Cisplatin-Induced Nausea and Vomiting: Effect of Herbal Medicines
Abstract
1. Introduction
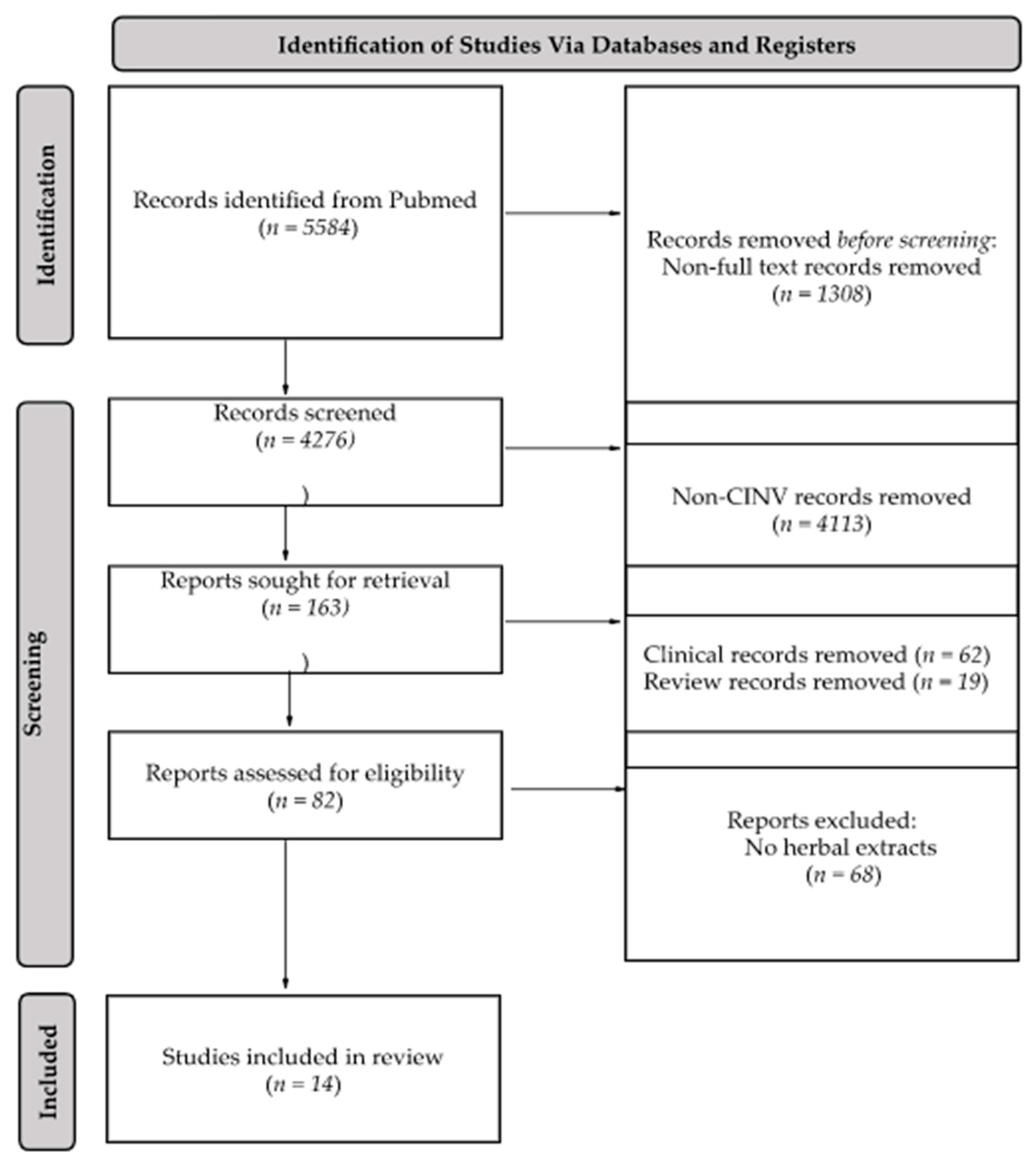
2. Results
2.1. Kaolin Consumption
2.2. Retching and Vomiting
2.3. Food Intake
2.4. Body Weight
2.5. Herbal Medicines Used in the Studies
2.6. Mechanisms of Action
3. Discussion
4. Materials and Methods
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Galanski, M.; Jakupec, M.A.; Keppler, B.K. Update of the preclinical situation of anticancer platinum complexes: Novel design strategies and innovative analytical approaches. Curr. Med. Chem. 2005, 12, 2075–2094. [Google Scholar] [CrossRef] [PubMed]
- Hannon, M.J. Metal-based anticancer drugs: From a past anchored in platinum chemistry to a post-genomic future of diverse chemistry and biology. Pure Appl. Chem. 2007, 79, 2243–2261. [Google Scholar] [CrossRef]
- Ho, G.Y.; Woodward, N.; Coward, J.I.G. Cisplatin versus carboplatin: Comparative review of therapeutic management in solid malignancies. Crit. Rev. Oncol./Hematol. 2016, 102, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Siddik, Z.H. Cisplatin: Mode of cytotoxic action and molecular basis of resistance. Oncogene 2003, 22, 7265–7279. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, R.B.; Solomon, M.J.; Varshavsky, A.; Lippard, S.J. In vivo effects of cis- and trans-diamminedichloroplatinum(II) on SV40 chromosomes: Differential repair, DNA-protein crosslinking, and inhibition of replication. Biochemistry 1985, 24, 7533–7540. [Google Scholar] [CrossRef]
- Achkar, I.W.; Abdulrahman, N.; Al-Sulaiti, H.; Joseph, J.M.; Uddin, S.; Mraiche, F. Cisplatin based therapy: The role of the mitogen activated protein kinase signaling pathway. J. Transl. Med. 2018, 16, 96. [Google Scholar] [CrossRef] [PubMed]
- Astolfi, L.; Ghiselli, S.; Guaran, V.; Chicca, M.; Simoni, E.; Olivetto, E.; Lelli, G.; Martini, A. Correlation of adverse effects of cisplatin administration in patients affected by solid tumours: A retrospective evaluation. Oncol. Rep. 2013, 29, 1285–1292. [Google Scholar] [CrossRef] [PubMed]
- Oun, R.; Moussa, Y.E.; Wheate, N.J. The side effects of platinum-based chemotherapy drugs: A review for chemists. Dalton Trans. 2018, 47, 6645–6653. [Google Scholar] [CrossRef]
- Manohar, S.; Leung, N. Cisplatin nephrotoxicity: A review of the literature. J. Nephrol. 2018, 31, 15–25. [Google Scholar] [CrossRef]
- Pabla, N.; Dong, Z. Cisplatin nephrotoxicity: Mechanisms and renoprotective strategies. Kidney Int. 2008, 73, 994–1007. [Google Scholar] [CrossRef]
- Ghonaim, E.; El-Haggar, S.; Gohar, S. Possible protective effect of pantoprazole against cisplatin-induced nephrotoxicity in head and neck cancer patients: A randomized controlled trial. Med. Oncol. 2021, 38, 108. [Google Scholar] [CrossRef] [PubMed]
- Abd Rashid, N.; Abd Halim, S.A.S.; Teoh, S.L.; Budin, S.B.; Hussan, F.; Adib Ridzuan, N.R.; Abdul Jalil, N.A. The role of natural antioxidants in cisplatin-induced hepatotoxicity. Biomed. Pharmacother. 2021, 144, 112328. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S. Cisplatin: The first metal based anticancer drug. Bioorg. Chem. 2019, 88, 102925. [Google Scholar] [CrossRef] [PubMed]
- Dranitsaris, G.; Molassiotis, A.; Clemons, M.; Roeland, E.; Schwartzberg, L.; Dielenseger, P.; Jordan, K.; Young, A.; Aapro, M. The development of a prediction tool to identify cancer patients at high risk for chemotherapy-induced nausea and vomiting. Ann. Oncol. 2017, 28, 1260–1267. [Google Scholar] [CrossRef] [PubMed]
