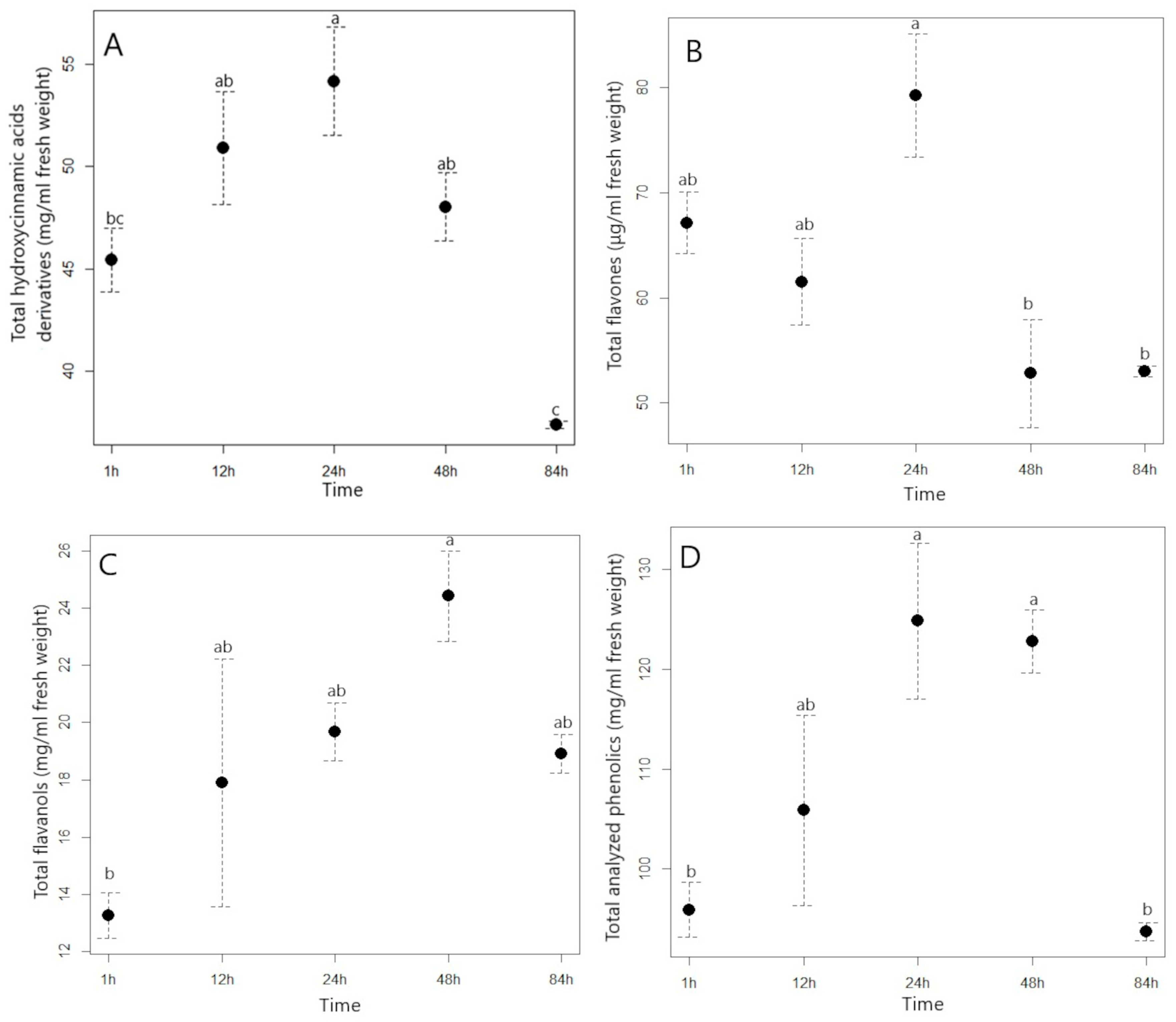
2.2. Effect of Solvent Type and Extraction Time on Phytochemical Extraction from Japanese Knotweed and American Pokeweed
3. Materials and Methods
3.1. Plant Material
3.2. Preparation of Aqueous Extracts
3.3. Seed Germination
3.4. Effect of Solvent on the Extraction Efficiency of Phenolic Compounds
3.5. Effect of Time on the Content of Phenolic Compounds in the Aqueous Extract
3.6. HPLC-DAD Analysis and Identification of Phenolic Compounds
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abgrall, C.; Forey, E.; Mignot, L.; Chauvat, M. Invasion by Fallopia japonica alters soil food webs through secondary metabolites. Soil Biol. Biochem. 2018, 127, 100–109. [Google Scholar] [CrossRef] [Green Version]
- Feng, Q.W.; Wang, B.; Chen, M.; Wu, P.; Lee, X.; Xing, Y. Invasive plants as potential sustainable feedstocks for biochar production and multiple applications: A review. Resour. Conserv. Recycl. 2021, 164, 105204. [Google Scholar] [CrossRef]
- Wang, C.X.; Zhu, M.X.; Chen, X.H.; Qu, B. Review on Allelopathy of Exotic Invasive Plants. In Proceedings of the 2nd SREE Conference on Chemical Engineering (CCE), Macao, China, 17–18 December 2011. [Google Scholar]
- Li, Z.R.; Amist, N.; Bai, L.Y. Allelopathy in sustainable weeds management. Allelopathy J. 2019, 48, 109–138. [Google Scholar] [CrossRef]
- Kalisz, S.; Kivlin, S.N.; Bialic-Murphy, L. Allelopathy is pervasive in invasive plants. Biol. Invasions 2021, 23, 367–371. [Google Scholar] [CrossRef]
- Inderjit; Duke, S.O. Ecophysiological aspects of allelopathy. Planta 2003, 217, 529–539. [Google Scholar] [CrossRef]
- Dahiya, S.; Kumar, S.; Khedwal, R.S.; Jakhar, S.R. Allelopathy for sustainable weed management. J. Pharmacogn. Phytochem. 2017, 6, 832–837. [Google Scholar]
- Gniazdowska, A.; Bogatek, R. Allelopathic interactions between plants. Multi site action of allelochemicals. Acta Physiol. Plant. 2005, 27, 395–407. [Google Scholar] [CrossRef]
- Schandry, N.; Becker, C. Allelopathic Plants: Models for Studying Plant-Interkingdom Interactions. Trends Plant Sci. 2020, 25, 176–185. [Google Scholar] [CrossRef] [Green Version]
- Muzolf-Panek, M.; Stuper-Szablewska, K. Comprehensive study on the antioxidant capacity and phenolic profiles of black seed and other spices and herbs: Effect of solvent and time of extraction. J. Food Meas. Charact. 2021, 15, 4561–4574. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Hirun, S.; Roach, P.D.; Bowyer, M.C.; Phillips, P.A.; Scarlett, C.J. Effect of extraction conditions on total phenolic compounds and antioxidant activities of Carica papaya leaf aqueous extracts. J. Herb. Med. 2013, 3, 104–111. [Google Scholar] [CrossRef]
