Luffa cylindrica Intercropping with Semen cassiae—A Production Practice of Improving Land Use in Soil Contaminated with Arsenic
Abstract
:1. Introduction
2. Results
2.1. Field Experiment
2.1.1. Biomass and Yield of Plants
2.1.2. Levels of As in Different Parts of the Plants and the BCA
2.2. Pot Experiment
2.2.1. Biomass and Yield of L. cylindrica and S. cassiae
2.2.2. Concentration of As in Different Parts of L. cylindrica and S. cassiae and the BCA
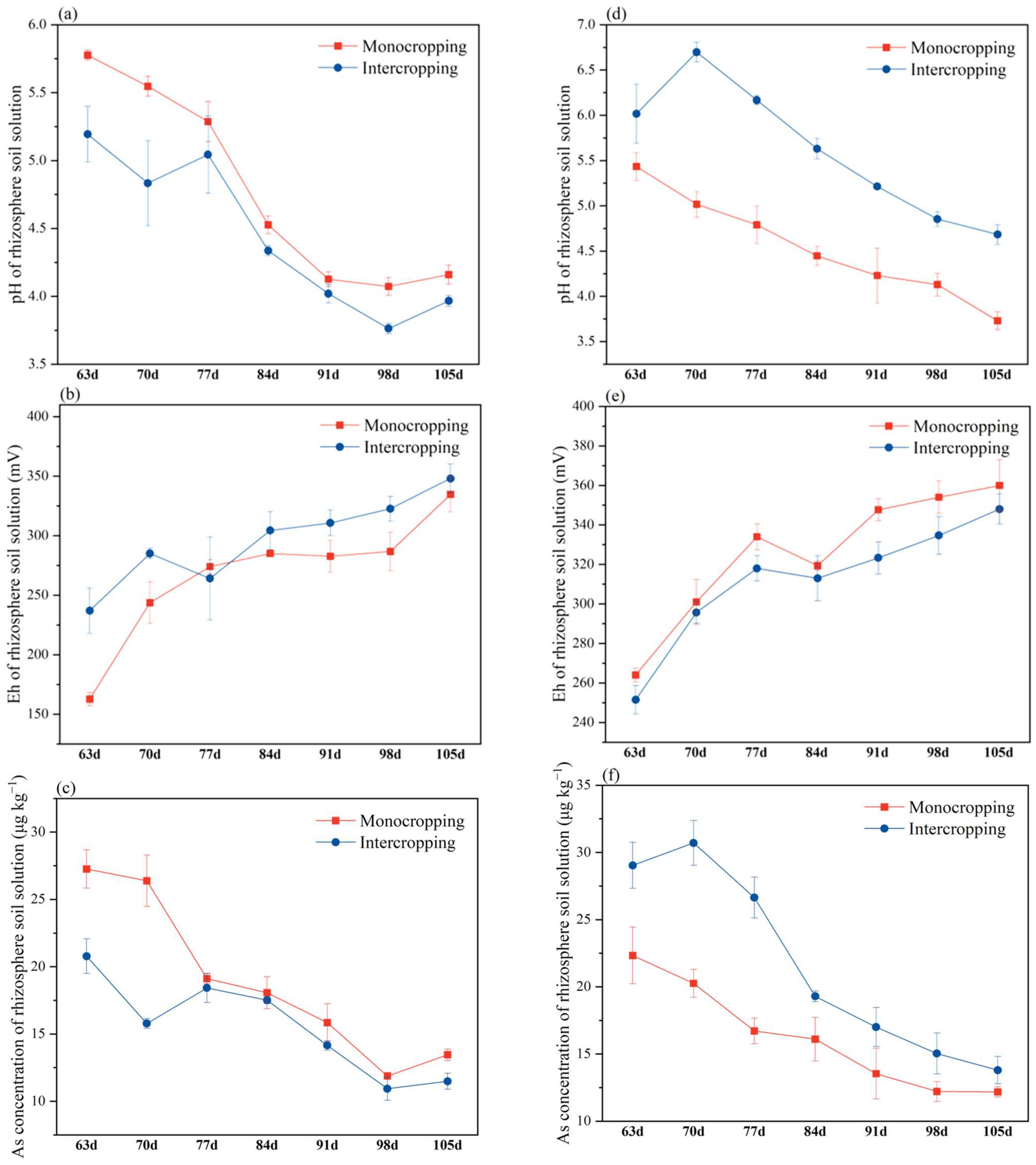
2.2.3. Concentration of As and the pH and Eh of the Rhizosphere Soil Solution
3. Materials and Methods
3.1. Soil Characterization
3.2. The L. cylindrica and S. cassiae Seedlings
3.3. Experimental Design
3.4. Sampling and Analysis
3.4.1. Sampling and Analysis
3.4.2. Determination of As
Total As Concentration in L. cylindrica and S. cassiae
Determination of the As Concentration, pH, and Oxidation-Reduction Potential (Eh) in the Rhizosphere Soil Solution
3.5. Data Analysis
3.5.1. Bioconcentration Amount (BCA)
3.5.2. Land Equivalent Ratio (LER)
3.5.3. Metal Removal Equivalent Ratio (MRER)
4. Discussions
4.1. Growth Status of L. cylindrica and S. cassiae
4.2. Levels of As in L. cylindrica and S. cassiae
4.3. Changes in the pH, Eh, and As Content in the Rhizosphere Soil Solution of L. cylindrica and S. cassiae
5. Conclusions and Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wiggenhauser, M.; Bigalke, M.; Imseng, M.; Müller, M.; Keller, A.; Murphy, K.; Kreissig, K.; Rehkämper, M.; Wilcke, W.; Frossard, E. Cadmium Isotope Fractionation in Soil–Wheat Systems. Environ. Sci. Technol. 2016, 50, 9223–9231. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cui, X.; Cheng, H.; Chen, F.; Wang, J.; Zhao, X.; Lin, C.; Pu, X. A review of soil cadmium contamination in China including a health risk assessment. Environ. Sci. Pollut. Res. 2015, 22, 16441–16452. [Google Scholar] [CrossRef] [PubMed]
- Ministry of Environment Protection of China. National Soil Pollution Survey Bulletin; Ministry of Environment Protection of China: Beijing, China, 2014.