- Lorusso, V.; Russo, A.; Giotta, F.; Codega, P. Management of Chemotherapy-Induced Nausea and Vomiting (CINV): A Short Review on the Role of Netupitant-Palonosetron (NEPA). Core Evid. 2020, 15, 21–29. [Google Scholar] [CrossRef]
- Inoue, M.; Shoji, M.; Shindo, N.; Otsuka, K.; Miura, M.; Shibata, H. Cohort study of consistency between the compliance with guidelines for chemotherapy-induced nausea and vomiting and patient outcome. BMC Pharm. Toxicol 2015, 16, 5. [Google Scholar] [CrossRef][Green Version]
- Cohen, L.; de Moor, C.A.; Eisenberg, P.; Ming, E.E.; Hu, H. Chemotherapy-induced nausea and vomiting: Incidence and impact on patient quality of life at community oncology settings. Support Care Cancer 2007, 15, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Ballatori, E.; Roila, F. Impact of nausea and vomiting on quality of life in cancer patients during chemotherapy. Health Qual. Life Outcomes 2003, 1, 46. [Google Scholar] [CrossRef] [PubMed]
- Krikorian, S.; Pories, S.; Tataronis, G.; Caughey, T.; Chervinsky, K.; Lotz, M.; Shen, A.H.; Weissmann, L. Adherence to oral chemotherapy: Challenges and opportunities. J. Oncol. Pharm. Pract. 2019, 25, 1590–1598. [Google Scholar] [CrossRef]
- Rao, K.V.; Faso, A. Chemotherapy-induced nausea and vomiting: Optimizing prevention and management. Am. Health Drug Benefits 2012, 5, 232. [Google Scholar] [PubMed]
- Adel, N. Overview of chemotherapy-induced nausea and vomiting and evidence-based therapies. Am. J. Manag. Care 2017, 23, S259–S265. [Google Scholar] [PubMed]
- Piechotta, V.; Adams, A.; Haque, M.; Scheckel, B.; Kreuzberger, N.; Monsef, I.; Jordan, K.; Kuhr, K.; Skoetz, N. Antiemetics for adults for prevention of nausea and vomiting caused by moderately or highly emetogenic chemotherapy: A network meta-analysis. Cochrane Database Syst. Rev. 2021, 9, 1–18. [Google Scholar] [CrossRef]
- Wolfe, R.C.; Bequette, J. Dopamine Receptor Antagonists for the Prevention and Treatment of Postoperative Nausea and Vomiting. J. Perianesth. Nurs. 2021, 36, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Viale, P.H.; Grande, C.; Moore, S. Efficacy and cost: Avoiding undertreatment of chemotherapy-induced nausea and vomiting. Clin. J. Oncol. Nurs. 2012, 16, E133–E141. [Google Scholar] [CrossRef] [PubMed]
- Schwartzberg, L.; Barbour, S.Y.; Morrow, G.R.; Ballinari, G.; Thorn, M.D.; Cox, D. Pooled analysis of phase III clinical studies of palonosetron versus ondansetron, dolasetron, and granisetron in the prevention of chemotherapy-induced nausea and vomiting (CINV). Support Care Cancer 2014, 22, 469–477. [Google Scholar] [CrossRef]
- Hesketh, P.J.; Grunberg, S.M.; Gralla, R.J.; Warr, D.G.; Roila, F.; de Wit, R.; Chawla, S.P.; Carides, A.D.; Ianus, J.; Elmer, M.E.; et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: A multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin--the Aprepitant Protocol 052 Study Group. J. Clin. Oncol. 2003, 21, 4112–4119. [Google Scholar] [CrossRef]
- Köseoglu, V.; Kürekçi, A.E.; Sarici, U.; Atay, A.A.; Ozcan, O. Comparison of the efficacy and side-effects of ondansetron and metoclopramide-diphenhydramine administered to control nausea and vomiting in children treated with antineoplastic chemotherapy: A prospective randomized study. Eur. J. Pediatr. 1998, 157, 806–810. [Google Scholar] [CrossRef]