- Naczk, M.; Shahidi, F. Extraction and analysis of phenolics in food. J. Chromatogr. A 2004, 1054, 95–111. [Google Scholar] [CrossRef]
- Mojzer, E.B.; Hrncic, M.K.; Skerget, M.; Knez, Z.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soural, I.; Vrchotova, N.; Triska, J.; Balik, J.; Hornik, S.; Curinova, P.; Sykora, J. Various extraction methods for obtaining stilbenes from grape cane of Vitis vinifera L. Molecules 2015, 20, 6093–6112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roby, M.H.H.; Sarhan, M.A.; Selim, K.A.H.; Khalel, K.I. Evaluation of antioxidant activity, total phenols and phenolic compounds in thyme (Thymus vulgaris L.), sage (Salvia officinalis L.), and marjoram (Origanum majorana L.) extracts. Ind. Crops Prod. 2013, 43, 827–831. [Google Scholar] [CrossRef]
- Sepahpour, S.; Selamat, J.; Manap, M.Y.A.; Khatib, A.; Razis, A.F.A. Comparative analysis of chemical composition, antioxidant activity and quantitative characterization of some phenolic compounds in selected herbs and spices in different solvent extraction systems. Molecules 2018, 23, 402. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokrani, A.; Madani, K. Effect of solvent, time and temperature on the extraction of phenolic compounds and antioxidant capacity of peach (Prunus persica L.) fruit. Sep. Purif. Technol. 2016, 162, 68–76. [Google Scholar] [CrossRef]
- Canadas, R.; Gonzalez-Miquel, M.; Gonzalez, E.J.; Diaz, I.; Rodriguez, M. Evaluation of bio-based solvents for phenolic acids extraction from aqueous matrices. J. Mol. Liq. 2021, 338, 116930. [Google Scholar] [CrossRef]
- Lachowicz, S.; Oszmianski, J.; Wojdylo, A.; Cebulak, T.; Hirnle, L.; Siewinski, M. UPLC-PDA-Q/TOF-MS identification of bioactive compounds and on-line UPLC-ABTS assay in Fallopia japonica Houtt and Fallopia sachalinensis (F. Schmidt) leaves and rhizomes grown in Poland. Eur. Food Res. Technol. 2019, 245, 691–706. [Google Scholar] [CrossRef] [Green Version]
- Vrchotova, N.; Sera, B. Allelopathic properties of knotweed rhizome extracts. Plant Soil Environ. 2008, 54, 301–303. [Google Scholar] [CrossRef] [Green Version]
- Soln, K.; Znidarsic, N.; Koce, J.D. Root growth inhibition and ultrastructural changes in radish root tips after treatment with aqueous extracts of Fallopia japonica and F. xbohemica rhizomes. Protoplasma 2022, 259, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Marinas, I.C.; Oprea, E.; Geana, E.I.; Luntraru, C.M.; Gird, C.E.; Chifiriuc, M.C. Chemical composition, antimicrobial and antioxidant activity of Phytolacca americana L. fruits and leaves extracts. Farmacia 2021, 69, 883–889. [Google Scholar] [CrossRef]
