Consequences of Arsenic Contamination on Plants and Mycoremediation-Mediated Arsenic Stress Tolerance for Sustainable Agriculture
Abstract
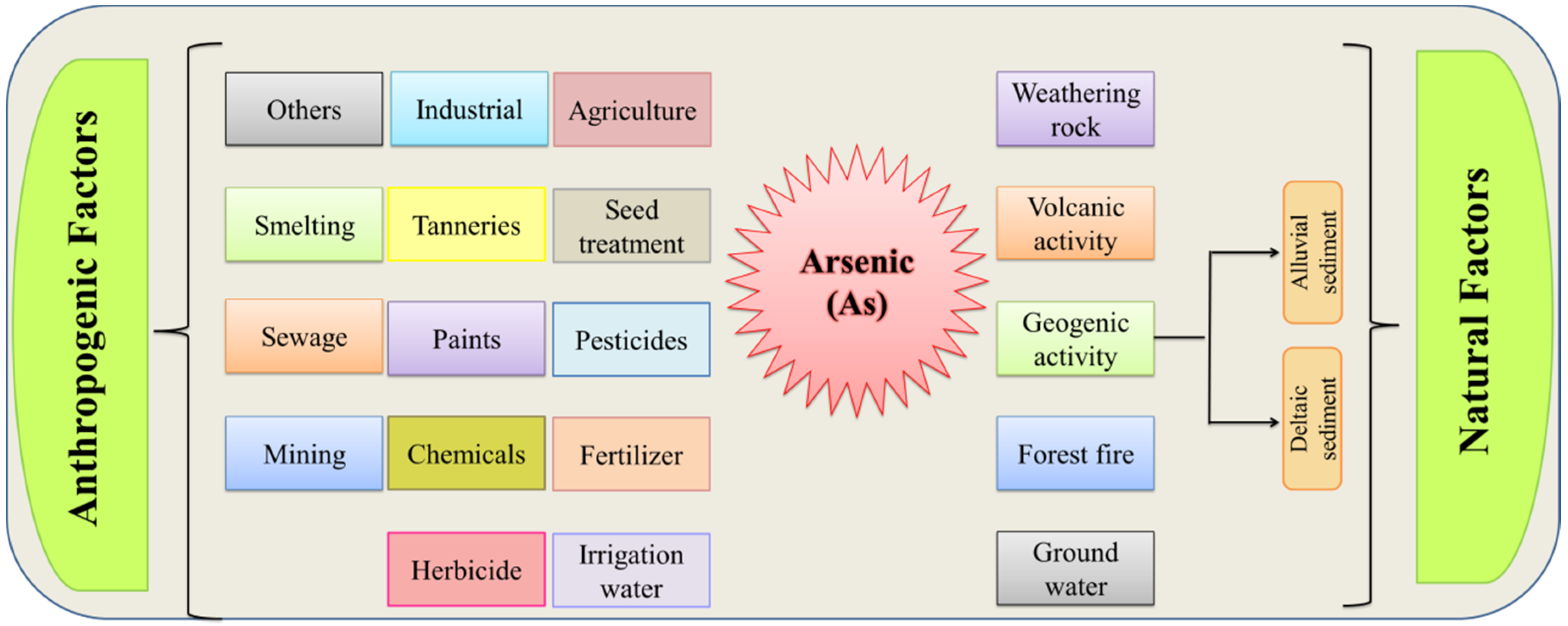
:1. Introduction
2. Effects of Arsenic Contamination on Microbial Dynamics and Crops
Mechanism of Arsenic Metabolism, Transport, and Detoxification in Food Crops
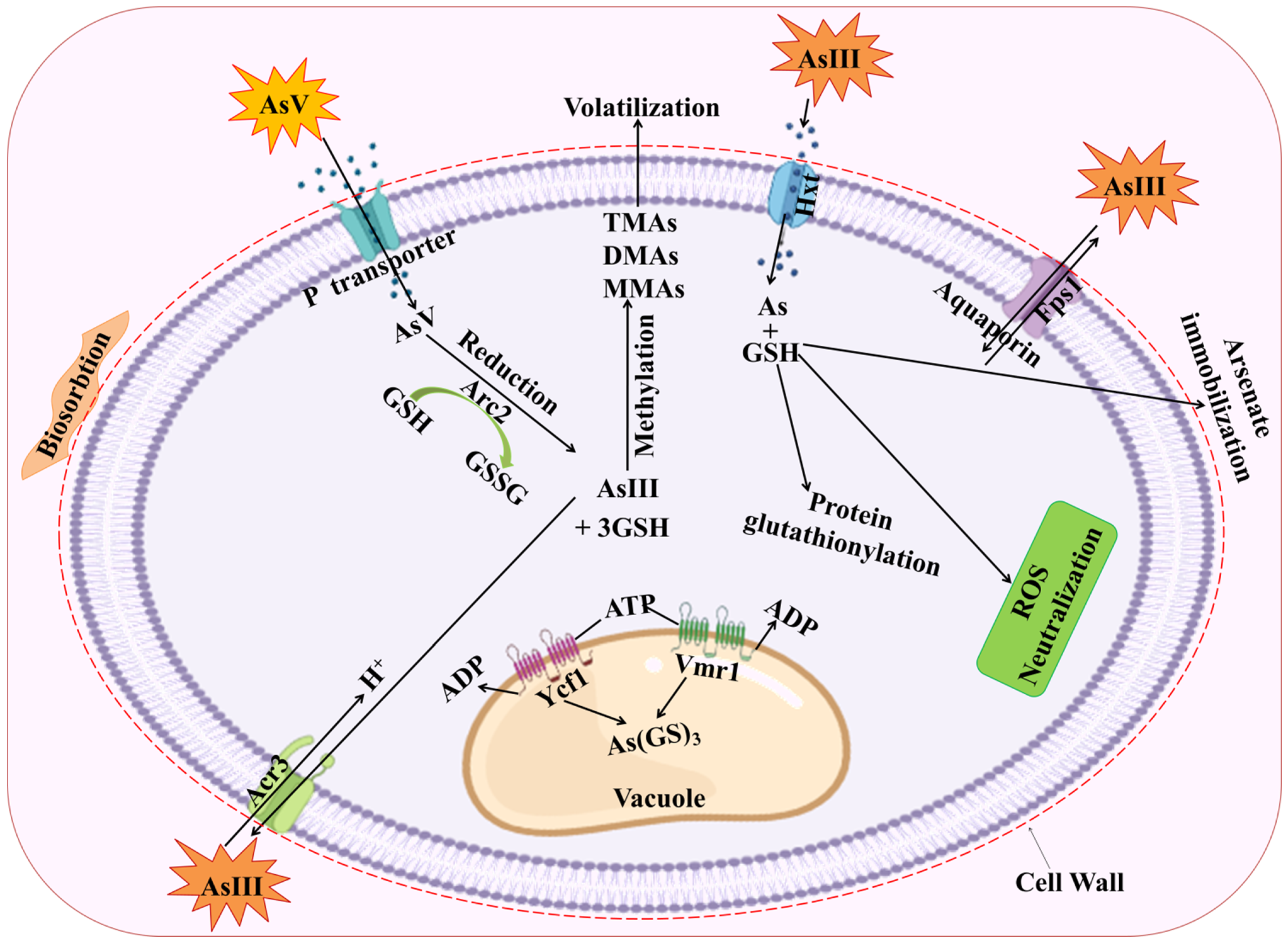
3. Arsenic Detoxification Mechanism Using as Tolerant Fungi and Its Mitigation via Glutathione Biosynthesis
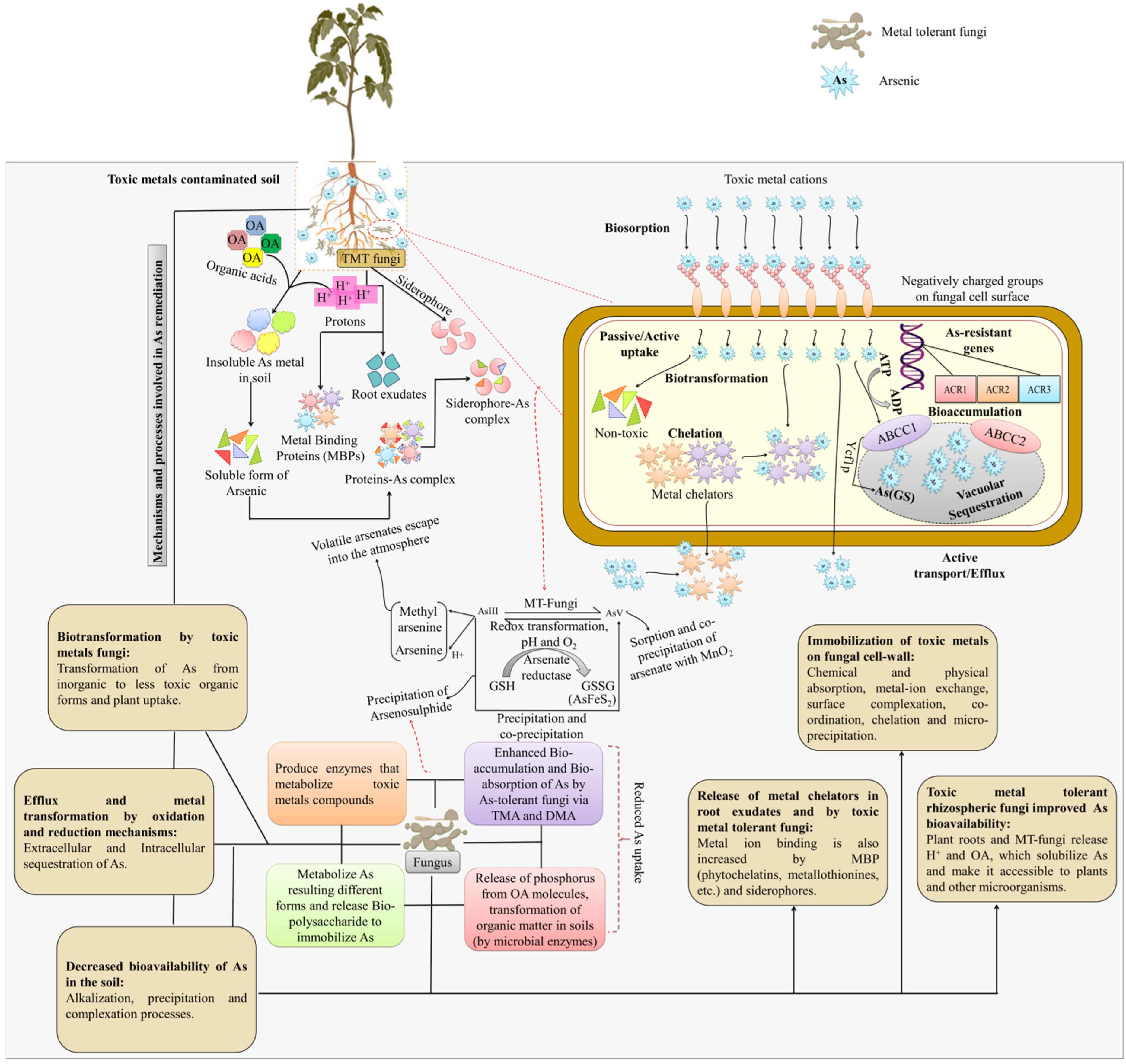
4. Mycorrhizae-Based Mitigation of Arsenic
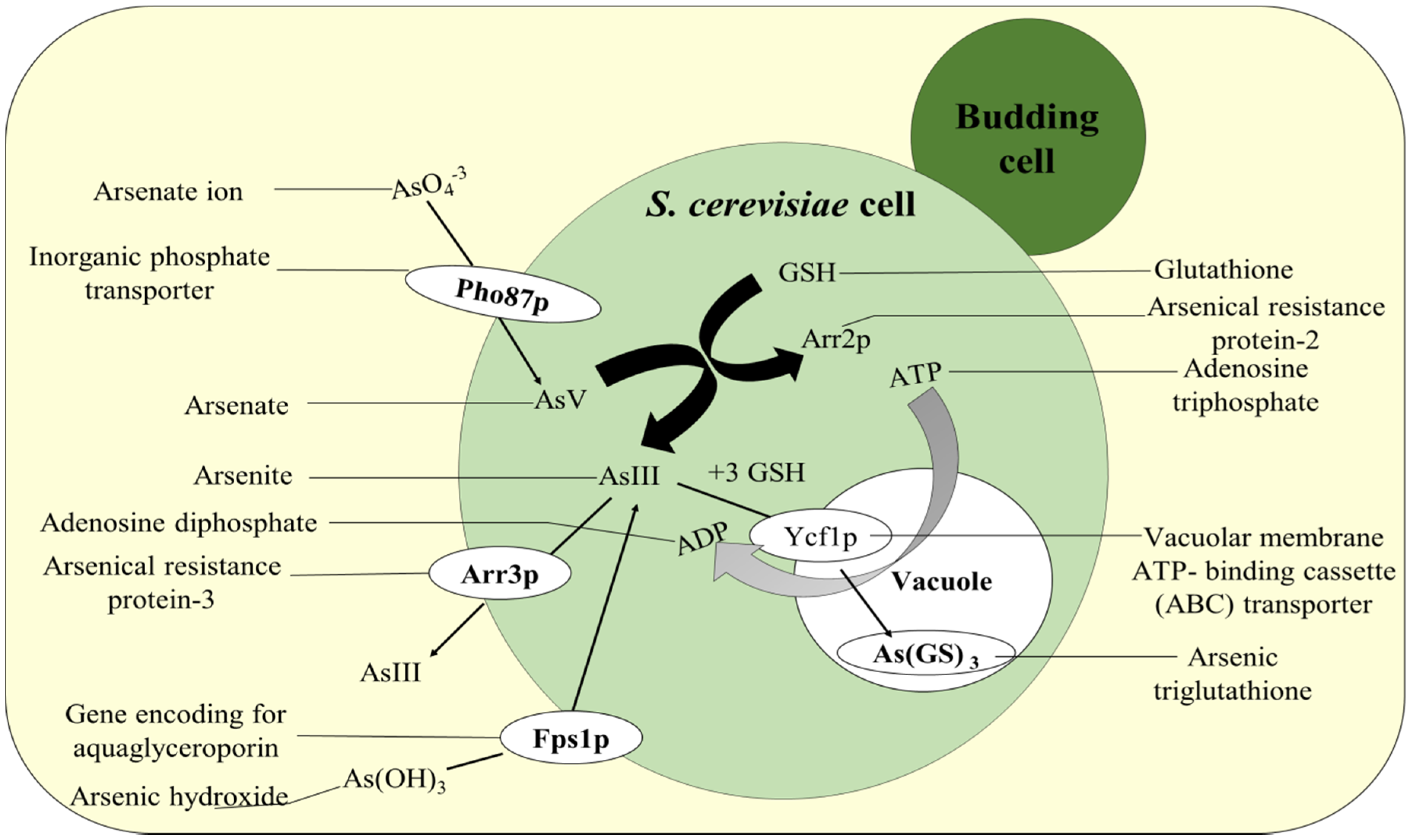
5. Mechanism of Recombinant Yeast and Fungi-Induced Arsenic Remediation
5.1. Biosorption and Bioaccumulation
5.2. Chelation of Metals
5.2.1. Organic Acids
5.2.2. Compounds That Chelate Metals
5.2.3. Metal Exclusion through Efflux Transport
5.3. Cell Surface Precipitation
5.4. Bioaugmentation and Biostimulation
5.5. Biovolatilization and Methylation
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cui, J.; Li, Y.; Jin, Q.; Li, F. Silica nanoparticles inhibit arsenic uptake into rice suspension cells: Via improving pectin synthesis and the mechanical force of the cell wall. Environ. Sci. Nano 2020, 7, 162–171. [Google Scholar] [CrossRef]
- Kumar, S.; Prasad, S.; Yadav, K.K.; Shrivastava, M.; Gupta, N.; Nagar, S.; Bach, Q.V.; Kamyab, H.; Khan, S.A.; Yadav, S.; et al. Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches—A review. Environ. Res. 2019, 179, 108792. [Google Scholar] [CrossRef] [PubMed]
- Arslan, B.; Djamgoz, M.B.A.; Akün, E. Arsenic: A review on exposure pathways, accumulation, mobility and transmission into the human food chain. Rev. Environ. Contam. Toxicol. 2017, 243, 27–51. [Google Scholar] [PubMed]
- Sodhi, K.K.; Kumar, M.; Agrawal, P.K.; Singh, D.K. Perspectives on arsenic toxicity, carcinogenicity and its systemic remediation strategies. Environ. Technol. Innov. 2019, 16, 100462. [Google Scholar] [CrossRef]
- Chowdhary, P.; Bharagava, R.N.; Mishra, S.; Khan, N. Role of industries in water scarcity and its adverse effects on environment and human health. In Environmental Concerns and Sustainable Development; Springer: Singapore, 2020; pp. 235–256. [Google Scholar] [CrossRef]
- Briffa, J.; Sinagra, E.; Blundell, R. Heavy metal pollution in the environment and their toxicological effects on humans. Heliyon 2020, 6, e04691. [Google Scholar] [CrossRef]
- Kumar, V.; Dwivedi, S.K. Mycoremediation of heavy metals: Processes, mechanisms, and affecting factors. Environ. Sci. Pollut. Res. 2021, 28, 10375–10412. [Google Scholar] [CrossRef]
- Hassan, A.; Pariatamby, A.; Ossai, I.C.; Hamid, F.S. Bioaugmentation assisted mycoremediation of heavy metal and/metalloid landfill contaminated soil using consortia of filamentous fungi. Biochem. Eng. J. 2020, 157, 107550. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, X. On the potential of biological treatment for arsenic contaminated soils and groundwater. J. Environ. Manag. 2009, 90, 2367–2376. [Google Scholar] [CrossRef]
- Sher, S.; Rehman, A. Use of heavy metals resistant bacteria—A strategy for arsenic bioremediation. Appl. Microbiol. Biotechnol. 2019, 103, 6007–6021. [Google Scholar] [CrossRef]
- Hassan, A.; Pariatamby, A.; Ahmed, A.; Auta, H.S.; Hamid, F.S. Enhanced bioremediation of heavy metal contaminated landfill soil using filamentous fungi consortia: A demonstration of bioaugmentation potential. Water. Air. Soil Pollut. 2019, 230, 215. [Google Scholar] [CrossRef]
- Naseem, M.; Raghuwanshi, R.; Verma, P.C.; Srivastava, P.K. Mycoremediation- Effective strategy to ameliorate arsenic toxicity. In Fungi Bio-Prospects in Sustainable Agriculture, Environment and Nano-Technology; Academic Press: Cambridge, MA, USA, 2021; pp. 433–458. [Google Scholar]
- Cánovas, D.; De Lorenzo, V. Osmotic stress limits arsenic hypertolerance in Aspergillus sp. P37. FEMS Microbiol. Ecol. 2007, 61, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, P.K.; Vaish, A.; Dwivedi, S.; Chakrabarty, D.; Singh, N.; Tripathi, R.D. Biological removal of arsenic pollution by soil fungi. Sci. Total Environ. 2011, 409, 2430–2442. [Google Scholar] [CrossRef] [PubMed]
- Maheswari, S.; Murugesan, A.G. Remediation of arsenic in soil by Aspergillus nidulans isolated from an arsenic-contaminated site. Environ. Technol. 2009, 30, 921–926. [Google Scholar] [CrossRef]
- Singh, S.; Jha, P.; Jobby, R. Fungi: A promising tool for bioremediation of toxic heavy metals. In Bioremediation for Environmental Sustainability; Elsevier: Amsterdam, The Netherlands, 2021; pp. 123–144. [Google Scholar] [CrossRef]
- Soares Guimarães, L.H.; Segura, F.R.; Tonani, L.; von-Zeska-Kress, M.R.; Rodrigues, J.L.; Calixto, L.A.; Silva, F.F.; Batista, B.L. Arsenic volatilization by Aspergillus sp. and Penicillium sp. isolated from rice rhizosphere as a promising eco-safe tool for arsenic mitigation. J. Environ. Manag. 2019, 237, 170–179. [Google Scholar] [CrossRef] [PubMed]
- Dixit, R.; Malaviya, D.; Pandiyan, K.; Singh, U.B.; Sahu, A.; Shukla, R.; Singh, B.P.; Rai, J.P.; Sharma, P.K.; Lade, H.; et al. Bioremediation of heavy metals from soil and aquatic environment: An overview of principles and criteria of fundamental processes. Sustainability 2015, 7, 2189–2212. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Li, T.; Yang, L. Mechanisms of removing pollutants from aqueous solutions by microorganisms and their aggregates: A review. Bioresour. Technol. 2012, 107, 10–18. [Google Scholar] [CrossRef]
- Das, A.; Osborne, J.W. Bioremediation of Heavy Metals. In Nanotechnology, Food Security and Water Treatment; Gothandam, K., Ranjan, S., Dasgupta, N., Ramalingam, C., Lichtfouse, E., Eds.; Environmental chemistry for a sustainable world; Springer: Berlin, Germany, 2018; pp. 277–311. [Google Scholar]
- Leitão, A.L. Potential of Penicillium species in the bioremediation field. Int. J. Environ. Res. Public Health 2009, 6, 1393–1417. [Google Scholar] [CrossRef] [Green Version]
- Talukdar, D.; Jasrotia, T.; Sharma, R.; Jaglan, S.; Kumar, R.; Vats, R.; Kumar, R.; Mahnashi, M.H.; Umar, A. Evaluation of novel indigenous fungal consortium for enhanced bioremediation of heavy metals from contaminated sites. Environ. Technol. Innov. 2020, 20, 101050. [Google Scholar] [CrossRef]
- Huang, C.; Huang, C.P. Application of Aspergillus oryze and Rhizopus oryzae for Cu(II) removal. Water Res. 1996, 30, 1985–1990. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.S.; Jo, J.H.; Park, J.M. Mechanism of hexavalent chromium removal by dead fungal biomass of Aspergillus niger. Water Res. 2005, 39, 533–540. [Google Scholar] [CrossRef]
- Das, S.K.; Das, A.R.; Guha, A.K. A study on the adsorption mechanism of mercury on Aspergillus versicolor biomass. Environ. Sci. Technol. 2007, 41, 8281–8287. [Google Scholar] [CrossRef] [PubMed]
