Tissue Culture in Ornamentals: Cultivation Factors, Propagation Techniques, and Its Application
Abstract
1. Introduction
2. In Vitro Cultivation Factors
2.1. Carbohydrate Supplements as Carbon Sources in Culture Media
2.2. Plant Growth Regulators, Inhibitors, and Elicitors in Culture Media
2.3. Light-Emitting Diodes over Conventional Light
3. Standard Techniques Involved in Plantlet Generation In Vitro
3.1. Callus Culture
3.2. Protoplast Culture
3.3. Somatic Embryogenesis
3.4. Protocorm-like Body
4. Application of In Vitro Techniques in Ornamentals
4.1. Plant Improvement by the Application of In Vitro Embryo Rescue
4.2. Plant Improvement by Somatic Hybridization and In Vitro Pollination
4.3. Production of Synthetic Seeds
4.4. In Vitro Ploidy Manipulation
5. Future Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bhojwani, S.S.; Dantu, P.K. Micropropagation. In Plant Tissue Culture: An Introductory Text; Bhojwani, S.S., Dantu, P.K., Eds.; Springer: New Delhi, India, 2013; Chapter 17; pp. 245–274. [Google Scholar]
- White, P.R. Potentially unlimited growth of excised tomato root tips in a liquid medium. Plant Physiol. 1934, 9, 585–600. [Google Scholar] [CrossRef] [PubMed]
- White, P.R. Accessory salts in the nutrition of excised tomato roots. Plant Physiol. 1938, 13, 391–398. [Google Scholar] [CrossRef] [PubMed]
- White, P.R. Glycine in the nutrition of excised tomato roots. Plant Physiol. 1939, 14, 527–538. [Google Scholar] [CrossRef] [PubMed][Green Version]
- White, P.R. A Handbook of Plant Tissue Culture; The Jaques Cattell Press: Lancaster, PA, USA, 1943; pp. 1–277. [Google Scholar]
- Murashige, T.; Skoog, F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- White, P.R. The Cultivation of Animal and Plant Cells; Ronald Press, Co.: New York, NY, USA, 1963; p. 239. [Google Scholar]
- Linsmaier, E.M.; Skoog, F. Organic growth factor requirements of tobacco tissue cultures. Physiol. Plant 1965, 18, 100–127. [Google Scholar] [CrossRef]
- Gamborg, O.L.; Miller, R.A.; Ojima, K. Nutrient requirements of suspension culture of soybean root cells. Exp. Cell. Res. 1968, 50, 15–158. [Google Scholar] [CrossRef]
- Nitsch, J.P.; Nitsch, C. Haploid plants from pollen grains. Science 1969, 163, 85–87. [Google Scholar] [CrossRef]
- Madke, S.S.; Cherian, K.J.; Badere, R.S. A modified Murashige and Skoog media for efficient multiple shoot induction in G. arborea Roxb. J. For. Res. 2014, 25, 557–564. [Google Scholar] [CrossRef]
- Enoki, S.; Takahara, Y. Application of a modified MS medium for tissue culture with cutting in Phalaenopsis-comparison with other conventional media with regard to the survival rate and varietal differences in cultural characteristics. J. Sci. High Technol. Agric. (Shokubutsu Kankyo Kogaku) 2014, 26, 109–117. [Google Scholar] [CrossRef]
- Yaseen, M.; Ahmad, T.; Sablok, G.; Standardi, A.; Hafiz, I.H. Review: Role of carbon sources for in vitro plant growth and development. Mol. Biol. Rep. 2013, 40, 2837–2849. [Google Scholar] [CrossRef]
- Calamar, A.; De Klerk, G.J. Effect of sucrose on adventitious root regeneration in apple. Plant Cell Tissue Organ Cult. 2002, 70, 207–212. [Google Scholar] [CrossRef]
- Kozai, T.; Kubota, C.; Jeong, B.R. Environmental control for the large-scale production of plants through in vitro techniques. Plant Cell Tissue Organ Cult. 1997, 51, 49–56. [Google Scholar] [CrossRef]
- Borisjuk, L.; Walenta, S.; Rollerschek, H.; Mueller-Klieser, W.; Wobus, U.; Weber, H. Spatial analysis of plant metabolism: Sucrose imaging within Vicia faba in cotyledons reveals specific developmental patterns. Plant J. 2003, 29, 521–530. [Google Scholar] [CrossRef]
- Stepan-Sarkissian, G.; Fowler, M.W. Carbohydrates by suspension cultures. Plant Physiol. 1977, 59, 151–181. [Google Scholar]
- Neto, V.B.D.P.; Otoni, W.C. Carbon sources and their osmotic potential in plant tissue culture: Does it matter? Sci. Hortic. 2003, 97, 193–202. [Google Scholar] [CrossRef]
- Tokuhara, K.; Mii, M. Highly-efficient somatic embryogenesis from cell suspension cultures of Phalaenopsis orchids by adjusting carbohydrate sources. Vitr. Cell Dev. Biol. Plant 2003, 39, 635–639. [Google Scholar] [CrossRef]
- Liu, T.H.A.; Lin, J.J.; Wu, R.Y. The effects of using trehalose as a carbon source on the proliferation of Phalaenopsis and Doritaenopsis protocorm-like-bodies. Plant Cell Tissue Organ Cult. 2006, 86, 125–129. [Google Scholar] [CrossRef]
- Gibson, S.I. Plant sugar-response pathways. Part of a complex regulatory web. Plant Physiol. 2000, 124, 1532–1539. [Google Scholar] [CrossRef]
- Baskaran, P.; Jayabalan, N. Role of basal media, carbon sources and growth regulators in micropropagation of Eclipta alba—A valuable medicinal herb. Curr. Appl. Sci. Technol. 2005, 5, 469–482. [Google Scholar]
- Javed, F.; Ikram, S. Effect of sucrose induced osmotic stress on callus growth and biochemical aspects of two wheat genotypes. Pak. J. Bot. 2008, 40, 1487–1495. [Google Scholar]
- Saad, A.I.; Elshahed, A.M. Plant tissue culture media. In Recent Advances in Plant In Vitro Culture; Leva, A., Rinaldi, L.M.R., Eds.; IntechOpen: London, UK, 2012; Chapter 2; pp. 1–13. [Google Scholar]
- Yamaguchi, H.; Sasaki, K.; Shikata, M.; Aida, R.; Ohtsubo, N. Trehalose drastically extends the in vitro vegetative culture period and facilitates maintenance of Torenia fournieri plants. Plant Biotechnol. 2011, 28, 263–266. [Google Scholar] [CrossRef]
- Mehraj, H.; Alam, M.M.; Habiba, S.U.; Mehbub, H. LEDs combined with CHO sources and CCC priming PLB regeneration of Phalaenopsis. Horticulturae 2019, 5, 34. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A. Ornamental chrysanthemums: Improvement by biotechnology. Plant Cell Tissue Organ Cult. 2004, 79, 1–18. [Google Scholar] [CrossRef]
- Hong, P.I.; Chen, J.T.; Chang, W.C. Promotion of direct somatic embryogenesis of Oncidium by adjusting carbon sources. Biol. Plant. 2008, 52, 597–600. [Google Scholar] [CrossRef]
- Blanc, G.; Lardet, L.; Martin, A.; Jacob, J.L.; Carron, M.P. Differential carbohydrate metabolism conducts morphogenesis in embryogenic callus of Hevea brasiliensis (Mull. Arg.). J. Exp. Bot. 2002, 53, 1453–1462. [Google Scholar] [CrossRef]
- Capellades, M.; Lemeur, R.; Debergh, P. Effects of sucrose on starch accumulation and rate of photosynthesis in Rosa cultured in vitro. Plant Cell Tissue Organ Cult. 1991, 25, 21–26. [Google Scholar] [CrossRef]
- Mehbub, H.; Shimasaki, K.; Mehraj, H. Low concentration of anti-auxin and anti-fungal agent accelerates the PLB regeneration of Dendrobium okinawense under green LED. Plants 2022, 11, 1082. [Google Scholar] [CrossRef]
- Jo, E.A.; Tewari, R.K.; Hahn, E.J.; Paek, K.Y. In vitro sucrose concentration affects growth and acclimatization of Alocasia amazonica plantlets. Plant Cell Tissue Organ Cult. 2009, 96, 307–315. [Google Scholar] [CrossRef]
- Shahzad, A.; Parveen, S.; Sharma, S.; Shaheen, A.; Saeed, T.; Yadav, V.; Akhtar, R.; Ahmad, Z.; Upadhyay, A. Plant tissue culture: Applications in plant improvement and conservation. In Plant Biotechnology: Principles and Applications; Abdin, M., Kiran, U., Ali, A., Eds.; Springer: Singapore, 2017; Chapter 2; pp. 37–72. [Google Scholar]
- Che, P.; Lall, S.; Howell, S.H. Developmental steps in acquiring competence for shoot development in Arabidopsis tissue culture. Planta 2007, 226, 1183–1194. [Google Scholar] [CrossRef]
- Atta, R.; Laurens, L.; Boucheron-Dubuisson, E.; Guivarc’h, A.; Carnero, E.; Giraudat-Pautot, V.; Rech, P.; Chriqui, D. Pluripotency of Arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyl explants grown in vitro. Plant J. 2009, 57, 626–644. [Google Scholar] [CrossRef]
- Marhavý, P.; Montesinos, J.C.; Abuzeineh, A.; Van Damme, D.; Vermeer, J.E.; Duclercq, J.; Rakusová, H.; Nováková, P.; Friml, J.; Geldner, N.; et al. Targeted cell elimination reveals an auxin-guided biphasic mode of lateral root initiation. Genes Dev. 2016, 30, 471–483. [Google Scholar] [CrossRef]
- Blakesley, D.; Weston, G.; Hall, J. The role of endogenous auxin in root initiation. Plant Growth Regul. 1991, 10, 341–353. [Google Scholar] [CrossRef]
- Roy, J.; Banerjee, N. Induction of callus and plant regeneration from shoot-tip explants of Dendrobium fimbriatum Lindl. var. oculatum Hk. f. Sci. Hortic. 2003, 97, 333–340. [Google Scholar] [CrossRef]
- Benková, E.; Michniewicz, M.; Sauer, M.; Teichmann, T.; Seifertová, D.; Jürgens, G.; Friml, J. Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 2003, 115, 591–602. [Google Scholar] [CrossRef]
- Wang, Y.H.; Irving, H.R. Developing a model of plant hormone interactions. Plant Signal. Behav. 2011, 6, 494–500. [Google Scholar] [CrossRef]
- Ludwig-Müller, J. Auxin conjugates: Their role for plant development and in the evolution of land plants. J. Exp. Bot. 2011, 62, 1757–1773. [Google Scholar] [CrossRef]
- Simon, S.; Petrášek, J. Why plants need more than one type of auxin. Plant Sci. 2011, 180, 454–460. [Google Scholar] [CrossRef]
- Schmülling, T. Cytokinin. In Encyclopedia of Biological Chemistry, 2nd ed.; Lennarz, J.W., Lane, D.M., Eds.; Academic Press: Cambridge, MA, USA, 2013; pp. 627–631. [Google Scholar]
- Thimann, K.V.; Bonner, J. The mechanism of the action of the growth substance of plants. Proc. R. Soc. Lond. Ser. B 1933, 113, 126–149. [Google Scholar]
- Mares, D.J.; Marschner, H.; Krauss, A. Effect of gibberellic acid on growth and carbohydrate metabolism of developing tubers of potato (Solanum tuberosum L.). Physiol. Plant 1981, 52, 267–274. [Google Scholar] [CrossRef]
- Wang, H.; Li, H.; Liu, F.; Xiao, L. Chlorocholine chloride application effects on photosynthetic capacity and photoassimilates partitioning in potato (Solanum tuberosum L.). Sci. Hortic. 2009, 119, 113–116. [Google Scholar] [CrossRef]
- Wen, Z.Z.; Lin, Y.; Liu, Y.Q.; Wang, M.; Wang, Y.Q.; Liu, W. Effects of paclobutrazol in vitro on transplanting efficiency and root tip development of Dendrobium nobile. Biol. Plant 2013, 57, 576–580. [Google Scholar] [CrossRef]
- Gimenes, R.; Pivetta, K.F.L.; Mazzini-Guedes, R.B.; Ferraz, M.V.; Pereira, S.T.S.; Santos, Á.S.; de Faria, R.T.; de Almeida, L.C.P. Paclobutrazol on in vitro growth and development of Zygopetalum crinitum orchid, and on seedling acclimatization. Am. J. Plant Sci. 2018, 9, 1029–1036. [Google Scholar] [CrossRef]
- Murthy, H.N.; Lee, E.J.; Paek, K.Y. Production of secondary metabolites from cell and organ cultures: Strategies and approaches for biomass improvement and metabolite accumulation. Plant Cell Tissue Organ Cult. 2014, 118, 1–16. [Google Scholar] [CrossRef]
- Xu, A.; Zhan, J.C.; Huang, W.D. Effects of ultraviolet C, methyl jasmonate and salicylic acid, alone or in combination, on stilbene biosynthesis in cell suspension cultures of Vitis vinifera L. cv. Cabernet Sauvignon. Plant Cell Tissue Organ Cult. 2015, 122, 197–211. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Kosson, R.; Treder, J. Polyamines and methyl jasmonate in bulb formation of in vitro propagated tulips. Plant Cell Tissue Organ Cult. 2015, 123, 591–605. [Google Scholar] [CrossRef]
- Cardoso, J.C.; Teixeira da Silva, J.A. Micropropagation of gerbera using chlorine dioxide (ClO2) to sterilize the culture medium. Vitr. Cell Dev. Biol. Plant 2011, 48, 362–368. [Google Scholar] [CrossRef]
- Tian, C.; Xie, Z.; Zhao, Y.; Zhang, Z.; Xue, T.; Sheng, W.; Zhao, F.; Duan, Y. Microgram-grade concentration of chlorine dioxide induces one-step plant regeneration in chrysanthemum. Vitr. Cell Dev. Biol. Plant 2022, 1–7. [Google Scholar] [CrossRef]
- Rajapakse, N.C.; Shahak, Y. Light-quality manipulation by horticulture industry. In Annual Plant Reviews, Volume 30: Light and Plant Development IV. Applied Aspects of Photomorphogenesis; Whitelam, G.C., Halliday, K.J., Eds.; Blackwell Publishing Ltd.: Hoboken, NJ, USA, 2007; Chapter 12; pp. 290–312. [Google Scholar]
- Bello-Bello, J.J.; Perez-Sato, J.A.; Cruz-Cruz, C.A.; Martinez-Estrada, E. Light-emitting diodes: Progress in plant micropropagation. In Chlorophyll; Jacob-Lopes, E., Zepka, L.Q., Queiroz, M.I., Eds.; IntechOpen: London, UK, 2017; Chapter 6; pp. 93–103. [Google Scholar]
- Yeow, L.C.; Chew, B.L.; Sreeramanan, S. Elevation of secondary metabolites production through light-emitting diodes (LEDs) illumination in protocorm-like bodies (PLBs) of Dendrobium hybrid orchid rich in phytochemicals with therapeutic effects. Biotechnol. Rep. 2020, 27, e00497. [Google Scholar] [CrossRef]
- Hanus-Fajerska, E.; Wojciechowska, R. Impact of light-emitting diodes (LEDs) on propagation of orchids in tissue culture. In Light Emitting Diodes for Agriculture; Dutta Gupta, S., Ed.; Springer: Singapore, 2017; Chapter 13; pp. 305–320. [Google Scholar]
- Tanaka, M.; Takamura, T.; Watanabe, H.; Endo, M.; Yanagi, T.; Okamoto, K. In vitro growth of Cymbidium plantlets cultured under superbright red and blue light-emitting diodes (LEDs). J. Hort. Sci. Biotech. 1998, 73, 39–44. [Google Scholar] [CrossRef]
- Huan, L.V.T.; Tanaka, M. Callus induction from protocorm-like body segments and plant regeneration in Cymbidium (Orchidaceae). J. Hortic. Sci. Biotechnol. 2004, 79, 406–410. [Google Scholar] [CrossRef]
- Goins, G.D.; Yorio, N.C.; Sanwo, M.; Brown, C.S. Photomorphogenesis photosynthesis, and seed yield of wheat plants grown under red light-emitting diodes (LED) with and without supplement blue lighting. J. Exp. Bot. 1997, 312, 1407–1413. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Li, J.; Li, B.; He, T. Effects of light quality on growth and development of procorm-like bodied of Dendrobium officinale in vitro. Plant Cell Tissue Organ Cult. 2011, 105, 329–335. [Google Scholar] [CrossRef]
- Habiba, S.U.; Shimasaki, K.; Ahasan, M.M.; Alam, M.M. Effects of different light quality on growth and development of protocorm-like bodies (PLBs) in Dendrobium kingianum cultured in vitro. Bangladesh Res. Public J. 2014, 10, 223–227. [Google Scholar]
- Xu, Z.G.; Cui, J.; Di, X.R. Effects of different spectral energy distribution on tissue culture of Oncidium in vitro. Int. J. Autom. Comput. 2009, 31, 45–50. [Google Scholar]
- Ona, A.F.; Shimasaki, K.; Emteas, M.A.; Uddin, A.F.M.J. Effects of different LED lights on the organogenesis of a Cymbidium cultivar. Environ. Control Biol. 2021, 59, 197–201. [Google Scholar] [CrossRef]
- Haberlandt, G. Culturversuehe mit isolierten Pflanzenzellen. Sitzungsber. Akad. Wiss. Wien Math. Nat. 1902, 111, 69–92. [Google Scholar]
- Fehér, A. Callus, dedifferentiation, totipotency, somatic embryogenesis: What these terms mean in the era of molecular plant biology? Front. Plant Sci. 2019, 10, 536. [Google Scholar] [CrossRef]
- Bhatia, S. Plant tissue culture. In Modern Applications of Plant Biotechnology in Pharmaceutical Sciences; Bhatia., S., Sharma, K., Dahiya, R., Bera, T., Eds.; Academic Press: Cambridge, MA, USA, 2015; pp. 31–107. [Google Scholar]
- Efferth, T. Biotechnology applications of plant callus cultures. Engineering 2019, 5, 50–59. [Google Scholar] [CrossRef]
- Naing, A.H.; Adedeji, O.S.; Kim, C.K. Protoplast technology in ornamentals: Current progress and potential applications on genetic improvement. Sci. Hortic. 2021, 283, 110043. [Google Scholar] [CrossRef]
- Thomas, A.; Pujari, I.; Shetty, V.; Joshi, M.B.; Rai, P.S.; Satyamoorthy, K.; Babu, V.S. Dendrobium protoplast co-culture promotes phytochemical assemblage in vitro. Protoplasma 2017, 254, 1517–1528. [Google Scholar] [CrossRef]
- Yousuf, S.; Ashraf, F.; Kazmi, S.K.; Khan, S.; Kayani, H.A. A study on the isolation of protoplasts from the callus of Lilium longiflorum Overig. Pak. J. Bot. 2015, 47, 2391–2396. [Google Scholar]
- Pati, P.K.; Sharma, M.; Ahuja, P.S. Rose protoplast isolation and culture and heterokaryonselection by immobilization in extra thin alginate film. Protoplasma 2008, 233, 165–171. [Google Scholar] [CrossRef]
