Hydrolats from Humulus lupulus and Their Potential Activity as an Organic Control for Varroa destructor
Abstract
:1. Introduction
2. Results
2.1. Chemical Composition
2.2. Mite and Bee Lethality Test
2.3. Attractivity Test
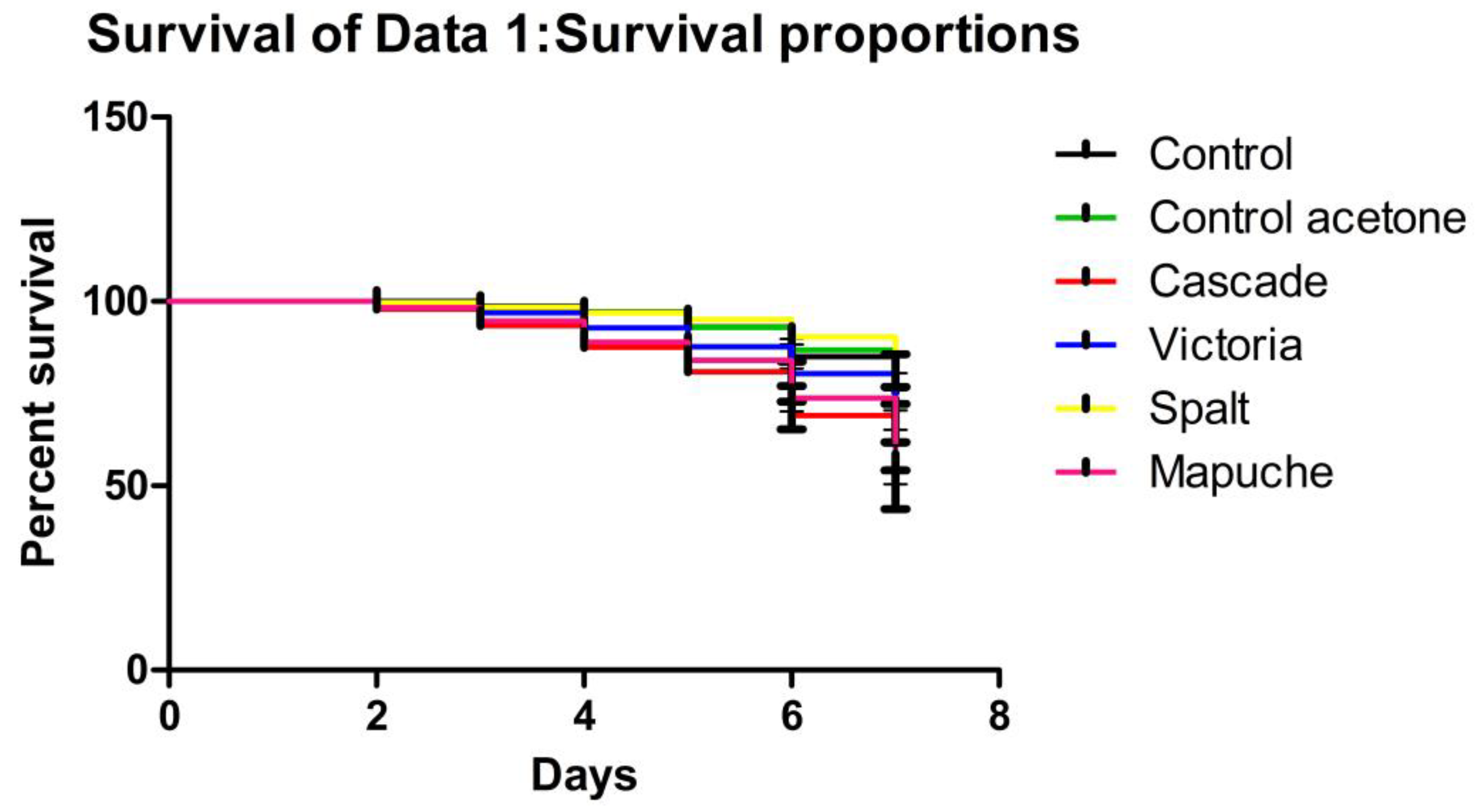
2.4. Larvae Lethality Test
3. Discussion
4. Materials and Methods
4.1. Mites and Bees
4.2. Plant Material
4.3. Volatile Compounds
GC-MS Analysis
4.4. Total Content of Phenolic Compounds
4.4.1. Total Flavonoid Content
4.4.2. Total Saponin Content
4.5. Mite and Bee Lethality Test
4.6. Attractivity Test
4.7. Honeybee Larvae Lethality Test
4.8. Statistical Analysis
4.8.1. Analysis of Mites and Bee Lethality Test
4.8.2. Analysis of Attractivity
4.8.3. Analysis of Honeybee Larvae Lethality Tests and Weight
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Linnaeus, C. Systema Naturae; Laurentii Salvii: Stockholm, Sweden, 1758; Volume 1, p. 532. [Google Scholar]
- Garibaldi, L.A.; Steffan-Dewenter Winfree, R.; Aizen, M.A.; Bommarco, R.; Cunningham SAKremen, C.; Carvalheiro, L.G.; Harder, L.D.; Afik, O. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef] [PubMed]
- Basualdo, M.; Cavigliasso, P.; Samuel de Avila, R.; Aldea-Sánchez, P.; Correa-Benítez, A.; Martínez Harms, J.; Ramos, A.K.; Rojas-Bravo, V.; Salvarrey, S. Current status and economic value of insect-pollinated dependent crops in Latin America. Ecol. Econ. 2022, 196, 107395. [Google Scholar] [CrossRef]
- Anderson, D.L.; Trueman, J.W.H. Varroa jacobsoni (Acari: Varroidae) is more than one species. Exp. Appl. Acarol. 2000, 24, 165–189. [Google Scholar] [CrossRef] [PubMed]
- De la Rúa, P.; Jaffé, R.; Dall’Olio, R.; Muñoz, I.; Serrano, J. Biodiversity, conservation and current threats to European honeybees. Apidologie 2009, 40, 263–284. [Google Scholar] [CrossRef] [Green Version]
- Maggi, M.; Tourn, E.; Negri, P.; Szawarski, N.; Marconi, A.; Gallez, L.; Medici, S.; Ruffonengo, S.; Brasesco, C.; De Feudis, L.; et al. A new formulation of oxalic acid for Varroa destructor control applied in Apis mellifera colonies in the presence of brood. Apidologie 2016, 47, 596–605. [Google Scholar] [CrossRef] [Green Version]
- Peng YS, C.; Fang, Y.; Xu, S.; Ge, L.; Nasr, M.E. Response of foster Asian honeybee (Apis cerana Fabr.) colonies to the brood of European honeybee (Apis mellifera L.) infested with parasitic mite, Varroa jacobsoni Oudemans. J. Invertebr. Pathol. 1987, 49, 259–264. [Google Scholar] [CrossRef]
- Milani, N.; Barbattini, R. Effectiveness of Apistan (fluvalinate) in the control of Varroa jacobsoni Andemans and its tolerance by Apis mellifera L. Apicoltura 1988, 3–58. [Google Scholar]
- Milani, N.; Iob, M. Plastic strips containing organophosphorus acaricides to control Varroa jacobsonii: A preliminary experiment. Am. Bee J. 1998. [Google Scholar]