- Kim, Y.O.; Johnson, J.D.; Lee, E.J. Phytotoxicity of Phytolacca americana leaf extracts on the growth, and physiological response of Cassia mimosoides. J. Chem. Ecol. 2005, 31, 2963–2974. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Moreno, N.; Volpe, F.; Moler, J.A.; Esparza, I.; Ancin-Azpilicueta, C. Impact of extraction conditions on the phenolic composition and antioxidant capacity of grape stem extracts. Antioxidants 2019, 8, 597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanoeva, J.P.; Balshikevska, E.; Stefova, M.; Tusevski, O.; Simic, S.G. Comparison of the effect of acids in solvent mixtures for extraction of phenolic compounds from Aronia melanocarpa. Nat. Prod. Commun. 2020, 15, 1–10. [Google Scholar] [CrossRef]
- Kekeçoğlu, M.; Sorucu, A. Determination of The effect of green extraction solvents on the phenolic acids and flavonoids of propolis. J. Res. Vet. Med. 2021, 40, 49–54. [Google Scholar] [CrossRef]
- Al-Muwaly, K.Y.; Al-Flayeh, K.A.; Ali, A.A. Antioxidant and free radical scavenging effects of Iraqi sumac (Rhus coriaria L). Baghdad Sci. J. 2013, 10, 921–932. [Google Scholar]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.P.D.; Forster-Carneiro, T.; Vazquez-Espinosa, M.; Gonzalez-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of flavonoids from natural sources using modern techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef]
- Grigonis, D.; Venskutonis, P.R.; Sivik, B.; Sandahl, M.; Eskilsson, C.S. Comparison of different extraction techniques for isolation of antioxidants from sweet grass (Hierochloe odorata). J. Supercrit. Fluids 2005, 33, 223–233. [Google Scholar] [CrossRef]
- Altiok, E.; Baycin, D.; Bayraktar, O.; Ulku, S. Isolation of polyphenols from the extracts of olive leaves (Olea europaea L.) by adsorption on silk fibroin. Sep. Purif. Technol. 2008, 62, 342–348. [Google Scholar] [CrossRef] [Green Version]
- Spigno, G.; Trarnelli, L.; De Faveri, D.M. Effects of extraction time, temperature and solvent on concentration and antioxidant activity of grape marc phenolics. J. Food Eng. 2007, 81, 200–208. [Google Scholar] [CrossRef]
- Alide, T.; Wangila, P.; Kiprop, A. Effect of cooking temperature and time on total phenolic content, total flavonoid content and total in vitro antioxidant activity of garlic. BMC Res. Notes 2020, 13, 564. [Google Scholar] [CrossRef]
- Sulaiman, I.S.C.; Basri, M.; Masoumi, H.R.F.; Chee, W.J.; Ashari, S.E.; Ismail, M. Effects of temperature, time, and solvent ratio on the extraction of phenolic compounds and the anti-radical activity of Clinacanthus nutans Lindau leaves by response surface methodology. Chem. Cent. J. 2017, 11, 54. [Google Scholar] [CrossRef] [Green Version]