- Adedeji, O.S.; Naing, A.H.; Kim, C.K. Protoplast isolation and shoot regeneration from protoplast-derived calli of Chrysanthemum cv. White ND. Plant Cell Tissue Organ Cult. 2020, 141, 571–581. [Google Scholar] [CrossRef]
- Kang, H.H.; Naing, A.H.; Kim, C.K. Protoplast isolation and shoot regeneration from protoplast-derived callus of Petunia hybrida Cv. Mirage Rose. Biology 2020, 9, 228. [Google Scholar] [CrossRef] [PubMed]
- Shiba, T.; Mii, M. Plant regeneration from mesophyll-and cell suspension-derived protoplasts of Dianthus acicularis and characterization of regenerated plants. Vitr. Cell Dev. Biol. Plant 2005, 41, 794. [Google Scholar] [CrossRef]
- Liqing, Z.; Bochu, W.; Jing, Z.; Lingxi, C.; Chuanyun, D.; Chuanren, D. Protoplast isolation of callus in Echinacea augustifolia. Colloids Surf. B Biointerfaces 2005, 44, 1–5. [Google Scholar] [CrossRef]
- Nassour, M.; Dorion, N. Plant regeneration from protoplasts of micropropagated Pelargonium x hortorum ‘Alain’: Effect of some environmental and medium factors on protoplast system efficiency. Plant Sci. 2002, 163, 169–176. [Google Scholar] [CrossRef]
- Nassour, M.; Chasseriaux, G.; Dorion, N. Optimization of protoplast-to-plant system for Pelargonium× hortorum ‘Alain’ and genetic stability of the regenerated plants. Plant Sci. 2003, 165, 121–128. [Google Scholar] [CrossRef]
- Rahmani, M.S.; Pijut, P.M.; Shabanian, N. Protoplast isolation and genetically true-to-type plant regeneration from leaf-and callus-derived protoplasts of Albizia julibrissin. Plant Cell Tissue Organ Cult. 2016, 127, 475–488. [Google Scholar] [CrossRef]
- Lang, I.; Sassmann, S.; Schmidt, B.; Komis, G. Plasmolysis: Loss of turgor and beyond. Plants 2014, 3, 583–593. [Google Scholar] [CrossRef]
- Pan, Z.G.; Liu, C.Z.; Zobayed, S.M.A.; Saxena, P.K. Plant regeneration from mesophyll protoplasts of Echinacea purpurea. Plant Cell Tissue Organ Cult. 2004, 77, 251–255. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, B.; Zhu, L. Conditioned culture for protoplasts isolated from Chrysanthemum: An efficient approach. Colloids Surf. B Biointerfaces 2005, 45, 113–119. [Google Scholar] [CrossRef] [PubMed]
- Duquenne, B.; Eeckhaut, T.; Werbrouck, S. Effect of enzyme concentrations on protoplast isolation and protoplast culture of Spathiphyllum and Anthurium. Plant Cell Tissue Organ Cult. 2007, 91, 165–173. [Google Scholar] [CrossRef]
- Pongchawee, K.; Na-Nakorn, U.; Lamseejan, S.; Poompuang, S.; Phansiri, S. Factors affecting the protoplast isolation and culture of Anubias nana Engler. Int. J. Bot. 2006, 2, 193–200. [Google Scholar] [CrossRef]
- Meyer, L.; Serek, M.; Winkelmann, T. Protoplast isolation and plant regeneration of different genotypes of Petunia and Calibrachoa. Plant Cell Tissue Organ Cult. 2009, 99, 27–34. [Google Scholar] [CrossRef]
- Li, J.; Liao, X.; Zhou, S.; Liu, S.; Jiang, L.; Wang, G. Efficient protoplast isolation and transient gene expression system for Phalaenopsis hybrid cultivar ‘Ruili Beauty’. Vitr. Cell Dev. Biol. Plant 2018, 54, 87–93. [Google Scholar] [CrossRef]
- Teo, C.K.H.; Neumann, K.H. The culture of protoplasts isolated from Renantanda Rosalind Cheok. Orchid Rev. 1978, 86, 156–158. [Google Scholar]
- Teo, C.K.H.; Neumann, K.H. The isolation and hybridization of protoplasts from orchids. Orchid Rev. 1978, 86, 186–189. [Google Scholar]
- Kobayashi, S.; Kameya, T.; Ichihashi, S. Plant regeneration from protoplasts derived from callus of Phalaenopsis. Plant Tiss. Cult. Lett. 1993, 10, 267–270. [Google Scholar] [CrossRef]
- Kunasakdakul, K.; Smitamana, P. Dendrobium Pratum Red protoplast. Thai J. Agric. Sci. 2003, 36, 1–8. [Google Scholar]
- Khentry, Y.; Paradornuvat, A.; Tantiwiwat, S.; Phansiri, S.; Thaveechai, N. Protoplast isolation and culture of Dendrobium Sonia “Bom 17”. Kasetsart J. (Nat. Sci.) 2006, 40, 361–369. [Google Scholar]
- Shrestha, B.R.; Tokuhara, K.; Mii, M. Plant regeneration from cell suspension-derived protoplasts of Phalaenopsis. Plant Cell Rep. 2007, 26, 719–725. [Google Scholar] [CrossRef] [PubMed]
- Tee, C.S.; Lee, P.S.; Kiong, A.L.P.; Mahmood, M. Optimisation of protoplast isolation protocols using in vitro leaves of Dendrobium crumenatum (pigeon orchid). Afr. J. Agric. Res. 2011, 5, 2685–2693. [Google Scholar]
- Cui, J.; Mackenzie, K.K.; Eeckhaut, T.; Müller, R.; Lütken, H. Protoplast isolation and culture from Kalanchoë species: Optimization of plant growth regulator concentration for efficient callus production. Plant Cell Tissue Organ Cult. 2019, 138, 287–297. [Google Scholar] [CrossRef]
- Furuta, H.; Shinoyama, H.; Nomura, Y.; Maeda, M.; Makara, K. Production of intergeneric somatic hybrids of chrysanthemum [Dendranthema × grandiflorum (Ramat.) Kitamura] and wormwood (Artemisia sieversiana JF Ehrh. ex. Willd) with rust (Puccinia horiana Henning) resistance by electrofusion of protoplasts. Plant Sci. 2004, 166, 695–702. [Google Scholar] [CrossRef]
- Steward, F.C.; Mapes, M.O.; Mears, K. Growth and organized development of cultured cells. II. Organization in cultures grown from freely suspended cells. Am. J. Bot. 1958, 45, 705–708. [Google Scholar] [CrossRef]
- Reinert, J. Über die kontrolle der morphogenese und die induktion von adventivembryonen an gewebekulturen aus karotten. Planta 1959, 53, 318–333. [Google Scholar] [CrossRef]
- Backs-Hüsemann, D.; Reinert, J. Embryobildung durch isolierte Einzelzellen aus Gewebekulturen vonDaucus carota. Protoplasma 1970, 70, 49–60. [Google Scholar] [CrossRef]
- Hossain, M.M.; Kant, R.; Van, P.T.; Winarto, B.; Zeng, S.; Teixeira da Silva, J.A. The application of biotechnology to orchids. Crit. Rev. Plant Sci. 2013, 32, 69–139. [Google Scholar]
- Mujib, A. Somatic Embryogenesis in Ornamentals and Its Applications; Springer: New Delhi, India, 2016; Volume 267, pp. 1–267. [Google Scholar]
- Nic-Can, G.I.; Galaz-Ávalos, R.M.; De-la-Peña, C.; AlcazarMagaña, A.; Wrobel, K.; Loyola-Vargas, V.M. Somatic embryogenesis: Identified factors that lead to embryogenic repression. a case of species of the same genus. PLoS ONE 2015, 10, e0126414. [Google Scholar] [CrossRef]
- Loyola-Vargas, V.M.; Ochoa-Alejo, N. Somatic Embryogenesis: Fundamental Aspects and Applications; Springer: Cham, Switzerland, 2018; pp. 1–296. [Google Scholar]
- Mahendran, G.; Bai, V.N. Direct somatic embryogenesis and plant regeneration from seed derived protocorms of Cymbidium bicolor Lindl. Sci. Hortic. 2012, 135, 40–44. [Google Scholar] [CrossRef]
- Deb, C.R.; Pongener, A. Studies on the in vitro regenerative competence of aerial roots of two horticultural important Cymbidium species. J. Plant Biochem. Biotechnol. 2012, 21, 235–241. [Google Scholar] [CrossRef]
- Chang, C.; Chang, W.C. Plant regeneration from callus of Cymbidium ensifolium var ‘Misericors’. Plant Cell Rep. 1998, 17, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Teixeira da Silva, J.A.; Chan, M.-T.; Sanjaya; Chai, M.-L.; Tanaka, M. Priming abiotic factors for optimal hybrid Cymbidium (Orchidaceae) PLB and callus induction, plantlet formation, and their subsequent cytogenetic stability analysis. Sci. Hortic. 2006, 109, 368–378. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Singh, N.; Tanaka, M. Priming biotic factors for optimal protocorm-like body and callus induction in hybrid Cymbidium (Orchidaceae), and assessment of cytogenetic stability in regenerated plantlets. Plant Cell Tissue Organ Cult. 2006, 84, 135–144. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Winarto, B. Somatic embryogenesis in two orchid genera (Cymbidium, Dendrobium). In In Vitro Embryogenesis in Higher Plants. Methods in Molecular Biology; Germana, M., Lambardi, M., Eds.; Humana Press: Totowa, NJ, USA, 2016; Volume 1359, pp. 371–386. [Google Scholar]
- Ishii, Y.; Takamura, T.; Goi, M.; Tanaka, M. Callus induction and somatic embryogenesis of Phalaenopsis. Plant Cell Rep. 1998, 17, 446–450. [Google Scholar] [CrossRef]
- Chen, J.T.; Chang, W.C. Direct somatic embryogenesis and plant regeneration from leaf explants of Phalaenopsis amabilis. Biol. Plant. 2006, 50, 169–173. [Google Scholar] [CrossRef]
- Gow, W.P.; Chen, J.T.; Chang, W.C. Enhancement of direct somatic embryogenesis and plantlet growth from leaf explants of Phalaenopsis by adjusting culture period and explant length. Acta Physiol. Plant. 2010, 32, 621–627. [Google Scholar] [CrossRef]
- Gow, W.P.; Chen, J.T.; Chang, W.C. Influence of growth regulators on direct embryo formation from leaf explants of Phalaenopsis orchids. Acta Physiol. Plant. 2008, 30, 507–512. [Google Scholar] [CrossRef]
- Gow, W.P.; Chen, J.T.; Chang, W.C. Effects of genotype, light regime, explant position and orientation on direct somatic embryogenesis from leaf explants of Phalaenopsis orchids. Acta Physiol. Plant. 2009, 31, 363–369. [Google Scholar] [CrossRef]
- Niknejad, A.; Kadir, M.A.; Kadzimin, S.B. In vitro plant regeneration from protocorms-like bodies (PLBs) and callus of Phalaenopsis gigantea (Epidendroideae: Orchidaceae). Afr. J. Biotechnol. 2011, 10, 11808–11816. [Google Scholar]
- Feng, J.H.; Chen, J.T. A novel in vitro protocol for inducing direct somatic embryogenesis in Phalaenopsis aphrodite without taking explants. Sci. World J. 2014, 7, 263642. [Google Scholar]
- Chen, J.T.; Chang, C.; Chang, W.C. Direct somatic embryogenesis on leaf explants of Oncidium Gower Ramsey and subsequent plant regeneration. Plant Cell Rep. 1999, 19, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.T.; Chang, W.C. Effects of tissue culture conditions and explant characteristics on direct somatic embryogenesis in Oncidium ‘Gower Ramsey’. Plant Cell Tissue Organ Cult. 2002, 69, 41–44. [Google Scholar] [CrossRef]
- Su, Y.J.; Chen, J.T.; Chang, W.C. Efficient and repetitive production of leaf-derived somatic embryos of Oncidium. Biol. Plant. 2006, 50, 107–110. [Google Scholar] [CrossRef]
- Hong, P.I.; Chen, J.T.; Chang, W.C. Effects of salicylic and acetylsalicylic acid on direct somatic embryogenesis in Oncidium. J. Plant Biochem. Biotechnol. 2008, 17, 149–153. [Google Scholar] [CrossRef]
- Shen, H.J.; Chen, J.T.; Chung, H.H.; Chang, W.C. Plant regeneration via direct somatic embryogenesis from leaf explants of Tolumnia Louise Elmore ‘Elsa’. Bot. Stud. 2018, 59, 4. [Google Scholar] [CrossRef]
- Chung, H.H.; Chen, J.T.; Chang, W.C. Cytokinins induce direct somatic embryogenesis of Dendrobium Chiengmai Pink and subsequent plant regeneration. In Vitro Cell. Dev. Biol. Plant 2005, 41, 765–769. [Google Scholar] [CrossRef]
- Chung, H.H.; Chen, J.T.; Chang, W.C. Plant regeneration through direct somatic embryogenesis from leaf explants of Dendrobium. Biol. Plant. 2007, 51, 346–350. [Google Scholar] [CrossRef]
- Asghar, S.; Ahmad, T.; Hafiz, I.A.; Yaseen, M. In vitro propagation of orchid (Dendrobium nobile) var. Emma White. Afr. J. Biotechnol. 2011, 10, 3097–3103. [Google Scholar]
- Parthibhan, S.; Rao, M.V.; Teixeira da Silva, J.A.; Kumar, T.S. Somatic embryogenesis from stem thin cell layers of Dendrobium aqueum. Biol. Plant. 2018, 62, 439–450. [Google Scholar] [CrossRef]
- Islam, S.S.; Bhattacharjee, B. Plant regeneration through somatic embryogenesis from leaf and root explants of Rhynchostylis retusa (L.) Blume. Appl. Biol. Res. 2015, 17, 158–165. [Google Scholar] [CrossRef]
- Wu, K.L.; Zeng, S.J.; Teixeira da Silva, J.A.; Chen, Z.L.; Zhang, J.X.; Yang, Y.S.; Duan, J. Efficient regeneration of Renanthera Tom Thumb ‘Qilin’ from leaf explants. Sci. Hortic. 2012, 135, 194–201. [Google Scholar] [CrossRef]
- Hong, P.I.; Chen, J.T.; Chang, W.C. Plant regeneration via protocormlike body formation and shoot multiplication from seed-derived callus of a maudiae type slipper orchid. Acta Physiol. Plant. 2008, 30, 755–759. [Google Scholar] [CrossRef]
- Long, B.; Niemiera, A.X.; Cheng, Z.Y.; Long, C.L. In vitro propagation of four threatened Paphiopedilum species (Orchidaceae). Plant Cell Tissue Organ Cult. 2010, 101, 151–162. [Google Scholar] [CrossRef]
- Cheruvathur, M.K.; Abraham, J.; Mani, B.; Thomas, T.D. Adventitious shoot induction from cultured internodal explants of Malaxis acuminata D. Don, a valuable terrestrial medicinal orchid. Plant Cell Tissue Organ Cult. 2010, 101, 163–170. [Google Scholar] [CrossRef]
- Mahendran, G.; Bai, V.N. Direct somatic embryogenesis of Malaxis densiflora (A. Rich.) Kuntze. J. Genet. Eng. Biotechnol. 2016, 14, 77–81. [Google Scholar] [CrossRef]
- Moradi, S.; Daylami, S.D.; Arab, M.; Vahdati, K. Direct somatic embryogenesis in Epipactis veratrifolia, a temperate terrestrial orchid. J. Hortic. Sci. Biotechnol. 2017, 92, 88–97. [Google Scholar] [CrossRef]
- Manokari, M.; Priyadharshini, S.; Shekhawat, M.S. Direct somatic embryogenesis using leaf explants and short term storage of synseeds in Spathoglottis plicata Blume. Plant Cell Tissue Organ Cult. 2021, 145, 321–331. [Google Scholar] [CrossRef]
- Bhadra, S.K.; Hossain, M.M. In vitro germination and micropropagation of Geodorum densiflorum (Lam.) Schltr., an endangered orchid species. Plant Tissue Cult. 2003, 13, 165–171. [Google Scholar]
- Sherif, N.A.; Benjamin, J.H.F.; Kumar, T.S.; Rao, M.V. Somatic embryogenesis, acclimatization and genetic homogeneity assessment of regenerated plantlets of Anoectochilus elatus Lindl., an endangered terrestrial jewel orchid. Plant Cell Tissue Organ Cult. 2018, 132, 303–316. [Google Scholar] [CrossRef]
- Zeng, S.J.; Chen, Z.L.; Wu, K.L.; Bai, C.K.; Zhang, J.X.; Teixeira da Silva, J.A.; Duan, J. Asymbiotic seed germination, induction of calli and protocorm-like bodies, and in vitro seedling development of the rare and endangered Nothodoritis zhejiangensis Chinese orchid. HortScience 2011, 46, 460–465. [Google Scholar] [CrossRef]
- Azadi, P.; Kermani, M.J.; Samiei, L. Somatic embryogenesis in Rosa hybrida. In Step Wise Protocols for Somatic Embryogenesis of Important Woody Plants; Jain, S., Gupta, P., Eds.; Springer: Cham, Switzerland, 2018; Volume II, pp. 161–170. [Google Scholar]
- Pati, P.K.; Sharma, M.; Sood, A.; Ahuja, P.S. Direct shoot regeneration from leaf explants of Rosa damascena Mill. Vitr. Cell Dev. Biol. Plant 2004, 40, 192–195. [Google Scholar] [CrossRef]
- Tanaka, K.; Kanno, Y.; Kudo, S.; Suzuki, M. Somatic embryogenesis and plant regeneration in chrysanthemum (Dendranthema grandiflorum (Ramat.) Kitamura). Plant Cell Rep. 2000, 19, 946–953. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A.; Lema-Rumińska, J.; Tymoszuk, A.; Kulpa, D. Regeneration from chrysanthemum flowers: A review. Acta Physiol. Plant. 2015, 37, 67–77. [Google Scholar]
- Khosravi, S.; Azghandi, A.V.; Hadad, R.; Mojtahedi, N. In vitro micrpropagation of Lilium longiflorum. J. Agric. Res. Seed Plant 2007, 23, 159–168. [Google Scholar]
- Bakhshaie, M.; Babalar, M.; Mirmasoumi, M.; Khalighi, A. Somatic embryogenesis and plant regeneration of Lilium ledebourii (Baker) Boiss., an endangered species. Plant Cell Tissue Organ Cult. 2010, 102, 229–235. [Google Scholar] [CrossRef]
- Zhang, J.; Gai, M.; Li, X.; Li, T.; Sun, H. Somatic embryogenesis and direct as well as indirect organogenesis in Lilium pumilum DC. Fisch., an endangered ornamental and medicinal plant. Biosci. Biotechnol. Biochem. 2016, 80, 1898–1906. [Google Scholar] [CrossRef]
- Fu, L.; Zhu, Y.; Li, M.; Wang, C.; Sun, H. Autopolyploid induction via somatic embryogenesis in Lilium distichum Nakai and Lilium cernuum Komar. Plant Cell Tissue Organ Cult. 2019, 139, 237–248. [Google Scholar] [CrossRef]
- Priyadharshini, S.; Manokari, M.; Shekhawat, M.S. In vitro conservation strategies for the critically endangered Malabar river lily (Crinum malabaricum Lekhak & Yadav) using somatic embryogenesis and synthetic seed production. S. Afr. J. Bot. 2020, 135, 172–180. [Google Scholar]
- Yan, R.; Sun, Y.; Sun, H. Current status and future perspectives of somatic embryogenesis in Lilium. Plant Cell Tissue Organ Cult. 2020, 143, 229–240. [Google Scholar] [CrossRef]