- Rosenkranz, P.; Aumeier, P.; Ziegelmann, B. Biology and control of Varroa destructor. J. Invertebr. Pathol. 2010, 103, S96–S119. [Google Scholar] [CrossRef]
- Johnson, R.M.; Pollock, H.S.; Berenbaum, M.R. Synergistic interactions between in-hive miticides in Apis mellifera. J. Econ. Entomol. 2009, 102, 474–479. [Google Scholar] [CrossRef]
- Boncristiani, H.; Underwood, R.; Schwarz, R.; Evans, J.D.; Pettis, J. Direct effect of acaricides on pathogen loads and gene expression levels in honey bees Apis mellifera. J. Insect Physiol. 2012, 58, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Tihelka, E. Effects of synthetic and organic acaricides on honey bee health: A review. Slov. Vet. Res. 2018, 55, 114–140. [Google Scholar] [CrossRef]
- Medici, S.K.; Sarlo, E.G.; Porrini, M.P.; Braunstein, M.; Eguaras, M.J. Genetic variation and widespread dispersal of Nosema ceranae in Apis mellifera apiaries from Argentina. Parasitol. Res. 2012, 110, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Medici, S.K.; Maggi, M.D.; Sarlo, E.G.; Ruffinengo, S.; Marioli, J.M.; Eguaras, M.J. The presence of synthetic acaricides in beeswax and its influence on the development of resistance in Varroa destructor. J. Apic. Res. 2015, 54, 267–274. [Google Scholar] [CrossRef]
- Dai, P.; Jack, C.J.; Mortensen, A.N.; Bustamante, T.A.; Ellis, J.D. Chronic toxicity of amitraz, coumaphos and fluvalinate to Apis mellifera L. larvae reared in vitro. Sci Rep. 2018, 8, 5635. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Liao, C.; He, X.; Zhang, L.; Yan, W.; Zeng, Z. Sublethal fluvalinate negatively affect the development and flight capacity of honeybee (Apis mellifera L.) workers. Environ. Res. 2022, 203, 111836. [Google Scholar] [CrossRef]
- Spreafico, M.; Eördegh, F.R.; Bernardinelli, I.; Colombo, M. First detection of strains of Varroa destructor resistant to coumaphos. Results of laboratory tests and field trials. Apidologie 2001, 32, 49–55. [Google Scholar] [CrossRef] [Green Version]
- Pettis, J.S. A scientific note on Varroa destructor resistance to coumaphos in the United States. Apidologie 2004, 35, 91–92. [Google Scholar] [CrossRef] [Green Version]
- Martin, S.J. Acaricide (pyrethroid) resistance in Varroa destructor. Bee World 2004, 85, 67–69. [Google Scholar] [CrossRef]
- Maggi, M.D.; Sardella, N.H.; Ruffinengo, S.R.; Eguaras, M.J. Morphotypes of Varroa destructor collected in Apis mellifera colonies from different geographic locations of Argentina. Parasitol. Res. 2009, 105, 1629–1636. [Google Scholar] [CrossRef]