- Cui, M.; Lee, Y.; Choi, J.; Kim, J.; Han, Z.; Son, Y.; Khim, J. Evaluation of stabilizing materials for immobilization of toxic heavy metals in contaminated agricultural soils in China. J. Clean. Prod. 2018, 193, 748–758. [Google Scholar] [CrossRef]
- Ma, L.Q.; Komar, K.M.; Tu, C.; Zhang, W.; Cai, Y.; Kennelley, E.D. A fern that hyperaccumulates arsenic. Nature 2001, 409, 579. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, P.R.; Butcher, D.J. Phytoremediation of arsenic by two hyperaccumulators in a hydroponic environment. Microchem. J. 2007, 85, 297–300. [Google Scholar] [CrossRef]
- Singh, N.; Raj, A.; Khare, P.B.; Tripathi, R.D.; Jamil, S. Arsenic accumulation pattern in 12 Indian ferns and assessing the potential of Adiantum capillus-veneris, in comparison to Pteris vittata, as arsenic hyperaccumulator. Bioresour. Technol. 2010, 101, 8960–8968. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Chen, T.; Yang, J. Intercropped Pteris vittata L. and Morus alba L. presents a safe utilization mode for arsenic-contaminated soil. Sci. Total Environ. 2017, 579, 1467–1475. [Google Scholar] [CrossRef]
- Ma, J.; Lei, E.; Lei, M.; Liu, Y.; Chen, T. Remediation of Arsenic contaminated soil using malposed intercropping of Pteris vittata L. and maize. Chemosphere 2018, 194, 737–744. [Google Scholar] [CrossRef]
- Xue, Y.; Xia, H.; Christie, P.; Zhang, Z.; Li, L.; Tang, C. Crop acquisition of phosphorus, iron and zinc from soil in cereal/legume intercropping systems: A critical review. Ann. Bot. 2016, 117, 363–377. [Google Scholar] [CrossRef]
- Ning, C.; Qu, J.; He, L.; Yang, R.; Chen, Q.; Luo, S.; Cai, K. Improvement of yield, pest control and Si nutrition of rice by rice-water spinach intercropping. Field Crops Res. 2017, 208, 34–43. [Google Scholar] [CrossRef]
- Li, C.; Hoffland, E.; Kuyper, T.W.; Yu, Y.; Li, H.; Zhang, C.; Zhang, F.; Werf, W. Yield gain, complementarity and competitive dominance in intercropping in China: A meta-analysis of drivers of yield gain using additive partitioning. Eur. J. Agron. 2020, 113, 125987. [Google Scholar] [CrossRef]
- Monti, M.; Pellicanò, A.; Santonoceto, C.; Preiti, G.; Pristeri, A. Yield components and nitrogen use in cereal-pea intercrops in Mediterranean environment. Field Crops Res. 2016, 196, 379–388. [Google Scholar] [CrossRef]