- Maxion-Bergemann, S.; Wolf, M.; Bornhöft, G.; Matthiessen, P.F.; Wolf, U. Complementary and alternative medicine costs—A systematic literature review. Forsch Komplementmed 2006, 13 (Suppl. 2), 42–45. [Google Scholar] [CrossRef]
- Hoenders, H.J.; Willgeroth, F.C.; Appelo, M.T. Western and alternative medicine: A comparison of paradigms and methods. J. Altern. Complement. Med. 2008, 14, 894–896. [Google Scholar] [CrossRef]
- Su, S.Y.; Muo, C.H.; Morisky, D.E. Use of Chinese medicine correlates negatively with the consumption of conventional medicine and medical cost in patients with uterine fibroids: A population-based retrospective cohort study in Taiwan. BMC Complement. Altern. Med. 2015, 15, 129. [Google Scholar] [CrossRef]
- Asnaashari, S.; Dastmalchi, S.; Javadzadeh, Y. Gastroprotective effects of herbal medicines (roots). Int. J. Food Prop. 2018, 21, 902–920. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.W.; Ha, N.Y.; Kim, J.; Ryu, H.S. Herbal Therapies in Functional Gastrointestinal Disorders: A Narrative Review and Clinical Implication. Front. Psychiatry 2020, 11, 601. [Google Scholar] [CrossRef] [PubMed]
- Palatty, P.L.; Haniadka, R.; Valder, B.; Arora, R.; Baliga, M.S. Ginger in the prevention of nausea and vomiting: A review. Crit. Rev. Food Sci. Nutr. 2013, 53, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Cheng, Q.; Feng, X.; Chen, S.; Li, Y.; Zhang, G.; Nie, K. The Antiemetic Effect of Xiao-Ban-Xia-Tang Formula against Cisplatin-Induced Emesis is Mediated through Inhibition of NLRP3 Inflammasome Activation in a Rat Pica Model. Evid.-Based Complement. Altern. Med. 2020, 2020, 19. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Q.; Feng, X.; Meng, Q.; Li, Y.; Chen, S.; Wang, G.; Nie, K. [6]-gingerol Ameliorates Cisplatin-Induced Pica by Regulating the TPH/MAO-A/SERT/5-HT/5-HT(3) Receptor System in Rats. Drug Des. Dev. Ther. 2020, 14, 4085–4099. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Z.; Basila, D.; Aung, H.H.; Mehendale, S.R.; Chang, W.-T.; McEntee, E.; Guan, X.; Yuan, C.-S. Effects of Ganoderma lucidum extract on chemotherapy-induced nausea and vomiting in a rat model. Am. J. Chin. Med. 2005, 33, 807–815. [Google Scholar] [CrossRef]
- Mehendale, S.; Aung, H.; Wang, A.; Yin, J.J.; Wang, C.Z.; Xie, J.T.; Yuan, C.S. American ginseng berry extract and ginsenoside Re attenuate cisplatin-induced kaolin intake in rats. Cancer Chemother. Pharmacol. 2005, 56, 63–69. [Google Scholar] [CrossRef]
- Mehendale, S.R.; Aung, H.H.; Yin, J.J.; Lin, E.; Fishbein, A.; Wang, C.Z.; Xie, J.T.; Yuan, C.S. Effects of antioxidant herbs on chemotherapy-induced nausea and vomiting in a rat-pica model. Am. J. Chin. Med. 2004, 32, 897–905. [Google Scholar] [CrossRef]
- Aung, H.H.; Dey, L.; Mehendale, S.; Xie, J.T.; Wu, J.A.; Yuan, C.S. Scutellaria baicalensis extract decreases cisplatin-induced pica in rats. Cancer Chemother. Pharmacol. 2003, 52, 453–458. [Google Scholar] [CrossRef]
- Raghavendran, H.R.; Rekha, S.; Shin, J.W.; Kim, H.G.; Wang, J.H.; Park, H.J.; Choi, M.K.; Cho, J.H.; Son, C.G. Effects of Korean ginseng root extract on cisplatin-induced emesis in a rat-pica model. Food Chem. Toxicol. 2011, 49, 215–221. [Google Scholar] [CrossRef]