- Maggi, M.; Damiani, N.; Ruffinengo, S.; De Jong, D.; Principal, J.; Eguaras, M. Brood cell size of Apis mellifera modifies the reproductive behavior of Varroa destructor. Exp. Appl. Acarol. 2010, 50, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Maggi, M.; Gende, L.; Russo, K.; Fritz, R.; Eguaras, M. Bioactivity of Rosmarinus officinalis essential oils against Apis mellifera, Varroa destructor and Paenibacillus larvae related to the drying treatment of the plant material. Nat. Prod. Res. 2011, 25, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Mitton, G.A.; Quintana, S.; Giménez Martínez, P.; Mendoza, Y.; Ramallo, G.; Brasesco, C.; Villalba, A.; Eguaras, M.J.; Maggi, M.D.; Ruffinengo, S.R. First record of resistance to flumethrin in a Varroa population from Uruguay. J. Apic. Res. 2016, 55, 422–427. [Google Scholar] [CrossRef]
- Mitton, G.A.; Szawarski, N.; Ramos, F.; Fuselli, S.; Meroi Arcerito, F.R.; Eguaras, M.J.; Ruffinengo, S.R.; Maggi, M.D. Varroa destructor: When reversion to coumaphos resistance does not happen. J. Apic. Res. 2018, 57, 536–540. [Google Scholar] [CrossRef]
- Giovenazzo, P.; Dubreuil, P. Evaluation of spring organic treatments against Varroa destructor (Acari: Varroidae) in honey bee Apis mellifera (Hymenoptera: Apidae) colonies in eastern Canada. Exp. Appl. Acarol. 2011, 55, 65–76. [Google Scholar] [CrossRef]
- Satta, A.; Floris, I.; Eguaras, M.; Cabras, P.; Garau, V.L.; Melis, M. Formic acid-based treatments for control of Varroa destructor in a Mediterranean area. J. Econ. Entomol. 2005, 98, 267–273. [Google Scholar] [CrossRef]
- Isman, M.B. Plant essential oils for pest and disease management. Crop Prot. 2000, 19, 603–608. [Google Scholar] [CrossRef]
- Ruffinengo, S.R.; Maggi, M.D.; Marcangeli, J.A.; Eguaras, M.J.; Principal, J.; Barrios, C.; De Piano, F.; Mitton, G. Integrated Pest Management to control Varroa destructor and its implications to Apis mellifera colonies. Zootec. Trop. 2014, 32, 149–168. [Google Scholar]
- Jack, C.J.; Ellis, J.D. Integrated Pest Management Control of Varroa destructor (Acari: Varroidae), the Most Damaging Pest of (Apis mellifera L.(Hymenoptera: Apidae)) Colonies. J. Insect Sci. 2021, 21, 6. [Google Scholar] [CrossRef]
- Ruffinengo, S.; Eguaras, M.; Floris, I.; Faverin, C.; Bailac, P.; Ponzi, M. LD50 and repellent effects of essential oils from Argentinian wild plant species on Varroa destructor. J. Econ. Entomol. 2005, 98, 651–655. [Google Scholar] [CrossRef]
- Conti, B.; Bocchino, R.; Cosci, F.; Ascrizzi, R.; Flamini, G.; Bedini, S. Essential oils against Varroa destructor: A soft way to fight the parasitic mite of Apis mellifera. J. Apic. Res. 2020, 59, 774–782. [Google Scholar] [CrossRef]
- Wallace, R.J. Antimicrobial properties of plant secondary metabolites. Proc. Nutr. Soc. 2004, 63, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Aivazi, A.A.; Vijayan, V.A. Larvicidal activity of oak Quercus infectoria Oliv. (Fagaceae) gall extracts against Anopheles stephensi Liston. Parasitol. Res. 2009, 104, 1289–1293. [Google Scholar] [CrossRef] [PubMed]
- Banchio, A.J.; Brady, J.F. Accelerated stokesian dynamics: Brownian motion. J. Chem. Phys. 2003, 118, 10323–10332. [Google Scholar] [CrossRef] [Green Version]