- Vergara-Salinas, J.R.; Perez-Jimenez, J.; Torres, J.L.; Agosin, E.; Perez-Correa, J.R. Effects of temperature and time on polyphenolic content and antioxidant activity in the pressurized hot water extraction of deodorized thyme (Thymus vulgaris). J. Agric. Food Chem. 2012, 60, 10920–10929. [Google Scholar] [CrossRef]
- Lovric, V.; Putnik, P.; Kovacevic, D.B.; Jukic, M.; Dragovic-Uzelac, V. Effect of microwave-assisted extraction on the phenolic compounds and antioxidant capacity of blackthorn flowers. Food Technol. Biotechnol. 2017, 55, 243–250. [Google Scholar] [CrossRef]
- Park, J.J.; Lee, W.Y. Adsorption and desorption characteristics of a phenolic compound from Ecklonia cava on macroporous. Food Chem. 2021, 338, 128150. [Google Scholar] [CrossRef]
- Ma, C.Y.; Tao, G.J.; Tang, J.; Lou, Z.X.; Wang, H.X.; Gu, X.H.; Hu, L.M.; Yin, M.L. Preparative separation and purification of rosavin in Rhodiola rosea by macroporous adsorption resins. Sep. Purif. Technol. 2009, 69, 22–28. [Google Scholar] [CrossRef]
- Romero-Perez, A.I.; Lamuela-Raventos, R.M.; Andres-Lacueva, C.; de la Torre-Boronat, M.C. Method for the quantitative extraction of resveratrol and piceid isomers in grape berry skins. Effect of powdery mildew on the stilbene content. J. Agric. Food Chem. 2001, 49, 210–215. [Google Scholar] [CrossRef]
- Hapsari, S.; Yohed, I.; Kristianita, R.A.; Jadid, N.; Aparamarta, H.W.; Gunawan, S. Phenolic and flavonoid compounds extraction from Calophyllum inophyllum leaves. Arab. J. Chem. 2022, 15, 103666. [Google Scholar] [CrossRef]
- Chigayo, K.; Mojapelo, P.E.L.; Mnyakeni-Moleele, S.; Misihairabgwi, J.M. Phytochemical and antioxidant properties of different solvent extracts of Kirkia wilmsii tubers. Asian Pac. J. Trop. Biomed. 2016, 6, 1037–1043. [Google Scholar] [CrossRef] [Green Version]
- Mikulic-Petkovsek, M.; Koron, D.; Rusjan, D. The impact of food processing on the phenolic content in products made from juneberry (Amelanchier lamarckii) fruits. J. Food Sci. 2020, 85, 386–393. [Google Scholar] [CrossRef] [PubMed]
- Mikulic-Petkovsek, M.; Stampar, F.; Veberic, R.; Sircelj, H. Wild Prunus fruit species as a rich source of bioactive compounds. J. Food Sci. 2016, 81, C1928–C1937. [Google Scholar] [CrossRef] [PubMed]


| Phenolic Group | Methanol Extract | Water Extract |
|---|---|---|
| Total hydroxybenzoic acids derivatives | 0.00 ± 0.00 | 273.74 ± 24.17 |
| Gallic acid | / | 16.367 ± 1366 |
| Galloylhexoside 1 | / | 83.49 ± 7.64 |
| Galloylhexoside 2 | / | 26.57 ± 3.41 |
| Total hydroxycinnamic acid derivatives | 18,757.57 ± 2016.84 b | 28,600.34 ± 592.94 a |
| 3-feruloylquinic acid | 4.71 ± 0.55 a | 8.28 ± 2.66 a |