- Sathyanath, R.; Hanumantha Rao, B.R.; Kim, H.G.; Cho, J.H.; Son, C.G. Saponin and non-saponin fractions of red ginseng ameliorate cisplatin-induced pica in rats. Pharm. Biol. 2013, 51, 1052–1060. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Bi, P.; Zhang, G.; Li, Y.; Chen, S.; Nie, K. Forsythiae Fructus aqueous extract attenuates cisplatin-induced kaolin consumption (pica) by inhibiting NLRP3 inflammasome activation in rats. Biosci. Biotechnol. Biochem. 2021, 85, 2054–2064. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Qian, W.; Qian, Q.; Zhang, W.; Cai, X. Gingerol inhibits cisplatin-induced acute and delayed emesis in rats and minks by regulating the central and peripheral 5-HT, SP, and DA systems. J. Nat. Med. 2020, 74, 353–370. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.-Q.; Yang, Y.-H.; Zhang, G.-L.; Meng, Q.; Cheng, Q.-Q.; Nie, K. RNA-Seq reveals inflammatory mechanisms of Xiao-Ban-Xia-Tang decoction to ameliorate cisplatin-induced emesis in a rat pica model. Biomed. Pharmacother. 2020, 131, 110699. [Google Scholar] [CrossRef]
- Qian, Q.; Chen, W.; Yue, W.; Yang, Z.; Liu, Z.; Qian, W. Antiemetic effect of Xiao-Ban-Xia-Tang, a Chinese medicinal herb recipe, on cisplatin-induced acute and delayed emesis in minks. J. Ethnopharmacol. 2010, 128, 590–593. [Google Scholar] [CrossRef]
- Qian, Q.H.; Yue, W.; Wang, Y.X.; Yang, Z.H.; Liu, Z.T.; Chen, W.H. Gingerol inhibits cisplatin-induced vomiting by down regulating 5-hydroxytryptamine, dopamine and substance P expression in minks. Arch. Pharmacal Res. 2009, 32, 565–573. [Google Scholar] [CrossRef]
- Ullah, I.; Subhan, F.; Rudd, J.A.; Rauf, K.; Alam, J.; Shahid, M.; Sewell, R.D. Attenuation of cisplatin-induced emetogenesis by standardized Bacopa monnieri extracts in the pigeon: Behavioral and neurochemical correlations. Planta Med. 2014, 80, 1569–1579. [Google Scholar] [CrossRef]
- Kanoski, S.E.; Rupprecht, L.E.; Fortin, S.M.; De Jonghe, B.C.; Hayes, M.R. The role of nausea in food intake and body weight suppression by peripheral GLP-1 receptor agonists, exendin-4 and liraglutide. Neuropharmacology 2012, 62, 1916–1927. [Google Scholar] [CrossRef]
- De Jonghe, B.C.; Lawler, M.P.; Horn, C.C.; Tordoff, M.G. Pica as an adaptive response: Kaolin consumption helps rats recover from chemotherapy-induced illness. Physiol. Behav. 2009, 97, 87–90. [Google Scholar] [CrossRef]
- Mehler, W.R. Observations on the connectivity of the parvicellular reticular formation with respect to a vomiting center. Brain Behav. Evol. 1983, 23, 63–80. [Google Scholar] [CrossRef]
- Takeda, N.; Hasegawa, S.; Morita, M.; Matsunaga, T. Pica in rats is analogous to emesis: An animal model in emesis research. Pharmacol. Biochem. Behav. 1993, 45, 817–821. [Google Scholar] [CrossRef] [PubMed]
- Lai, S.-W.; Lin, J.-H.; Lai, S.-S.; Wu, Y.-L. Influence of Ganoderma lucidum on blood biochemistry and immunocompetence in horses. Am. J. Chin. Med. 2004, 32, 931–940. [Google Scholar] [CrossRef] [PubMed]
- Tanihata, S.; Igarashi, H.; Suzuki, M.; Uchiyama, T. Cisplatin-induced early and delayed emesis in the pigeon. Br. J. Pharmacol. 2000, 130, 132–138. [Google Scholar] [CrossRef][Green Version]
- Zhang, F.; Wang, L.; Yang, Z.H.; Liu, Z.T.; Yue, W. Value of mink vomit model in study of anti-emetic drugs. World J. Gastroenterol. 2006, 12, 1300–1302. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Min, D.; Kim, B.; Ko, S.-G.; Kim, W. Effect and Mechanism of Herbal Medicines on Cisplatin-Induced Anorexia. Pharmaceuticals 2022, 15, 208. [Google Scholar] [CrossRef]