- Ciccia, G.; Coussio, J.; Mongelli, E. Insecticidal activity against Aedes aegypti larvae of some medicinal South American plants. J. Ethnopharmacol. 2000, 72, 185–189. [Google Scholar] [CrossRef]
- Jbilou, R.; Ennabili, A.; Sayah, F. Insecticidal activity of four medicinal plant extracts against Tribolium castaneum (Herbst)(Coleoptera: Tenebrionidae). Afr. J. Biotechnol. 2006, 5, 10. [Google Scholar]
- Bedini, S.; Flamini, G.; Girardi, J.; Cosci, F.; Conti, B. Not just for beer: Evaluation of spent hops (Humulus lupulus L.) as a source of eco-friendly repellents for insect pests of stored foods. J. Pest Sci. 2015, 88, 583–592. [Google Scholar] [CrossRef]
- Bedini, S.; Flamini, G.; Cosci, F.; Ascrizzi, R.; Benelli, G.; Conti, B. Cannabis sativa and Humulus lupulus essential oils as novel control tools against the invasive mosquito Aedes albopictus and fresh water snail Physella acuta. Ind. Crop. Prod. 2016, 85, 318–323. [Google Scholar] [CrossRef] [Green Version]
- DeGrandi-Hoffman, G.; Ahumada, F.; Probasco, G.; Schantz, L. The effects of beta acids from hops (Humulus lupulus) on mortality of Varroa destructor (Acari: Varroidae). Exp. Appl. Acarol. 2012, 58, 407–421. [Google Scholar] [CrossRef] [Green Version]
- Rademacher, E.; Harz, M.; Schneider, S. The development of HopGuard® as a winter treatment against Varroa destructor in colonies of Apis mellifera. Apidologie 2015, 46, 748–759. [Google Scholar] [CrossRef]
- Reher, T.; Van Kerckvoorde, V.; Verheyden, L.; Wenseleers, T.; Beliën, T.; Bylemans, D.; Martens, J.A. Evaluation of hop (Humulus lupulus) as a repellent for the management of Drosophila suzukii. Crop Prot. 2019, 124, 104839. [Google Scholar] [CrossRef]
- Iglesias, A.; Mitton, G.; Szawarski, N.; Cooley, H.; Ramos, F.; Meroi Arcerito, F.R.; Brasesco, C.; Ramirez, C.; Gende, L.; Eguaras, M.J.; et al. Essential oils from Humulus lupulus as novel control agents against Varroa destructor. Ind. Crop. Prod. 2020, 158, 113043. [Google Scholar] [CrossRef]
- Iglesias, A.; Gimenez Martinez, P.; Ramirez, C.; Mitton, G.; Meroi Acerito, F.R.; Fangio, M.F.; Churio, M.S.; Fuselli, S.; Fanovich, A.; Eguaras, M.; et al. Valorization of hop leaves for development of eco-friendly bee pesticides. Apidologie 2021, 52, 186–198. [Google Scholar] [CrossRef]
- Zanoli, P.; Zavatti, M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Sägesser, M.; Deinzer, M. HPLC-ion spray-tandem mass spectrometry of flavonol glycosides in hops. J. Am. Soc. Brew. Chem. 1996, 54, 129–134. [Google Scholar] [CrossRef]
- Gorissen, H.; Bellinck, C.; Vancraenenbroeck, R.; Lontie, R. Separation and identification of (+)-gallocatechine in hops. Arch. Int. Physiol. Biochim. 1968, 76, 932–934. [Google Scholar]
- Malizia, R.A.; Molli, J.S.; Cardell, D.A.; Grau, R.J.A. Essential oil of hop cones (Humulus lupulus L.). J. Essent. Oil Res. 1999, 11, 13–15. [Google Scholar] [CrossRef]