- de Almeida, N.V.; Rivas, E.B.; Cardoso, J.C. Somatic embryogenesis from flower tepals of Hippeastrum aiming regeneration of virus-free plants. Plant Sci. 2022, 317, 111191. [Google Scholar] [CrossRef] [PubMed]
- Gaber, M.K.; Barakat, A.A. Micropropagation and somatic embryogenesis induction of Gardenia jasminoides plants. Alex. Sci. Exch. J. 2019, 40, 190–202. [Google Scholar] [CrossRef]
- Yumbla-Orbes, M.; da Cruz, A.C.F.; Pinheiro, M.V.M.; Rocha, D.I.; Batista, D.S.; Koehler, A.D.; Barbosa, J.G.; Otoni, W.C. Somatic embryogenesis and de novo shoot organogenesis can be alternatively induced by reactivating pericycle cells in Lisianthus (Eustoma grandiflorum (Raf.) Shinners) root explants. Vitr. Cell Dev. Biol. Plant 2017, 53, 209–218. [Google Scholar] [CrossRef]
- Yumbla-Orbes, M.; Rocha, D.I.; de Matos, E.M.; Koehler, A.D.; Pinheiro, M.V.M.; Batista, D.S.; Freitas, D.M.S.; da Cruz, A.C.; Barbosa, J.G.; Viccini, L.F.; et al. Somatic embryogenesis induced from vascular tissues in leaf explants of Lisianthus (Eustoma grandiflorum (Raf.) Shinn) generates true-to-type diploid plants. Vegetos 2020, 33, 135–144. [Google Scholar] [CrossRef]
- Nhut, D.T.; Tuan, N.S.; Ngoc, H.M.; Uyen, P.N.; Don, N.T.; Mai, N.T.; Teixeira da Silva, J.A. Somatic embryogenesis induction from in vitro leaf cultures of Lisianthus (Eustoma grandiflorum (Raf.) Shinn.). Propag. Ornam. Plants 2006, 6, 121–127. [Google Scholar]
- Ruffoni, B.; Bassolino, L. Somatic embryogenesis in Lisianthus (Eustoma russellianum Griseb.). In In Vitro Embryogenesis in Higher Plants, Methods in Molecular Biology Series; Maria, A.G., Maurizio, L., Eds.; Humana Press: Totowa, NJ, USA, 2016; Volume 1359, Chapter 17; pp. 359–370. [Google Scholar]
- Iantcheva, A. Somatic embryogenesis and genetic transformation of carnation (Dianthus caryophyllus L.). In Somatic Embryogenesis in Ornamentals and Its Applications; Mujib, A., Ed.; Springer: New Delhi, India, 2016; Chapter 7; pp. 107–120. [Google Scholar]
- Vieitez, A.M.; Barciela, J. Somatic embryogenesis and plant regeneration from embryonic tissues of Camellia japonica L. Plant Cell Tissue Organ Cult. 1990, 21, 267–274. [Google Scholar] [CrossRef]
- Ponsamuel, J.; Samson, N.P.; Ganeshan, P.S.; Sathyaprakash, V.; Abraham, G.C. Somatic embryogenesis and plant regeneration from the immature cotyledonary tissues of cultivated tea (Camellia sinensis (L).O. Kuntze). Plant Cell Rep. 1996, 16, 210–214. [Google Scholar] [CrossRef]
- Lü, J.; Chen, R.; Zhang, M.; Teixeira da Silva, J.A.; Ma, G. Plant regeneration via somatic embryogenesis and shoot organogenesis from immature cotyledons of Camellia nitidissima. J. Plant Physiol. 2013, 170, 1202–1211. [Google Scholar] [CrossRef]
- San José, M.C.; Couselo, J.L.; Martínez, M.T.; Mansilla, P.; Corredoira, E. Somatic embryogenesis in Camellia japonica L.: Challenges and future prospects. In Somatic Embryogenesis in Ornamentals and Its Applications; Mujib, A., Ed.; Springer: New Delhi, India, 2016; Chapter 6; pp. 91–105. [Google Scholar]
- Gladfelter, H.J.; Johnston, J.; Wilde, H.D.; Markle, S.A. Somatic embryogenesis and cryopreservation of Stewartia species. Plant Cell Tissue Organ Cult. 2021, 144, 211–221. [Google Scholar] [CrossRef]
- Sivanesan, I.; Jeong, B.R. Optimizing factors affecting somatic embryogenesis in Cineraria. In Somatic Embryogenesis in Ornamentals and Its Applications; Mujib, A., Ed.; Springer: New Delhi, India, 2016; Chapter 4; pp. 55–65. [Google Scholar]
- Choffe, K.L.; Victor, J.M.; Muruch, S.J.; Saxena, P.K. In vitro regeneration of Echinacea purpurea L.: Direct somatic embryogenesis and indirect shoot organogenesis in petiole culture. Vitr. Cell Dev. Biol. Plant 2000, 36, 30–36. [Google Scholar] [CrossRef]
- Dehestani-Ardakani, M.; Hejazi, M.; Aliabad, K.K. Indirect somatic embryogenesis of purple coneflower (Echinacea purpurea (L.) Moench): A medicinal-ornamental plant: Evaluation of antioxidant enzymes activity and histological study. Mol. Biol. Rep. 2020, 47, 6621–6633. [Google Scholar] [CrossRef] [PubMed]
- Sivanesan, I.; Son, M.S.; Jana, S.; Jeong, B.R. Secondary somatic embryogenesis in Crocus vernus (L.) Hill. Propag. Ornam. Plants 2012, 12, 163–170. [Google Scholar]
- Mitrofanova, I.; Ivanova, N.; Kuzmina, T.; Mitrofanova, O.; Zubkova, N. In vitro regeneration of clematis plants in the Nikita Botanical Garden via somatic embryogenesis and organogenesis. Front. Plant Sci. 2021, 12, 541171. [Google Scholar] [CrossRef]
- Verma, S.K.; Das, A.K.; Cingoz, G.S.; Uslu, E.; Gurel, E. Influence of nutrient media on callus induction, somatic embryogenesis and plant regeneration in selected Turkish crocus species. Biotechnol. Rep. 2016, 10, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Sevindik, B.; Mendi, Y.Y. Somatic embryogenesis in Crocus sativus L. In In Vitro Embryogenesis in Higher Plants, Methods in Molecular Biology Series; Germana, M.A., Lambardi, K., Eds.; Humana Press: Totowa, NJ, USA, 2016; Chapter 16; pp. 351–357. [Google Scholar]
- Mandegaran, Z.; Sieber, V.K. Somatic embryogenesis in Clematis integrifolia × C. viticella. Plant Cell Tissue Organ Cult. 2000, 62, 163–165. [Google Scholar] [CrossRef]
- Mitrofanova, I.V.; Galaev, A.V.; Sivolap, Y.M. Investigation of molecular-genetic heterogeneity of clematis plants (Clematis L.) obtained by organogenesis and somatic embryogenesis in vitro. Tsitol. Genet. 2003, 37, 12–26. [Google Scholar]
- Hosoi, Y.; Maruyama, T.E. Somatic embryogenesis in Sawara cypress (Chamaecyparis pisifera Sieb. et Zucc.). In Somatic Embryogenesis in Ornamentals and Its Applications; Mujib, A., Ed.; Springer: New Delhi, India, 2016; Chapter 6; pp. 41–53. [Google Scholar]
- Tagipur, M.E.; Seker, G.; Teixeira da Silva, J.A.; Mendi, Y.Y. Somatic embryogenesis, cryopreservation, and in vitro mutagenesis in Cyclamen. In Somatic Embryogenesis in Ornamentals and Its Applications; Mujib, A., Ed.; Springer: New Delhi, India, 2016; Chapter 10; pp. 155–167. [Google Scholar]
- Sivanesan, I.; Lim, M.Y.; Jeong, B.R. Somatic embryogenesis and plant regeneration from leaf and petiole explants of Campanula punctata Lam. var. rubriflora Makino. Plant Cell Tissue Organ Cult. 2011, 107, 365–369. [Google Scholar] [CrossRef]
- Pipino, L.; Braglia, L.; Giovannini, A.; Fascella, G.; Mercuri, A. In vitro regeneration of Passiflora species with ornamental value. Propag. Ornam. Plants 2008, 8, 47–49. [Google Scholar]
- Correa, C.M.; de Oliveira, G.N.; Astariata, L.V.; Santarem, E.R. Plant regeneration through somatic embryogenesis of yacon [Smallanthus sonchifolius (Poepp. and Endl.) H. Robinson]. Braz. Arch. Biol. Technol. 2009, 52, 549–554. [Google Scholar] [CrossRef]
- Salma, U.; Kundu, S.; Ali, M.N.; Mandal, N. Somatic embryogenesis-mediated plant regeneration of Eclipta alba (L.) Hassk. and its conservation through synthetic seed technology. Acta Physiol. Plant. 2019, 41, 103. [Google Scholar] [CrossRef]
- Podwyszyńska, M.; Marasek-Ciolakowska, A. Micropropagation of tulip via somatic embryogenesis. Agronomy 2020, 10, 1857. [Google Scholar] [CrossRef]
- Mujib, A.; Ali, M.; Isah, T.; Dipti, T. Somatic embryo mediated mass production of Catharanthus roseus in culture vessel (bioreactor)—A comparative study. Saudi J. Biol. Sci. 2014, 21, 442–449. [Google Scholar] [CrossRef]
- Jana, S.; Sivanesan, I.; Lim, M.Y.; Jeong, B.R. In vitro zygotic embryo germination and somatic embryogenesis through cotyledonary explants of Paeonia lactiflora Pall. Kor. Soc. Floricult. Sci. 2013, 21, 17–22. [Google Scholar] [CrossRef]
- Du, Y.; Cheng, F.; Zhong, Y. Induction of direct somatic embryogenesis and shoot organogenesis and histological study in tree peony (Paeonia sect. Moutan). Plant Cell Tissue Organ Cult. 2020, 141, 557–570. [Google Scholar] [CrossRef]
- Kuehnle, A.R.; Chen, F.C.; Sugii, N. Somatic embryogenesis and plant regeneration in Anthurium andraeanum hybrids. Plant Cell Rep. 1992, 11, 438–442. [Google Scholar] [CrossRef]
- Pinheiro, M.V.M.; Martins, F.B.; da Cruz, A.C.F.; de Carvalho, A.C.P.P.; Ventrella, M.C.; Otoni, W.C. Somatic embryogenesis in anthurium (Anthurium andraeanum cv. Eidibel) as affected by different explants. Acta Sci. Agron. 2014, 36, 87–98. [Google Scholar] [CrossRef][Green Version]
- Teixeira da Silva, J.A.; Dobránszki, J.; Winarto, B.; Zeng, S. Anthurium in vitro: A review. Sci. Hortic. 2015, 186, 266–298. [Google Scholar] [CrossRef]
- Bhattacharya, C.; Dam, A.; Karmakar, J.; Bandyopadhyay, T.K. Direct somatic embryogenesis and genetic homogeneity assessment of regenerated plants of Anthurium andraeanum Linden cv. Fantasia. Vitr. Cell Dev. Biol. Plant 2016, 52, 512–519. [Google Scholar] [CrossRef]
- Wang, G.; Xu, C.; Yan, S.; Xu, B. An efficient somatic embryo liquid culture system for potential use in large-scale and synchronic production of Anthurium andraeanum seedlings. Front. Plant Sci. 2019, 10, 29. [Google Scholar] [CrossRef]
- Fiuk, A.; Rybczyński, J.J. Morphogenic capability of Gentiana kurroo Royle seedling and leaf explants. Acta Physiol. Plant. 2008, 30, 157–166. [Google Scholar] [CrossRef]
- Fiuk, A.; Rybczyński, J.J. The effect of several factors on somatic embryogenesis and plant regeneration in protoplast cultures of Gentiana kurroo (Royle). Plant Cell Tissue Organ Cult. 2007, 91, 263–271. [Google Scholar] [CrossRef]
- Wu, H.J.; Wang, X.X.; Li, Y.; Zhang, D.G.; Zhang, B.W.; Xin, Y. Propagation of Gentiana macrophylla (Pall) from hairy root explants via indirect somatic embryogenesis and gentiopicroside content in obtained plants. Acta Physiol. Plant. 2011, 33, 2229–2237. [Google Scholar] [CrossRef]
- Vinterhalter, B.; Mitić, N.; Vinterhalter, D.; Uzelac, B.; Krstić-Milošević, D. Somatic embryogenesis and in vitro shoot propagation of Gentiana utriculosa. Biologia 2016, 71, 139–148. [Google Scholar] [CrossRef]
- da Silva, V.; Eeswara, J.P. Induction of somatic embryogenesis from leaf explants of Exacum trinervium (L.) Druce (Binara). J. Natl. Sci. Found. Sri Lanka 2022, 50, 27–33. [Google Scholar] [CrossRef]
- Mahendran, D.; Kavi Kishor, P.B.; Geetha, N.; Venkatachalam, P. Phycomolecule-coated silver nanoparticles and seaweed extracts induced high-frequency somatic embryogenesis and plant regeneration from Gloriosa superba L. J. Appl. Phycol. 2018, 30, 1425–1436. [Google Scholar] [CrossRef]
- Balamurugan, V.; Amal, T.C.; Karthika, P.; Selvakumar, S.; Vasanth, K. Somatic embryogenesis and plant regeneration in Gloriosa superba L.: An endangered medicinal plant. In In Vitro Plant Breeding Towards Novel Agronomic Traits; Kumar, M., Muthusamy, A., Kumar, V., Bhalla-Sarin, N., Eds.; Springer: Singapore, 2019; Chapter 2; pp. 27–42. [Google Scholar]
- Ren, Z.; Lv, X.; Zhang, D.; Xia, Y. Efficient somatic embryogenesis and bulblet regeneration of the endangered bulbous flower Griffinia liboniana. Plant Cell Tissue Organ Cult. 2018, 135, 523–533. [Google Scholar] [CrossRef]
- Vejsadová, H.; Matiska, P.; Obert, B.; Ürgeová, E.; Preťová, A. Somatic embryogenesis in Phlox paniculata—Histological analysis. Biologia 2016, 71, 763–768. [Google Scholar] [CrossRef]
- Simonović, A.D.; Trifunović-Momčilov, M.; Filipović, B.K.; Marković, M.P.; Bogdanović, M.D.; Subotić, A.R. Somatic embryogenesis in Centaurium erythraea Rafn—Current status and perspectives: A review. Plants 2021, 10, 70. [Google Scholar] [CrossRef]
- Kumar, V.; Moyo, M.; Van Staden, J. Enhancing plant regeneration of Lachenalia viridiflora, a critically endangered ornamental geophyte with high floricultural potential. Sci. Hortic. 2016, 211, 263–268. [Google Scholar] [CrossRef]
- von Aderkas, P.; Label, P.; Lelu, M.A. Charcoal affects early development and hormonal concentrations of somatic embryos of hybrid larch. Tree Physiol. 2002, 22, 431–434. [Google Scholar] [CrossRef] [PubMed]
- Nunes, S.; Marum, L.; Farinha, N.; Pereira, V.T.; Almeida, T.; Sousa, D.; Mano, N.; Figueiredo, J.; Dias, M.C.; Santos, C. Somatic embryogenesis of hybrid Pinus elliottii var. elliottii × P. caribaea var. hondurensis and ploidy assessment of somatic plants. Plant Cell Tissue Organ Cult. 2018, 132, 71–84. [Google Scholar] [CrossRef]
- Abrahamsson, M.; Clapham, D.; Arnold, S. Somatic embryogenesis in Scots pine (L.). In Step Wise Protocols for Somatic Embryogenesis of Important Woody Plants, Forestry Sciences; Jain, S.M., Gupta, P., Eds.; Springer: Cham, Switzerland, 2018; Volume 84, pp. 123–133. [Google Scholar]
- Aalifar, M.; Arab, M.; Aliniaeifard, S.; Dianati, S.; Mehrjerdi, M.Z.; Limpens, E.; Serek, M. Embryogenesis efficiency and genetic stability of Dianthus caryophyllus embryos in response to different light spectra and plant growth regulators. Plant Cell Tissue Organ Cult. 2019, 139, 479–492. [Google Scholar] [CrossRef]
- Maruyama, T.E.; Hosoi, Y. Progress in somatic embryogenesis of Japanese pines. Front. Plant Sci. 2019, 10, 31. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Garay, B.; Gutiérrez-Mora, A.; Acosta-Duefias, B. Somatic embryogenesis of Agave victoria-reginae Moore. Plant Cell Tissue Organ Cult. 1996, 46, 85–87. [Google Scholar] [CrossRef]
- Tejavathi, D.H.; Rajanna, M.D.; Sowmya, R.; Gayathramma, K. Induction of somatic embryos from cultures of Agave vera-cruz Mill. Vitr. Cell Dev. Biol. Plant 2007, 43, 423–428. [Google Scholar] [CrossRef]
- Portillo, L.; Santacruz-Ruvalcaba, F.; Gutiérrez-Mora, A.; Rodríguez-Garay, B. Somatic embryogenesis in Agave tequilana Weber cultivar azul. Vitr. Cell Dev. Biol. Plant 2007, 43, 569–575. [Google Scholar] [CrossRef]
- Reyes-Diaz, J.I.; Arzate-Fernández, A.M.; Pina-Escutia, J.L.; Vázquez-García, L.M. Media culture factors affecting somatic embryogenesis in Agave angustifolia Haw. Ind. Crops Prod. 2017, 108, 81–85. [Google Scholar] [CrossRef]
- Kim, D.H.; Sivanesan, I. Somatic embryogenesis in Hosta minor (Baker) Nakai. Propag. Ornam. Plants 2017, 19, 24–29. [Google Scholar]
- Morel, G.M. Producing virus-free cymbidiums. Amer. Orchid Soc. Bull. 1960, 29, 495–497. [Google Scholar]
- Lee, Y.I.; Hsu, S.T.; Yeung, E.C. Orchid protocorm-like bodies are somatic embryos. Am. J. Bot. 2013, 100, 2121–2213. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, J.C.; Zanello, C.A.; Chen, J.T. An overview of orchid protocorm-like bodies: Mass propagation, biotechnology, molecular aspects, and breeding. Int. J. Mol. Sci. 2020, 21, 985. [Google Scholar] [CrossRef] [PubMed]
- Chugh, S.; Guha, S.; Rao, I.U. Micropropagation of orchids: A review on the potential of different explants. Sci. Hortic. 2009, 122, 507–520. [Google Scholar] [CrossRef]
- Yam, T.W.; Arditti, J. History of orchid propagation: A mirror of the history of biotechnology. Plant Biotechnol. Rep. 2009, 3, 1–56. [Google Scholar] [CrossRef]
- Yeung, E.C. A perspective on orchid seed and protocorm development. Bot. Stud. 2017, 58, 33. [Google Scholar] [CrossRef] [PubMed]
- Habiba, S.U.; Shimasaki, K.; Hasan, K.M.; Mehraj, H.; Alam, M.M.; Sharma, S.; Ahasan, M.M. Very low and high temperature act as stress factor on organogenesis in protocorm-like bodies (PLBs) of Dendrobium kingianum. World Appl. Sci. J. 2016, 34, 278–282. [Google Scholar]
- Habiba, S.U.; Shimasaki, K.; Ahasan, M.M.; Alam, M.M. Effect of 6-benzylaminopurine (BA) and hyaluronic acid (HA) under white light emitting diode (LED) on organogenesis in protocorm-like bodies (PLBs) of Dendrobium kingianum. Am. Eurasian J. Agric. Environ. Sci. 2014, 14, 605–609. [Google Scholar]
- Habiba, S.U.; Shimasaki, K.; Ahasan, M.M.; Kamal, M.M.; Alam, M.M. 5-aminolevulinic acid regulates growth and development of protocorm-like bodies (PLBs) in Dendrobium kingianum cultured in vitro. Middle East J. Sci. Res. 2014, 22, 279–283. [Google Scholar]
- Habiba, S.U.; Shimasaki, K.; Ahasan, M.M.; Uddin, A.F.M.J. Effect of two bio polysaccharides on organogenesis of PLBs in Dendrobium kingianum cultured in vitro. Acta Hortic. 2017, 1167, 127–132. [Google Scholar] [CrossRef]