- Devi, R.; Behera, B.; Raza, M.B.; Mangal, V.; Altaf, M.A.; Kumar, R.; Kumar, A.; Tiwari, R.K.; Lal, M.K.; Singh, B. An insight into microbes mediated heavy metal detoxification in plants: A review. J. Soil Sci. Plant Nutr. 2021, 22, 914–936. [Google Scholar] [CrossRef]
- Tarfeen, N.; Nisa, K.U.; Hamid, B.; Bashir, Z.; Yatoo, A.M.; Dar, M.A.; Mohiddin, F.A.; Amin, Z.; Ahmad, R.A.; Sayyed, R.Z. Microbial remediation: A promising tool for reclamation of contaminated sites with special emphasis on heavy metal and pesticide pollution: A review. Processes 2022, 10, 1358. [Google Scholar] [CrossRef]
- Hussein, K.A.; Hassan, S.H.A.; Joo, J.H. Potential capacity of Beauveria bassiana and Metarhizium anisopliae in the biosorption of Cd2+ and Pb2+. J. Gen. Appl. Microbiol. 2011, 57, 347–355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, X.; Fang, W.; Tong, J.; Liu, S.; Wu, H.; Shi, J. Metarhizium robertsii as a promising microbial agent for rice in situ cadmium reduction and plant growth promotion. Chemosphere 2022, 305, 135427. [Google Scholar] [CrossRef]
- Nurzhan, A.; Tian, H.; Nuralykyzy, B.; He, W. Soil enzyme activities and enzyme activity indices in long-term arsenic-contaminated soils. Eurasian Soil Sci. 2022, 55, 1425–1435. [Google Scholar] [CrossRef]
- Abbas, G.; Murtaza, B.; Bibi, I.; Shahid, M.; Niazi, N.K.; Khan, M.I.; Amjad, M.; Hussain, M. Arsenic uptake, toxicity, detoxification, and speciation in plants: Physiological, biochemical, and molecular aspects. Int. J. Environ. Res. Public Health 2018, 15, 59. [Google Scholar] [CrossRef] [Green Version]
- Bibi, I.; Hussain, K.; Amen, R.; Hasan, I.M.U.; Shahid, M.; Bashir, S.; Niazi, N.K.; Mehmood, T.; Asghar, H.N.; Nawaz, M.F.; et al. The potential of microbes and sulfate in reducing arsenic phytoaccumulation by maize (Zea mays L.) plants. Environ. Geochem. Health 2021, 43, 5037–5051. [Google Scholar] [CrossRef]
- Bora, F.D.; Bunea, C.I.; Chira, R.; Bunea, A. Assessment of the quality of polluted areas in northwest Romania based on the content of elements in different organs of Grapevine (Vitis vinifera L.). Molecules 2020, 25, 750. [Google Scholar] [CrossRef] [Green Version]
- Allevato, E.; Stazi, S.R.; Marabottini, R.; D’Annibale, A. Mechanisms of arsenic assimilation by plants and countermeasures to attenuate its accumulation in crops other than rice. Ecotoxicol. Environ. Saf. 2019, 185, 109701. [Google Scholar] [CrossRef]
- Khanna, K.; Jamwal, V.L.; Gandhi, S.G.; Ohri, P.; Bhardwaj, R. Metal resistant PGPR lowered Cd uptake and expression of metal transporter genes with improved growth and photosynthetic pigments in Lycopersicon esculentum under metal toxicity. Sci. Rep. 2019, 9, 5855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Zhang, S.; Shan, X.; Zhu, Y.G. Toxicity of arsenate and arsenite on germination, seedling growth and amylolytic activity of wheat. Chemosphere 2005, 61, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef] [PubMed]
- Kuehr, S.; Kosfeld, V.; Schlechtriem, C. Bioaccumulation assessment of nanomaterials using freshwater invertebrate species. Environ. Sci. Eur. 2021, 33, 9. [Google Scholar] [CrossRef]
- Duxbury, J.M.; Panaullah, G.M. Remediation of Arsenic for Agriculture Sustainability, Food Security and Health in Bangladesh; FAO: Rome, Italy, 2007; pp. 1–28. [Google Scholar]
- Irem, S.; Islam, E.; Maathuis, F.; Niazi, N.K.; Li, T. Assessment of potential dietary toxicity and arsenic accumulation in two contrasting rice genotypes: Effect of soil amendments. Chemosphere 2019, 225, 104–114. [Google Scholar] [CrossRef]
- Wu, C.; Ye, Z.; Shu, W.; Zhu, Y.; Wong, M. Arsenic accumulation and speciation in rice are affected by root aeration and variation of genotypes. J. Exp. Bot. 2011, 62, 2889–2898. [Google Scholar] [CrossRef] [Green Version]
- Joseph, T.; Dubey, B.; McBean, E.A. Human health risk assessment from arsenic exposures in Bangladesh. Sci. Total Environ. 2015, 527, 552–560. [Google Scholar] [CrossRef]
- Mosa, K.A.; Kumar, K.; Chhikara, S.; Mcdermott, J.; Liu, Z.; Musante, C.; White, J.C.; Dhankher, O.P. Members of rice plasma membrane intrinsic proteins subfamily are involved in arsenite permeability and tolerance in plants. Transgenic Res. 2012, 21, 1265–1277. [Google Scholar] [CrossRef]
- Deng, F.; Zeng, F.; Chen, G.; Feng, X.; Riaz, A.; Wu, X.; Gao, W.; Wu, F.; Holford, P.; Chen, Z.H. Metalloid hazards: From plant molecular evolution to mitigation strategies. J. Hazard. Mater. 2021, 409, 124495. [Google Scholar] [CrossRef]
- Deng, F.L.; Liu, X.; Chen, Y.S.; Rathinasabapathi, B.; Rensing, C.; Chen, J.; Bi, J.; Xian, P.; Ma, L.N.Q. Aquaporins mediated arsenite transport in plants: Molecular mechanisms and applications in crop improvement. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1613–1639. [Google Scholar] [CrossRef]
- Lindsay, E.R.; Maathuis, F.J.M. New molecular mechanisms to reduce arsenic in crops. Trends Plant Sci. 2017, 22, 1016–1026. [Google Scholar] [CrossRef] [PubMed]
- Song, W.Y.; Yamaki, T.; Yamaji, N.; Ko, D.; Jung, K.H.; Fujii-Kashino, M.; An, G.; Martinoia, E.; Lee, Y.; Feng, J. A rice ABC transporter, OsABCC1, reduces arsenic accumulation in the grain. Proc. Natl. Acad. Sci. USA 2014, 111, 15699–15704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riyazuddin, R.; Nisha, N.; Ejaz, B.; Khan, M.I.R.; Kumar, M.; Ramteke, P.W.; Gupta, R. A comprehensive review on the heavy metal toxicity and sequestration in plants. Biomolecules 2021, 12, 43. [Google Scholar] [CrossRef] [PubMed]
- Sundaram, S.; Wu, S.; Ma, L.Q.; Rathinasabapathi, B. Expression of a Pteris vittata glutaredoxin PvGRX5 in transgenic Arabidopsis thaliana increases plant arsenic tolerance and decreases arsenic accumulation in the leaves. Plant. Cell Environ. 2009, 32, 851–858. [Google Scholar] [CrossRef]
- Upadhyay, M.K.; Shukla, A.; Yadav, P.; Srivastava, S. A review of arsenic in crops, vegetables, animals and food products. Food Chem. 2019, 276, 608–618. [Google Scholar] [CrossRef]
- Shi, G.L.; Zhu, S.; Meng, J.R.; Qian, M.; Yang, N.; Lou, L.Q.; Cai, Q.S. Variation in arsenic accumulation and translocation among wheat cultivars: The relationship between arsenic accumulation, efflux by wheat roots and arsenate tolerance of wheat seedlings. J. Hazard. Mater. 2015, 289, 190–196. [Google Scholar] [CrossRef]
- Adomako, E.E.; Williams, P.N.; Deacon, C.; Meharg, A.A. Inorganic arsenic and trace elements in Ghanaian grain staples. Environ. Pollut. 2011, 159, 2435–2442. [Google Scholar] [CrossRef]
- Williams, P.N.; Villada, A.; Deacon, C.; Raab, A.; Figuerola, J.; Green, A.J.; Feldmann, J.; Meharg, A.A. Greatly enhanced arsenic shoot assimilation in rice leads to elevated grain levels compared to wheat and barley. Environ. Sci. Technol. 2007, 41, 6854–6859. [Google Scholar] [CrossRef]