- Eri, S.; Khoo, B.K.; Lech, J.; Hartman, T.G. Direct thermal desorptiongas chromatography and gas chromatography-mass spectrometry profiling of hop (Humulus lupulus L.) essential oils in support of varietal characterization. J. Agric. Food Chem. 2000, 48, 1140–1149. [Google Scholar] [CrossRef]
- Knez Hrnčič, M.; Španinger, E.; Košir, I.J.; Knez, Ž.; Bren, U. Hop compounds: Extraction techniques, chemical analyses, antioxidative, antimicrobial, and anticarcinogenic effects. Nutrients 2019, 11, 257. [Google Scholar] [CrossRef] [Green Version]
- Babaz, Y.; Guezoul, O.; Bouras, N. Evaluation under Laboratory Conditions of the Efficacy of Four Extracts of Spontaneous Plants from the Mzab Valley (Algeria) against the Date Palm Mite (Oligonychus afrasiaticus). Tunis. J. Plant Prot. 2021, 16, 29–41. [Google Scholar] [CrossRef]
- Catão, H.C.R.M.; Aquino, C.F.; Sales, N.D.L.P.; da Silva Rocha, F.; Caixeta, F.; Civil, N. Hydrolats and extracts vegetable action on quality of stored castor bean seeds in non-controlled conditions. Rev. Bras. Cienc. Agrar. 2018, 13, 1–7. [Google Scholar] [CrossRef]
- Aćimović, M.G.; Tešević, V.V.; Smiljanić, K.T.; Cvetković, M.T.; Stanković, J.M.; Kiprovski, B.M.; Sikora, V.S. Hydrolates: By-products of essential oil distillation: Chemical composition, biological activity and potential uses. Adv. Technol. 2020, 9, 54–70. [Google Scholar] [CrossRef]
- Paolini, J.; Leandri, C.; Desjobert, J.M.; Barboni, T.; Costa, J. Comparison of liquid–liquid extraction with headspace methods for the characterization of volatile fractions of commercial hydrolats from typically Mediterranean species. J. Chromatogr. A. 2008, 1193, 37–49. [Google Scholar] [CrossRef] [PubMed]
- Vilarem, C.; Piou, V.; Vogelweith, F.; Vétillard, A. Varroa destructor from the laboratory to the field: Control, biocontrol and ipm perspectives—A review. Insects 2021, 12, 800. [Google Scholar] [CrossRef]
- Zaitoon, A.A. Evaluation of certain plant extracts for the control of parasitic bee mites, Varroa jacobsoni. J. Pest. Cont. Environ. Sci. 2001, 9, 77–88. [Google Scholar]
- Damiani, N.; Gende, L.B.; Maggi, M.D.; Palacios, S.; Marcangeli, J.A.; Eguaras, M.J. Repellent and acaricidal effects of botanical extracts on Varroa destructor. Parasitol. Res. 2011, 108, 79–86. [Google Scholar] [CrossRef]
- Armah, C.N.; Mackie, A.R.; Roy, C.; Price, K.; Osbourn, A.E.; Bowyer, P.; Ladha, S. The membrane-permeabilizing effect of avenacin A-1 involves the reorganization of bilayer cholesterol. Biophys. J. 1999, 76, 281–290. [Google Scholar] [CrossRef]
- De Geyter, E.; Swevers, L.; Soin, T.; Geelen, D.; Smagghe, G. Saponins do not affect the ecdysteroid receptor complex but cause membrane permeation in insect culture cell lines. J. Insect Physiol. 2012, 58, 18–23. [Google Scholar] [CrossRef]
- Mahmoudi, R.; Amini, K.; Hosseinirad, H.; Valizadeh, S.; Kabudari, A.; Aali, E. Phytochemistry and insecticidal effect of different parts of Melissa officinalis on Tetranychus urticae. Res. J. Pharmacogn. 2017, 4, 49–56. [Google Scholar]