- Habiba, S.U.; Shimasaki, K.; Ahasan, M.M.; Uddin, A.F.M.J. Effect of ethylene precursor 1-aminocyclopropane-1-carboxylic acid and ethylene inhibitor, silver thiosulfateon organogenesis of PLBs in Dendrobium kingianum cultured in vitro. Acta Hortic. 2017, 1167, 133–138. [Google Scholar] [CrossRef]
- Habiba, S.U.; Shimasaki, K.; Ahasan, M.M. Effects of ethrel on organogenesis of protocorm-like bodies in Dendrobium kingianum in vitro. Plant Tissue Cult. Biotech. 2018, 28, 141–146. [Google Scholar] [CrossRef]
- Sultana, K.S.; Hasan, K.M.; Hasan, K.M.; Sultana, S.; Mehraj, H.; Ahasan, M.; Shimasaki, K.; Habiba, S.U. Effect of two elicitors on organogenesis in protocorm-like-bodies (PLBs) of Phalaenopsis ‘Fmk02010’ cultured in vitro. World Appl. Sci. J. 2015, 33, 1528–1532. [Google Scholar]
- Sultana, K.S.; Hasan, K.M.; Hasan, K.M.; Sultana, S.; Mehraj, H.; Ahasan, M.; Shimasaki, K.; Habiba, S.U. Effect of hyaluronic acid (HA) on organogenesis in protocorm-like bodies (PLBs) of Phalaenopsis ‘Fmk02010’ cultured in vitro. Am. Eurasian J. Agric. Environ. Sci. 2015, 15, 1721–1724. [Google Scholar]
- Mehraj, H.; Shimasaki, K. In vitro PLBs organogenesis of Phalaenopsis using different concentrations of HA9 and HA12 combination. J. Biosci. Agric. Res. 2017, 12, 1036–1040. [Google Scholar] [CrossRef]
- Hannig, E. Zur physiologie pflanzlicher embryonen. I. Ueber die cultur von cruciferen-embryonen ausserhalb des embrysacks. Bot. Ztg. 1904, 62, 45–80. [Google Scholar]
- Dieterich, K. U¨ber kultur von Eembryonen ausserhalb des samens. Flora 1924, 117, 379–417. [Google Scholar]
- Laibach, F. Das taubwerden von bastardsamen und die kunstliche Aufzucht fruh absterbender bastardembryonen. Z. Bot. 1925, 17, 417–459. [Google Scholar]
- Raghavan, V. One hundred years of zygotic embryo culture investigations. Vitr. Cell Dev. Biol. Plant 2003, 39, 437–442. [Google Scholar] [CrossRef]
- Marasek-Ciolakowska, A.; Nishikawa, T.; Shea, D.J.; Okazaki, K. Breeding of lilies and tulips-Interspecific hybridization and genetic background. Breed. Sci. 2018, 68, 35–52. [Google Scholar] [CrossRef]
- Sharma, D.R.; Kaur, R.; Kumar, K. Embryo rescue in plants: A review. Euphytica 1996, 89, 325–337. [Google Scholar] [CrossRef]
- Cheng, X.; Chen, S.M.; Chen, F.D.; Fang, W.M.; Deng, Y.M.; She, L.F. Interspecific hybrids between Dendranthema morifolium (Ramat.) Kitamura and D. nankingense (Nakai) Tzvel. achieved using ovary rescue and their cold tolerance characteristics. Euphytica 2010, 172, 101–108. [Google Scholar] [CrossRef]
- Deng, Y.; Teng, N.; Chen, S.; Guan, Z.; Song, A.; Chang, Q. Reproductive barriers in the intergeneric hybridization between Chrysanthemum grandiflorum (Ramat.) Kitam. and Ajania przewalskii Poljak. (Asteraceae). Euphytica 2010, 174, 41–50. [Google Scholar] [CrossRef]
- Sahijram, L.; Rao, B.M. Hybrid embryo rescue in crop improvement. In Plant Biology and Biotechnology; Bahadur, B., Venkat Rajam, M., Sahijram, L., Krishnamurthy, K., Eds.; Springer: New Delhi, India, 2015; Chapter 18; pp. 363–384. [Google Scholar]
- Zulkarnain, Z.; Tapingkae, T.; Taji, A. Applications of in vitro techniques in plant breeding. In Advances in Plant Breeding Strategies: Breeding, Biotechnology and Molecular Tools; Al-Khayri, J.M., Jain, S.M., Johnson, D.V., Eds.; Springer: Cham, Switzerland, 2015; Chapter 10; pp. 293–328. [Google Scholar]
- Pramanik, K.; Sahoo, J.P.; Mohapatra, P.P.; Acharya, L.K.; Jena, C. Insights into the embryo rescue—A modern in-vitro crop improvement approach in horticulture. Plant Cell Biotechnol. Mol. Biol. 2021, 22, 20–33. [Google Scholar]
- Caser, M.; Dente, F.; Ghione, G.G.; Mansuino, A.; Giovannini, A.; Scariot, V. Shortening of selection time of Rosa hybrida by in vitro culture of isolated embryos and immature seeds. Propag. Ornam. Plants 2014, 14, 139–144. [Google Scholar]
- Yuan, M.S.; Wu, M.C.; Shii, C.T. Shortening breeding cycles of spider lilies (Lycoris spp.) through embryo culture and dikaryotype hybridization between Lycoris aurea and “a” karyotype species. Acta Hortic. 2003, 620, 345–352. [Google Scholar] [CrossRef]
- Deng, Y.; Chen, S.; Chen, F.; Cheng, X.; Zhang, F. The embryo rescue derived intergeneric hybrid between chrysanthemum and Ajania przewalskii shows enhanced cold tolerance. Plant Cell Rep. 2011, 30, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Chen, S.; Chen, F.; Deng, Y.; Fang, W.; Tang, F.; Liu, Z.; Shao, W. Creating novel chrysanthemum germplasm via interspecific hybridization and backcrossing. Euphytica 2011, 177, 45–53. [Google Scholar] [CrossRef]
- Sun, C.Q.; Chen, F.D.; Teng, N.J.; Liu, Z.L.; Fang, W.M.; Hou, X.L. Factors affecting seed set in the crosses between Dendranthema grandiflorum (Ramat.) Kitamura and its wild species. Euphytica 2009, 171, 181–192. [Google Scholar] [CrossRef]
- Sun, C.Q.; Chen, F.D.; Teng, N.J.; Liu, Z.L.; Fang, W.M.; Hou, X.L. Interspecific hybrids between Chrysanthemum grandiflorum (Ramat.) Kitamura and C. indicum (L.) Des Moul. and their drought tolerance evaluation. Euphytica 2010, 174, 51–60. [Google Scholar] [CrossRef]
- Zhu, W.Y.; Jiang, J.F.; Chen, S.M.; Wang, L.; Xu, L.L.; Wang, H.B.; Li, P.L.; Guan, Z.Y.; Chen, F.D. Intergeneric hybrid between Chrysanthemum × morifolium and Artemisia japonica achieved via embryo rescue shows salt tolerance. Euphytica 2013, 191, 109–119. [Google Scholar] [CrossRef]
- Deng, Y.M.; Chen, S.M.; Lu, A.M.; Chen, F.D.; Tang, F.P.; Guan, Z.Y.; Teng, N.J. Production and characterisation of the intergeneric hybrids between Dendranthema morifolium and Artemisia vulgaris exhibiting enhanced resistance to chrysanthemum aphid (Macrosiphoniella sanbourni). Planta 2010, 231, 693–703. [Google Scholar] [CrossRef]
- Tang, F.; Chen, F.; Chen, S.; Teng, N.; Fang, W. Intergeneric hybridization and relationship of genera within the tribe Anthemideae Cass. (I. Dendranthema crissum (kitam.) kitam. × Crossostephium chinense (L.) Makino). Euphytica 2009, 169, 133–140. [Google Scholar] [CrossRef]
- Röper, A.C.; Lütken, H.; Hegelund, J.N.; Petersen, K.K.; Christensen, B.; Müller, R. Effect of different ovule isolation times on the embryo development of Campanula hybrids. Acta Hortic. 2012, 953, 161–166. [Google Scholar] [CrossRef]
- Holeman, D.J. Simple Embryo Culture for Plant Breeders: A Manual of Technique for the Extraction and In-Vitro Germination of Mature Plant Embryos with Emphasis on the Rose, 1st ed.; Rose Hybridizers Association: Hartford, CT, USA, 2009; pp. 1–34. [Google Scholar]
- Shen, X.; Gmitter, F.G.; Grosser, J.W. Immature embryo rescue and culture. In Plant Embryo Culture, Methods in Molecular Biology Series; Thorpe, T., Yeung, E., Eds.; Humana Press: Totowa, NJ, USA, 2011; Volume 710, Chapter 7; pp. 75–92. [Google Scholar]
- Abdolmohammadi, M.; Kermani, M.J.; Zakizadeh, H.; Hamidoghli, Y. In vitro embryo germination and interploidy hybridization of rose (Rosa sp). Euphytica 2014, 198, 255–264. [Google Scholar] [CrossRef]
- Puangkrit, T.; Nontaswatsri, C. Intersubgeneric hybridization between Paracurcuma and Eucurcuma via embryo rescue. Acta Hortic. 2014, 1025, 37–42. [Google Scholar] [CrossRef]
- Wang, Q.; Zhang, Y.; Kawabata, S.; Li, Y. Double fertilization and embryogenesis of Eustoma grandiflorum. J. Jap. Soc. Hortic. Sci. 2011, 80, 351–357. [Google Scholar] [CrossRef][Green Version]
- Marasek-Ciolakowska, A.; Sochacki, D.; Marciniak, P. Breeding aspects of selected ornamental bulbous crops. Agronomy 2021, 11, 1709. [Google Scholar] [CrossRef]
- Li, Z.; Pinkham, L.; Campbell, N.F.; Espinosa, A.C.; Conev, R. Development of triploid daylily (Hemerocallis) germplasm by embryo rescue. Euphytica 2009, 169, 313–318. [Google Scholar] [CrossRef]
- Yao, J.L.; Cohen, D. Production of triploid Zantedeschia hybrids using embryo rescue. N. Z. J. Crop Hortic. Sci. 1996, 24, 297–301. [Google Scholar] [CrossRef]
- Burchi, G.; Mercuri, A.; Bianchini, C.; Bregliano, R.; Schiva, T. New interspecific hybrids of Alstroemeria obtained through in vitro embryo-rescue. Acta Hortic. 2000, 508, 233–236. [Google Scholar] [CrossRef]
- Bridgen, M.; Kollman, E.; Lu, C. Interspecific hybridization of Alstroemeria for the development of new, ornamentals. Acta Hortic. 2009, 836, 73–78. [Google Scholar] [CrossRef]
- Lim, S.S.; Lee, S.I.; Kang, S.C.; Kim, J.B. Alstroemeria plants and its biotechnological applications. J. Plant Biotechnol. 2012, 39, 219–224. [Google Scholar] [CrossRef]
- Aros, D.; Suazo, M.; Rivas, C.; Zapata, P.; Úbeda, C.; Bridgen, M. Molecular and morphological characterization of new interspecific hybrids of alstroemeria originated from A. caryophylleae scented lines. Euphytica 2019, 215, 93. [Google Scholar] [CrossRef]
- Kato, J.; Mii, M. Production of interspecific hybrid plants in Primula. In Plant Cell Culture Protocols, Methods in Molecular Biology Series; Loyola-Vargas, V.M., Vázquez-Flota, F., Eds.; Humana Press: Totowa, NJ, USA, 2006; Volume 318, Chapter 21; pp. 253–262. [Google Scholar]
- Amano, J.; Kato, J.; Nakano, M.; Mii, M. Production of inter-section hybrids between Primula filchnerae and P. sinensis through ovule culture. Sci. Hortic. 2006, 110, 223–227. [Google Scholar] [CrossRef]
- Benega-Garcia, R.; Cisneros, A.; Schneider, B.; Tel-Zur, N. Gynogenesis in the vine cacti Hylocereus and Selenicereus (Cactaceae). Plant Cell Rep. 2009, 28, 719–726. [Google Scholar] [CrossRef]
- Cisneros, A.; Tel-Zur, N. Embryo rescue and plant regeneration following interspecific crosses in the genus Hylocereus (Cactaceae). Euphytica 2010, 174, 73–82. [Google Scholar] [CrossRef]
- Cisneros, A.; Garcia, R.B.; Tel-Zur, N. Creation of novel interspecific-interploid Hylocereus hybrids (Cactaceae) via embryo rescue. Euphytica 2013, 189, 433–443. [Google Scholar] [CrossRef]
- Nishihara, M.; Tasaki, K.; Sasaki, N.; Takahashi, H. Development of basic technologies for improvement of breeding and cultivation of Japanese gentian. Breed. Sci. 2018, 68, 14–24. [Google Scholar] [CrossRef]
- Takamura, Y.; Asano, C.; Hikage, T.; Hatakeyama, K.; Takahata, Y. Production of interspecific hybrids between Japanese gentians and wild species of Gentiana. Breed. Sci. 2019, 69, 680–687. [Google Scholar] [CrossRef]
- Takamura, Y.; Takahashi, R.; Hikage, T.; Hatakeyama, K.; Takahata, Y. Production of haploids and doubled haploids from unfertilized ovule culture of various wild species of gentians (Gentiana spp.). Plant Cell Tissue Organ Cult. 2021, 146, 505–514. [Google Scholar] [CrossRef]
- Nishimoto, S.I.; Shimizu, K.; Hashimoto, F.; Sakata, Y. Interspecific hybrids of Camellia chrysantha × C. japonica by ovule culture. J. Jpn. Soc. Hortic. Sci. 2003, 72, 236–242. [Google Scholar] [CrossRef]
- Chen, Y.M.; Mii, M. Inter-sectional hybrids obtained from reciprocal crosses between Begonia semperflorens (section Begonia) and B. ‘Orange Rubra’ (section Gaerdita × section Pritzelia). Breed. Sci. 2012, 62, 113–123. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morgan, E.; Burge, G.; Seelye, J.; Hopping, M.E.; Grant, J.E.; Warren, A.G.F.; Brundell, D. Wide crosses in the Colchicaceae: Sandersonia aurantiaca (Hook.) × Littonia modesta (Hook.). Euphytica 2001, 121, 343–348. [Google Scholar] [CrossRef]
- Sato, S.; Katoh, N.; Yoshida, H.; Iwai, S.; Hagimori, M. Production of doubled haploid plants of carnation (Dianthus caryophyllus L.) by pseudo fertilized ovule culture. Sci. Hortic. 2000, 83, 301–310. [Google Scholar] [CrossRef]
- Nimura, M.; Kato, J.; Mii, M.; Morioka, M. Unilateral compatibility and genotypic difference in crossability in interspecific hybridization between Dianthus caryophyllus L. and Dianthus japonicus Thunb. Theor. Appl. Genet. 2003, 106, 1164–1170. [Google Scholar] [CrossRef]
- Kishi, F.; Kagami, Y.; Shinohara, M.; Hatano, S.; Tsurushima, H. Production of interspecific hybrid in Gypsophila by ovule-embryo culture. Euphytica 1994, 74, 85–90. [Google Scholar] [CrossRef]
- Eeckhaut, T.; De Keyser, E.; Van Huylenbroeck, J.; de Riek, J.; van Bockstaele, E. Application of embryo rescue after interspecific crosses in the genus Rhododendron. Plant Cell Tissue Organ Cult. 2007, 89, 29–35. [Google Scholar] [CrossRef]
- Ishizaka, H. Interspecific hybridization by embryo rescue in the genus Cyclamen. Plant Biotechnol. 2008, 25, 511–519. [Google Scholar] [CrossRef][Green Version]
- Nomura, Y.; Maeda, M.; Tsuchiya, T.; Makara, K. Efficient production of interspecific hybrids between Allium chinense and edible Allium spp. through ovary culture and pollen storage. Breed. Sci. 1994, 44, 151–155. [Google Scholar] [CrossRef]
- Dubouzet, J.G.; Arisumi, K.I.; Takeomi, E.; Maeda, M.; Sakata, Y. Studies on the development of new ornamental Allium through interspecific hybridization III. Hybridization of autumn-flowering species through pull-style pollination, cutflower culture and embryo rescue. Mem. Fac. Agric. Kagoshima Univ. 1994, 30, 35–42. [Google Scholar]
- Wilcock, C.; Neiland, R. Pollination failure in plants: Why it happens and when it matters. Trends Plant Sci. 2002, 7, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T. Reproductive barrier and genomic imprinting in the endosperm of flowering plants. Genes Genet. Syst. 2007, 82, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Murthy, K.S.R.; Kondamudi, R.; Rao, P.V.C.; Pullaiah, T. In vitro flowering—A review. J. Agric. Technol. 2012, 8, 1517–1536. [Google Scholar]
- Yamashita, Y.; Terada, R.; Nishibayashi, S.; Shimamoto, K. Asymmetric somatic hybrids of Brassica: Partial transfer of B. campestris genome into B. oleracea by cell fusion. Theor. Appl. Genet. 1989, 77, 189–194. [Google Scholar] [CrossRef]
- Trick, H.; Zelcer, A.; Bates, G.W. Chromosome elimination in asymmetric somatic hybrids: Effect of gamma dose and time in culture. Theor. Appl. Genet. 1994, 88, 965–972. [Google Scholar] [CrossRef] [PubMed]
- Anthony, P.; Marchant, R.; Blackhall, N.W.; Power, J.B.; Davey, M.R. Chemical fusion of protoplasts. In Plant Tissue Culture Manual; Lindsey, K., Ed.; Springer: Berlin, Germany, 1995; Chapter 1; pp. 1–15. [Google Scholar]
- Smith, H.H.; Kao, K.N.; Combatti, N.C. Interspecific hybridization by protoplast fusion in Nicotiana. Confirmation and extension. J. Hered. 1976, 67, 123–128. [Google Scholar] [CrossRef]
- Dudits, D.; Fejer, O.; Hadlaczky, G.; Koncz, C.; Lazar, G.B.; Horvath, G. Intergeneric gene transfer mediated by protoplast fusion. Mol. Gen. Genet. 1980, 179, 283–288. [Google Scholar] [CrossRef]
- Wang, J.; Jiang, J.; Wang, Y. Protoplast fusion for crop improvement and breeding in China. Plant Cell Tissue Organ Cult. 2013, 112, 131–142. [Google Scholar] [CrossRef]
- Ranghoo-Sanmukhiya, V.M. Somaclonal variation and methods used for its detection. In Propagation and Genetic Manipulation of Plants; Siddique, I., Ed.; Springer: Singapore, 2021; Chapter 1; pp. 1–18. [Google Scholar]
- Kanchanapoom, K.; Jantaro, S.; Rakchad, D. Isolation and fusion of protoplasts from mesophyll cells of Dendrobium pompadour. ScienceAsia 2001, 27, 29–34. [Google Scholar] [CrossRef]
- Nakano, M.; Mii, M. Somatic hybridization between Dianthus chinensis and D. barbatus through protoplast fusion. Theor. Appl. Genet. 1993, 86, 1–5. [Google Scholar] [CrossRef]
- Tomiczak, K.; Sliwinska, E.; Rybczyński, J.J. Protoplast fusion in the genus Gentiana: Genomic composition and genetic stability of somatic hybrids between Gentiana kurroo Royle and G. cruciata L. Plant Cell Tissue Organ Cult. 2017, 131, 1–14. [Google Scholar] [CrossRef]
- Tomiczak, K. Molecular and cytogenetic description of somatic hybrids between Gentiana cruciata L. and G. tibetica King. J. Appl. Genet. 2020, 61, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, K.; Miyabe, Y.; Nagaike, H.; Yabuya, T.; Adachi, T. Production of somatic hybrid plants between Iris ensata Thunb. and I. germanica. Euphytica 1999, 107, 105–113. [Google Scholar] [CrossRef]