- Marwa, E.M.M.; Meharg, A.A.; Rice, C.M. Risk assessment of potentially toxic elements in agricultural soils and maize tissues from selected districts in Tanzania. Sci. Total Environ. 2012, 416, 180–186. [Google Scholar] [CrossRef]
- Williams, P.N.; Islam, M.R.; Adomako, E.E.; Raab, A.; Hossain, S.A.; Zhu, Y.G.; Feldmann, J.; Meharg, A.A. Increase in rice grain arsenic for regions of Bangladesh irrigating paddies with elevated arsenic in groundwaters. Environ. Sci. Technol. 2006, 40, 4903–4908. [Google Scholar] [CrossRef]
- Boivin, M.; Bourdeau, N.; Barnabé, S.; Desgagné-Penix, I. Sprout suppressive molecules effective on Potato (Solanum tuberosum) tubers during storage: A review. Am. J. Potato Res. 2020, 97, 451–463. [Google Scholar] [CrossRef]
- Signes-Pastor, A.J.; Cottingham, K.L.; Carey, M.; Sayarath, V.; Palys, T.; Meharg, A.A.; Folt, C.L.; Karagas, M.R. Infants’ dietary arsenic exposure during transition to solid food. Sci. Rep. 2018, 8, 7114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, P.; Samal, A.C.; Majumdar, J.; Santra, S.C. Arsenic contamination in rice, wheat, pulses, and vegetables: A study in an arsenic affected area of West Bengal, India. Water Air Soil Pollut. 2010, 213, 3–13. [Google Scholar] [CrossRef]
- Rahman, M.M.; Asaduzzaman, M.; Naidu, R. Consumption of arsenic and other elements from vegetables and drinking water from an arsenic-contaminated area of Bangladesh. J. Hazard. Mater. 2013, 262, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Roychowdhury, T.; Tokunaga, H.; Ando, M. Survey of arsenic and other heavy metals in food composites and drinking water and estimation of dietary intake by the villagers from an arsenic-affected area of West Bengal, India. Sci. Total Environ. 2003, 308, 15–35. [Google Scholar] [CrossRef] [PubMed]
- Niego, A.G.; Rapior, S.; Thongklang, N.; Raspé, O.; Jaidee, W.; Lumyong, S.; Hyde, K.D. Macrofungi as a nutraceutical source: Promising bioactive compounds and market value. J. Fungi 2021, 7, 397. [Google Scholar] [CrossRef]
- Seyfferth, A.L.; McClatchy, C.; Paukett, M. Arsenic, lead, and cadmium in U.S. mushrooms and substrate in relation to dietary exposure. Environ. Sci. Technol. 2016, 50, 9661–9670. [Google Scholar] [CrossRef]
- Kokkoris, V.; Massas, I.; Polemis, E.; Koutrotsios, G.; Zervakis, G.I. Accumulation of heavy metals by wild edible mushrooms with respect to soil substrates in the Athens metropolitan area (Greece). Sci. Total Environ. 2019, 685, 280–296. [Google Scholar] [CrossRef]
- Braeuer, S.; Borovička, J.; Goessler, W. A unique arsenic speciation profile in Elaphomyces spp. (“deer truffles”)—Trimethylarsine oxide and methylarsonous acid as significant arsenic compounds. Anal. Bioanal. Chem. 2018, 410, 2283–2290. [Google Scholar] [CrossRef] [Green Version]
- Hoque, E.; Fritscher, J. Multimetal bioremediation and biomining by a combination of new aquatic strains of Mucor hiemalis. Sci. Rep. 2019, 9, 10318. [Google Scholar] [CrossRef] [Green Version]
- Oladipo, O.G.; Awotoye, O.O.; Olayinka, A.; Bezuidenhout, C.C.; Maboeta, M.S. Heavy metal tolerance traits of filamentous fungi isolated from gold and gemstone mining sites. Braz. J. Microbiol. 2018, 49, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Yin, C.; Abbas, N.; Mao, Z.; Zhang, Y. Multiple heavy metal tolerance and removal by an earthworm gut fungus Trichoderma brevicompactum QYCD-6. Sci. Rep. 2020, 10, 6940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bui, V.C.; Franken, P. Acclimatization of Rhizophagus irregularis enhances Zn tolerance of the fungus and the mycorrhizal plant partner. Front. Microbiol. 2018, 9, 3156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vinuradha, R.; Kumutha, K.; Binodh, A.K. Accumulation of cadmium in maize roots inoculated with root organ culture of Rhizophagus irregularis improving cadmium tolerance through activation of antioxidative defense enzymes. J. Appl. Biol. Biotechnol. 2022, 10, 84–93. [Google Scholar] [CrossRef]
- Zhan, F.; Li, B.; Jiang, M.; Li, T.; He, Y.; Li, Y.; Wang, Y. Effects of arbuscular mycorrhizal fungi on the growth and heavy metal accumulation of bermudagrass [Cynodon dactylon (L.) Pers.] grown in a lead–zinc mine wasteland. Int. J. Phytoremediat. 2019, 21, 849–856. [Google Scholar] [CrossRef]
- Singh, G.; Pankaj, U.; Chand, S.; Verma, R.K. Arbuscular mycorrhizal fungi-assisted phytoextraction of toxic metals by Zea mays L. from tannery sludge. Soil Sediment Contam. Int. J. 2019, 28, 729–746. [Google Scholar] [CrossRef]
- Mondal, N.K.; Samanta, A.; Dutta, S.; Chattoraj, S. Optimization of Cr(VI) biosorption onto Aspergillus niger using 3-level Box-Behnken design: Equilibrium, kinetic, thermodynamic and regeneration studies. J. Genet. Eng. Biotechnol. 2017, 15, 151–160. [Google Scholar] [CrossRef]
- Mohammadian, E.; Babai Ahari, A.; Arzanlou, M.; Oustan, S.; Khazaei, S.H. Tolerance to heavy metals in filamentous fungi isolated from contaminated mining soils in the Zanjan Province, Iran. Chemosphere 2017, 185, 290–296. [Google Scholar] [CrossRef]
- Baldrian, P. Interactions of heavy metals with white-rot fungi. Enzyme Microb. Technol. 2003, 32, 78–91. [Google Scholar] [CrossRef]
- Igiri, B.E.; Okoduwa, S.I.R.; Idoko, G.O.; Akabuogu, E.P.; Adeyi, A.O.; Ejiogu, I.K. Toxicity and bioremediation of heavy metals contaminated ecosystem from tannery wastewater: A review. J. Toxicol. 2018, 2018, 2568038. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.Y.; Li, S.W.; Leng, Y.; Kang, X.H. Structural and functional responses of bacterial and fungal communities to multiple heavy metal exposure in arid loess. Sci. Total Environ. 2020, 723, 138081. [Google Scholar] [CrossRef] [PubMed]
- Matschullat, J. Arsenic in the geosphere—A review. Sci. Total Environ. 2000, 249, 297–312. [Google Scholar] [CrossRef]
- O’Day, P.A. Chemistry and Mineralogy of Arsenic. Elements 2006, 2, 77–83. [Google Scholar] [CrossRef]
- Khullar, S.; Sudhakara Reddy, M. Ectomycorrhizal fungi and its role in metal homeostasis through metallothionein and glutathione mechanisms. Curr. Biotechnol. 2018, 7, 231–241. [Google Scholar] [CrossRef]
- Anderson, M.E. Glutathione: An overview of biosynthesis and modulation. Chem. Biol. Interact. 1998, 111–112, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zeng, X.; Williams, P.N.; Gao, X.; Zhang, L.; Zhang, J.; Shan, H.; Su, S. Arsenic resistance in fungi conferred by extracellular bonding and vacuole-septa compartmentalization. J. Hazard. Mater. 2021, 401, 123370. [Google Scholar] [CrossRef]
- Persson, B.L.; Lagerstedt, J.O.; Pratt, J.R.; Pattison-Granberg, J.; Lundh, K.; Shokrollahzadeh, S.; Lundh, F. Regulation of phosphate acquisition in Saccharomyces cerevisiae. Curr. Genet. 2003, 43, 225–244. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Cao, C.L.; Liu, Y.L.; Wang, J.; Li, J.; Li, S.Y.; Deng, Y. Identification of the genetic requirements for zinc tolerance and toxicity in Saccharomyces cerevisiae. G3 Genes|Genomes|Genetics 2020, 10, 479–488. [Google Scholar] [CrossRef]