- Oh, M.S.; Yang, J.Y.; Kim, M.G.; Lee, H.S. Acaricidal activities of β-caryophyllene oxide and structural analogues derived from Psidium cattleianum oil against house dust mites. Pest Manag. Sci. 2014, 70, 757–762. [Google Scholar] [CrossRef]
- de Melo, J.P.R.; da Camara, C.A.G.; da Silva Lima, G.; de Moraes, M.M.; Alves, P.B. Acaricidal properties of the essential oil from Aristolochia trilobata and its major constituents against the two-spotted spider mite (Tetranychus urticae). Can. J. Plant Sci. 2018, 98, 1342–1348. [Google Scholar] [CrossRef]
- Ribeiro, N.C.; Camara, C.A.; Melo, J.P.; Moraes, M.M. Acaricidal properties of essential oils from agro-industrial waste products from citric fruit against Tetranychus urticae. J. Appl. Entomol. 2019, 143, 731–743. [Google Scholar] [CrossRef]
- Bava, R.; Castagna, F.; Piras, C.; Palma, E.; Cringoli, G.; Musolino, V.; Lupia, C.; Perri, M.R.; Statti, G.; Britti, D.; et al. In Vitro Evaluation of Acute Toxicity of Five Citrus spp. Essential Oils towards the Parasitic Mite Varroa destructor. Pathogens 2021, 10, 1182. [Google Scholar] [CrossRef] [PubMed]
- Brasesco, C.; Gende, L.; Negri, P.; Szawarski, N.; Iglesias, A.; Eguaras, M.; Ruffinengo, S.; Maggi, M. Assessing in Vitro acaricidal effect and joint action of a binary mixture between essential oil compounds (Thymol, Phellandrene, Eucalyptol, Cinnamaldehyde, Myrcene, Carvacrol) over ectoparasitic mite Varroa destructor (Acari: Varroidae). J. Apic. Sci. 2017, 61, 203–215. [Google Scholar] [CrossRef] [Green Version]
- Colin, M.B.; Ciavarella, F.; Otero-Colina, G.; Belzunces, L.P. A method for characterizing the biological activity of essential oil against Varroa jacobsoni. In New Perspectives on Varroa; Matheson, A., Ed.; Internacional Bee Research Association: Cardiff, Wales, 1994; pp. 109–114. [Google Scholar]
- Fernández, N.J.; Damiani, N.; Podaza, E.A.; Martucci, J.F.; Fasce, D.; Quiroz, F.; Meretta, P.E.; Quintana, S.; Eguaras, M.J.; Gende, L.B. Laurus nobilis L. Extracts against Paenibacillus larvae: Antimicrobial activity, antioxidant capacity, hygienic behavior and colony strength. Saudi J. Biol. Sci. 2019, 26, 906–912. [Google Scholar] [CrossRef]
- Larran, S.; Ringuelet, J.A.; Carranza, M.R.; Henning, C.P.; Re, M.S.; Cerimele, E.L.; Urrutía, M.I. In vitro fungistatic effect of essential oils against Ascosphaera apis. J. Essent. Oil Res. 2001, 13, 122–124. [Google Scholar] [CrossRef]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am. J. Enol. Vitic. 1965, 16, 144–158. [Google Scholar]
- Lamuela-Raventós, R.M. Folin–Ciocalteu method for the measurement of total phenolic content and antioxidant capacity. In Measurement of Antioxidant Activity & Capacity: Recent Trends and Applications, 1st ed.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2018. [Google Scholar] [CrossRef]