- Horita, M.; Morohashi, H.; Komai, F. Production of fertile somatic hybrid plants between Oriental hybrid lily and Lilium × formolongi. Planta 2003, 217, 597–601. [Google Scholar] [CrossRef] [PubMed]
- Power, J.B.; Berry, S.F.; Chapman, J.V.; Cocking, E.C. Somatic hybridization of sexually incompatible petunias: Petunia parodii, Petunia parviflora. Theor. Appl. Genet. 1980, 57, 1–4. [Google Scholar] [CrossRef]
- Rode, C.; Winkelmann, T.; Meyer, L.; Debener, T. The ethylene 2 receptor gene as a robust molecular marker for intergeneric somatic hybrids between Petunia and Calibrachoa. Plant Breed. 2010, 129, 448–453. [Google Scholar]
- Kästner, U.; Klocke, E.; Abel, S. Regeneration of protoplasts after somatic hybridisation of Hydrangea. Plant Cell Tissue Organ Cult. 2017, 129, 359–373. [Google Scholar] [CrossRef]
- Prange, A.N.S.; Bartsch, M.; Meiners, J.; Serek, M.; Winkelmann, T. Interspecific somatic hybrids between Cyclamen persicum and C. coum, two sexually incompatible species. Plant Cell Rep. 2012, 31, 723–735. [Google Scholar] [CrossRef]
- Al-Atabee, J.S.; Mulligan, B.J.; Power, J.B.; Afkhami-Sarvestani, R.; Serek, M.; Winkelmann, T. Interspecific somatic hybrids of Rudbeckia hirta and R. Laciniata (Compositae). Plant Cell Rep. 1990, 8, 517–520. [Google Scholar] [CrossRef]
- Afkhami-Sarvestani, R.; Serek, M.; Winkelmann, T. Protoplast isolation and culture from Streptocarpus, followed by fusion with Saintpaulia ionantha protoplasts. Europ. J. Hort. Sci. 2012, 77, S249–S260. [Google Scholar]
- Chin, C.K.; Lee, Z.H.; Mubbarakh, S.A.; Antony, J.J.J.; Chew, B.L.; Subramaniam, S. Effects of plant growth regulators and activated charcoal on somaclonal variations of protocorm-like bodies (PLBs) of Dendrobium Sabin Blue orchid. Biocatal. Agric. Biotechnol. 2019, 22, 101426. [Google Scholar] [CrossRef]
- Qahtan, A.A.; Abdel-Salam, E.M.; Alatar, A.A.; Wang, Q.C.; Faisal, M. An introduction to synthetic seeds: Production, techniques, and applications. In Synthetic Seeds; Faisal, M., Alatar, A.A., Eds.; Springer: Cham, Switzerland, 2019; Chapter 1; pp. 1–20. [Google Scholar]
- Maqsood, M.; Khusrau, M.; Mujib, A.; Kaloo, Z.A. Synthetic seed technology in some ornamental and medicinal plants: An overview. In Propagation and Genetic Manipulation of Plants; Siddique, I., Ed.; Springer: Singapore, 2021; Chapter 2; pp. 19–31. [Google Scholar]
- Touchell, D.H.; Palmer, I.E.; Ranney, T.G. In vitro ploidy manipulation for crop improvement. Front. Plant Sci. 2020, 11, 722. [Google Scholar] [CrossRef] [PubMed]
- Dhooghe, E.; van Laere, K.; Eeckhaut, T.; Leus, L.; van Huylenbroeck, J. Mitotic chromosome doubling of plant tissues in vitro. Plant Cell Tissue Organ Cult. 2011, 104, 359–373. [Google Scholar] [CrossRef]
- Habibi, M.; Shukurova, M.K.; Watanabe, K.N. Testing two chromosome doubling agents for in vitro tetraploid induction on ginger lilies, Hedychium gardnerianum Shepard ex Ker Gawl and Hedychium coronarium J. Koenig. Vitr. Cell Dev. Biol. Plant 2022, 58, 489–497. [Google Scholar] [CrossRef]
- Cai, X.; Cao, Z.; Xu, S.; Deng, Z. Induction, regeneration and characterization of tetraploids and variants in ‘Tapestry’ caladium. Plant Cell Tissue Organ Cult. 2015, 120, 689–700. [Google Scholar] [CrossRef]
- Talebi, S.F.; Saharkhiz, M.J.; Kermani, M.J.; Sharafi, Y.; Raouf Fard, F. Effect of different antimitotic agents on polyploid induction of anise hyssop (Agastache foeniculum L.). Caryologia 2017, 70, 184–193. [Google Scholar] [CrossRef]
- Miguel, T.P.; Leonhardt, K.W. In vitro polyploid induction of orchids using oryzalin. Sci. Hortic. 2011, 130, 314–319. [Google Scholar] [CrossRef]
- Giovannini, A.; Laura, M.; Nesi, B.; Savona, M.; Cardi, T. Genes and genome editing tools for breeding desirable phenotypes in ornamentals. Plant Cell Rep. 2021, 40, 461–478. [Google Scholar] [CrossRef]
- Koetle, M.J.; Finniea, J.F.; Balázsab, E.; Staden, J.V. A review on factors affecting the Agrobacterium-mediated genetic transformation in ornamental monocotyledonous geophytes. S. Afr. J. Bot. 2015, 98, 37–44. [Google Scholar] [CrossRef]
- Bull, T.; Michelmore, R. Molecular determinants of in vitro plant regeneration: Prospects for enhanced manipulation of lettuce (Lactuca sativa L.). Front. Plant Sci. 2022, 13, 1211. [Google Scholar] [CrossRef]
- Bednarek, P.T.; Orłowska, R. Plant tissue culture environment as a switch-key of (epi)genetic changes. Plant Cell Tissue Organ Cult. 2020, 140, 245–257. [Google Scholar] [CrossRef]
- Ghosh, A.; Igamberdiev, A.U.; Debnath, S.C. Tissue culture-induced DNA methylation in crop plants: A review. Mol. Biol. Rep. 2021, 48, 823–841. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, H.; Sugimoto, K.; Tarr, P.T.; Temman, H.; Kadokura, S.; Inui, Y.; Sakamoto, T.; Sasaki, T.; Aida, M.; Suzuki, T.; et al. Primed histone demethylation regulates shoot regenerative competency. Nat. Commun. 2019, 10, 1786. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Xu, W.; Wang, C.; Yi, X.; Zhang, W.; Su, Z. Differential deposition of H2A.Z in combination with histone modifications within related genes in Oryza sativa callus and seedling. Plant J. 2017, 89, 264–277. [Google Scholar] [CrossRef]
- Azman, A.S.; Mhiri, C.; Grandbastien, M.A.; Tam, S.M. Transposable elements and the detection of somaclonal variation in plant tissue culture: A review. Malays. Appl. Biol. 2014, 43, 1–12. [Google Scholar]
- Mitra, G.C.; Prasad, R.N.; Choudhury, R.A. Inorganic salts & differentiation of protocorms in seed callus of an orchid & correlated changes in its free amino acid content. Indian J. Exp. Biol. 1976, 14, 350–351. [Google Scholar]
- Knudson, L. A new nutrient solution for the germination of orchid seed. Amer. Orchid Soc. Bull. 1946, 15, 214–217. [Google Scholar]
- Van der Salm, T.P.; Van der Toorn, C.J.; Ten Cate, C.H.H.; Dubois, L.A.; De Vries, D.P.; Dons, H.J. Importance of the iron chelate formula for micropropagation of Rosa hybrida L. ‘Moneyway’. Plant Cell Tissue Organ Cult. 1994, 37, 73–77. [Google Scholar] [CrossRef]
- Driver, J.A.; Kuniyuki, A.H. In vitro propagation of Paradox walnut rootstock. Hort. Sci. 1984, 19, 507–509. [Google Scholar]
- Teixeira da Silva, J.A. Response of hybrid Cymbidium (Orchidaceae) protocorm-like bodies to 26 plant growth regulators. Bot. Lith. 2014, 20, 3–13. [Google Scholar]
- Nayak, N.R.; Chand, P.K.; Rath, S.P.; Patnaik, S.N. Influence of some plant growth regulators on the growth and organogenesis of Cymbidium aloifolium (L.) Sw. seed-derived rhizomes in vitro. Vitr. Cell Dev. Biol. Plant 1998, 34, 185. [Google Scholar] [CrossRef]
- Nayak, N.R.; Sahoo, S.; Patnaik, S.; Rath, S.P. Establishment of thin cross section (TCS) culture method for rapid micropropagation of Cymbidium aloifolium (L.) Sw. and Dendrobium nobile Lindl. (Orchidaceae). Sci. Hortic. 2002, 94, 107–116. [Google Scholar] [CrossRef]
- Lukatkin, A.S.; Mokshin, E.V.; Bolshakova, E.V.; Teixeira da Silva, J.A. Effects of inorganic salts concentration and alternative plant growth regulators on the in vitro organogenesis of a new hybrid Cymbidium. BioTechnologia 2019, 100, 279–288. [Google Scholar] [CrossRef]
- Kaewjampa, N.; Shimasaki, K.; Nahar, S.J. Hyaluronic acid can be a new plant growth regulator for hybrid Cymbidium micropropagation. Plant Tissue Cult. Biotech. 2012, 22, 59–64. [Google Scholar] [CrossRef]
- Nahar, S.J.; Shimasaki, K.; Haque, S.M. Chondroitin sulfate can be a new plant growth regulator for Cymbidium micropropagation. Acta Hortic. 2013, 1014, 449–455. [Google Scholar] [CrossRef]
- Tao, J.; Yu, L.; Kong, F.; Zhao, D. Effects of plant growth regulators on in vitro propagation of Cymbidium faberi Rolfe. Afr. J. Biotechnol. 2011, 10, 15639–15646. [Google Scholar] [CrossRef]
- Nahar, S.J.; Shimasaki, K.; Huang, C.L.; Naruemol, K. Effect of plant growth regulators on organogenesis in protocorm-like body (PLBs) of Cymbidium dayanum in vitro. ARPN J. Agric. Biol. Sci. 2011, 6, 28–33. [Google Scholar]
- Pant, B.; Swar, S. Micropropagation of Cymbidium iridioides. Nepal J. Sci. Technol. 2011, 12, 91–96. [Google Scholar] [CrossRef]
- Islam, S.S.; Islam, T.; Bhattacharjee, B.; Mondal, T.K.; Subramaniam, S. In vitro pseudobulb based micropropagation for mass development of Cymbidium finlaysonianum Lindl. Emir. J. Food Agric. 2015, 27, 469–474. [Google Scholar] [CrossRef]
- Khatun, H.; Khatun, M.; Biswas, M.; Kabir, M.; Al-Amin, M. In vitro growth and development of Dendrobium hybrid orchid. Bangladesh J. Agric. Res. 2010, 35, 507–514. [Google Scholar] [CrossRef][Green Version]
- Khatun, M.; Khatun, H.; Khanam, D.; Al-Amin, M. In vitro root formation and plantlet development in Dendrobium orchid. Bangladesh J. Agric. Res. 2010, 35, 257–265. [Google Scholar] [CrossRef]
- Martin, K.P.; Madassery, J. Rapid in vitro propagation of Dendrobium hybrids through direct shoot formation from foliar explants, and protocorm-like bodies. Sci. Hortic. 2006, 108, 95–99. [Google Scholar] [CrossRef]
- Goswami, K.; Yasmin, S.; Nasiruddin, K.M.; Khatun, F.; Akte, J. In vitro regeneration of Dendrobium sp. of orchid using leaf tip as explant. J. Environ. Sci. Nat. Resour. 2015, 8, 75–78. [Google Scholar] [CrossRef]
- Hossen, M.M.; Saha, S.; Khatun, F. Effects of plant growth regulators on in vitro growth and development of orchid (Dendrobium sp.) from protocorm like bodies (PLBs). J. Bangladesh Agric. Univ. 2021, 193, 294–301. [Google Scholar] [CrossRef]
- Luo, J.P.; Wang, Y.; Zha, X.Q.; Huang, L. Micropropagation of Dendrobium densiflorum Lindl. ex Wall. through protocorm-like bodies: Effects of plant growth regulators and lanthanoids. Plant Cell Tissue Organ Cult. 2008, 93, 333. [Google Scholar] [CrossRef]
- Pradhan, S.; Paudel, Y.P.; Pant, B. Efficient regeneration of plants from shoot tip explants of Dendrobium densiflorum Lindl., a medicinal orchid. Afr. J. Biotechnol. 2013, 12, 1378–1383. [Google Scholar]
- Sheela, V.L.; Sarada, S.; Anitha, S. Development of protocorm-like bodies and shoots in Dendrobium cv. Sonia following gamma irradiation. J. Trop. Agric. 2006, 44, 86–87. [Google Scholar]
- Bhattacharyya, P.; Kumaria, S.; Tandon, P. High frequency regeneration protocol for Dendrobium nobile: A model tissue culture approach for propagation of medicinally important orchid species. S. Afr. J. Bot. 2016, 104, 232–243. [Google Scholar] [CrossRef]
- Malabadi, R.B.; Mulgund, G.S.; Kallappa, N. Micropropagation of Dendrobium nobile from shoot tip sections. J. Plant Physiol. 2005, 162, 473–478. [Google Scholar] [CrossRef]
- Tikendra, L.; Potshangbam, A.M.; Dey, A.; Devi, T.R.; Sahoo, M.R.; Nongdam, P. RAPD, ISSR, and SCoT markers based genetic stability assessment of micropropagated Dendrobium fimbriatum Lindl. var. oculatum Hk. f.- an important endangered orchid. Physiol. Mol. Biol. Plants 2021, 27, 341–357. [Google Scholar] [CrossRef]
- Bhowmik, T.K.; Rahman, M.M. Micropropagation of commercially important orchid Dendrobium palpebrae Lindl. through in vitro developed pseudobulb culture. J. Adv. Biotechnol. Exp. Ther. 2020, 3, 225–232. [Google Scholar] [CrossRef]
- Shiau, Y.J.; Nalawade, S.M.; Hsia, C.N.; Mulabagal, V.; Tsay, H.S. In vitro propagation of the Chinese medicinal plant, Dendrobium candidum wall. ex lindl., from axenic nodal segments. Vitr. Cell Dev. Biol. Plant 2005, 41, 666–670. [Google Scholar] [CrossRef]
- Zhao, P.; Wu, F.; Feng, F.S.; Wang, W.J. Protocorm-like body (PLB) formation and plant regeneration from the callus culture of Dendrobium candidum Wall ex Lindl. Vitr. Cell Dev. Biol. Plant 2008, 44, 178–185. [Google Scholar] [CrossRef]
- Longchar, T.B.; Deb, C.R. Optimization of in vitro propagation protocol of Dendrobium heterocarpum Wall. ex. Lindl. and clonal genetic fidelity assessment of the regenerates: An orchid of horticultural and medicinal importance. S. Afr. J. Bot. 2022, 149, 67–78. [Google Scholar] [CrossRef]
- Pant, B.; Thapa, D. In vitro mass propagation of an epiphytic orchid, Dendrobium primulinum Lindl. through shoot tip culture. Afr. J. Biotechnol. 2012, 11, 9970–9974. [Google Scholar]
- Tikendra, L.; Koijam, A.S.; Nongdam, P. Molecular markers based genetic fidelity assessment of micropropagated Dendrobium chrysotoxum Lindl. Meta Gene 2019, 20, 100562. [Google Scholar] [CrossRef]
- Hajong, S.; Kumaria, S.; Tandon, P. Effect of plant growth regulators on regeneration potential of axenic nodal segments of Dendrobium chrysanthum Wall. ex Lindl. J. Agric. Sci. Tech. 2013, 15, 1425–1435. [Google Scholar]
- Bhattacharyya, P.; Kumaria, S.; Job, N.; Tandon, P. Phyto-molecular profiling and assessment of antioxidant activity within micropropagated plants of Dendrobium thyrsiflorum: A threatened, medicinal orchid. Plant Cell Tissue Organ Cult. 2015, 122, 535–550. [Google Scholar] [CrossRef]
- Zhao, D.; Hu, G.; Chen, Z.; Shi, Y.; Zheng, L.; Tang, A.; Long, C. Micropropagation and in vitro flowering of Dendrobium wangliangii: A critically endangered medicinal orchid. J. Med. Plants Res. 2013, 7, 2098–2110. [Google Scholar]
- Chen, B.; Trueman, S.J.; Li, J.; Li, Q.; Fan, H.; Zhang, J. Micropropagation of the endangered medicinal orchid, Dendrobium officinale. Life Sci. J. 2014, 11, 526–530. [Google Scholar]
- Nasiruddin, K.M.; Begum, R.; Yasmin, S. Protocorm like bodies and plantlet regeneration from Dendrobium formosum leaf callus. Asian J. Plant Sci. 2003, 2, 955–957. [Google Scholar] [CrossRef][Green Version]
- Riva, S.S.; Islam, A.; Hoque, M.E. In vitro regeneration and rapid multiplication of Dendrobium bensoniae, an indigenous ornamental orchid. Agriculturists 2016, 14, 24–31. [Google Scholar] [CrossRef][Green Version]
- Khatun, K.; Nath, U.K.; Rahman, M.S. Tissue culture of Phalaenopsis: Present status and future prospects. J. Adv. Biotechnol. Exp. Therap. 2020, 3, 273–285. [Google Scholar] [CrossRef]
- Zanello, C.A.; Cardoso, J.C. PLBs induction and clonal plantlet regeneration from leaf segment of commercial hybrids of Phalaenopsis. J. Hortic. Sci. Biotechnol. 2019, 94, 627–631. [Google Scholar] [CrossRef]
- Mose, W.; Daryono, B.S.; Indrianto, A.; Purwantoro, A.; Semiarti, E. Direct somatic embryogenesis and regeneration of an Indonesian orchid Phalaenopsis amabilis (L.) Blume under a variety of plant growth regulators, light regime, and organic substances. Jordan J. Biol. Sci. 2020, 13, 509–518. [Google Scholar]
- Balilashaki, K.; Vahedi, M.; Karimi, R. In vitro direct regeneration from node and leaf explants of Phalaenopsis cv. ‘Surabaya’. Plant Tissue Cult. Biotech. 2015, 25, 193–205. [Google Scholar] [CrossRef]
- Kuo, H.L.; Chen, J.T.; Chang, W.C. Efficient plant regeneration through direct somatic embryogenesis from leaf explants of Phalaenopsis ‘Little Steve’. Vitr. Cell Dev. Biol. Plant 2005, 41, 453–456. [Google Scholar] [CrossRef]
- Sinha, P.; Jahan, M.A.A. Clonal propagation of Phalaenopsis amabilis (L.) BL. cv. ‘Golden Horizon’ through In vitro culture of leaf segments. Bangladesh J. Sci. Ind. Res. 2011, 46, 163–168. [Google Scholar] [CrossRef]
- Rittirat, S.; Kongruk, S.; Te-chato, S. Induction of protocorm-like bodies (PLBs) and plantlet regeneration from wounded protocorms of Phalaenopsis cornucervi (Breda) Blume & Rchb. f. Int. J. Agric. Technol. 2012, 8, 2397–2407. [Google Scholar]
- Kalimuthu, K.; Senthilkumar, R.; Vijayakumar, S. In vitro micropropagation of orchid, Oncidium sp. (Dancing Dolls). Afr. J. Biotechnol. 2007, 6, 1171–1174. [Google Scholar]
- Chen, J.T.; Chang, W.C. TIBA affects the induction of direct somatic embryogenesis from leaf explants of Oncidium. Plant Cell Tissue Organ Cult. 2004, 79, 315–320. [Google Scholar] [CrossRef]
- Mata-Rosas, M.; Baltazar-García, R.J.; Chávez-Avila, V.M. In vitro regeneration through direct organogenesis from protocorms of Oncidium tigrinum Llave & Lex. (Orchidaceae), an endemic and threatened Mexican species. Hort. Sci. 2011, 46, 1132–1135. [Google Scholar]