- Nyawade, S.O.; Karanja, N.N.; Gachene, C.K.K.; Gitari, H.I.; Schulte-Geldermann, E.; Parker, M. Optimizing soil nitrogen balance in a potato cropping system through legume intercropping. Nutr. Cycl. Agroecosyst. 2020, 117, 43–59. [Google Scholar] [CrossRef] [Green Version]
- Banik, P.; Sharma, R.C. Yield and resource utilization efficiency in baby corn—legume-intercropping system in the Eastern Plateau of India. J. Sustain. Agric. 2009, 33, 379–395. [Google Scholar] [CrossRef]
- Huang, S.Y.; Zhuo, C.; Du, X.Y.; Li, H.S. Remediation of arsenic-contaminated paddy soil by intercropping aquatic vegetables and rice. Int. J. Phytoremed. 2021, 23, 1021–1029. [Google Scholar] [CrossRef]
- Carvalho, C.F.M.D.; Viana, D.G.; Pires, F.R.; Filho, F.B.E.; Bonomo, R.; Martins, L.F.; Cruz, L.B.S.; Nascimento, M.C.P.; Cargnelutti Filho, A.; Rocha Júnior, P.R.D. Phytoremediation of barium-affected flooded soils using single and intercropping cultivation of aquatic macrophytes. Chemosphere 2019, 214, 10–16. [Google Scholar] [CrossRef]
- De Conti, L.; Ceretta, C.A.; Melo, G.W.B.; Tiecher, T.L.; Silva, L.O.S.; Garlet, L.P.; Mimmo, T.; Cesco, S.; Brunetto, G. Intercropping of young grapevines with native grasses for phytoremediation of Cu-contaminated soils. Chemosphere 2019, 216, 147–156. [Google Scholar] [CrossRef] [Green Version]
- De Conti, L.; Cesco, S.; Mimmo, T.; Pii, Y.; Valentinuzzi, F.; Melo, G.W.B.; Ceretta, C.A.; Trentin, E.; Marques, A.C.R.; Brunetto, G. Iron fertilization to enhance tolerance mechanisms to copper toxicity of ryegrass plants used as cover crop in vineyards. Chemosphere 2020, 243, 125298. [Google Scholar] [CrossRef]
- Wan, X.; Lei, M.; Chen, T. Review on remediation technologies for arsenic-contaminated soil. Front. Environ. Sci. Eng. 2020, 14, 1–14. [Google Scholar] [CrossRef]
- Wang, J.; Cai, M.; Zheng, J.; Li, D.; Wang, X.; Zhang, Q. Investigating the intercropping effects of Sedum plumbizincicola and Luffa cylindrical on soil cadmium fractions and cadmium uptake by Luffa cylindrica. J. Agro-Environ. Sci. 2016, 35, 2292–2298. [Google Scholar]