- Bun-ya, M.; Harashima, S.; Oshima, Y. Putative GTP-binding protein, Gtr1, associated with the function of the Pho84 inorganic phosphate transporter in Saccharomyces cerevisiae. Mol. Cell. Biol. 1992, 12, 2958–2966. [Google Scholar] [CrossRef]
- Bun-ya, M.; Shikata, K.; Nakade, S.; Yompakdee, C.; Harashima, S.; Oshima, Y. Two new genes, PHO86 and PHO87, involved in inorganic phosphate uptake in Saccharomyces cerevisiae. Curr. Genet. 1996, 29, 344–351. [Google Scholar] [CrossRef]
- Pearson, S.A.; Cowan, J.A. Glutathione-coordinated metal complexes as substrates for cellular transporters. Metallomics 2021, 13, 15. [Google Scholar] [CrossRef] [PubMed]
- Roy, M.; Giri, A.K.; Dutta, S.; Mukherjee, P. Integrated phytobial remediation for sustainable management of arsenic in soil and water. Environ. Int. 2015, 75, 180–198. [Google Scholar] [CrossRef] [PubMed]
- Wysocki, R.; Tamás, M.J. Saccharomyces cerevisiae as a model organism for elucidating arsenic tolerance mechanisms. In Cellular Effects of Heavy Metals; Bánfalvi, G., Ed.; Springer: Berlín, Germany; Springer: Dordrecht, The Netherlands, 2011; pp. 87–112. [Google Scholar]
- Cánovas, D.; Mukhopadhyay, R.; Rosen, B.P.; De Lorenzo, V. Arsenate transport and reduction in the hyper-tolerant fungus Aspergillus sp. P37. Environ. Microbiol. 2003, 5, 1087–1093. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Shi, J.; Rosen, B.P. Purification and characterization of Acr2p, the Saccharomyces cerevisiae arsenate reductase. J. Biol. Chem. 2000, 275, 21149–21157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellion, M.; Courbot, M.; Jacob, C.; Blaudez, D.; Chalot, M. Extracellular and cellular mechanisms sustaining metal tolerance in ectomycorrhizal fungi. FEMS Microbiol. Lett. 2006, 254, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Anand, G.; Singh, N.; Kapoor, R. Arbuscular mycorrhiza augments arsenic tolerance in wheat (Triticum aestivum L.) by strengthening antioxidant defense system and thiol metabolism. Front. Plant Sci. 2017, 8, 906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verma, P.K.; Verma, S.; Meher, A.K.; Pande, V.; Mallick, S.; Bansiwal, A.K.; Tripathi, R.D.; Dhankher, O.P.; Chakrabarty, D. Overexpression of rice glutaredoxins (OsGrxs) significantly reduces arsenite accumulation by maintaining glutathione pool and modulating aquaporins in yeast. Plant Physiol. Biochem. 2016, 106, 208–217. [Google Scholar] [CrossRef]
- Ahsan, N.; Lee, D.G.; Alam, I.; Kim, P.J.; Lee, J.J.; Ahn, Y.O.; Kwak, S.S.; Lee, I.J.; Bahk, J.D.; Kang, K.Y.; et al. Comparative proteomic study of arsenic-induced differentially expressed proteins in rice roots reveals glutathione plays a central role during As stress. Proteomics 2008, 8, 3561–3576. [Google Scholar] [CrossRef]
- Khullar, S.; Reddy, M.S. Cadmium and arsenic responses in the ectomycorrhizal fungus Laccaria bicolor: Glutathione metabolism and its role in metal(loid) homeostasis. Environ. Microbiol. Rep. 2019, 11, 53–61. [Google Scholar] [CrossRef]
- Khullar, S.; Reddy, M.S. Arsenic toxicity and its mitigation in ectomycorrhizal fungus Hebeloma cylindrosporum through glutathione biosynthesis. Chemosphere 2020, 240, 124914. [Google Scholar] [CrossRef]
- Khullar, S.; Reddy, M.S. Ectomycorrhizal diversity and tree sustainability. In Microbial Diversity in Ecosystem Sustainability and Biotechnological Applications: Volume 2. Soil & Agroecosystems; Satyanarayana, T., Das, S.K., Johri, B.N., Eds.; Springer: Singapore, 2019; pp. 145–166. [Google Scholar]
- Mukherjee, A.; Das, D.; Mondal, S.K.; Biswas, R.; Das, T.K.; Boujedaini, N.; Khuda-Bukhsh, A.R. Tolerance of arsenate-induced stress in Aspergillus niger, a possible candidate for bioremediation. Ecotoxicol. Environ. Saf. 2010, 73, 172–182. [Google Scholar] [CrossRef] [PubMed]
- Thorsen, M.; Lagniel, G.; Kristiansson, E.; Junot, C.; Nerman, O.; Labarre, J.; Tamás, M.J. Quantitative transcriptome, proteome, and sulfur metabolite profiling of the Saccharomyces cerevisiae response to arsenite. Physiol. Genomics 2007, 30, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Yadav, S.K. Heavy metals toxicity in plants: An overview on the role of glutathione and phytochelatins in heavy metal stress tolerance of plants. S. Afr. J. Bot. 2010, 76, 167–179. [Google Scholar] [CrossRef] [Green Version]
- Davison, K.; Côté, S.; Mader, S.; Miller, W.H. Glutathione depletion overcomes resistance to arsenic trioxide in arsenic-resistant cell lines. Leukemia 2003, 17, 931–940. [Google Scholar] [CrossRef] [Green Version]
- Stýblo, M.; Venkatratnam, A.; Fry, R.C.; Thomas, D.J. Origins, fate, and actions of methylated trivalent metabolites of inorganic arsenic: Progress and prospects. Arch. Toxicol. 2021, 95, 1547–1572. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Tiwari, S.; Patel, A.; Prasad, S.M. Arsenic contamination, speciation, toxicity and defense strategies in plants. Rev. Bras. Bot. 2021, 44, 1–10. [Google Scholar] [CrossRef]
- Klein, M.; Mamnun, Y.M.; Eggmann, T.; Schüller, C.; Wolfger, H.; Martinoia, E.; Kuchler, K. The ATP-binding cassette (ABC) transporter Bpt1p mediates vacuolar sequestration of glutathione conjugates in yeast. FEBS Lett. 2002, 520, 63–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cánovas, D.; Vooijs, R.; Schat, H.; De Lorenzo, V. The Role of Thiol Species in the Hypertolerance of Aspergillus sp. P37 to Arsenic. J. Biol. Chem. 2004, 279, 51234–51240. [Google Scholar] [CrossRef]
- Zhao, F.J.; Ma, J.F.; Meharg, A.A.; McGrath, S.P. Arsenic uptake and metabolism in plants. New Phytol. 2009, 181, 777–794. [Google Scholar] [CrossRef]
- Perego, P.; Howell, S.B. Molecular mechanisms controlling sensitivity to toxic metal ions in yeast. Toxicol. Appl. Pharmacol. 1997, 147, 312–318. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.S.; Park, J.M. The past, present, and future trends of biosorption. Biotechnol. Bioprocess Eng. 2010, 15, 86–102. [Google Scholar] [CrossRef]
- Timková, I.; Sedláková-Kaduková, J.; Pristaš, P. Biosorption and bioaccumulation abilities of Actinomycetes/Streptomycetes isolated from metal contaminated sites. Separations 2018, 5, 54. [Google Scholar] [CrossRef] [Green Version]
- Di, X.; Beesley, L.; Zhang, Z.; Zhi, S.; Jia, Y.; Ding, Y. Microbial arsenic methylation in soil and uptake and metabolism of methylated arsenic in plants: A review. Int. J. Environ. Res. Public Health 2019, 16, 5012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, K.M.; Nordstrom, D.K. Arsenic speciation and sorption in natural environments. Rev. Mineral. Geochem. 2014, 79, 185–216. [Google Scholar] [CrossRef]