- Cummings, D.E.; Overduin, J. Gastrointestinal regulation of food intake. J. Clin. Investig. 2007, 117, 13–23. [Google Scholar] [CrossRef]
- Shahid, F.; Farooqui, Z.; Khan, F. Cisplatin-induced gastrointestinal toxicity: An update on possible mechanisms and on available gastroprotective strategies. Eur. J. Pharmacol. 2018, 827, 49–57. [Google Scholar] [CrossRef]
- Hornby, P.J. Central neurocircuitry associated with emesis. Am. J. Med. 2001, 111, 106–112. [Google Scholar] [CrossRef]
- Ranganath, P.; Einhorn, L.; Albany, C. Management of chemotherapy induced nausea and vomiting in patients on multiday cisplatin based combination chemotherapy. BioMed Res. Int. 2015, 2015, 8. [Google Scholar] [CrossRef]
- Darmani, N.A.; Ray, A.P. Evidence for a re-evaluation of the neurochemical and anatomical bases of chemotherapy-induced vomiting. Chem. Rev. 2009, 109, 3158–3199. [Google Scholar] [CrossRef]
- Hosoi, T.; Okuma, Y.; Matsuda, T.; Nomura, Y. Novel pathway for LPS-induced afferent vagus nerve activation: Possible role of nodose ganglion. Auton. Neurosci. 2005, 120, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Bonaz, B.; Sinniger, V.; Pellissier, S. Anti-inflammatory properties of the vagus nerve: Potential therapeutic implications of vagus nerve stimulation. J. Physiol. 2016, 594, 5781–5790. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Tchounwou, P.B. Cisplatin in cancer therapy: Molecular mechanisms of action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef]
- Roila, F.; Molassiotis, A.; Herrstedt, J.; Aapro, M.; Gralla, R.; Bruera, E.; Clark-Snow, R.; Dupuis, L.; Einhorn, L.; Feyer, P. 2016 MASCC and ESMO guideline update for the prevention of chemotherapy-and radiotherapy-induced nausea and vomiting and of nausea and vomiting in advanced cancer patients. Ann. Oncol. 2016, 27, v119–v133. [Google Scholar] [CrossRef]
- Lin, M.-T.; Ko, J.-L.; Liu, T.-C.; Chao, P.-T.; Ou, C.-C. Protective effect of D-methionine on body weight loss, anorexia, and nephrotoxicity in cisplatin-induced chronic toxicity in rats. Integr. Cancer Ther. 2018, 17, 813–824. [Google Scholar] [CrossRef] [PubMed]
- Semwal, R.B.; Semwal, D.K.; Combrinck, S.; Viljoen, A.M. Gingerols and shogaols: Important nutraceutical principles from ginger. Phytochemistry 2015, 117, 554–568. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Zhang, T.; Sun, H.; Liu, G. Ginsenoside Rg3 (Shenyi capsule) combined with chemotherapy for digestive system cancer in China: A meta-analysis and systematic review. Evid.-Based Complement. Altern. Med. 2019, 2019, 19. [Google Scholar] [CrossRef]
- He, M.; Huang, X.; Liu, S.; Guo, C.; Xie, Y.; Meijer, A.H.; Wang, M. The Difference between White and Red Ginseng: Variations in Ginsenosides and Immunomodulation. Planta Med. 2018, 84, 845–854. [Google Scholar] [CrossRef]
- Shin, H.R.; Kim, J.Y.; Yun, T.K.; Morgan, G.; Vainio, H. The cancer-preventive potential of Panax ginseng: A review of human and experimental evidence. Cancer Causes Control 2000, 11, 565–576. [Google Scholar] [CrossRef]
- Gupta, K.; Walton, R.; Kataria, S.P. Chemotherapy-Induced Nausea and Vomiting: Pathogenesis, Recommendations, and New Trends. Cancer Treat. Res. Commun. 2021, 26, 100278. [Google Scholar] [CrossRef]