- Woisky, R.G.; Salatino, A. Analysis of propolis: Some parameters and procedures for chemical quality control. Analysis of propolis: Some parameters and procedures for chemical quality control. J. Apic. Res. 1998, 37, 99–105. [Google Scholar] [CrossRef]
- Le, A.V.; Parks, S.E.; Nguyen, M.H.; Roach, P.D. Improving the Vanillin-Sulphuric Acid Method for Quantifying Total Saponins. Technologies 2018, 6, 84. [Google Scholar] [CrossRef] [Green Version]
- Maggi, M.D.; Ruffinengo, S.R.; Gende, L.B.; Eguaras, M.J.; Sardella, N.H. LC50 baseline levels of amitraz, coumaphos, fluvalinate and flumethrin in populations of Varroa destructor from Buenos Aires Province, Argentina. J. Apic. Res. 2008, 47, 292–295. [Google Scholar] [CrossRef]
- Damiani, N.; Maggi, M.D.; Gende, L.B.; Faverin, C.; Eguaras, M.J.; Marcangeli, J.A. Evaluation of the toxicity of a propolis extract on Varroa destructor (Acari: Varroidae) and Apis mellifera (Hymenoptera: Apidae). J. Apic. Res. 2010, 49, 257–264. [Google Scholar] [CrossRef]
- Damiani, N.; Fernández, N.J.; Porrini, M.P.; Gende, L.B.; Álvarez, E.; Buffa, F.; Brasesco, C.; Maggi, M.D.; Marcangeli, J.A.; Eguaras, M.J. Laurel leaf extracts for honeybee pest and disease management: Antimicrobial, microsporicidal, and acaricidal activity. Parasitol. Res. 2014, 113, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Medrzycki, P.; Giffard, H.; Aupinel, P.; Belzunces, L.P.; Chauzat, M.P.; Claßen, C.; Colin, M.E.; Dupont, T.; Girolami, V.; Johnson, R.; et al. Standard methods for toxicology research in Apis mellifera. J. Apic. Res. 2013, 52, 1–60. [Google Scholar] [CrossRef] [Green Version]
- Aupinel, P.; Fortini, D.; Dufour, H.; Tasei, J.; Michaud, B.; Odoux, J.; Pham-Delegue, M. Improvement of artificial feeding in a standard in vitro method for rearing Apis mellifera larvae. Bull. Insectol. 2005, 58, 107. [Google Scholar]
- Smodiš Škerl, M.I.; Rivera-Gomis, J.; Tlak Gajger, I.; Bubnič, J.; Talakić, G.; Formato, G.; Baggio, A.; Mutinelli, F.; Tollenaers, W.; Laget, D.; et al. Efficacy and Toxicity of VarroMed® Used for Controlling Varroa destructor Infestation in Different Seasons and Geographical Areas. Appl. Sci. 2021, 11, 8564. [Google Scholar] [CrossRef]
- Hewlett, P.S.; Plackett, R.L. An Introduction to the Interpretation of Quantal Responses in Biology [Pesticides]; University Park Press: University Park, PA, USA, 1979. [Google Scholar]
- Stokes, M.E.; Davis, C.S.; Koch, G.G. Categorical Data Analysis Using the SAS System; SAS Institute. Inc.: Cary, NC, USA, 1995; pp. 34–35. [Google Scholar]

| Compound | KI (Exp) | KI (lit) | Mapuche | Victoria | Cascade | Spalt |
|---|---|---|---|---|---|---|
| % | % | % | ||||
| Pentyl Acetate | 859 | 859 | 20.07 | |||
| Beta-Linalool | 1088 | 1086 | 7.31 | 10.09 | 49.49 | 44.20 |
| Trans-Linalool Oxide | 1100 | 1102 | 1.51 | 1.95 | 5.79 | |