- Mayer, J.L.S.; Stancato, G.C.; Appezzato-Da-Glória, B. Direct regeneration of protocorm-like bodies (PLBs) from leaf apices of Oncidium flexuosum Sims (Orchidaceae). Plant Cell Tissue Organ Cult. 2010, 103, 411–416. [Google Scholar] [CrossRef]
- Bhattacharjee, B.; Islam, S.S. Effects of plant growth regulators on multiple shoot induction in Vanda tessellata (Roxb.) Hook. Ex G. Don an endangered medicinal orchid. Int. J. Sci. Nat. 2014, 5, 707–712. [Google Scholar]
- Roy, A.R.; Patel, R.S.; Patel, V.V.; Sajeev, S.; Deka, B.C. Asymbiotic seed germination, mass propagation and seedling development of Vanda coerulea Griff ex. Lindl. (Blue Vanda): An in vitro protocol for an endangered orchid. Sci. Hortic. 2011, 128, 325–331. [Google Scholar] [CrossRef]
- Decruse, S.W.; Gangaprasad, A.; Seeni, S.; Menon, V.S. A protocol for shoot multiplication from foliar meristem of Vanda spathulata (L.) Spreng. Indian J. Exp. Biol. 2003, 41, 924–927. [Google Scholar]
- Naing, A.H.; Myint, K.T.; Hwang, Y.J.; Park, I.S.; Chung, J.D.; Lim, K.B. Micropropagation and conservation of the wild medicinal orchid, Coelogyne cristata. Horti. Environ. Biotechnol. 2010, 51, 109–114. [Google Scholar]
- Singh, N.; Kumaria, S. A combinational phytomolecular-mediated assessment in micropropagated plantlets of Coelogyne ovalis Lindl.: A horticultural and medicinal orchid. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2020, 90, 455–466. [Google Scholar] [CrossRef]
- Kaur, S.; Bhutani, K.K. In vitro mass propagation of ornamentally and medicinally important Coelogyne flaccida Lindl. through pseudobulb segments. Plant Tissue Cult. Biotech. 2013, 23, 39–47. [Google Scholar] [CrossRef]
- Kalyan, K.D.; Sil, S. Protocorm-like bodies and plant regeneration from foliar explants of Coelogyne flaccida, a horticulturally and medicinally important endangered orchid of eastern himalaya. Lankesteriana 2015, 15, 151–158. [Google Scholar]
- Aung, W.T.; Bang, K.S.; Yoon, S.A.; Ko, B.; Bae, J.H. Effects of different natural extracts and plant growth regulators on plant regeneration and callus induction from pseudobulbs explants through in vitro seed germination of endangered orchid Bulbophyllum auricomum Lindl. J. Bio. Environ. Con. 2022, 31, 133–141. [Google Scholar] [CrossRef]
- Prasad, G.; Seal, T.; Mao, A.A.; Vijayan, D.; Lokho, A. Assessment of clonal fidelity and phytomedicinal potential in micropropagated plants of Bulbophyllum odoratissimum-An endangered medicinal orchid of Indo Burma megabiodiversity hotspot. S. Afr. J. Bot. 2021, 141, 487–497. [Google Scholar] [CrossRef]
- Ng, C.Y.; Saleh, N.M. In vitro propagation of Paphiopedilum orchid through formation of protocorm-like bodies. Plant Cell Tissue Organ Cult. 2011, 105, 193–202. [Google Scholar] [CrossRef]
- Masnoddin, M.; Repin, R.; Abd Aziz, Z. Micropropagation of an endangered Borneo orchid, Paphiopedilum rothschildianum callus using temporary immersion bioreactor system. Thai Agric. Res. J. 2016, 34, 161–171. [Google Scholar]
- Coello, C.Y.; Miceli, C.L.; Orantes, C.; Dendooven, L.; Gutiérrez, F.A. Plant growth regulators optimization for in vitro cultivation of the orchid Guarianthe skinneri (Bateman) Dressier & WE Higgins. Gayana Bot. 2010, 67, 19–26. [Google Scholar]
- Baker, A.; Kaviani, B.; Nematzadeh, G.; Negahdar, N. Micropropagation of Orchis catasetum—A rare and endangered orchid. Acta Sci. Pol. Hortorum Cultus 2014, 13, 197–205. [Google Scholar]
- Chauhan, S.; Promila Pathak, A.; Sharma, S.K. Teepol regeneration of Eulophia dabia through rhizome explants and flowering: A study in vitro. J. Orchid Soc. India 2015, 29, 61–65. [Google Scholar]
- Sopalun, K.; Thammasiri, K.; Ishikawa, K. Micropropagation of the Thai orchid Grammatophyllum speciosum blume. Plant Cell Tissue Organ Cult. 2010, 101, 143–150. [Google Scholar] [CrossRef]
- Jainol, J.E.; Gansau, J.A. Effect of growth regulators and explant orientation on shoot tip culture of Borneo endemic orchid, Dimorphorchis lowii. Trans. Sci. Technol. 2016, 3, 306–312. [Google Scholar]
- Acemi, A. Chitosan versus plant growth regulators: A comparative analysis of their effects on in vitro development of Serapias vomeracea (Burm.f.) Briq. Plant Cell Tissue Organ Cult. 2020, 141, 327–338. [Google Scholar] [CrossRef]
- Sunitibala, D.Y.; Neelashree, N. Micropropagation of the monopodial orchid, Rhynchostylis retusa (L.). Int. J. Life Sci. 2018, 6, 181–186. [Google Scholar]
- Gantait, S.; Sinniah, U.R. Rapid micropropagation of monopodial orchid hybrid (Aranda Wan Chark Kuan ‘Blue’ × Vanda coerulea Grifft. ex. Lindl.) through direct induction of protocorm-like bodies from leaf segments. Plant Growth Regul. 2012, 68, 129–140. [Google Scholar] [CrossRef]
- Paudel, M.R.; Pant, B. In vitro micropropagation of rare orchid (Esmeralda clarkei Rchb. f.) from shoot tip section. Int. J. Biol. Pharm. Allied Sci. 2012, 1, 1587–1597. [Google Scholar]
- Sherif, N.A.; Senthil Kumar, T.; Rao, M.V. DNA barcoding and genetic fidelity assessment of micropropagated Aenhenrya rotundifolia (Blatt.) C.S. Kumar and F.N. Rasm.: A critically endangered jewel orchid. Physiol. Mol. Biol. Plants 2020, 26, 2391–2405. [Google Scholar] [CrossRef] [PubMed]
- Bustam, B.M.; Dixon, K.; Bunn, E. Ex situ germplasm preservation and plant regeneration of a threatened terrestrial orchid, Caladenia huegelii, through micropropagation and cryopreservation. Aust. J. Bot. 2016, 64, 659–663. [Google Scholar] [CrossRef]
- Picolotto, D.R.N.; Paiva, V.B.D.; Barros, F.D.; Padilha, D.R.C.; Cruz, A.C.F.D.; Otoni, W.C. Micropropagation of Cyrtopodium paludicolum (Orchidaceae) from root tip explants. Crop Breed. Appl. Biotechnol. 2017, 17, 191–197. [Google Scholar] [CrossRef][Green Version]
- Saleh-E-In, M.M.; Bhattacharyya, P.; Van Staden, J. Chemical composition and cytotoxic activity of the essential oil and oleoresins of in vitro micropropagated Ansellia africana Lindl: A vulnerable medicinal orchid of Africa. Molecules 2021, 26, 4556. [Google Scholar] [CrossRef]
- Chookoh, N.; Chiu, Y.T.; Chang, C.; Hu, W.H.; Dai, T.E. Micropropagation of Tolumnia orchids through induction of protocorm-like bodies from leaf segments. Hort. Sci. 2019, 54, 1230–1236. [Google Scholar] [CrossRef]
- Guo, W.L.; Chang, Y.C.A.; Kao, C.Y. Protocorm-like bodies initiation from root tips of Cyrtopodium paranaense (Orchidaceae). Hort. Sci. 2010, 45, 1365–1368. [Google Scholar] [CrossRef]
- Xu, J.; Beleski, D.G.; Vendrame, W.A. Effects of culture methods and plant growth regulators on in vitro propagation of Brassavola nodosa (L.) Lindl. hybrid. Vitr. Cell Dev. Biol. Plant 2022. [Google Scholar] [CrossRef]
- Ncube, B.; Finnie, J.F.; Van Staden, J. In vitro regeneration of Cyrtanthus species: Ornamental plants with medicinal benefits. Vitr. Cell Dev. Biol. Plant 2015, 51, 42–51. [Google Scholar] [CrossRef]
- Matand, K.; Shoemake, M.; Li, C. High frequency in vitro regeneration of adventitious shoots in daylilies (Hemerocallis sp) stem tissue using thidiazuron. BMC Plant Biol. 2020, 20, 31. [Google Scholar] [CrossRef] [PubMed]
- Youssef, N.M.; Shaaban, S.A.; Ghareeb, Z.F.; Taha, L.S. In vitro bulb formation of direct and indirect regeneration of Lilium orientalis cv. “Starfighter” plants. Bull. Natl. Res. Cent. 2019, 43, 211. [Google Scholar] [CrossRef]
- Javaheri, N.; Kaviani, B. Effect of hormonal combination of auxin and cytokinin on micropropagation of eastern lily (Lilium oriental hybrid ‘Casablanca’) plant using bulb scale explant. J. Hort. Sci. 2022, 36, 57–69. [Google Scholar]
- Han, B.H.; Yae, B.W.; Yu, H.J.; Peak, K.Y. Improvement of in vitro micropropagation of Lilium oriental hybrid ‘Casablanca’by the formation of shoots with abnormally swollen basal plates. Sci. Hortic. 2005, 103, 351–359. [Google Scholar] [CrossRef]
- Ptak, A.; Bach, A. Somatic embryogenesis in tulip (Tulipa gesneriana L.) flower stem cultures. Vitr. Cell Dev. Biol. Plant 2007, 43, 35–39. [Google Scholar] [CrossRef]
- Kritskaya, T.A.; Kashin, A.S.; Kasatkin, M.Y. Micropropagation and somaclonal variation of Tulipa suaveolens (Liliaceae) in vitro. Russ. J. Dev. Biol. 2019, 50, 209–215. [Google Scholar] [CrossRef]
- Maślanka, M.; Bach, A. Induction of bulb organogenesis in in vitro cultures of tarda tulip (Tulipa tarda Stapf.) from seed-derived explants. Vitr. Cell Dev. Biol. Plant 2014, 50, 712–721. [Google Scholar] [CrossRef]
- Ibrahim, M.A.; Draaj, I.A. The effect of explant source and cytokinin concentration on the direct bulb formation of tulip (Tulipa gesnerina L.) by plant tissue culture technique. Plant Cell Biotechnol. Mol. Biol. 2020, 21, 111–119. [Google Scholar]
- Wang, G.Y.; Yuan, M.F.; Hong, Y. In vitro flower induction in roses. Vitr. Cell Dev. Biol. Plant 2002, 38, 513–518. [Google Scholar] [CrossRef]
- Oo, K.T.; Lwin, K.M.; Khai, A.A. In vitro micropropagation of rose (Hybrid Rosa spp.) through plant tissue culture technique. J. Sci. Innov. Res. 2021, 10, 1–4. [Google Scholar] [CrossRef]
- Tawfik, A.A.; Ibrahim, O.H.M.; Abdul-Hafeez, E.Y.; Ibrahim, S.A. Optimizing micropropagation protocol for Rosa hybrida cv. Eiffel Tower with improved in vitro rooting ability. Egypt. J. Hort. 2018, 45, 323–335. [Google Scholar]
- Tirkey, D.S.; Nirala, D.P.; Kumari, D.P.N.P. Micropropagation from nodal explants of rose (Rosa hybrida L.) at different concentration of BAP (6-Benzyl Amino Purine). Int. J. Chem. Stud. 2019, SP6, 427–430. [Google Scholar]
- Khaskheli, A.J.; Khaskheli, M.I.; Khaskheli, M.A.; Shar, T.; Ahmad, W.; Lighari, U.A.; Khaskheli, M.A.; Khaskheli, A.A.; Makan, F.H. Proliferation, multiplication and improvement of micro-propagation system for mass clonal production of rose through shoot tip culture. Am. J. Plant Sci. 2018, 9, 296–310. [Google Scholar] [CrossRef]
- Kanchanapoom, K.; Posayapisit, N.; Kanchanapoom, K. In vitro flowering from cultured nodal explants of rose (Rosa hybrida L.). Not. Bot. Horti Agrobot. Cluj Napoca 2009, 37, 261–263. [Google Scholar]
- Kanchanapoom, K.; Sakpeth, P.; Kanchanapoom, L. In vitro flowering of shoots regenerated from cultured nodal expiants of Rosa hybrida cv. ‘Heirloom’. ScienceAsia 2010, 36, 161–164. [Google Scholar] [CrossRef]
- Afrin, S.; Rahman, M.A.; Khalekuzzaman, M.; Hasan, M.M.; Fahim, A.H.F.; Alam, M.A. Study on in vitro micropropagation of Rosa sp. Bangladesh J. Agric. 2022, 47, 66–74. [Google Scholar] [CrossRef]
- Wojtania, A.; Matysiak, B. In vitro propagation of Rosa ‘Konstancin’ (R. rugosa × R. beggeriana), a plant with high nutritional and pro-health value. Folia Hort. 2018, 30, 259–267. [Google Scholar] [CrossRef]
- Ali, M.; Baloch, S.K.; Seema, N.; Yaeen, S.; Kaleri, A.A.; Kaleri, R.R.; Nizamani, G.S.; Subhapoto, G.F.; Kaleri, M.A.; Shahani, F.; et al. Influence of phytohormones on callus indication and micropropagation on rose (Rosa indica L.). J. Basic Appl. Sci. 2018, 14, 9–11. [Google Scholar]
- Shabbir, A.; Hameed, N.; Ali, A.; Bajwa, R. Effect of different cultural conditions on micropropagation of rose (Rosa indica L.). Pak. J. Bot. 2009, 41, 2877–2882. [Google Scholar]
- Chhalgri, M.A.; Khan, M.T.; Nizamani, G.S.; Yasmeen, S.; Khan, I.A.; Aslam, M.M.; Rajpar, A.A.; Tayyaba, T.; Nizamani, F.; Nizamani, M.R.; et al. Effect of plant growth hormones on shoot and root regeneration in rose under in vitro conditions. Adv. Life Sci. 2020, 8, 93–97. [Google Scholar]
- Quynh, N.P.D.; Pha, N.T. Effect of culture media on micropropagation and in vitro flowering of red Eden Rose (Rosa ‘Red Eden’). Dong Thap Univ. J. Sci. 2020, 9, 93–99. [Google Scholar]
- Maheswari, N.U.; Vaishnavi, K. In vitro micropropagation of Rosa damascena Mill. Asian J. Multidimen. Res. 2018, 7, 107–114. [Google Scholar]
- Nikbakht, A.; Kafi, M.; Mirmasoudi, M.; Babalar, M. Micropropagation of damask rose (Rosa damascena Mill.) cvs Azaran and Ghamsar. Int. J. Agric. Biol. 2005, 7, 535–538. [Google Scholar]
- Kumar, A.; Sood, A.; Palni, U.; Gupta, A.; Palni, L.M. Micropropagation of Rosa damascena Mill. from mature bushes using thidiazuron. J. Hort. Sci. Biotechnol. 2001, 76, 30–34. [Google Scholar] [CrossRef]
- Alsemaan, T. Micro-propagation of Damask rose (Rosa damascena Mill.) cv. Almarah. Int. J. Agric. Res. 2013, 8, 172–177. [Google Scholar] [CrossRef]
- Jabbarzadeh, Z.; Khosh-Khui, M. Factors affecting tissue culture of Damask rose (Rosa damascena Mill.). Sci. Hortic. 2005, 105, 475–482. [Google Scholar] [CrossRef]
- Mirzaei, S.; Zare, A.G.; Jafary, S. Evaluating micropropagation of Kashan Damask rose, Yasooj aromatic rose and their hybrid. Int. J. Environ. Agric. Biotech. 2019, 4, 1407–1413. [Google Scholar]
- Tibkwang, A.; Junkasiraporn, S.; Chotikadachanarong, K. Effects of cytokinnin and sucrose on tissue culture of Rosa chinensis Jacq. var. minima Voss. Burapha Sci. J. 2018, 23, 712–721. [Google Scholar]
- Kumar, M.; Sirohi, U.; Malik, S.; Kumar, S.; Ahirwar, G.K.; Chaudhary, V.; Yadav, M.K.; Singh, J.; Kumar, A.; Pal, V.; et al. Methods and factors influencing in vitro propagation efficiency of ornamental tuberose (Polianthes species): A systematic review of recent developments and future prospects. Horticulturae 2022, 8, 998. [Google Scholar] [CrossRef]
- Ali, M.R.; Akand, M.; Homayra, H.; Mehraj, H.; Uddin, A.F.M.J. In vitro regeneration and rapid multiplication of tuberose. Int. J. Bus. Soc. Sci. Res. 2015, 3, 35–38. [Google Scholar]
- Ali, M.R.; Mehraj, H.; Uddin, A.F.M.J. Kinetin (KIN) and indole-3-acetic acid (IAA) on in vitro shoot and root initiation of tuberose. Int. J. Sust. Agril. Technol. 2014, 10, 1–4. [Google Scholar]
- Daneshvar, M.H.; Havil, M.; Lotfi Jalal-Abadi, A. Micropropagation of Polianthes tuberosa L. through direct organogenesis. J. Plant Prod. 2022, 45. [Google Scholar]
- Singh, K.; Madhavan, J.; Sadhukhan, R.; Chandra, S.; Rao, U.; Mandal, P.K. Production of nematode free plantlets in Polianthes tuberosa using in vitro culture techniques. Hortic. Environ. Biotechnol. 2020, 61, 929–937. [Google Scholar] [CrossRef]
- Gajbhiye, S.S.; Tripathi, M.K.; Vidya, M.S.; Singh, M.; Baghel, B.S.; Tiwari, S. Direct shoot organogenesis from cultured stem disc explants of tuberose (Polianthes tuberosa Linn.). J. Agric. Technol. 2011, 7, 695–709. [Google Scholar]
- Raghuvanshi, S.; Tripathi, M.K.; Vidhya-Sankar, M.; Singh, O.P. Establishment of low-cost effective protocol for massive in vitro propagation in Polianthes tuberosa Linn. Plant Cell Biotech. Mol. Biol. 2013, 14, 49–59. [Google Scholar]
- Sangavai, C.; Chellapandi, P. In vitro propagation of a tuberose plant (Polianthes tuberosa L.). Electron. J. Biol. 2008, 4, 98–101. [Google Scholar]
- Khanchana, K.; Kannan, M.; Hemaprabha, K.; Ganga, M. Standardization of protocol for sterilization and in vitro regeneration in tuberose (Polianthes tuberosa L.). Int. J. Chem. Stu. 2019, 7, 236–241. [Google Scholar]
- Surendranath, R.; Ganga, M.; Jawaharlal, M. In vitro propagation of tuberose. Environ. Ecol. 2015, 34, 2556–2560. [Google Scholar]
- Hernández-Mendoza, F.; Carrillo-Castañeda, G.; García-Gaytán, V.; Pedraza-Santos, M.; de la Cruz-Torres, E.; Mendoza-Castillo, M. In vitro plant regeneration of Polianthes tuberosa L. from leaf and flower buds tissue. Trop. Subtrop. Agroecosyst. 2021, 24, 55. [Google Scholar]
- Memon, N.; Qasim, M.; Jaskani, M.J.; Ahmad, R. In vitro cormel production of gladiolus. Pak. J. Agric. Sci. 2010, 47, 115–123. [Google Scholar]
- Torabi-Giglou, M.; Hajieghrari, B. In vitro study on regeneration of Gladiolus grandiflorus corm calli as affected by plant growth regulators. Pak. J. Biol. Sci. 2008, 1, 1147–1154. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tripathi, M.K.; Malviya, R.K.; Vidhyashankar, M.; Patel, R.P. Effect of plant growth regulators on in vitro morphogenesis in gladiolus (Gladiolus hybridus Hort.) from cultured corm slice. Int. J. Agric. Tech. 2017, 13, 583–599. [Google Scholar]