- Ye, J.; Rensing, C.; Rosen, B.P.; Zhu, Y.G. Arsenic biomethylation by photosynthetic organisms. Trends Plant Sci. 2012, 17, 155–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, S.L.; Singh, S.; Dasilva, N.A.; Chen, W. Co-expression of Arabidopsis thaliana phytochelatin synthase and Treponema denticola cysteine desulfhydrase for enhanced arsenic accumulation. Biotechnol. Bioeng. 2012, 109, 605–608. [Google Scholar] [CrossRef] [PubMed]
- Qiao, J.T.; Li, X.M.; Hu, M.; Li, F.B.; Young, L.Y.; Sun, W.M.; Huang, W.; Cui, J.H. Transcriptional activity of arsenic-reducing bacteria and genes regulated by lactate and biochar during arsenic transformation in flooded paddy soil. Environ. Sci. Technol. 2018, 52, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Y.; Costa, M. PI3K/Akt/mTOR signaling pathway and the biphasic effect of arsenic in carcinogenesis. Mol. Pharmacol. 2018, 94, 784–792. [Google Scholar] [CrossRef] [PubMed]
- Govarthanan, M.; Mythili, R.; Selvankumar, T.; Kamala-Kannan, S.; Kim, H. Myco-phytoremediation of arsenic- and lead-contaminated soils by Helianthus annuus and wood rot fungi, Trichoderma sp. isolated from decayed wood. Ecotoxicol. Environ. Saf. 2018, 151, 279–284. [Google Scholar] [CrossRef]
- Singh, M.; Srivastava, P.K.; Verma, P.C.; Kharwar, R.N.; Singh, N.; Tripathi, R.D. Soil fungi for mycoremediation of arsenic pollution in agriculture soils. J. Appl. Microbiol. 2015, 119, 1278–1290. [Google Scholar] [CrossRef] [Green Version]
- Zeng, X.; Su, S.; Jiang, X.; Li, L.; Bai, L.; Zhang, Y. Capability of pentavalent arsenic bioaccumulation and biovolatilization of three fungal strains under laboratory conditions. Clean-Soil Air Water 2010, 38, 238–241. [Google Scholar] [CrossRef]
- Ditusa, S.F.; Fontenot, E.B.; Wallace, R.W.; Silvers, M.A.; Steele, T.N.; Elnagar, A.H.; Dearman, K.M.; Smith, A.P. A member of the phosphate transporter 1 (Pht1) family from the arsenic-hyperaccumulating fern Pteris vittata is a high-affinity arsenate transporter. New Phytol. 2016, 209, 762–772. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Christophersen, H.M.; Pope, S.; Smith, F.A. Arsenic uptake and toxicity in plants: Integrating mycorrhizal influences. Plant Soil 2010, 327, 1–21. [Google Scholar] [CrossRef]
- Colpaert, J.V.; Wevers, J.H.L.; Krznaric, E.; Adriaensen, K. How metal-tolerant ecotypes of ectomycorrhizal fungi protect plants from heavy metal pollution. Ann. For. Sci. 2011, 68, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Ren, B.H.; Wu, S.L.; Sun, Y.Q.; Lin, G.; Chen, B.D. Arbuscular mycorrhizal symbiosis influences arsenic accumulation and speciation in Medicago truncatula L. in arsenic-contaminated soil. Chemosphere 2015, 119, 224–230. [Google Scholar] [CrossRef]
- de los Angeles Beltrán-Nambo, M.; Rojas-Jacuinde, N.; Martínez-Trujillo, M.; Jaramillo-López, P.F.; Romero, M.G.; Carreón-Abud, Y. Differential strategies of two species of arbuscular mycorrhizal fungi in the protection of maize plants grown in chromium-contaminated soils. BioMetals 2021, 34, 1247–1261. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Molecular mechanisms of metal hyperaccumulation in plants. New Phytol. 2009, 181, 759–776. [Google Scholar] [CrossRef]
- Garg, N.; Chandel, S. Role of arbuscular mycorrhiza in arresting reactive oxygen species (ROS) and strengthening antioxidant defense in Cajanus cajan (L.) Mill sp. nodules under salinity (NaCl) and cadmium (Cd) stress. Plant Growth Regul. 2015, 75, 521–534. [Google Scholar] [CrossRef]
- Guo, L.D. Presidential address: Recent advance of mycorrhizal research in China. Mycology 2018, 9, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Shri, M.; Singh, P.K.; Kidwai, M.; Gautam, N.; Dubey, S.; Verma, G.; Chakrabarty, D. Recent advances in arsenic metabolism in plants: Current status, challenges and highlighted biotechnological intervention to reduce grain arsenic in rice. Metallomics 2019, 11, 519–532. [Google Scholar] [CrossRef]
- Singh, P.K.; Indoliya, Y.; Chauhan, A.S.; Singh, S.P.; Singh, A.P.; Dwivedi, S.; Tripathi, R.D.; Chakrabarty, D. Nitric oxide mediated transcriptional modulation enhances plant adaptive responses to arsenic stress. Sci. Rep. 2017, 7, 3592. [Google Scholar] [CrossRef] [PubMed]
- Saeed, M.; Quraishi, U.M.; Malik, R.N. Arsenic uptake and toxicity in wheat (Triticum aestivum L.): A review of multi-omics approaches to identify tolerance mechanisms. Food Chem. 2021, 355, 129607. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, F.; Ashraf, M. Antioxidants as modulators of arsenic-induced oxidative stress tolerance in plants: An overview. J. Hazard. Mater. 2021, 427, 127891. [Google Scholar] [CrossRef] [PubMed]
- Abu-Elsaoud, A.M.; Nafady, N.A.; Abdel-Azeem, A.M. Arbuscular mycorrhizal strategy for zinc mycoremediation and diminished translocation to shoots and grains in wheat. PLoS ONE 2017, 12, e0188220. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Xu, Y.; Ding, W.; Li, Y.; Xu, H. Mycoremediation of manganese and phenanthrene by Pleurotus eryngii mycelium enhanced by Tween 80 and saponin. Appl. Microbiol. Biotechnol. 2016, 100, 7249–7261. [Google Scholar] [CrossRef] [PubMed]
- Albert, Q.; Leleyter, L.; Lemoine, M.; Heutte, N.; Rioult, J.P.; Sage, L.; Baraud, F.; Garon, D. Comparison of tolerance and biosorption of three trace metals (Cd, Cu, Pb) by the soil fungus Absidia cylindrospora. Chemosphere 2018, 196, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Kapahi, M.; Sachdeva, S. Mycoremediation potential of Pleurotus species for heavy metals: A review. Bioresour. Bioprocess. 2017, 4, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, W.K.; Wildeboer, D.; Garelick, H.; Purchase, D. Mycoremediation of heavy metal/metalloid-contaminated soil: Current understanding and future prospects. In Fungal Applications in Sustainable Environmental Biotechnology; Springer: Cham, Switzerland, 2016; pp. 249–272. [Google Scholar] [CrossRef]
- Benguenab, A.; Chibani, A. Biodegradation of petroleum hydrocarbons by filamentous fungi (Aspergillus ustus and Purpureocillium lilacinum) isolated from used engine oil contaminated soil. Acta Ecol. Sin. 2021, 41, 416–423. [Google Scholar] [CrossRef]
- Alonso, L.M.; Kleiner, D.; Ortega, E. Spores of the mycorrhizal fungus Glomus mosseae host yeasts that solubilize phosphate and accumulate polyphosphates. Mycorrhiza 2008, 18, 198–204. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, F.; Chen, J.; Sun, G. Arsenic removal from contaminated soil via biovolatilization by genetically engineered bacteria under laboratory conditions. J. Environ. Sci. 2011, 23, 1544–1550. [Google Scholar] [CrossRef]
- Verma, S.; Verma, P.K.; Chakrabarty, D. Arsenic Bio-volatilization by engineered yeast promotes rice growth and reduces arsenic accumulation in grains. Int. J. Environ. Res. 2019, 13, 475–485. [Google Scholar] [CrossRef]
- Kaushal, J.; Khatri, M.; Arya, S.K. A treatise on organophosphate pesticide pollution: Current strategies and advancements in their environmental degradation and elimination. Ecotoxicol. Environ. Saf. 2021, 207, 111483. [Google Scholar] [CrossRef] [PubMed]