| 3-caffeoylquinic acid | 751.44 ± 131.32 b | 1752.37 ± 68.72 a |
| 3-p-coumaroylquinic acid | 73.19 ± 25.99 a | 109.65 ± 5.82 a |
| 4-caffeoylquinic acid | 72.13 ± 7.49 b | 110.05 ± 2.12 a |
| 4-p-coumaroylquinic acid | 28.55 ± 13.44 a | 47.60 ± 7.06 a |
| 5-p-coumaroylquinic acid 1 | 31.15 ± 7.38 a | 18.62 ± 3.88 a |
| 5-p-coumaroylquinic acid 2 | 71.04 ± 10.04 a | 54.50 ± 6.21 a |
| 5-caffeoylquinic acid 1 | 396.09 ± 61.43 a | 414.68 ± 13.65 a |
| 5-caffeoylquinic acid 2 | 73.68 ± 11.78 b | 135.15 ± 5.14 a |
| Caftaric acid 1 | 16,805.52 ± 1744.96 b | 25,640.47 ± 494.09 a |
| Caftaric acid 2 | 44.26 ± 7.08 b | 81.19 ± 3.09 a |
| Caffeic acid hexoside | 35.24 ± 11.91 a | 31.53 ± 1.92 a |
| cis-coutaric acid | 33.86 ± 8.02 a | 20.24 ± 4.22 a |
| Dicaffeoylquinic acid 1 | 57.01 ± 1.74 a | 3.86 ± 0.47 b |
| Dicaffeoylquinic acid 2 | 9.69 ± 1.98 a | 1.54 ± 0.63 b |
| Ferulic acid pentoside | 99.32 ± 14.04 a | 76.20 ± 8.68 a |
| trans-coutaric acid | 158.63 ± 15.72 a | 84.45 ± 13.04 b |
| p-coumaric acid | 2.71 ± 0.64 a | 1.62 ± 0.34 a |
| p-coumaric acid hexoside | 9.36 ± 3.16 a | 8.37 ± 0.51 a |
| Total flavanols | 26,898.14 ± 2305.39 a | 21,607.29 ± 1812.44 a |
| Epicatechin | 579.84 ± 92.73 b | 1063.67 ± 40.43 a |
| Catechin hexoside | 753.68 ± 307.75 a | 552.90 ± 21.23 a |
| Catechin gallate | 62.75 ± 3.47 a | 18.54 ± 4.17 b |
| Procyanidin dimer 1 | 368.62 ± 105.33 b | 831.28 ± 17.67 a |
| Procyanidin dimer 2 | 3966.48 ± 466.60 b | 8588.65 ± 847.48 a |
| Procyanidin dimer 3 | 1380.08 ± 76.25 a | 407.87 ± 91.61 b |
| Procyanidin tetramer 1 | 59.87 ± 9.57 b | 109.82 ± 4.17 a |
| Procyanidin tetramer 2 | 5033.12 ± 496.56 a | 1579.94 ± 110.01 b |
| Procyanidin tetramer 3 | 1157.61 ± 114.73 a | 555.98 ± 61.67 b |
| Procyanidin tetramer 4 | 591.46 ± 32.68 a | 174.80 ± 39.26 b |
| Procyanidin trimer 1 | 369.32 ± 57.28 a | 386.66 ± 12.73 a |
| Procyanidin trimer 2 | 118.99 ± 14.00 b | 257.66 ± 25.42 a |
| Procyanidin trimer 3 | 3390.53 ± 422.49 a | 2297.43 ± 286.87 a |
| Procyanidin trimer 4 | 384.65 ± 54.38 a | 295.10 ± 33.61 a |
| Procyanidin trimer 5 | 83.62 ± 11.82 a | 64.15 ± 7.31 a |
| Procyanidin trimer 6 | 2841.72 ± 471.16 a | 1929.60 ± 302.69 a |
| Procyanidin trimer 7 | 5755.80 ± 394.09 a | 2493.25 ± 93.01 b |
| Flavones | 85.98 ± 2.63 a | 5.23 ± 0.26 b |
| Apigenin hexoside | 85.98 ± 2.63 a | 5.23 ± 0.26 b |
| Total flavonols | 17,630.81 ± 524.29 a | 1223.46 ± 103.51 b |
| Isorhamnetin hexoside | 3.22 ± 0.20 a | 0.21 ± 0.03 b |
| Kaempferol hexoside | 40.17 ± 1.42 a | 3.48 ± 0.39 b |
| Kaempferol-3-rhamnoside | 165.97 ± 7.31 a | 7.92 ± 1.11 b |
| Kaempferol-3-rutinoside | 6.94 ± 0.28 a | 0.41 ± 0.03 b |