- Chien, M.; Yang, C.; Huang, C.; Chen, C. Investigation of aflatoxins contamination in herbal materia medica in a Taiwan pharmaceutical factory. J. Food Drug Anal. 2018, 26, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Hu, J.; Zhu, X.; Zhuang, Y.; Yin, F.; Qin, K.; Cai, B. Qualitative analysis of multiple compounds in raw and prepared Semen Cassiae coupled with multiple statistical strategies. J. Sep. Sci. 2017, 40, 4718–4729. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Tewolde, H.; Liu, H.; Ren, T.; Liu, E. Nitrogen uptake and transfer in broad bean and garlic strip intercropping systems. J. Integr. Agric. 2018, 17, 220–230. [Google Scholar] [CrossRef] [Green Version]
- Food and Agriculture Organization. World Reference Base for Soil Resources; Food and Agriculture Organization: Rome, Italy, 2014. [Google Scholar]
- Kang, Z.; Zhang, W.; Qin, J.; Li, S.; Yang, X.; Wei, X.; Li, H. Yield advantage and cadmium decreasing of rice in intercropping with water spinach under moisture management. Ecotoxicol. Environ. Saf. 2020, 190, 110102. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qin, J.; Li, J.; Lai, Z.; Li, H. Upland rice intercropping with Solanum nigrum inoculated with arbuscular mycorrhizal fungi reduces grain Cd while promoting phytoremediation of Cd-contaminated soil. J. Hazard. Mater. 2021, 406, 124325. [Google Scholar] [CrossRef]
- Chen, W.; Kang, Z.; Yang, Y.; Li, Y.; Qiu, R.; Qin, J.; Li, H. Interplanting of rice cultivars with high and low Cd accumulation can achieve the goal of “repairing while producing” in Cd-contaminated soil. Sci. Total Environ. 2022, 851, 158229. [Google Scholar] [CrossRef]
- Li, L.C.A.U.; Yang, S.C.; Li, X.L.; Zhang, F.S.; Christie, P. Interspecific complementary and competitive interactions between intercropped maize and faba bean. Plant Soil 1999, 212, 105–114. [Google Scholar] [CrossRef]
- Zeng, L.; Lin, X.; Zhou, F.; Qin, J.; Li, H. Biochar and crushed straw additions affect cadmium absorption in cassava-peanut intercropping system. Ecotoxicol. Environ. Saf. 2019, 167, 520–530. [Google Scholar] [CrossRef]
- Tang, L.; Hamid, Y.; Zehra, A.; Sahito, Z.A.; He, Z.; Beri, W.T.; Khan, M.B.; Yang, X. Fava bean intercropping with Sedum alfredii inoculated with endophytes enhances phytoremediation of cadmium and lead co-contaminated field. Environ. Pollut. 2020, 265, 114861. [Google Scholar] [CrossRef]