- Hage-Ahmed, K.; Rosner, K.; Steinkellner, S. Arbuscular mycorrhizal fungi and their response to pesticides. Pest Manag. Sci. 2019, 75, 583–590. [Google Scholar] [CrossRef] [PubMed]
- Raffa, C.M.; Chiampo, F. Bioremediation of agricultural soils polluted with pesticides: A review. Bioengineering 2021, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.P.; Serrano, C.; Pires, T.; Mestrinho, E.; Dias, L.; Teixeira, D.M.; Caldeira, A.T. Degradation of terbuthylazine, difenoconazole and pendimethalin pesticides by selected fungi cultures. Sci. Total Environ. 2012, 435–436, 402–410. [Google Scholar] [CrossRef]
- Silambarasan, S.; Abraham, J. Mycoremediation of endosulfan and its metabolites in aqueous medium and soil by Botryosphaeria laricina JAS6 and Aspergillus tamarii JAS9. PLoS ONE 2013, 8, e77170. [Google Scholar] [CrossRef] [Green Version]
- Gajendiran, A.; Abraham, J. Biomineralisation of fipronil and its major metabolite, fipronil sulfone, by Aspergillus glaucus strain AJAG1 with enzymes studies and bioformulation. 3 Biotech 2017, 7, 212. [Google Scholar] [CrossRef]
- Balsano, E.; Esterhuizen-Londt, M.; Hoque, E.; Lima, S.P. Responses of the antioxidative and biotransformation enzymes in the aquatic fungus Mucor hiemalis exposed to cyanotoxins. Biotechnol. Lett. 2017, 39, 1201–1209. [Google Scholar] [CrossRef]
- Balsano, E.; Esterhuizen-Londt, M.; Hoque, E.; Pflugmacher, S. Toxin resistance in aquatic fungi poses environmentally friendly remediation possibilities: A study on the growth responses and biosorption potential of Mucor hiemalis EH5 against Cyanobacterial toxins. Int. J. Water Wastewater Treat. 2015, 1, 1–9. [Google Scholar] [CrossRef]
- Al-Mamoori, A.M.J.; Al-Shammari, R.H.H.; Al-amari, M.J.Y.; Al-Juboori, M.M.K. Removal of Anabaena sp. bloom and microcystin-LR by coculturing with Mucor rouxii pellets. Aquat. Ecosyst. Health Manag. 2020, 23, 267–273. [Google Scholar] [CrossRef]
- Bankole, P.O.; Adekunle, A.A.; Obidi, O.F.; Olukanni, O.D.; Govindwar, S.P. Degradation of indigo dye by a newly isolated yeast, Diutina rugosa from dye wastewater polluted soil. J. Environ. Chem. Eng. 2017, 5, 4639–4648. [Google Scholar] [CrossRef]
- Xue, W.N.; Peng, Y.B. Study on environmental materials with Aspergillus niger as adsorbent for sequestering Pb(II) from aqueous solution. In Advanced Materials Research; Trans Tech Publications Ltd.: Bäch, Switzerland, 2013; pp. 119–123. [Google Scholar]
- Vala, A.K. Tolerance and removal of arsenic by a facultative marine fungus Aspergillus candidus. Bioresour. Technol. 2010, 101, 2565–2567. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.C.; Rosen, B.P. New mechanisms of bacterial arsenic resistance. Biomed. J. 2016, 39, 5–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukhopadhyay, R.; Rosen, B.P.; Phung, L.T.; Silver, S. Microbial arsenic: From geocycles to genes and enzymes. FEMS Microbiol. Rev. 2002, 26, 311–325. [Google Scholar] [CrossRef]
- Chojnacka, K. Biosorption and bioaccumulation—the prospects for practical applications. Environ. Int. 2010, 36, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Gavrilescu, M. Removal of heavy metals from the environment by biosorption. Eng. Life Sci. 2004, 4, 219–232. [Google Scholar] [CrossRef]
- Zouboulis, A.I.; Loukidou, M.X.; Matis, K.A. Biosorption of toxic metals from aqueous solutions by bacteria strains isolated from metal-polluted soils. Process Biochem. 2004, 39, 909–916. [Google Scholar] [CrossRef]
- Al-Makishah, N.H.; Taleb, M.A.; Barakat, M.A. Arsenic bioaccumulation in arsenic-contaminated soil: A review. Chem. Pap. 2020, 74, 2743–2757. [Google Scholar] [CrossRef]
- Dahoumane, S.A.; Mechouet, M.; Wijesekera, K.; Filipe, C.D.M.; Sicard, C.; Bazylinski, D.A.; Jeffryes, C. Algae-mediated biosynthesis of inorganic nanomaterials as a promising route in nanobiotechnology-A review. Green Chem. 2017, 19, 552–587. [Google Scholar] [CrossRef]
- Liang, J.; Diao, H.; Song, W.; Li, L. Tolerance and Bioaccumulation of arsenate by Aspergillus oryzae TLWK-09 isolated from arsenic-contaminated soils. Water Air Soil Pollut. 2018, 229, 169. [Google Scholar] [CrossRef]
- Ghosh, M.; Shen, J.; Rosen, B.P. Pathways of As(III) detoxification in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA 1999, 96, 5001–5006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tripathi, A.K.; Harsh, N.S.K.; Gupta, N. Fungal treatment of industrial effluents: A mini-review. Life Sci. J. 2007, 4, 1097–8135. [Google Scholar]
- Giller, K.E.; Witter, E.; McGrath, S.P. Heavy metals and soil microbes. Soil Biol. Biochem. 2009, 41, 2031–2037. [Google Scholar] [CrossRef]
- Badsha, M.A.H.; Khan, M.; Wu, B.; Kumar, A.; Lo, I.M.C. Role of surface functional groups of hydrogels in metal adsorption: From performance to mechanism. J. Hazard. Mater. 2021, 408, 124463. [Google Scholar] [CrossRef] [PubMed]
- Simonescu, C.M.; Lavric, V.; Musina, A.; Antonescu, O.M.; Culita, D.C.; Marinescu, V.; Tardei, C.; Oprea, O.; Pandele, A.M. Experimental and modeling of cadmium ions removal by chelating resins. J. Mol. Liq. 2020, 307, 112973. [Google Scholar] [CrossRef]
- Ahonen-Jonnarth, U.; Van Hees, P.A.W.; Lundström, U.S.; Finlay, R.D. Organic acids produced by mycorrhizal Pinus sylvestris exposed to elevated aluminium and heavy metal concentrations. New Phytol. 2000, 146, 557–567. [Google Scholar] [CrossRef]
- Jarosz-Wilkolazka, A.; Gadd, G.M. Oxalate production by wood-rotting fungi growing in toxic metal-amended medium. Chemosphere 2003, 52, 541–547. [Google Scholar] [CrossRef]
- Priyadarshini, E.; Priyadarshini, S.S.; Cousins, B.G.; Pradhan, N. Metal-fungus interaction: Review on cellular processes underlying heavy metal detoxification and synthesis of metal nanoparticles. Chemosphere 2021, 274, 129976. [Google Scholar] [CrossRef]
- Ott, T.; Fritz, E.; Polle, A.; Schützendübel, A. Characterisation of antioxidative systems in the ectomycorrhiza-building basidiomycete Paxillus involutus (Bartsch) Fr. and its reaction to cadmium. FEMS Microbiol. Ecol. 2002, 42, 359–366. [Google Scholar] [CrossRef]
- Fogarty, R.V.; Tobin, J.M. Fungal melanins and their interactions with metals. Enzyme Microb. Technol. 1996, 19, 311–317. [Google Scholar] [CrossRef]
- Gadd, G.M.; de Rome, L. Biosorption of copper by fungal melanin. Appl. Microbiol. Biotechnol. 1988, 29, 610–617. [Google Scholar] [CrossRef]
- Morselt, A.F.W.; Smits, W.T.M.; Limonard, T. Histochemical demonstration of heavy metal tolerance in ectomycorrhizal fungi on JSTOR. Plant Soil 1986, 96, 417–420. [Google Scholar] [CrossRef]
- Courbot, M.; Diez, L.; Ruotolo, R.; Chalot, M.; Leroy, P. Cadmium-responsive thiols in the ectomycorrhizal fungus Paxillus involutus. Appl. Environ. Microbiol. 2004, 70, 7413–7417. [Google Scholar] [CrossRef] [Green Version]