- Janelsins, M.C.; Tejani, M.A.; Kamen, C.; Peoples, A.R.; Mustian, K.M.; Morrow, G.R. Current pharmacotherapy for chemotherapy-induced nausea and vomiting in cancer patients. Expert Opin. Pharmacother. 2013, 14, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, B.L. Delayed Chemotherapy-Induced Nausea and Vomiting: Pathogenesis, Incidence, and Current Management. Front. Pharmacol. 2017, 8, 19. [Google Scholar] [CrossRef]
- Simon, A.; Darcsi, A.; Kéry, Á.; Riethmüller, E. Blood-brain barrier permeability study of ginger constituents. J. Pharm. Biomed. Anal. 2020, 177, 112820. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.; McCully, C.L.; Murphy, R.F.; Bacher, J.; Balis, F.M.; Fox, E. Extracellular fluid concentrations of cisplatin, carboplatin, and oxaliplatin in brain, muscle, and blood measured using microdialysis in nonhuman primates. Cancer Chemother. Pharmacol. 2010, 65, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, H.; Windeck, T.; Ploch, M.; Verspohl, E.J. Mode of action of gingerols and shogaols on 5-HT3 receptors: Binding studies, cation uptake by the receptor channel and contraction of isolated guinea-pig ileum. Eur. J. Pharmacol. 2006, 530, 136–143. [Google Scholar] [CrossRef]
- Jin, Z.; Lee, G.; Kim, S.; Park, C.-S.; Park, Y.S.; Jin, Y.-H. Ginger and its pungent constituents non-competitively inhibit serotonin currents on visceral afferent neurons. Korean J. Physiol. Pharmacol. Off. J. Korean Physiol. Soc. Korean Soc. Pharmacol. 2014, 18, 149. [Google Scholar] [CrossRef]
- Pertz, H.H.; Lehmann, J.; Roth-Ehrang, R.; Elz, S. Effects of ginger constituents on the gastrointestinal tract: Role of cholinergic M3 and serotonergic 5-HT3 and 5-HT4 receptors. Planta Med. 2011, 77, 973–978. [Google Scholar] [CrossRef]
- Helms, S. Cancer prevention and therapeutics: Panax ginseng. Altern. Med. Rev. 2004, 9, 259–274. [Google Scholar]
- Vuksan, V.; Sung, M.-K.; Sievenpiper, J.L.; Stavro, P.M.; Jenkins, A.L.; Di Buono, M.; Lee, K.-S.; Leiter, L.A.; Nam, K.Y.; Arnason, J.T. Korean red ginseng (Panax ginseng) improves glucose and insulin regulation in well-controlled, type 2 diabetes: Results of a randomized, double-blind, placebo-controlled study of efficacy and safety. Nutr. Metab. Cardiovasc. Dis. 2008, 18, 46–56. [Google Scholar] [CrossRef]
- Kim, S.Y.; Seo, S.K.; Choi, Y.M.; Jeon, Y.E.; Lim, K.J.; Cho, S.; Choi, Y.S.; Lee, B.S. Effects of red ginseng supplementation on menopausal symptoms and cardiovascular risk factors in postmenopausal women: A double-blind randomized controlled trial. Menopause 2012, 19, 461–466. [Google Scholar] [CrossRef]
- Yabut, J.M.; Crane, J.D.; Green, A.E.; Keating, D.J.; Khan, W.I.; Steinberg, G.R. Emerging Roles for Serotonin in Regulating Metabolism: New Implications for an Ancient Molecule. Endocr. Rev. 2019, 40, 1092–1107. [Google Scholar] [CrossRef] [PubMed]
- Takeda, H.; Sadakane, C.; Hattori, T.; Katsurada, T.; Ohkawara, T.; Nagai, K.; Asaka, M. Rikkunshito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology 2008, 134, 2004–2013. [Google Scholar] [CrossRef]
- Yakabi, K.; Kurosawa, S.; Tamai, M.; Yuzurihara, M.; Nahata, M.; Ohno, S.; Ro, S.; Kato, S.; Aoyama, T.; Sakurada, T.; et al. Rikkunshito and 5-HT2C receptor antagonist improve cisplatin-induced anorexia via hypothalamic ghrelin interaction. Regul. Pept. 2010, 161, 97–105. [Google Scholar] [CrossRef]