- Kang, Z.; Gong, M.; Li, Y.; Chen, W.; Yang, Y.; Qin, J.; Li, H. Low Cd-accumulating rice intercropping with Sesbania cannabina L. reduces grain Cd while promoting phytoremediation of Cd-contaminated soil. Sci. Total Environ. 2021, 800, 149600. [Google Scholar] [CrossRef]
- Mensah, A.K.; Shaheen, S.M.; Rinklebe, J.; Heinze, S.; Marschner, B. Phytoavailability and uptake of arsenic in ryegrass affected by various amendments in soil of an abandoned gold mining site. Environ. Res. 2022, 214, 113729. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization; World Health Organization. List of Contaminants and Their Maximum Levels in Foods, 1st ed.; Food and Agriculture Organization: Geneva, Switzerland; World Health Organization: Geneva, Switzerland, 1984; Volume 17. [Google Scholar]
- Wan, Y.; Camara, A.Y.; Huang, Q.; Yu, Y.; Wang, Q.; Li, H. Arsenic uptake and accumulation in rice (Oryza sativa L.) with selenite fertilization and water management. Ecotoxicol. Environ. Saf. 2018, 156, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, L.; Rong, Q.; Luo, J.; Zhang, H.; Jones, K.C. Assessing the Impact of Atrazine on the Availability of Arsenic in Soils Using DGT Technique. Bull. Environ. Contam. Toxicol. 2020, 109, 616–622. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Jiménez, E.; Six, L.; Williams, P.N.; Smolders, E. Inorganic species of arsenic in soil solution determined by microcartridges and ferrihydrite-based diffusive gradient in thin films (DGT). Talanta 2013, 104, 83–89. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, P.; Yan, H.; Zhang, H.; Cheng, W.; Duan, G.; Zhu, Y. NH4H2PO4-extractable arsenic provides a reliable predictor for arsenic accumulation and speciation in pepper fruits (Capsicum annum L.). Environ. Pollut. 2019, 251, 651–658. [Google Scholar] [CrossRef]
- De, N.; Datta, S.C. Relationship between Phosphorus Sorption and Soil Acidity as Affected by Bicarbonate and Silicate Ions. Commun. Soil Sci. Plant Anal. 2007, 38, 679–694. [Google Scholar] [CrossRef]