| Quercetin acetyl hexoside | 0.12 ± 0.02 a | 0.03 ± 0.00 b |
| Quercetin dihexoside | 89.52 ± 6.56 a | 30.77 ± 1.00 b |
| Quercetin-3-arabinofuranoside | 931.80 ± 30.98 a | 10.77 ± 0.57 b |
| Quercetin-3-arabinopyranoside | 245.01 ± 15.10 a | 15.69 ± 1.98 b |
| Quercetin-3-galactoside | 473.79 ± 20.21 a | 46.56 ± 5.25 b |
| Quercetin-3-glucoside | 517.85 ± 18.25 a | 44.85 ± 4.97 b |
| Quercetin-3-rhamnoside | 146,36.70 ± 447.69 a | 951.59 ± 84.85 b |
| Quercetin-3-rutinoside | 151.41 ± 6.68 a | 85.70 ± 12.42 b |
| Quercetin-3-xyloside | 346.18 ± 14.07 a | 20.47 ± 1.76 b |
| Myricetin-3-rhamnoside | 22.15 ± 1.91 a | 5.02 ± 0.32 b |
| Quinones | 2.06 ± 0.42 a | 0.41 ± 0.08 b |
| Emodin hexoside | 2.06 ± 0.42 a | 0.41 ± 0.08 b |
| Total stilbenes | 3404.14 ± 307.54 a | 1471.23 ± 126.74 b |
| Astringin | 13.70 ± 2.27 a | 8.50 ± 0.99 a |
| cis-Resveratroloside | 19.64 ± 0.65 a | 0.24 ± 0.03 b |
| Piceatannol hexoside 1 | 345.69 ± 34.16 a | 325.46 ± 49.51 a |
| Piceatannol hexoside 2 | 23.76 ± 1.31 a | 8.16 ± 0.82 b |
| trans-Resveratroloside | 1188.06 ± 65.64 a | 407.98 ± 40.86 b |
| trans-Piceid 1 | 1628.03 ± 217.67 a | 673.22 ± 92.44 b |
| trans-Piceid 2 | 185.25 ± 15.95 a | 47.68 ± 4.17 b |
| Plants | Concentration (g/mL) | Labels | Germination (%) | Shoot Length (mm) | Root Length (mm) |
|---|---|---|---|---|---|
| Control J. knotweed | 97.50 ± 1.71 a | 28.03 ± 1.09 a | 20.57 ± 0.88 a | ||
| 0.2 0.15 | 1 2 | 50.00 ± 10.21 b 60.00 ± 7.91 b | 13.38 ± 1.09 b 14.35 ± 0.96 b | 1.03 ± 0.25 b 1.56 ± 0.30 b |
| Compound | Methanol Extract | Water Extract |
|---|---|---|
| Total betalains | 1200.55 ± 170.07 | 0.00 ± 0.00 |
| Apiosylisobetanin | 116.91 ± 9.70 | / |
| Apiosylisobetanin | 93.32 ± 25.75 | / |
| Betanin isomer 1 | 97.19 ± 16.18 | / |
| Betanin isomer 2 | 562.09 ± 47.72 | / |
| Betanin isomer 3 | 331.04 ± 79.84 | / |
| Total hydroxycinamic acid derivatives | 2471.39 ± 185.17 a | 2355.75 ± 127.97 a |
| 3-p-coumaroylquinic acid | 47.53 ± 3.52 a | 45.88 ± 7.55 a |
| 3-feruloylquinic acid | 21.63 ± 1.45 a | 19.22 ± 1.24 a |
| 3-caffeoylquinic acid | 452.80 ± 29.44 a | 459.32 ± 44.51 a |
| 4-caffeoylquinic acid | 1456.87 ± 98.95 a | 1248.71 ± 81.36 a |
| 4-p-coumaroylquinic acid | 34.65 ± 1.56 b | 60.84 ± 7.32 a |
| 5-caffeoylquinic acid 1 | 291.78 ± 101.72 a | 322.34 ± 59.49 a |
| 5-caffeoylquinic acid 2 | 42.28 ± 2.11 a | 58.70 ± 10.04 a |
| 5-p-coumaroylquinic acid 1 | 53.67 ± 3.39 a | 69.34 ± 11.47 a |
| 5-p-coumaroylquinic acid 2 | 11.82 ± 2.59 a | 31.55 ± 8.35 a |
| Dicaffeoylquinic acid 1 | 11.16 ± 0.80 a | 1.74 ± 0.06 b |
| Dicaffeoylquinic acid 2 | 32.62 ± 1.41 a | 17.32 ± 1.50 b |