- Akar, T.; Tunali, S.; Kiran, I. Botrytis cinerea as a new fungal biosorbent for removal of Pb(II) from aqueous solutions. Biochem. Eng. J. 2005, 25, 227–235. [Google Scholar] [CrossRef]
- Wilson, D.; Citiulo, F.; Hube, B. Zinc exploitation by pathogenic fungi. PLoS Pathog. 2012, 8, e1003034. [Google Scholar] [CrossRef] [Green Version]
- Devirgiliis, C.; Murgia, C.; Danscher, G.; Perozzi, G. Exchangeable zinc ions transiently accumulate in a vesicular compartment in the yeast Saccharomyces cerevisiae. Biochem. Biophys. Res. Commun. 2004, 323, 58–64. [Google Scholar] [CrossRef]
- Fomina, M.; Hillier, S.; Charnock, J.M.; Melville, K.; Alexander, I.J.; Gadd, G.M. Role of oxalic acid overexcretion in transformations of toxic metal minerals by Beauveria caledonica. Appl. Environ. Microbiol. 2005, 71, 371–381. [Google Scholar] [CrossRef] [Green Version]
- Ge, W.; Zamri, D.; Mineyama, H.; Valix, M. Bioaccumulation of heavy metals on adapted Aspergillus foetidus. Adsorption 2011, 17, 901–910. [Google Scholar] [CrossRef]
- Sintuprapa, W.; Thiravetyan, P.; Tanticharoen, M. A possible mechanism of Zn2+ uptake by living cells of Penicillium sp. Biotechnol. Lett. 2000, 22, 1709–1712. [Google Scholar] [CrossRef]
- Xu, X.; Xia, L.; Zhu, W.; Zhang, Z.; Huang, Q.; Chen, W. Role of Penicillium chrysogenum XJ-1 in the detoxification and bioremediation of cadmium. Front. Microbiol. 2015, 6, 1422. [Google Scholar] [CrossRef] [Green Version]
- Rao, R.; Rashmi, K.; Naveena, J.; Latha, L.; Mohan, M. Bioremediation of toxic metal ions using biomass of Aspergillus fumigatus from fermentative waste. Indian J. Biotechnol. 2005, 4, 139–143. [Google Scholar]
- Sharples, J.M.; Meharg, A.A.; Chambers, S.M.; Cairney, J.W.G. Mechanism of arsenate resistance in the ericoid mycorrhizal fungus Hymenoscyphus ericae. Plant Physiol. 2000, 124, 1327–1334. [Google Scholar] [CrossRef] [Green Version]
- Farooq, U.; Kozinski, J.A.; Khan, M.A.; Athar, M. Biosorption of heavy metal ions using wheat based biosorbents—A review of the recent literature. Bioresour. Technol. 2010, 101, 5043–5053. [Google Scholar] [CrossRef]
- Liu, N.; Luo, S.; Yang, Y.; Zhang, T.; Jin, J.; Liao, J. Biosorption of americium-241 by Saccharomyces cerevisiae. J. Radioanal. Nucl. Chem. 2002, 252, 187–191. [Google Scholar] [CrossRef]
- Rosen, B.P. Transport and detoxification systems for transition metals, heavy metals and metalloids in eukaryotic and prokaryotic microbes. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2002, 133, 689–693. [Google Scholar] [CrossRef]
- Shen, J.; Hsu, C.M.; Kang, B.K.; Rosen, B.P.; Bhattacharjee, H. The Saccharomyces cerevisiae Arr4p is involved in metal and heat tolerance. BioMetals 2003, 16, 369–378. [Google Scholar] [CrossRef]
- Singh, S.; Lee, W.; DaSilva, N.A.; Mulchandani, A.; Chen, W. Enhanced arsenic accumulation by engineered yeast cells expressing Arabidopsis thaliana phytochelatin synthase. Biotechnol. Bioeng. 2008, 99, 333–340. [Google Scholar] [CrossRef]
- Koonsom, T.; Inthorn, D.; Thiravetyan, P. Effect of kaolin on arsenic accumulation in rice plants (Oryza sativa L.) grown in arsenic contaminated soils. Environ. Eng. Res. 2014, 19, 241–245. [Google Scholar] [CrossRef]
- Chen, P.; Li, J.; Wang, H.Y.; Zheng, R.L.; Sun, G.X. Evaluation of bioaugmentation and biostimulation on arsenic remediation in soil through biovolatilization. Environ. Sci. Pollut. Res. 2017, 24, 21739–21749. [Google Scholar] [CrossRef]
- Singh, N.; Marwa, N.; Mishra, S.K.; Mishra, J.; Verma, P.C.; Rathaur, S.; Singh, N. Brevundimonas diminuta mediated alleviation of arsenic toxicity and plant growth promotion in Oryza sativa L. Ecotoxicol. Environ. Saf. 2016, 125, 25–34. [Google Scholar] [CrossRef]
- Tripathi, P.; Khare, P.; Barnawal, D.; Shanker, K.; Srivastava, P.K.; Tripathi, R.D.; Kalra, A. Bioremediation of arsenic by soil methylating fungi: Role of Humicola sp. strain 2WS1 in amelioration of arsenic phytotoxicity in Bacopa monnieri L. Sci. Total Environ. 2020, 716, 136758. [Google Scholar] [CrossRef]
- Tiwari, M.; Sharma, D.; Dwivedi, S.; Singh, M.; Tripathi, R.D.; Trivedi, P.K. Expression in Arabidopsis and cellular localization reveal involvement of rice NRAMP, OsNRAMP1, in arsenic transport and tolerance. Plant Cell Environ. 2014, 37, 140–152. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Yan, H.; Chen, Y.; Shen, H.; Xu, W.; Zhang, H.; Shi, L.; Zhu, Y.G.; Ma, M. An aquaporin PvTIP4;1 from Pteris vittata may mediate arsenite uptake. New Phytol. 2016, 209, 746–761. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Han, Y.H.; Cao, Y.; Zhu, Y.G.; Rathinasabapathi, B.; Ma, L.Q. Arsenic transport in rice and biological solutions to reduce arsenic risk from rice. Front. Plant Sci. 2017, 8, 268. [Google Scholar] [CrossRef] [Green Version]
- Song, W.Y.; Park, J.; Mendoza-Cózatl, D.G.; Suter-Grotemeyer, M.; Shima, D.; Hörtensteiner, S.; Geisler, M.; Weder, B.; Rea, P.A.; Rentsch, D.; et al. Arsenic tolerance in Arabidopsis is mediated by two ABCC-type phytochelatin transporters. Proc. Natl. Acad. Sci. USA 2010, 107, 21187–21192. [Google Scholar] [CrossRef] [Green Version]
- Gasic, K.; Korban, S.S. Transgenic Indian mustard (Brassica juncea) plants expressing an Arabidopsis phytochelatin synthase (AtPCS1) exhibit enhanced As and Cd tolerance. Plant Mol. Biol. 2007, 64, 361–369. [Google Scholar] [CrossRef]
- Li, Y.; Dhankher, O.P.; Carreira, L.; Lee, D.; Chen, A.; Schroeder, J.I.; Balish, R.S.; Meagher, R.B. Overexpression of phytochelatin synthase in Arabidopsis leads to enhanced arsenic tolerance and cadmium hypersensitivity. Plant Cell Physiol. 2004, 45, 1787–1797. [Google Scholar] [CrossRef] [Green Version]
- Picault, N.; Cazalé, A.C.; Beyly, A.; Cuiné, S.; Carrier, P.; Luu, D.T.; Forestier, C.; Peltier, G. Chloroplast targeting of phytochelatin synthase in Arabidopsis: Effects on heavy metal tolerance and accumulation. Biochimie 2006, 88, 1743–1750. [Google Scholar] [CrossRef]
- Mandal, A. Transgenic tobacco plants expressing ACR2 gene of Arabidopsis thaliana exhibit reduced accumulation of arsenics and increased tolerance to arsenate. In Proceedings of the Global Summit on Plant Science, San Antonio, TX, USA, 21–23 September 2015; p. 32. [Google Scholar]
- Guo, J.; Dai, X.; Xu, W.; Ma, M. Overexpressing GSH1 and AsPCS1 simultaneously increases the tolerance and accumulation of cadmium and arsenic in Arabidopsis thaliana. Chemosphere 2008, 72, 1020–1026. [Google Scholar] [CrossRef]
- Reisinger, S.; Schiavon, M.; Terry, N.; Pilon-Smits, E.A.H. Heavy Metal tolerance and accumulation in Indian mustard (Brassica Juncea L.) expressing bacterial γ-glutamylcysteine synthetase or glutathione synthetase. Int. J. Phytoremediat. 2008, 10, 440–454. [Google Scholar] [CrossRef]
- Wojas, S.; Clemens, S.; SkŁodowska, A.; Maria Antosiewicz, D. Arsenic response of AtPCS1- and CePCS-expressing plants—Effects of external As(V) concentration on As-accumulation pattern and NPT metabolism. J. Plant Physiol. 2010, 167, 169–175. [Google Scholar] [CrossRef]