- Deshmukh, V.D.; Kharde, A.V.; Talekar, B.K. Interactive effects of BA and IAA on shoot proliferation of gladiolus (Gladiolus grandiflorus) var. White Prosperity. J. Oriental. Res. Madras 2021, XC, II–VII. [Google Scholar]
- Mateen, R.M. Development and optimization of micro-propagation, in vitro methodology for gladiolus. BioSci. Rev. 2019, 1, 21–36. [Google Scholar] [CrossRef]
- Devi, P.; Kumar, P.; Sengar, R.S.; Yadav, M.K.; Kumar, M.; Singh, S.K.; Singh, S. In-vitro multiple shoots production from cormel shoot buds in gladiolus (Gladiolus hybrida). Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1345–1350. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Sharma, V.; Mishra, A.; Singh, S.; Kumar, P. In vitro regeneration of gladiolus (Gladiolus hybrida L.): Optimization of growth media and assessment of genetic fidelity. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 2900–2909. [Google Scholar] [CrossRef]
- Wang, H.Y.; He, S.L.; Tanaka, M.; Van, P.T.; Teixeira da Silva, J.A. Effect of IBA concentration, carbon source, substrate, and light source on root induction ability of tree peony (Paeonia suffruticosa Andr.) plantlets in vitro. Europ. J. Hort. Sci. 2012, 77, S122–S128. [Google Scholar]
- Parida, R.; Mohanty, S.; Nayak, S. In vitro propagation of Hedychium coronarium Koen. through axillary bud proliferation. Plant Biosyst. 2013, 147, 905–912. [Google Scholar] [CrossRef]
- Jalali, N.; Naderi, R.; Shahi-Gharahlar, A.; Teixeira da Silva, J.A. Tissue culture of Cyclamen spp. Sci. Hortic. 2012, 137, 11–19. [Google Scholar] [CrossRef]
- İzgü, T.; Sevindik, B.; Çürük, P.; Şimşek, Ö.; Kaçar, Y.A.; Teixeira da Silva, J.A.; Mendi, Y. Development of an efficient regeneration protocol for four Cyclamen species endemic to Turkey. Plant Cell Tissue Organ Cult. 2016, 127, 95–113. [Google Scholar] [CrossRef]
- Yamaner, Ö.; Erdag, B. Direct shoot formation and microtuberization from aseptic seedlings of Cyclamen mirabile Hildebr. Biotechnology 2008, 7, 328–332. [Google Scholar] [CrossRef][Green Version]
- Abu-Qaoud, H. Direct regeneration in Cyclamen persicum Mill. using seedling tissues. An Najah Univ. J. Res.-A 2004, 18, 147–156. [Google Scholar]
- Kanwar, J.K.; Kumar, S. Influence of growth regulators and explants on shoot regeneration in carnation. Hort. Sci. 2009, 36, 140–146. [Google Scholar] [CrossRef]
- Lukatkin, A.S.; Mokshin, E.V.; Teixeira da Silva, J.A. Use of alternative plant growth regulators and carbon sources to manipulate Dianthus caryophyllus L. shoot induction in vitro. Rend. Fis. Acc. Lincei 2017, 28, 583–588. [Google Scholar] [CrossRef]
- Thokchom, R.; Maitra, S. Micropopagation of Anthurium andreanum cv. Jewel from leaf explants. J. Crop Weed 2017, 13, 23–27. [Google Scholar]
- Cardoso, J.C.; Habermann, G. Adventitious shoot induction from leaf segments in Anthurium andreanum is affected by age of explant, leaf orientation and plant growth regulator. Hortic. Environ. Biotechnol. 2014, 55, 56–62. [Google Scholar] [CrossRef]
- Gu, A.; Liu, W.; Ma, C.; Cui, J.; Henny, R.J.; Chen, J. Regeneration of Anthurium andraeanum from leaf explants and evaluation of microcutting rooting and growth under different light qualities. Hort. Sci. 2012, 47, 88–92. [Google Scholar] [CrossRef]
- Ülfer, R.; Türkoğlu, N.; Özdemir, F.A. Micropropagation of Zinnia elegans L. Int. J. Agric. For. Life Sci. 2020, 4, 161–166. [Google Scholar]
- Mei-Yin, C.; Sani, H. In vitro plantlet regeneration from nodal explant and callus induction of Vernonia amygdalina Delile. J. Plant Sci. 2018, 6, 1–6. [Google Scholar]
- Khalafalla, M.M.; Elgaali, E.I.; Ahmed, M.M. In vitro multiple shoot regeneration from nodal explants of Vernonia amygdalina-an important medicinal plant. Afr. Crop Sci. Confer. Proceed. 2007, 8, 747–752. [Google Scholar]
- Nasib, A.; Ali, K.; Khan, S. In vitro propagation of croton (Codiaeum variegatum). Pak. J. Bot. 2008, 40, 99–104. [Google Scholar]
- Marconi, P.L.; Radice, S. Organogenesis and somatic embryogenesis in Codiaeum variegatum (L.) Blume cv. "Corazón de Oro". Vitr. Cell Dev. Biol. Plant 1997, 33, 258–262. [Google Scholar] [CrossRef]
- Wei, A.; Xu, Y.; Yang, N.; Jiang, L.; Hu, J.; Yang, H.; Cai, C.; Chen, J.; Chen, G.; Pan, D. In vitro propagation of Codiaeum variegatum ‘Golden Queen’. Chinese J. Trop. Crops 2019, 40, 724–730. [Google Scholar]
- Bakheet, G.I.; Soliman, S.S.; Abdelkader, M.A.I.; Elashtokhy, M.M.A. Effects of different croton (Codiaeum variegatum L.) genotypes and growth regulators on callus induction, micro propagation and antibacterial activities. Zagazig J. Agric. Res. 2018, 45, 331–347. [Google Scholar] [CrossRef]
- Waseem, K.; Jilani, M.S.; Jaskani, M.J.; Khan, M.S.; Kiran, M.; Khan, G.U. Significance of different plant growth regulators on the regeneration of chrysanthemum plantlets (Dendranthema morifolium L.) through shoot tip culture. Pak. J. Bot. 2011, 43, 1843–1848. [Google Scholar]
- Naing, A.H.; Jeon, S.M.; Han, J.S.; Lim, S.H.; Lim, K.B.; Kim, C.K. Factors influencing in vitro shoot regeneration from leaf segments of Chrysanthemum. C. R. Biol. 2014, 337, 383–390. [Google Scholar] [CrossRef]
- Naing, A.H.; Park, K.I.; Chung, M.Y.; Lim, K.B.; Kim, C.K. Optimization of factors affecting efficient shoot regeneration in Chrysanthemum cv. Shinma. Braz. J. Bot. 2016, 39, 975–984. [Google Scholar] [CrossRef]
- Kazeroonian, R.; Mousavi, A.; Jari, S.K.; Tohidfar, M. Factors influencing in vitro organogenesis of Chrysanthemum morifolium cv. ‘Resomee Splendid’. Iranian J. Biotech. 2018, 16, e1454. [Google Scholar] [CrossRef]
- Parzymies, M.; Dąbski, M.; Pogorzelec, M.; Kozak, D.; Durlak, W.; Dudkiewicz, M. Rooting of a trumpet creeper (Campsis radicans (L.) seem.) microshoots in presence of auxins. Acta Sci. Pol. Hortorum Cultus 2014, 13, 187–196. [Google Scholar]
- Liberman, R.; Shahar, L.; Nissim-Levi, A.; Evenor, D.; Reuveni, M.; Oren-Shamir, M. Shoot regeneration from leaf explants of Brunfelsia calycina. Plant Cell Tissue Organ Cult. 2010, 100, 345–348. [Google Scholar] [CrossRef]
- Duhoky, M.M.; Al-Mizory, L.S. In vitro micropropagation of selected Bougainvillea sp. through callus induction. J. Agric. Vet. Sci. 2014, 6, 1–6. [Google Scholar]
- Papafotiou, M.; Skylourakis, A. In vitro propagation of Callistemon citrinus. Acta Hortic. 2010, 885, 267–270. [Google Scholar] [CrossRef]
- Farooq, I.; Qadri, Z.A.; Rather, Z.A.; Nazki, I.T.; Banday, N.; Rafiq, S.; Mansoor, S. Optimization of an improved, efficient and rapid in vitro micropropagation protocol for Petunia hybrida Vilm. Cv. “Bravo”. Saudi J. Biol. Sci. 2021, 28, 3701–3709. [Google Scholar] [CrossRef] [PubMed]
- Habas, R.R.; Turker, M.; Ozdemir, F.A. In vitro multiple shoot regeneration from Petunia hybrida. Turkish JAF Sci. Technol. 2019, 7, 1554–1560. [Google Scholar] [CrossRef]
- Panigrahi, J.; Dholu, P.; Shah, T.J.; Gantait, S. Silver nitrate-induced in vitro shoot multiplication and precocious flowering in Catharanthus roseus (L.) G. Don, a rich source of terpenoid indole alkaloids. Plant Cell Tissue Organ Cult. 2018, 132, 579–584. [Google Scholar] [CrossRef]
- Hoda, E. In vitro regeneration and somaclonal variation of Catharanthus roseus Don. using leaf and internodal explants. Alex. Sci. Exch. J. 2013, 34, 452–459. [Google Scholar]
- Plessis, H.J.D.; Nikolova, R.V.; Egan, B.A.; Kleynhans, R. Preliminary study on in vitro shoot culture of Hibiscus coddii subsp. barnardii, an indigenous South African flowering plant. Ornam. Hortic. 2021, 27, 408–416. [Google Scholar] [CrossRef]
- Seo, S.G.; Ryu, S.H.; Zhou, Y.; Kim, S.H. Development of an efficient protocol for high-frequency regeneration system in Hibiscus syriacus L. J. Plant Biotechnol. 2017, 44, 164–170. [Google Scholar] [CrossRef]
- Kumaria, S.; Kehie, M.; Bhowmik, S.S.D.; Singh, M.; Tandon, P. In vitro regeneration of Begonia rubrovenia var. meisneri CB Clarke—A rare and endemic ornamental plant of Meghalaya, India. Indian J. Biotechnol. 2012, 11, 300–303. [Google Scholar]
- Govindaraju, S.; Arulselvi, P.I. Effect of cytokinin combined elicitors (l-phenylalanine, salicylic acid and chitosan) on in vitro propagation, secondary metabolites and molecular characterization of medicinal herb—Coleus aromaticus Benth (L). J. Saudi Soc. Agric. Sci. 2018, 17, 435–444. [Google Scholar] [CrossRef]
- Saito, H.; Nakano, M. Plant regeneration from suspension cultures of Hosta sieboldiana. Plant Cell Tissue Organ Cult. 2002, 71, 23–28. [Google Scholar] [CrossRef]
- Choi, H.; Yang, J.C.; Ryu, S.H.; Yoon, S.M.; Kim, S.Y.; Lee, S.Y. In vitro multiplication of Hosta Tratt. species native to Korea by shoot-tip culture. Korean J. Plant Resour. 2019, 32, 53–62. [Google Scholar]
- Pe, P.P.W.; Naing, A.H.; Soe, M.T.; Kang, H.; Park, K.I.; Kim, C.K. Establishment of meristem culture for virus-free and genetically stable production of the endangered plant Hosta capitata. Sci. Hortic. 2020, 272, 109591. [Google Scholar] [CrossRef]
- Ku, B.S.; Cho, M.S. In vitro multiplication of Hosta plantaginea ‘Joseon’ by shoot-tip culture. Flower Res. J. 2016, 24, 328–336. [Google Scholar] [CrossRef]
- Song, K.; Kim, D.H.; Sivanesan, I. Effect of plant growth regulators on micropropagation of Hosta minor (Baker) Nakai through shoot tip culture. Propag. Ornam. Plants 2020, 20, 57–62. [Google Scholar]
- Sharma, U.; Kataria, V.; Shekhawat, N.S. In vitro propagation, ex vitro rooting and leaf micromorphology of Bauhinia racemosa Lam.: A leguminous tree with medicinal values. Physiol. Mol. Biol. Plants 2017, 23, 969–977. [Google Scholar] [CrossRef]
- Acemi, A.; Bayrak, B.; Çakır, M.; Demiryürek, E.; Gün, E.; Gueddari, N.E.E.; Özen, F. Comparative analysis of the effects of chitosan and common plant growth regulators on in vitro propagation of Ipomoea purpurea (L.) Roth from nodal explants. Vitr. Cell Dev. Biol. Plant 2018, 54, 537–544. [Google Scholar] [CrossRef]
- Kher, M.M.; Nataraj, M.; Parmar, H.D.; Buchad, H. Micropropagation of Merremia quinquefolia (L.) Hallier F. from nodal explants. J. Hortic. Res. 2015, 23, 13–16. [Google Scholar] [CrossRef]
- Timofeeva, S.N.; Elkonin, L.A.; Tyrnov, V.S. Micropropagation of Laburnum anagyroides Medic. through axillary shoot regeneration. Vitr. Cell Dev. Biol. Plant 2014, 50, 561–567. [Google Scholar] [CrossRef]
- Chavan, J.J.; Nimbalkar, M.S.; Adsul, A.A.; Kamble, S.S.; Gaikwad, N.B.; Dixit, G.B.; Gurav, R.V.; Bapat, V.A.; Yadav, S.R. Micropropagation and in vitro flowering of endemic and endangered plant Ceropegia attenuata Hook. J. Plant Biochem. Biotechnol. 2011, 20, 276–282. [Google Scholar] [CrossRef]
- Dhir, R.; Shekhawat, G.S. Ecorehabilitation and biochemical studies of Ceropegia bulbosa Roxb.: A threatened medicinal succulent. Acta Physiol. Plant 2014, 36, 1335–1343. [Google Scholar] [CrossRef]
- Dhir, R.; Shekhawat, G.S. Production, storability and morphogenic response of alginate encapsulated axillary meristems and genetic fidelity evaluation of in vitro regenerated Ceropegia bulbosa: A pharmaceutically important threatened plant species. Ind. Crops Prod. 2013, 47, 139–144. [Google Scholar] [CrossRef]
- Krishnareddy, P.V.; Pullaiah, T. In vitro conservation of Ceropegia elegans, an endemic plant of South India. Afr. J. Biotechnol. 2012, 11, 12443–12449. [Google Scholar] [CrossRef]
- Reddy, M.C.; Bramhachari, P.V.; Murthy, K.S.R. Optimized plant tissue culture protocol for in vitro morphogenesis of an endangered medicinal herb Ceropegia ensifolia Bedd. Trop. Subtrop. Agroecosystems 2015, 18, 95–101. [Google Scholar]
- Chavan, J.J.; Gaikwad, N.B.; Kshirsagar, P.R.; Umdale, S.D.; Bhat, K.V.; Dixit, G.B.; Yadav, S.R. Highly efficient in vitro proliferation and genetic stability analysis of micropropagated Ceropegia evansii by RAPD and ISSR markers: A critically endangered plant of Western Ghats. Plant Biosyst. 2015, 149, 442–450. [Google Scholar] [CrossRef]
- Chavan, J.J.; Nalawade, A.S.; Gaikwad, N.B.; Gurav, R.V.; Dixit, G.B.; Yadav, S.R. An efficient in vitro regeneration of Ceropegia noorjahaniae: An endemic and critically endangered medicinal herb of the Western Ghats. Physiol. Mol. Biol. Plants 2014, 20, 405–410. [Google Scholar] [CrossRef][Green Version]
- Chavan, J.J.; Gaikwad, N.B.; Yadav, S.R. High multiplication frequency and genetic stability analysis of Ceropegia panchganiensis, a threatened ornamental plant of Western Ghats: Conservation implications. Sci. Hortic. 2013, 161, 134–142. [Google Scholar] [CrossRef]
- Aslam, J.; Mujib, A.; Sharma, M.P. In vitro micropropagation of Dracaena sanderiana Sander ex Mast: An important indoor ornamental plant. Saudi J. Biol. Sci. 2013, 20, 63–68. [Google Scholar] [CrossRef]
- Doğan, S.; Çağlar, G.; Palaz, E.B. The effect of different applications on in vitro bulb development of an endemic hyacinth plant (Hyacinthus orientalis L. subsp. chionophyllus Wendelbo) grown in Turkey. Turkish JAF Sci. Technol. 2020, 8, 1713–1719. [Google Scholar] [CrossRef]
- Shen, X.; Kane, M.E.; Chen, J. Effects of genotype, explant source, and plant growth regulators on indirect shoot organogenesis in Dieffenbachia cultivars. Vitr. Cell Dev. Biol. Plant 2008, 44, 282–288. [Google Scholar] [CrossRef]
- Onsa, R.A.H.; Abdellatif, I.A.; Osman, M.G.; Abdullah, T.L. Effect of growth regulators in in vitro micropropagation of Ixora coccinea. Int. J. Sci. Res. Pub. 2018, 8, 144–149. [Google Scholar] [CrossRef]
- Wei, X.; Chen, J.; Zhang, C.; Wang, Z. In vitro shoot culture of Rhododendron fortunei: An important plant for bioactive phytochemicals. Ind. Crops Prod. 2018, 126, 459–465. [Google Scholar] [CrossRef]
- Veraplakorn, V. In vitro micropropagation and allelopathic effect of lantana (Lantana camara L.). Agric. Nat. Resour. 2017, 51, 478–484. [Google Scholar] [CrossRef]
- Vila, I.; Sales, E.; Ollero, J.; Munoz-Bertomeu, J.; Segura, J.; Arrillaga, I. Micropropagation of oleander (Nerium oleander L.). Hort. Sci. 2010, 45, 98–102. [Google Scholar] [CrossRef]
- Rahman, M.S.; Mouri, N.J.; Nandi, N.C.; Akter, S.; Khan, M.S. In vitro micropropagation of Jasminum grandiflorum L. Bangladesh J. Sci. Ind. Res. 2018, 53, 277–282. [Google Scholar] [CrossRef]
- Rathod, H.P.; Pohare, M.B.; Bhor, S.A.; Jadhav, K.P.; Batule, B.S.; Shahakar, S.B.; Wagh, S.G.; Wadekar, H.B.; Kelatkar, S.K.; Kulkarni, M.R. In vitro micro propagation of blue passion flower (Passiflora caerulea L.). Trends Biosci. 2014, 7, 3079–3082. [Google Scholar]
- Osburn, L.D.; Yang, X.; Li, Y.; Cheng, Z.M. Micropropagation of Japanese honeysuckle (Lonicera japonica) and Amur honeysuckle (L. maackii) by shoot tip culture. J. Environ. Hort. 2009, 27, 195–199. [Google Scholar] [CrossRef]
- Souza, E.H.; Soares, T.L.; Souza, F.V.D.; Santos-Serejo, J.A. Micropropagation of Heliconia rostrata and Heliconia bihai from mature zygotic embryos. Acta Hort. 2008, 865, 315–320. [Google Scholar] [CrossRef]
- Winhelmann, M.C.; Tedesco, M.; Lucchese, J.R.; Fior, C.S.; Schafer, G. In vitro propagation of Angelonia integerrima. Rodriguésia 2019, 70, e02232017. [Google Scholar] [CrossRef]
- Amer, E.M.; Fetouh, M.I.; Rasha, S.E. Micropropagation and acclimatization of Gardenia jasminoides Ellis. J. Biol. Chem. Environ. Sci. 2019, 14, 107–120. [Google Scholar]
- Minerva, G.; Kumar, S. Micropropagation of gerbera (Gerbera jamesonii Bolus). In Protocols for Micropropagation of Selected Economically-important Horticultural Plants; Lambardi, M., Ozudogru, E., Jain, S., Eds.; Humana Press: Totowa, NJ, USA, 2012; Chapter 24; pp. 305–316. [Google Scholar]
- Yalcın-Mendı, Y.; Unek, C.; Eldogan, S.; Akakacar, Y.; Serce, S.; Curuk, P.; Kocaman, E. The effects of different hormones on regeneration of gazania (Gazania rigens). Rom. Biotechnol. Lett. 2009, 14, 4728–4732. [Google Scholar]
- Haque, S.M.; Ghosh, B. In vitro completion of sexual life cycle: Production of R1 plants of Ipomoea quamoclit L. Propag. Ornam. Plants 2013, 13, 19–24. [Google Scholar]