- Zeng, Q.; Liao, B.; Jiang, Z.; Zhou, X.; Tang, C.; Zhong, N. Short-term changes of pH value and Al activity in acid soils after urea fertilization. J. Appl. Ecol. 2005, 16, 249–252. [Google Scholar]
- Jalali, M.; Najafi, S. Effect of pH on potentially toxic trace elements (Cd, Cu, Ni, and Zn) solubility in two native and spiked calcareous soils: Experimental and modeling. Commun. Soil Sci. Plant Anal. 2018, 49, 814–827. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhang, Y.; Zhou, D.; Qin, Y.; Liang, W. Profile distribution of micronutrients in an aquic brown soil as affected by land use. Plant Soil Environ. 2009, 55, 468–476. [Google Scholar] [CrossRef] [Green Version]
- Amofah, L.R.; Maurice, C.; Kumpiene, J.; Bhattacharya, P. The influence of temperature, pH/molarity and extractant on the removal of arsenic, chromium and zinc from contaminated soil. J. Soils Sediments 2011, 11, 1334–1344. [Google Scholar] [CrossRef]
- Li, J.; Kosugi, T.; Riya, S.; Hashimoto, Y.; Hou, H.; Terada, A.; Hosomi, M. Use of batch leaching tests to quantify arsenic release from excavated urban soils with relatively low levels of arsenic. J. Soils Sediments 2017, 17, 2136–2143. [Google Scholar] [CrossRef]
- Pérez-Sirvent, C.; Hernández-Pérez, C.; Martínez-Sánchez, M.J.; García-Lorenzo, M.L.; Bech, J. Metal uptake by wetland plants: Implications for phytoremediation and restoration. J. Soils Sediments 2017, 17, 1384–1393. [Google Scholar] [CrossRef]
- Li, N.; Hongwei, J.; Su, Y. Phytoremediation of arsenic contaminated soil based on drip irrigation and intercropping. Sci. Total Environ. 2020, 850, 157970. [Google Scholar] [CrossRef]

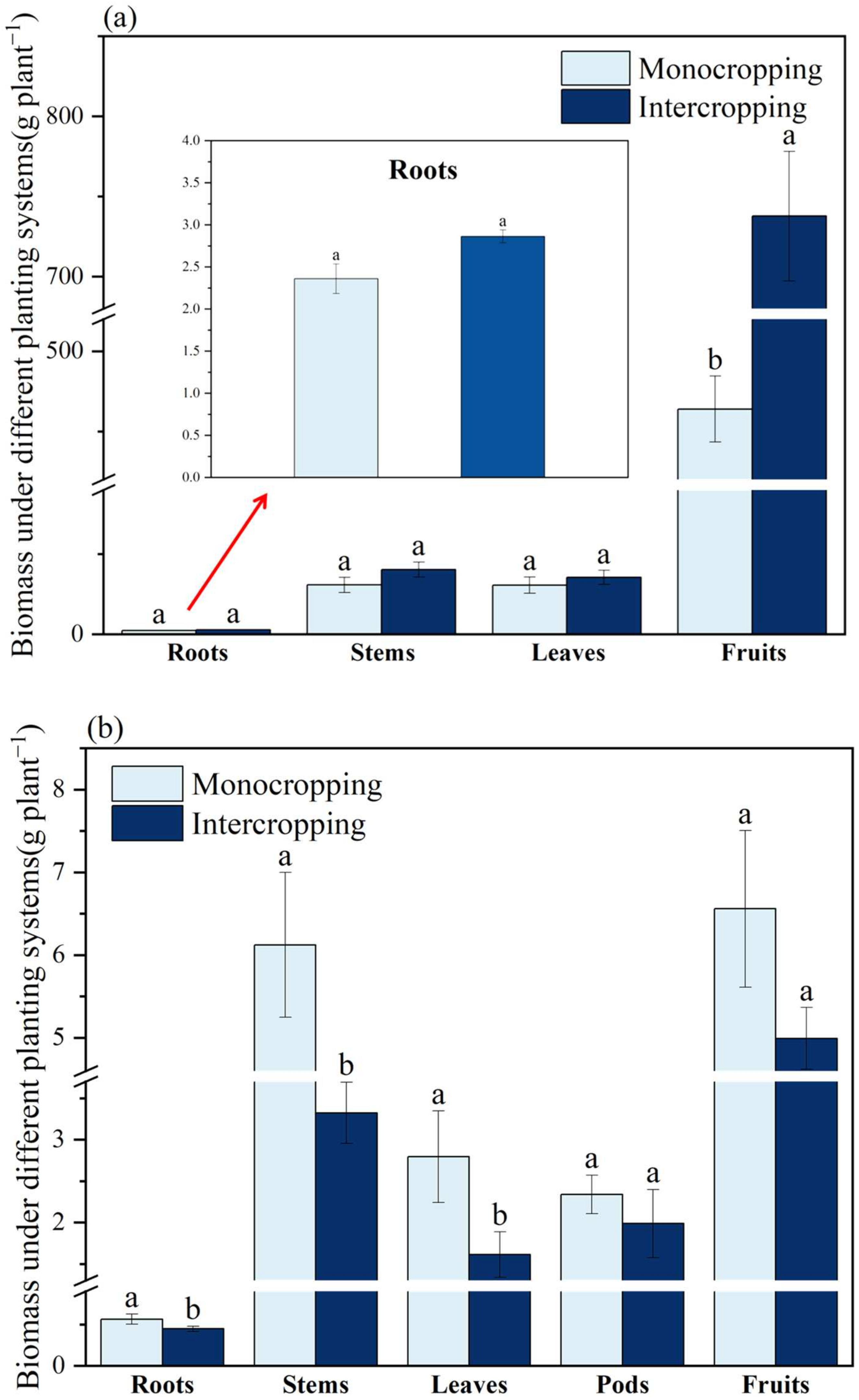

| Crops | Items | Organs | Plant Mode | |
|---|---|---|---|---|
| Monocropping | Intercropping | |||
| L. cylindrica | Biomass (g plant−1) | Roots | 2.33 ± 0.20 a | 2.98 ± 0.30 a |
| Stems | 19.84 ± 1.96 b | 37.32 ± 4.52 a | ||
| Leaves | 30.36 ± 0.61 b | 40.27 ± 3.14 a | ||
| Fruits | 1223.91 ± 61.83 b | 1558.75 ± 37.44 a | ||
| Yield (kg ha−1) | 61,195.63 ± 3091.49 a | 38,968.75 ± 936.07 b | ||
| LER | 1.03 | |||
| S. cassiae | Biomass (g plant−1) | Roots | 3.57 ± 0.34 a | 2.67 ± 0.11 a |