- Ohnishi, S.; Watari, H.; Kanno, M.; Ohba, Y.; Takeuchi, S.; Miyaji, T.; Oyamada, S.; Nomura, E.; Kato, H.; Sugiyama, T. Additive effect of rikkunshito, an herbal medicine, on chemotherapy-induced nausea, vomiting, and anorexia in uterine cervical or corpus cancer patients treated with cisplatin and paclitaxel: Results of a randomized phase II study (JORTC KMP-02). J. Gynecol. Oncol. 2017, 28, e44. [Google Scholar] [CrossRef] [PubMed]
- Ryan, J.L.; Heckler, C.E.; Roscoe, J.A.; Dakhil, S.R.; Kirshner, J.; Flynn, P.J.; Hickok, J.T.; Morrow, G.R. Ginger (Zingiber officinale) reduces acute chemotherapy-induced nausea: A URCC CCOP study of 576 patients. Support. Care Cancer 2012, 20, 1479–1489. [Google Scholar] [CrossRef] [PubMed]
| Herbal Medicine | Doses | Animals | Cisplatin | Kaolin Intake | Retching & Vomiting | Food Intake | Body Weight | Mechanism of Action | Authors |
|---|---|---|---|---|---|---|---|---|---|
| Xian-Bao- Xia-Tang (XBXT) | 1 & 4 g/kg (i.p.) | Male Mink | Single 6 mg/kg (i.p.) | - | ↑ | - | - | NK1R: ↓ | Qian et al., 2010 [45] |
| 1.6 g/kg (p.o.) | Male Wistar Rat | Single 6 mg/kg (i.p.) | ↓ | - | NS | NS | Ros, IL-1β, IL-18, NLRP3, Caspase-1: ↓ | Meng et al., 2020 [34] | |
| 1.6 g/kg (p.o.) | Male Wistar Rat | Single 6 mg/kg (i.p.) | ↓ | - | NS | NS | CD86, TNF, TRPV2, Map3k8, NLRP3 IL-1R1, IL-1β, IL-6, IL-34: ↓ | Li et al., 2020 [44] | |
| [6]-gingerol | 50, 100, 200 mg/kg (p.o.) | Male Mink | Single 7.5 mg/kg (i.p.) | - | ↓ | - | - | 5-HT, DA, Substance P: ↓ | Qian et al., 2009 [46] |
| 10, 20, 40 mg/kg (rat) & 50, 100, 200 mg/kg (mink) (p.o.) | Male Wistar Rat & Mink | Single 3 mg/kg (i.p., rat), 6 mg/kg (i.p., mink) | ↓ | ↓ | - | - | 5-HT, 5-HT3, TPH-1, -2, SP, NK1R, DA, D2R, TH: ↓ | Tian et al., 2020 [43] | |
| 50 & 100 mg/kg (p.o.) | Male SD Rat | Single 6 mg/kg (i.p.) | ↓ | - | NS | NS | 5-HT, 5-HT3A, TPH-1, -2: ↓ MAO-A: ↑ | Cheng et al., 2020 [35] | |
| Ginseng Radix (GR) | Pre-treatment: 25, 50, 100 mg/kg Post-treatment: 12.5, 25, 50 mg/kg (p.o.) | Male SD Rat | Single 6 or 7 mg/kg (i.p.) | ↓ | - | ↑ | ↑ | Pre-: Neutrophil, Lymphocytes, WBC: ↓ Post-: Hemoglobin, RBC: ↓ | Raghavendran et al., 2010 [40] |
| Steamed GR: 25, 50, 100 mg/kg GS: 5, 10 mg/kg GNS: 50, 100 mg/kg (p.o.) | Male SD Rats | Single 6 mg/kg (i.p.) | ↓ | - | NS | ↑ | Deformity (stomach, small intestine) 25 mg/kg: ↓ 50 & 100 mg/kg: NS | Sathyanath et al., 2013 [41] | |
| Scutellariae Radix (SR) | 1, 3, 10 mg/kg (i.p.) | Male Wistar Rat | Single 3, 5, 10 mg/kg (i.p.) | ↓ | - | ↑ | NS | - | Aung et al., 2004 [39] |
| 3, 10 mg/kg (i.p.) | Male Wistar Rat | Single 3 mg/kg (i.p.) | ↓ | - | - | - | - | Mehendale et al., 2004 [38] | |
| Berry of Panacis Quinquefolii Radix (BPQ) | 10, 50 mg/kg (i.p.) | Male Wistar Rat | Single 3 mg/kg (i.p.) | ↓ | - | - | - | - | Mehendale et al., 2004 [38] |
| 50, 100, 150 mg/kg (i.p.) | Male Wistar Rat | Single 3 mg/kg (i.p.) | ↓ | - | ↑ | - | - | Mehendale et al., 2005 [37] | |
| Ganoderma Lucidum (GL) | 1, 3, 10 mg/kg (i.p.) | Male Wistar Rat | Single 3 mg/kg (i.p.) | ↓ | - | ↑ | - | - | Wang et al., 2005 [36] |
| Bacopa monnieri (BM) | N-butanolic Fraction: 10, 20, 40 mg/kg & Methanolic Fraction: 5, 10, 20 mg/kg (i.m.) | Male & Female Pigeon | Single 7 mg/kg (i.v.) | - | ↓ | - | ↑ | NA: NS 5-HT, DA: ↓ | Ullah et al., 2014 [47] |
| - | ↓ | - | NS | ||||||
| Forsythiae Fructus (FF) | 1.7 g/kg (p.o.) | Male Wistar Rat | Single 6 mg/kg (i.p.) | - | ↓ | - | NS | Ros, IL-1β, IL-18, NLRP3, Caspase-1: ↓ | Meng et al., 2021 [42] |