- Kulus, D.; Miler, N. Application of plant extracts in micropropagation and cryopreservation of bleeding heart: An ornamental-medicinal plant species. Agriculture 2021, 11, 542. [Google Scholar] [CrossRef]
- Ghareeb, Z.F.; Taha, L.S. Micropropagation protocol for Antigonon leptopus an important ornamental and medicinal plant. J. Genet. Eng. Biotechnol. 2018, 16, 669–675. [Google Scholar] [CrossRef]
- Tour, J.; Ikram, U.; Bilal, M.; Ali, M.; Zaheer, U.; Nawaz, M.A. Efficient in vitro propagation of Amaranthus viridis L. using node explants. Acta Sci. Pol. Hortorum Cultus 2020, 19, 41–51. [Google Scholar]
- Bhatt, A.; Stanly, C.; Keng, C.L. In vitro propagation of five Alocasia species. Hortic. Bras. 2013, 31, 210–215. [Google Scholar] [CrossRef]
- Belokurova, V.; Lystvan, K.; Volga, D.; Vasylenko, M.; Kuchuk, M. In vitro culture and some biochemical characteristics of Fittonia albivenis (Lindl. ex Veitch) Brummitt. Agrbiodiv. Impr. Nut. Health Life Qual. 2019, 3, 186–194. [Google Scholar]
- Qu, L.; Chen, J.; Henny, R.J.; Huang, Y.; Caldwell, R.D.; Robinson, C.A. Thidiazuron promotes adventitious shoot regeneration from pothos (Epipremnum aureum) leaf and petiole explants. Vitr. Cell Dev. Biol. Plant 2014, 50, 561–567. [Google Scholar] [CrossRef]
- Hung, C.Y.; Zhang, J.; Bhattacharya, C.; Li, H.; Kittur, F.S.; Oldham, C.E.; Wei, X.; Burkey, K.O.; Chen, J.; Xie, J. Transformation of long-lived albino Epipremnum aureum ‘Golden Pothos’ and restoring chloroplast development. Front. Plant Sci. 2021, 12, 647507. [Google Scholar] [CrossRef]
- Khatri, P.; Rana, J.S.; Sindhu, A.; Jamdagni, P. Effect of additives on enhanced in-vitro shoot multiplication and their functional group identification of Chlorophytum borivilianum Sant. Et Fernand. SN Appl. Sci. 2019, 1, 1105. [Google Scholar] [CrossRef]
- Faisal, M.; Ahmad, N.; Anis, M.; Alatar, A.A.; Qahtan, A.A. Auxin-cytokinin synergism in vitro for producing genetically stable plants of Ruta graveolens using shoot tip meristems. Saudi J. Biol. Sci. 2018, 25, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Faisal, M.; Ahmad, N.; Anis, M. In vitro regeneration and mass propagation of Ruta graveolens L.—A multipurpose shrub. Hort. Sci. 2005, 40, 1478–1480. [Google Scholar] [CrossRef]
- Nowakowska, K.; Pacholczak, A.; Tepper, W. The effect of selected growth regulators and culture media on regeneration of Daphne mezereum L. ‘Alba’. Rend. Fis. Acc. Lincei 2019, 30, 197–205. [Google Scholar] [CrossRef]
- Nahar, S.J.; Shimasaki, K. Application of 5-aminolevulinic acid for the in vitro micropropagation of Cymbidium as a potential novel plant regulator. Environ. Control Biol. 2014, 52, 117–121. [Google Scholar] [CrossRef][Green Version]
- Nahar, S.J.; Syed, M.H.; Shimasaki, K. Organogenesis of Cymbidium orchid using elicitors. J. Plant Develop 2015, 22, 13–20. [Google Scholar]
- Teixeira da Silva, J.A. The effect of ethylene inhibitors (AgNO3, AVG), an ethylene-liberating compound (CEPA) and aeration on the formation of protocorm-like bodies of hybrid Cymbidium (Orchidaceae). Front. Biol. 2013, 8, 606–610. [Google Scholar] [CrossRef]
- Restanto, D.P.; Santoso, B.; Kriswanto, B.; Supardjono, S. The application of chitosan for protocorm like bodies (PLB) induction of orchid (Dendrobium sp) in vitro. Agric. Agric. Sci. Procedia 2016, 9, 462–468. [Google Scholar] [CrossRef][Green Version]
- Pornpienpakdee, P.; Singhasurasak, R.; Chaiyasap, P.; Pichyangkura, R.; Bunjongrat, R.; Chadchawan, S.; Limpanavech, P. Improving the micropropagation efficiency of hybrid Dendrobium orchids with chitosan. Sci. Hortic. 2010, 124, 490–499. [Google Scholar] [CrossRef]
- Nge, K.L.; Nwe, N.; Chandrkrachang, S.; Stevens, W.F. Chitosan as a growth stimulator in orchid tissue culture. Plant Sci. 2006, 170, 1185–1190. [Google Scholar] [CrossRef]
- Kananont, N.; Pichyangkura, R.; Chanprame, S.; Chadchawan, S.; Limpanavech, P. Chitosan specificity for the in vitro seed germination of two Dendrobium orchids (Asparagales: Orchidaceae). Sci. Hortic. 2010, 124, 239–247. [Google Scholar] [CrossRef]
- Soares, J.D.R.; Pasqual, M.; Rodrigues, F.A.; Villa, F.; Araujo, A.G.D. Silicon sources in the micropropagation of the Cattleya group orchid. Acta Sci. Agron. 2011, 33, 503–507. [Google Scholar]
- Matos, A.V.C.D.S.D.; Oliveira, B.S.D.; Oliveira, M.E.B.S.D.; Cardoso, J.C. AgNO3 improved micropropagation and stimulate in vitro flowering of rose (Rosa x hybrida) cv. Sena. Ornam. Hortic. 2020, 27, 33–40. [Google Scholar] [CrossRef]
- Cardoso, J.C. Silver nitrate enhances in vitro development and quality of shoots of Anthurium andraeanum. Sci. Hortic. 2019, 253, 358–363. [Google Scholar] [CrossRef]
- Zahara, M.; Datta, A.; Boonkorkaew, P.; Mishra, A. The effects of different media, sucrose concentrations and natural additives on plantlet growth of Phalaenopsis hybrid ‘Pink’. Braz. Arch. Biol. Technol. 2017, 60, 1–15. [Google Scholar] [CrossRef]
- Pyati, A.N.; Murthy, H.N.; Hahn, E.J.; Paek, K.Y. In vitro propagation of Dendrobium macrostachym Lindl.—A threatened orchid. Indian J. Exp. Biol. 2002, 40, 620–623. [Google Scholar]
- Pant, B.; Chand, K.; Paudel, M.R.; Joshi, P.R.; Thapa, B.B.; Park, S.Y.; Shakya, S.; Thakuri, L.S.; Rajbahak, S.; Sah, A.K.; et al. Micropropagation, antioxidant and anticancer activity of pineapple orchid: Dendrobium densiflorum Lindl. J. Plant Biochem. Biotechnol. 2022, 31, 399–409. [Google Scholar] [CrossRef]
- Sinha, P.; Roy, S.K. Regeneration of an indigenous orchid, Vanda teres (Roxb.) Lindl. through In vitro culture. Plant Tissue Cult. 2004, 14, 55–61. [Google Scholar]
- Samiei, L.; Pahnehkolayi, M.D.; Tehranifar, A.; Karimian, Z. Organic and inorganic elicitors enhance in vitro regeneration of Rosa canina. J. Genet. Eng. Biotechnol. 2021, 19, 60. [Google Scholar] [CrossRef]
- Zayed, R.; El-Shamy, H.; Berkov, S.; Bastida, J.; Codina, C. In vitro micropropagation and alkaloids of Hippeastrum vittatum. Vitr. Cell Dev. Biol. Plant 2011, 47, 695–701. [Google Scholar] [CrossRef]
- Le, V.T.; Tanaka, M. Effects of red and blue light-emitting diodes on callus induction, callus proliferation, and protocorm-like body formation from callus in Cymbidium orchid. Environ. Control Biol. 2004, 42, 57–64. [Google Scholar]
- Kaewjampa, N.; Shimasaki, K. Effects of green LED lighting on organogenesis and superoxide dismutase (SOD) activities in protocorm-like bodies (PLBs) of Cymbidium cultured in vitro. Environ. Control Biol. 2012, 50, 247–254. [Google Scholar] [CrossRef]
- Teixeira da Silva, J.A. The response of protocorm-like bodies of nine hybrid Cymbidium cultivars to light-emitting diodes. Environ. Exp. Biol. 2014, 12, 155–159. [Google Scholar]
- Wongnok, A.; Piluek, C.; Techasilpitak, T.; Tantivivat, S. Effects of light emitting diodes on micropropagation of Phalaenopsis orchids. Acta Hortic. 2008, 788, 149–156. [Google Scholar] [CrossRef]
- Billore, V.; Jain, M.; Suprasanna, P. Monochromic radiation through light-emitting diode (LED) positively augments in vitro shoot regeneration in Orchid (Dendrobium sonia). Can. J. Biotech. 2017, 1, 50. [Google Scholar] [CrossRef]
- Billore, V.; Mirajkar, S.J.; Suprasanna, P.; Jain, M. Gamma irradiation induced effects on in vitro shoot cultures and influence of monochromatic light regimes on irradiated shoot cultures of Dendrobium sonia orchid. Biotechnol. Rep. 2019, 22, e00343. [Google Scholar] [CrossRef] [PubMed]
- Cybularz-Urban, T.; Hanus-Fajerska, E.; Swiderski, A. Effect of light wavelength on in vitro organogenesis of a Cattleya hybrid. Acta Biol. Crac. Ser. Bot. 2007, 49, 113–118. [Google Scholar]
- Mengxi, L.; Zhigang, X.; Yang, Y.; Yijie, F. Effects of different spectral lights on Oncidium PLBs induction, proliferation, and plant regeneration. Plant Cell Tissue Organ Cult. 2011, 106, 1–10. [Google Scholar] [CrossRef]
- Luan, V.Q.; Huy, N.P.; Nam, N.B.; Huong, T.T.; Hien, V.T.; Hien, N.T.T.; Hai, N.T.; Thinh, D.K.; Nhut, D.T. Ex vitro and in vitro Paphiopedilum delenatii Guillaumin stem elongation under light-emitting diodes and shoot regeneration via stem node culture. Acta Physiol. Plant 2015, 37, 1–11. [Google Scholar] [CrossRef]
- Godo, T.; Fujiwara, K.; Guan, K.; Miyoshi, K. Effects of wavelength of LED-light on in vitro asymbiotic germination and seedling growth of Bletilla ochracea Schltr. (Orchidaceae). Plant Biotechnol. 2011, 28, 398–400. [Google Scholar] [CrossRef]
- Baque, A.M.; Shin, Y.K.; Elshmari, T.; Lee, E.J.; Paek, K.Y. Effect of light quality, sucrose and coconut water concentration on the microporpagation of Calanthe hybrids (‘Bukduseong’ ‘Hyesung’ and ‘Chunkwang’ ‘Hyesung’). Aust. J. Crop Sci. 2011, 5, 1247–1254. [Google Scholar]
- Shin, K.S.; Murthy, H.N.; Heo, J.W.; Hahn, E.J.; Paek, K.Y. The effect of light quality on the growth and development of in vitro cultured Doritaenopsis plants. Acta Physiol. Plant. 2008, 30, 339–343. [Google Scholar] [CrossRef]
- Favetta, V.; Colombo, R.C.; Mangili Júnior, J.F.; de Faria, R.T. Light sources and culture media in the in vitro growth of the Brazilian orchid Microlaelia lundii. Semin. Cienc. Agrar. 2017, 38, 1775–1784. [Google Scholar] [CrossRef]
- Azmi, N.S.; Ahmad, R.; Ibrahim, R. Fluorescent light (FL), red led and blue led spectrums effects on in vitro shoots multiplication. J. Teknol. 2016, 78, 6. [Google Scholar] [CrossRef][Green Version]
- Azmi, N.S.; Ahmad, R.; Ibrahim, R. Effects of red and blue (RB) LED on the in vitro growth of Rosa kordesii in multiplication phase. In 2nd International Conference on Agriculture and Biotechnology; IACSIT Press: Singapore, 2014; Volume 79. [Google Scholar]
- Miler, N.; Kulus, D.; Woźny, A.; Rymarz, D.; Hajzer, M.; Wierzbowski, K.; Nelke, R.; Szeffs, L. Application of wide-spectrum light-emitting diodes in micropropagation of popular ornamental plant species: A study on plant quality and cost reduction. Vitr. Cell Dev. Biol. Plant 2019, 55, 99–108. [Google Scholar] [CrossRef]
- Kurilčik, A.; Miklušytė-Čanova, R.; Dapkūnienė, S.; Žilinskaitė, S.; Kurilčik, G.; Tamulaitis, G.; Duchovskis, P.; Žukauskas, A. In vitro culture of Chrysanthemum plantlets using light-emitting diodes. Cent. Eur. J. Biol. 2008, 3, 161–167. [Google Scholar] [CrossRef]
- Kim, S.J.; Hahn, E.J.; Heo, J.W.; Paek, K.Y. Effects of LEDs on net photosynthetic rate, growth and leaf stomata of chrysanthemum plantlets in vitro. Sci. Hortic. 2004, 101, 143–151. [Google Scholar] [CrossRef]
- Cioć, M.; Kalisz, A.; Żupnik, M.; Pawłowska, B. Different LED light intensities and 6-benzyladenine concentrations in relation to shoot development, leaf architecture, and photosynthetic pigments of Gerbera jamesonii Bolus in vitro. Agronomy 2019, 9, 358. [Google Scholar] [CrossRef]
- Pawłowska, B.; Cioć, M.; Prokopiuk, B. How LED light rooting in vitro affected Gerbera acclimatization efficiency. Acta Hortic. 2018, 1201, 583–590. [Google Scholar] [CrossRef]
- Pawłowska, B.; Żupnik, M.; Szewczyk-Taranek, B.; Cioć, M. Impact of LED light sources on morphogenesis and levels of photosynthetic pigments in Gerbera jamesonii grown in vitro. Hortic. Environ. Biotechnol. 2018, 59, 115–123. [Google Scholar] [CrossRef]
- Martínez-Estrada, E.; Caamal-Velázquez, J.H.; Morales-Ramos, V.; Bello-Bello, J.J. Light emitting diodes improve in vitro shoot multiplication and growth of Anthurium andreanum Lind. Propag. Ornam. Plants 2016, 16, 3–8. [Google Scholar]
- Budiarto, K. Spectral quality affects morphogenesis on anthurium plantlet during in vitro culture. AGRIVITA J. Agric. Sci. 2010, 32, 234–240. [Google Scholar]
- Rodrigues, P.H.V.; Arruda, F.; Forti, V.A. Slow-grown in vitro conservation of Heliconia champneiana cv. Splash under different light spectra. Sci. Agric. 2018, 75, 163–166. [Google Scholar] [CrossRef]
- Cho, K.H.; Laux, V.Y.; Wallace-Springer, N.; Clark, D.G.; Folta, K.M.; Colquhoun, T.A. Effects of light quality on vegetative cutting and in vitro propagation of coleus (Plectranthus scutellarioides). Hort. Sci. 2019, 54, 926–935. [Google Scholar] [CrossRef]
- Dewir, Y.H.; Chakrabarty, D.; Kim, S.J.; Hahn, E.J.; Paek, K.Y. Effect of light-emitting diode on growth and shoot proliferation of Euphorbia millii and Spathiphyllum cannifolium. J. Kor. Soc. Hort. Sci. 2005, 46, 375–379. [Google Scholar]
- Lian, M.L.; Murthy, H.N.; Paek, K.Y. Effects of light emitting diodes (LEDs) on the in vitro induction and growth of bulblets of Lilium oriental hybrid ‘Pesaro’. Sci. Hortic. 2002, 94, 365–370. [Google Scholar] [CrossRef]
- Wu, H.C.; Lin, C.C. Red light-emitting diode light irradiation improves root and leaf formation in difficult-to-propagate Protea cynaroides L. plantlets in vitro. Hort. Sci. 2012, 47, 1490–1494. [Google Scholar] [CrossRef]
- Pinheiro, M.V.M.; Schmidt, D.; Diel, M.I.; Santos, J.D.; Thiesen, L.A.; Azevedo, G.C.V.D.; Holz, E. In vitro propagation of alpinia cultivars in different light sources. Ornam. Hortic. 2019, 25, 49–54. [Google Scholar] [CrossRef]
- Moon, H.K.; Park, S.Y.; Kim, Y.W.; Kim, C.S. Growth of Tsuru-rindo (Tripterospermum japonicum) cultured in vitro under various sources of light-emitting diode (LED) irradiation. J. Plant Biol. 2006, 49, 174–179. [Google Scholar] [CrossRef]
- Kwon, A.R.; Cui, H.Y.; Lee, H.; Lee, H.; Shin, H.; Kang, K.S.; Park, S.Y. Light quality affects shoot regeneration, cell division, and wood formation in elite clones of Populus euramericana. Acta Physiol. Plant 2015, 37, 65. [Google Scholar] [CrossRef]
- Nahar, S.J.; Haque, S.M.; Kazuhiko, S. Application of chondroitin sulfate on organogenesis of two Cymbidium spp. under different sources of lights. Not. Sci. Biol. 2016, 8, 156–160. [Google Scholar] [CrossRef][Green Version]
- Nahar, S.J.; Haque, S.M.; Shimasaki, K. Effect of light quality and plant growth regulator on organogenesis of orkid Cymbidium dayanum. Bangladesh J. Agric. Res. 2017, 42, 185–190. [Google Scholar] [CrossRef][Green Version]
- Gabryszewska, E.; Rudnicki, R. The influence of light quality on the shoot proliferation and rooting of Gerbera jamesonii in vitro. Acta Agrobot. 1995, 48, 105–111. [Google Scholar] [CrossRef][Green Version]
- Cioć, M.; Szewczyk, A.; Żupnik, M.; Kalisz, A.; Pawłowska, B. LED lighting affects plant growth, morphogenesis and phytochemical contents of Myrtus communis L. in vitro. Plant Cell Tissue Organ Cult. 2018, 132, 433–447. [Google Scholar] [CrossRef]
- Zielińska, S.; Piątczak, E.; Kozłowska, W.; Bohater, A.; Jezierska-Domaradzka, A.; Kolniak-Ostek, J.; Matkowski, A. LED illumination and plant growth regulators’ effects on growth and phenolic acids accumulation in Moluccella laevis L. in vitro cultures. Acta Physiol. Plant 2020, 42, 72. [Google Scholar] [CrossRef]


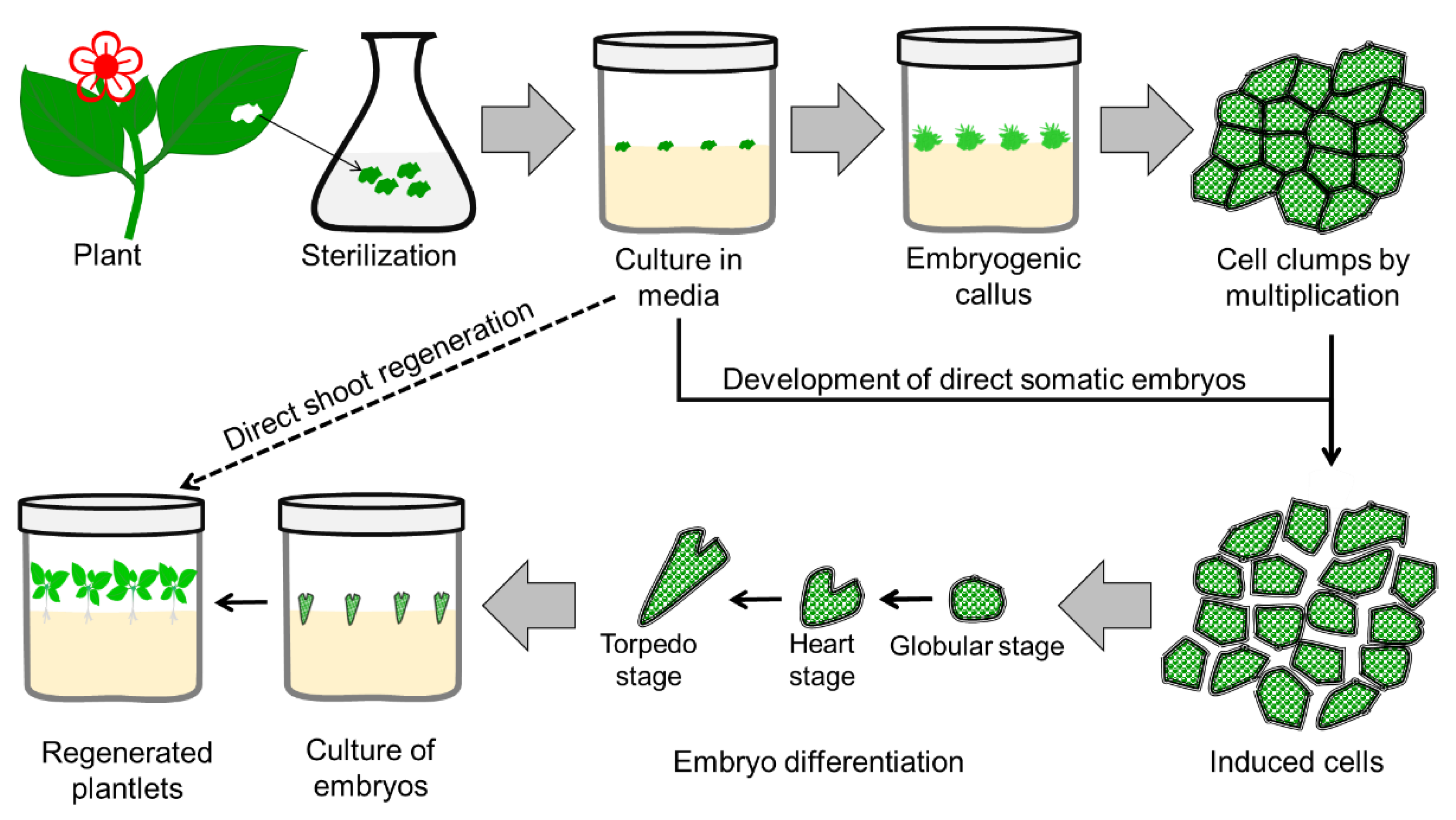

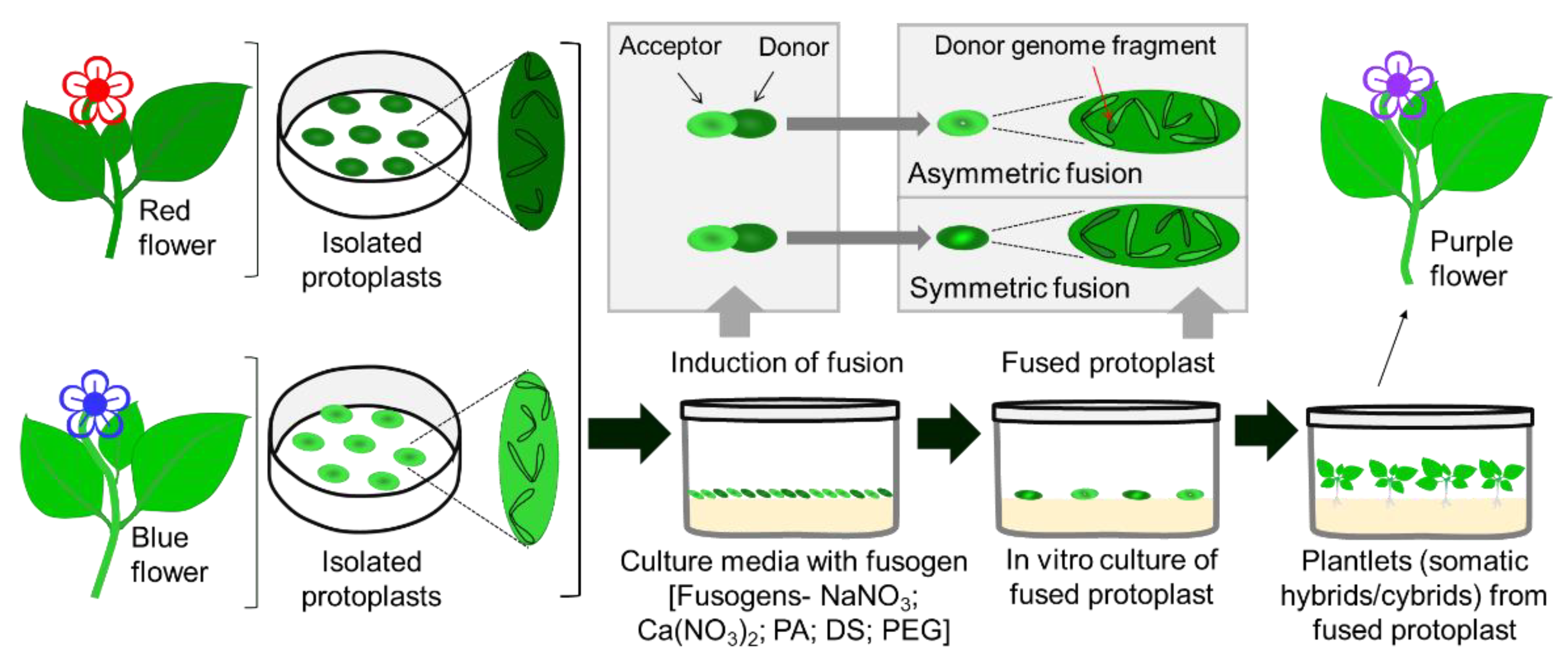


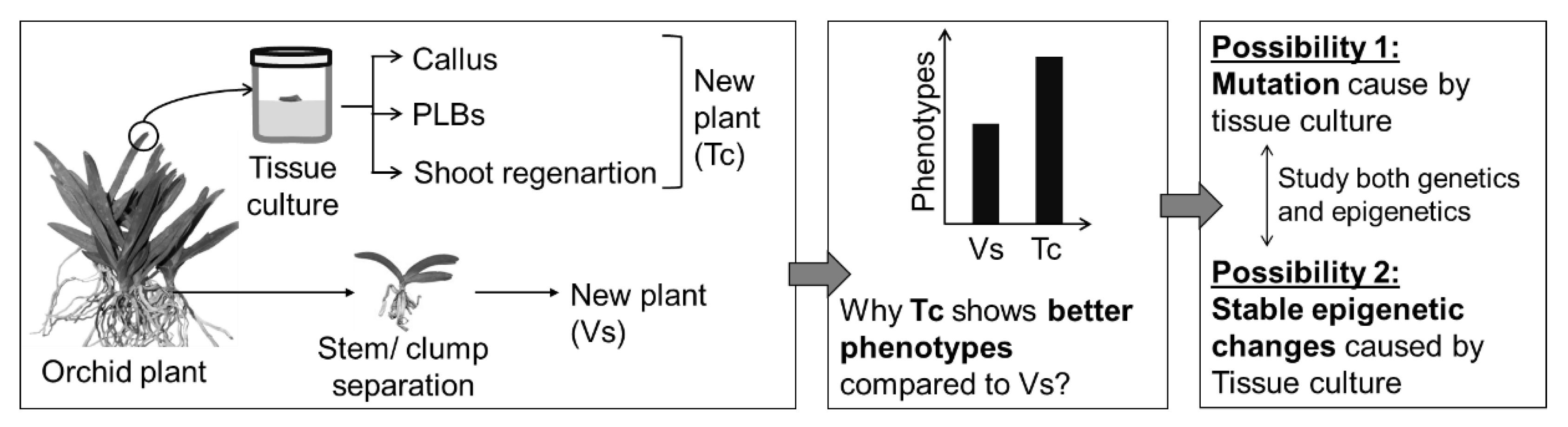
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mehbub, H.; Akter, A.; Akter, M.A.; Mandal, M.S.H.; Hoque, M.A.; Tuleja, M.; Mehraj, H. Tissue Culture in Ornamentals: Cultivation Factors, Propagation Techniques, and Its Application. Plants 2022, 11, 3208. https://doi.org/10.3390/plants11233208
Mehbub H, Akter A, Akter MA, Mandal MSH, Hoque MA, Tuleja M, Mehraj H. Tissue Culture in Ornamentals: Cultivation Factors, Propagation Techniques, and Its Application. Plants. 2022; 11(23):3208. https://doi.org/10.3390/plants11233208
Chicago/Turabian StyleMehbub, Hasan, Ayasha Akter, Mst. Arjina Akter, Mohammad Shamim Hasan Mandal, Md. Ashraful Hoque, Monika Tuleja, and Hasan Mehraj. 2022. "Tissue Culture in Ornamentals: Cultivation Factors, Propagation Techniques, and Its Application" Plants 11, no. 23: 3208. https://doi.org/10.3390/plants11233208
APA StyleMehbub, H., Akter, A., Akter, M. A., Mandal, M. S. H., Hoque, M. A., Tuleja, M., & Mehraj, H. (2022). Tissue Culture in Ornamentals: Cultivation Factors, Propagation Techniques, and Its Application. Plants, 11(23), 3208. https://doi.org/10.3390/plants11233208