| Stems | 18.89 ± 0.33 a | 12.94 ± 0.84 b | ||
| Leaves | 4.35 ± 0.33 a | 3.76 ± 0.60 a | ||
| Pods | 13.82 ± 1.72 a | 10.82 ± 1.24 a | ||
| Fruits | 34.76 ± 6.89 a | 27.23 ± 3.53 a | ||
| Yield (kg ha−1) | 1737.92 ± 344.62 a | 680.83 ± 88.37 b | ||
| Crops | Items | Organs | Plant | |
|---|---|---|---|---|
| Monocropping | Interplanting | |||
| L. cylindrica | As concentration (mg kg−1) | Roots | 5.18 ± 0.12 a | 4.00 ± 0.30 b |
| Stems | 1.76 ± 0.36 a | 0.88 ± 0.041 a | ||
| Leaves | 2.39 ± 0.09 a | 1.64 ± 0.07 b | ||
| Fruits | 0.08 ± 0.01 a | 0.04 ± 0.01 b | ||
| BCA (μg plant−1) | 210.12 ± 2.95 a | 166.58 ± 5.80 b | ||
| BCA (g ha−1) | 10.51 ± 0.15 a | 4.16 ± 0.15 b | ||
| S. cassiae | As concentration (mg kg−1) | Roots | 2.06 ± 0.41 b | 5.49 ± 0.78 a |
| Stems | 0.29 ± 0.01 a | 0.28 ± 0.03 a | ||
| Leaves | 0.36 ± 0.03 b | 1.60 ± 0.21 a | ||
| Pods | 0.11 ± 0.02 b | 0.82 ± 0.09 a | ||
| Fruits | 0.24 ± 0.02 b | 1.24 ± 0.18 a | ||
| BCA (μg plant−1) | 22.28 ± 2.0 b | 67.27 ± 10.68 a | ||
| BCA (g ha−1) | 1.11 ± 0.11 a | 1.68 ± 0.27 a | ||
| MRER | 2.34 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, W.; Yang, Y.; Meng, D.; Ying, J.; Huang, H.; Li, H. Luffa cylindrica Intercropping with Semen cassiae—A Production Practice of Improving Land Use in Soil Contaminated with Arsenic. Plants 2022, 11, 3398. https://doi.org/10.3390/plants11233398
Chen W, Yang Y, Meng D, Ying J, Huang H, Li H. Luffa cylindrica Intercropping with Semen cassiae—A Production Practice of Improving Land Use in Soil Contaminated with Arsenic. Plants. 2022; 11(23):3398. https://doi.org/10.3390/plants11233398
Chicago/Turabian StyleChen, Weizhen, Yanan Yang, Dele Meng, Jidong Ying, Huiyin Huang, and Huashou Li. 2022. "Luffa cylindrica Intercropping with Semen cassiae—A Production Practice of Improving Land Use in Soil Contaminated with Arsenic" Plants 11, no. 23: 3398. https://doi.org/10.3390/plants11233398
APA StyleChen, W., Yang, Y., Meng, D., Ying, J., Huang, H., & Li, H. (2022). Luffa cylindrica Intercropping with Semen cassiae—A Production Practice of Improving Land Use in Soil Contaminated with Arsenic. Plants, 11(23), 3398. https://doi.org/10.3390/plants11233398