| NI | 1120 | - | 1.43 | 2.47 | 4.05 | |
| (+)-Limonene Oxide | 1134 | 1138 | 2.21 | 2.27 | ||
| Isothujol | 1160 | 1157 | 1.32 | 1.44 | ||
| (+)-Alpha-Terpineol | 1185 | 1189 | 7.24 | 1.27 | 4.51 | |
| Methyl 8-Nonynoate | 1199 | 1200 | 2.08 | |||
| NI | 1277 | - | 2.05 | 2.87 | ||
| Limonene diepoxyde | 1294 | 1294 | 6.49 | 6.25 | ||
| 9-oxadiciclo[3.3.1]nonane-2,7-diol | 1346 | 1347 | 1.62 | 1.13 | ||
| Tetradecane | 1399 | 1399 | 17.43 | |||
| (−)Beta-Caryophyllene | 1431 | 1430 | 18.77 | |||
| Alpha-Caryophyllene | 1469 | 1463 | 9.60 | |||
| Globulol | 1576 | 1576 | 5.94 | 9.74 | ||
| Caryophyllene, Epoxide | 1597 | 1594 | 58.56 | 56.02 | 16.26 | 3.58 |
| NI | 1599 | - | 1.96 | |||
| Humuladienone | 1608 | 1607 | 2.44 | 2.45 | ||
| Octadecano | 1800 | 1800 | 11.13 | |||
| 2,6,10,14-tetrametilhexadecane | 1815 | 1815 | 10.75 | |||
| NI | 1860 | - | 1.88 | 2.42 |
| Hydrolats | Saponins | Flavonoids | Polyphenols | Antioxidant Capacity |
|---|---|---|---|---|
| AO µg/mL | Q µg/mL | AG µg/mL | TROLOX µg/mL | |
| Victoria | 648.7503 | 0.2507 | 133.2043 | 361.2587 |
| Cascade | 307.5998 | 0.0631 | 190.7868 | 217.3193 |
| Mapuche | 458.9114 | 0.0082 | 82.9924 | 157.8787 |
| Spalt | 129.9626 | 0.3261 | 210.7487 | 313.4731 |
| Hydrolats | Mites LC50 (µL/mL) | |
|---|---|---|
| 24 h | 48 h | |
| Cascade | 117.9 (47.6–292.0) | 35.2 (19.6–63.0) |
| Victoria | 16.1 (6.8–38.1) | 1.3 (0.7–2.2) |
| Spalt | 114.3 (25.9–503.7) | 21.5 (7.6–60.7) |
| Mapuche | 30.6 (9.5–98.5) | not estimated |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iglesias, A.E.; Fuentes, G.; Mitton, G.; Ramos, F.; Brasesco, C.; Manzo, R.; Orallo, D.; Gende, L.; Eguaras, M.; Ramirez, C.; et al. Hydrolats from Humulus lupulus and Their Potential Activity as an Organic Control for Varroa destructor. Plants 2022, 11, 3329. https://doi.org/10.3390/plants11233329
Iglesias AE, Fuentes G, Mitton G, Ramos F, Brasesco C, Manzo R, Orallo D, Gende L, Eguaras M, Ramirez C, et al. Hydrolats from Humulus lupulus and Their Potential Activity as an Organic Control for Varroa destructor. Plants. 2022; 11(23):3329. https://doi.org/10.3390/plants11233329
Chicago/Turabian StyleIglesias, Azucena Elizabeth, Giselle Fuentes, Giulia Mitton, Facundo Ramos, Constanza Brasesco, Rosa Manzo, Dalila Orallo, Liesel Gende, Martin Eguaras, Cristina Ramirez, and et al. 2022. "Hydrolats from Humulus lupulus and Their Potential Activity as an Organic Control for Varroa destructor" Plants 11, no. 23: 3329. https://doi.org/10.3390/plants11233329
APA StyleIglesias, A. E., Fuentes, G., Mitton, G., Ramos, F., Brasesco, C., Manzo, R., Orallo, D., Gende, L., Eguaras, M., Ramirez, C., Fanovich, A., & Maggi, M. (2022). Hydrolats from Humulus lupulus and Their Potential Activity as an Organic Control for Varroa destructor. Plants, 11(23), 3329. https://doi.org/10.3390/plants11233329