| p-coumaric acid hexoside | 12.99 ± 5.24 a | 18.69 ± 5.28 a |
| p-coumaric acid | 1.61 ± 0.10 a | 2.10 ± 0.35 a |
| Total flavanols | 735.67 ± 109.60 a | 827.82 ± 199.75 a |
| Epicatechin | 139.24 ± 6.96 a | 193.33 ± 33.06 a |
| Catechin | 62.83 ± 4.65 a | 60.66 ± 9.99 a |
| Catechin gallate | 213.44 ± 41.40 a | 229.53 ± 62.86 a |
| Catechin hexoside | 320.16 ± 62.10 a | 344.30 ± 94.29 a |
| Flavone | 5.17 ± 0.22 a | 2.85 ± 0.19 b |
| Apigenin dihexoside | 5.17 ± 0.22 a | 2.85 ± 0.19 b |
| Total flavonols | 4045.24 ± 168.42 a | 770.05 ± 21.32 b |
| Kaempferol glucuronyl dihexoside | 65.02 ± 3.30 a | 9.70 ± 1.19 b |
| Kaempferol glucuronyl pentoside hexoside | 60.72 ± 4.04 a | 4.29 ± 1.07 b |
| Kaempferol hexoside | 272.05 ± 19.39 a | 42.51 ± 1.57 b |
| Kaempferol-3-rutinoside | 291.74 ± 4.97 a | 80.32 ± 1.59 b |
| Kaempferol pentosyl hexoside | 1183.53 ± 39.52 a | 122.43 ± 1.55 b |
| Kaempferol rhamnosyl dihexoside | 91.50 ± 4.38 a | 37.66 ± 3.00 b |
| Quercetin-3-xyloside | 22.62 ± 1.35 b | 32.98 ± 3.30 a |
| Quercetin-3-arabinofuranoside | 4.08 ± 0.29 a | 0.64 ± 0.02 b |
| Quercetin dihexoside | 36.69 ± 3.95 a | 13.41 ± 3.17 b |
| Quercetin pentosyl hexoside 1 | 184.51 ± 9.12 a | 39.03 ± 1.75 b |
| Quercetin pentosyl hexoside 2 | 1087.10 ± 52.53 a | 193.92 ± 4.11 b |
| Quercetin rhamnosyl hexoside | 134.70 ± 6.66 a | 28.49 ± 1.28 b |
| Quercetin-3-glucoside | 611.00 ± 35.69 a | 164.68 ± 2.91 b |
| Plants | Concentration (g/mL) | Labels | Germination (%) | Shoot Length (mm) | Root Length (mm) |
|---|---|---|---|---|---|
| Control | 97.50 ± 1.71 a | 28.03 ± 1.09 a | 20.57 ± 0.88 a | ||
| A. pokeweed | 0.2 0.13 | 1 2 | 7.50 ± 1.44 b 15.00 ± 5.77 b | 6.50 ± 1.61 b 6.83 ± 0.91 b | 0.00 ± 0.00 b 0.00 ± 0.00 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikulic-Petkovsek, M.; Veberic, R.; Hudina, M.; Misic, E. HPLC-DAD-MS Identification and Quantification of Phenolic Components in Japanese Knotweed and American Pokeweed Extracts and Their Phytotoxic Effect on Seed Germination. Plants 2022, 11, 3053. https://doi.org/10.3390/plants11223053
Mikulic-Petkovsek M, Veberic R, Hudina M, Misic E. HPLC-DAD-MS Identification and Quantification of Phenolic Components in Japanese Knotweed and American Pokeweed Extracts and Their Phytotoxic Effect on Seed Germination. Plants. 2022; 11(22):3053. https://doi.org/10.3390/plants11223053
Chicago/Turabian StyleMikulic-Petkovsek, Maja, Robert Veberic, Metka Hudina, and Eva Misic. 2022. "HPLC-DAD-MS Identification and Quantification of Phenolic Components in Japanese Knotweed and American Pokeweed Extracts and Their Phytotoxic Effect on Seed Germination" Plants 11, no. 22: 3053. https://doi.org/10.3390/plants11223053