- Verma, P.K.; Verma, S.; Pande, V.; Mallick, S.; Tripathi, R.D.; Dhankher, O.P.; Chakrabarty, D. Overexpression of rice glutaredoxin OsGrx_C7 and OsGrx_C2.1 reduces intracellular arsenic accumulation and increases tolerance in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 740. [Google Scholar] [CrossRef] [Green Version]
- Tang, Z.; Lv, Y.; Chen, F.; Zhang, W.; Rosen, B.P.; Zhao, F.J. Arsenic Methylation in Arabidopsis thaliana Expressing an algal arsenite methyltransferase gene increases arsenic phytotoxicity. J. Agric. Food Chem. 2016, 64, 2674–2681. [Google Scholar] [CrossRef] [Green Version]
- Uchimiya, M.; Orlov, A.; Ramakrishnan, G.; Sistani, K. In situ and ex situ spectroscopic monitoring of biochar’s surface functional groups. J. Anal. Appl. Pyrolysis 2013, 102, 53–59. [Google Scholar] [CrossRef]
- Vithanage, M.; Herath, I.; Joseph, S.; Bundschuh, J.; Bolan, N.; Ok, Y.S.; Kirkham, M.B.; Rinklebe, J. Interaction of arsenic with biochar in soil and water: A critical review. Carbon N. Y. 2017, 113, 219–230. [Google Scholar] [CrossRef]


| Fungal Isolates | Time Taken in Remediation Process | Effect of Fungal Isolates on Soil | Mechanism of Action | Removal Efficiency (%) | References |
|---|---|---|---|---|---|
| A consortium of Ascomycota and Basidiomycota | 7 days | Enhanced soil quality, increased enzymatic activity | Volatilization/biomethylation | 31–77% | [8] |
| Trichoderma sp. MG and H. annuus | 6–8 days | Improves the soil enzymes activity, and also plant–fungi partnership for enhanced bioremediation of As | Bioaccumulation | In shoot 67%, in roots, 55%, | [116] |
| P. brevicaule and Aspergillus oryzae | 21 days | Improves the agricultural soil | Bioaccumulation and volatilization | 82% and 6.4 mg kg−1 | [117] |
| Lasiodiplodia sp. and Mycelia strain(FA-13) | 21 days | Enhances soil quality | Bioaccumulation/biosorption | 65.81% | [14] |
| P. janthinellum | 5 days | Improves the soil enzymes activity, and also plant–fungi partnership for enhanced bioremediation of As | Bioaccumulation | 87.0 µg g−1 | [118] |
| Location | Involved Functional Groups in Metal Uptake | References |
|---|---|---|
| Cell organelles | Cot1/Zrc1 transporters transfer to vacuoles. e.g., S. cerevisiae | [174] |
| Formation of vesicles (Zincosomes) e.g., S. cerevisiae | [175] | |
| Cytoplasm | Metal oxalates e.g., Beauveria caledonica | [176] |
| Precipitate of Cu and AsV e.g., Aspergillus foetidus | [177] | |
| PO3− precipitates e.g., Penicillium sp. PT1 | [178] | |
| Extracellular | Electronegative interaction with spores’ exterior surface. e.g., Mucor hiemalis | [65] |
| PO3− binding ligands e.g., M. hiemalis | [65] | |
| –OH and –C=O groups e.g., Penicillium chrysogenum XJ-1 | [179] | |
| CH-OH and OH/NH2 functional groups e.g., A. fumigates | [180] |
| Genes | Products | Gene Source | Principal Outcomes | References |
|---|---|---|---|---|
| OsNRAMP1 | Natural resistance-macrophage protein transporter | O. sativa | The transgenic line’s shoots and roots had a two-fold greater concentration of As than the WT. | [191] |
| PvTIP4;1 | Transporter of the TIP | Pterisvittata | Significant increase in As accumulation was linked to increasing vulnerability to As stress in transgenic lines as compared to the WT. | [192] |
| PvACR3;1 | Arsenic compound resistance 3 (AsIII antiporter) | P.vittata | In comparison to the WT line, the transgenic line displayed increased As retention in roots and decreased As translocation to shoots. | [193] |
| AtABCC1 and AtPCS1 | ATP binding cassette subfamily C transporter and PC synthase | A. thaliana | Overexpression of both genes at the same time resulted in enhanced As complexation by PCs and improved transport to the vacuole. | [194] |
| AtPCS1 | PC synthase | A. thaliana | The transgenic line has a much higher tolerance to As when compared to the WT. | [195] |
| AtPCS1 | PC synthase | A. thaliana | Arsenic resistance was observed in plants overexpressing AtPCS1 from a strong constitutive Arabidopsis actin regulatory sequence (A2). | [196] |
| AtPCs1 | PC synthase | A. thaliana | Plants that overexpressed AtPCS1 in the cytoplasm were more resistant to As than WT plants. When ATPCS1 was directed to the chloroplast, the effects were the total opposite. | [197] |
| ACR2 | Arsenate reductase | A. thaliana | The transformant has a higher tolerance to As and accumulates less of it than the WT. | [198] |
| GSH1 and AsPCS1 | γ-glutamylcysteine synthetase and Phytochelatin synthase | S. cerevisiae and Allium sativum | Tolerance to As has increased in both single-gene and double-gene transformants. Dual gene transformants outperform single gene transformants. | [199] |
| GSH1 and AsPCS1 | γ-glutamylcysteine synthetase and Phytochelatin synthase | Escherichiacoli constructs | Increased resistance to As in transgenics, but with a larger ability for accumulation than the WT. | [200] |
| AsPCS1 and CePCS1 | Phytochelatin synthase | A. thaliana and Caenorhabditis elegans | Increased in the lines that co-express both genes, there is As-tolerance. | [201] |
| AtPCS1 | PC synthase | P. vittata | Enhanced As tolerance in transgenic lines as a result of increased AsV reduction and improved AsIII efflux via aquaglyceroporin regulation. | [49] |
| OsGrx_C7 and OsGrx_C2.1 | Glutaredoxin | O. sativa | In comparison to the WT, transgenic expression of OsGrxs resulted in much lower As accumulation in seeds and shoots, as well as higher As tolerance. | [202] |
| CrarsM | SAM-methyltransferase | Chlamydomonas reinhardtii | The transgenic line had a considerable capacity to methylate As, however, this was accompanied by increased vulnerability to AsIII. | [203] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, A.; Dubey, P.; Kumar, M.; Roy, A.; Sharma, D.; Khan, M.M.; Bajpai, A.B.; Shukla, R.P.; Pathak, N.; Hasanuzzaman, M. Consequences of Arsenic Contamination on Plants and Mycoremediation-Mediated Arsenic Stress Tolerance for Sustainable Agriculture. Plants 2022, 11, 3220. https://doi.org/10.3390/plants11233220
Gupta A, Dubey P, Kumar M, Roy A, Sharma D, Khan MM, Bajpai AB, Shukla RP, Pathak N, Hasanuzzaman M. Consequences of Arsenic Contamination on Plants and Mycoremediation-Mediated Arsenic Stress Tolerance for Sustainable Agriculture. Plants. 2022; 11(23):3220. https://doi.org/10.3390/plants11233220
Chicago/Turabian StyleGupta, Anmol, Priya Dubey, Manoj Kumar, Aditi Roy, Deeksha Sharma, Mohammad Mustufa Khan, Atal Bihari Bajpai, Ravi Prakash Shukla, Neelam Pathak, and Mirza Hasanuzzaman. 2022. "Consequences of Arsenic Contamination on Plants and Mycoremediation-Mediated Arsenic Stress Tolerance for Sustainable Agriculture" Plants 11, no. 23: 3220. https://doi.org/10.3390/plants11233220