| Herbal Medicine/ Collected Locations | Preparation (Extraction) | Components |
|---|---|---|
| Bacopa monnieri/ Pakistan [47] | (Not Mentioned) Methanol | Bacoside A3, Bacopaside Ⅱ, Bacopsaponin C |
| Berry of Panacis Quinquefolii Radix/ United States [37,38] | 75% Ethanol | Protopanaxadiol Ginsenoside: Rb1, Rb2, Rc, Rd Protopanaxatriol Ginsenoside: Re, Rg1 |
| Forsythiae Fructus/ China [42] | 100% Water | Forsythiaside A (2.62%), Forsythin (0.28%) |
| Ganoderma lucidum/ China [36] | 5% Ethanol | Terpenoids (1.89%): Ganoderic acid A, Ganoderic acid C2, Ganodermanontriol Polysaccharides (15.8%) |
| Ginseng Radix/ Korea [40] | 100% Water | Protopanaxadiol Ginsenoside: Rb1 (5.14 mg/g of GR), Rb2 (3.60), Rb3 (6.33), Rc (2.61), Rd (0.43), Rg3 (1.08) Protopanaxatriol Ginsenoside: Re (2.21), Rg1 (7.22), Rg2 (0.67), Rh1 (0.58), Rh2 (0.02) |
| Scutellariae Radix/ China [38,39] | 100% Water | Wogonin (51.5%), Baicalein (35.6%), Skullcapflavon Ⅰ (4.8%), Skullcapflavon Ⅱ (8.3%) |
| Steamed Ginseng Radix/Korea [41] | 100% Water | Protopanaxadiol Ginsenoside: Rb1 (3.93 mg/g of GR), Rb2 (1.92), Rc (2.04), Rd (1.07), Rg3 (2.68) Protopanaxatriol Ginsenoside: Rf (0.97), Re (0.74), Rg1 (0.42), Rg2 (1.58), Rh1 (0.91) |
| XBXT (Pinelliae Tuber 2: Zingiberis rhizoma 1)/China [34,44,45] | 100% Water | Pinelliae Tuber: Ephedrine (0.309 mg/g of XBXT), Succinic acid (0.025) Zingiberis rhizoma: [6]-gingerol (0.0616), [6]-shogaol (0.0025) |
| Indicator | Cisplatin | Herbal Medicines | Location | ||
|---|---|---|---|---|---|
| Neurotransmitters | 5-HT/5-HT3R | ↑ | [6]-gingerol [35,43,46] | ↓ | Area postrema, Medulla oblongata, Ileum |
| DA | BM [47] | Area postrema, Brain stem, Small Intestine | |||
| NA | NS | NS | |||
| Neuropeptide | Substance P | ↑ | [6]-gingerol [43,46] | ↓ | Area Postrema, Ileum |
| NK1 Receptor | XBXT [45] | ↓ | Ileum, Area Postrema | ||
| Enzymes | TPH-1, -2 | ↑ | [6]-gingerol [35,43,44] | ↓ | Medulla Oblongata, Ileum |
| MAO-A | ↓ | ↑ | Medulla Oblongata, Ileum | ||
| Caspase-1 | ↑ | FAE [42] | ↓ | Antrum, Ileum | |
| Cytokine | IL -1R1, -1β, 6, -18, -34, | ↑ | XBXT [34] | ↓ | Serum |
| NLRP3 | Antrum, Ileum | ||||
| FAE [42] | |||||
| Hematological Parameters | Hemoglobin | ↑ | GR [40] | NS | Serum |
| Lymphocytes | ↓ | ||||
| Monocytes | NS | ||||
| Neutrophils | ↓ | ||||
| RBC | NS | ||||
| WBC | ↓ | ||||
| Histological Deformity | - | ↑ | XBXT [34,44] | ↓ | Antrum, Ileum |
| - | GR [40,41] | Stomach, Small Intestine | |||
| - | FAE [42] | Antrum, Ileum | |||
| Oxidative Stress | ROS | ↑ | XBXT [34] | ↓ | Serum, Antrum, Ileum |
| FAE [42] | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, Y.; Kim, B.; Kim, W. Cisplatin-Induced Nausea and Vomiting: Effect of Herbal Medicines. Plants 2022, 11, 3395. https://doi.org/10.3390/plants11233395
Shin Y, Kim B, Kim W. Cisplatin-Induced Nausea and Vomiting: Effect of Herbal Medicines. Plants. 2022; 11(23):3395. https://doi.org/10.3390/plants11233395
Chicago/Turabian StyleShin, Yuchan, Bonglee Kim, and Woojin Kim. 2022. "Cisplatin-Induced Nausea and Vomiting: Effect of Herbal Medicines" Plants 11, no. 23: 3395. https://doi.org/10.3390/plants11233395
APA StyleShin, Y., Kim, B., & Kim, W. (2022). Cisplatin-Induced Nausea and Vomiting: Effect of Herbal Medicines. Plants, 11(23), 3395. https://doi.org/10.3390/plants11233395






