Low Light Facilitates Cyclic Electron Flows around PSI to Assist PSII against High Temperature Stress
Abstract
1. Introduction
2. Results
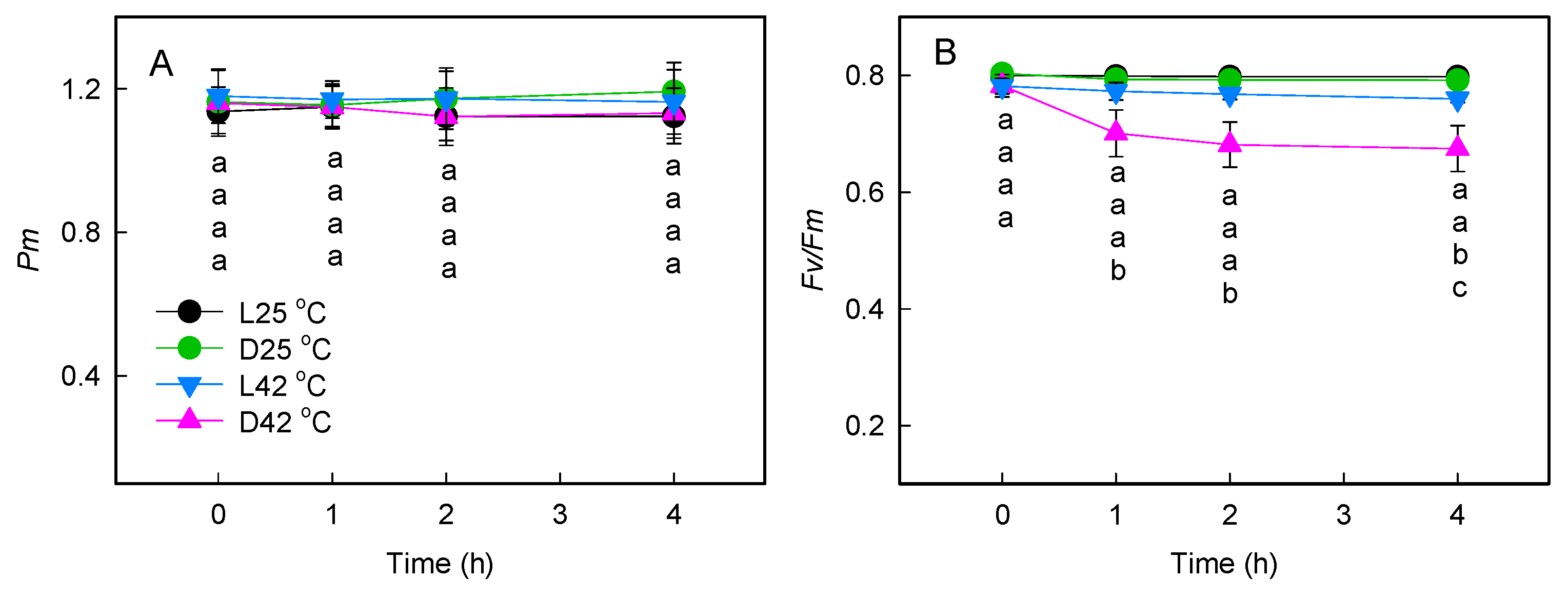
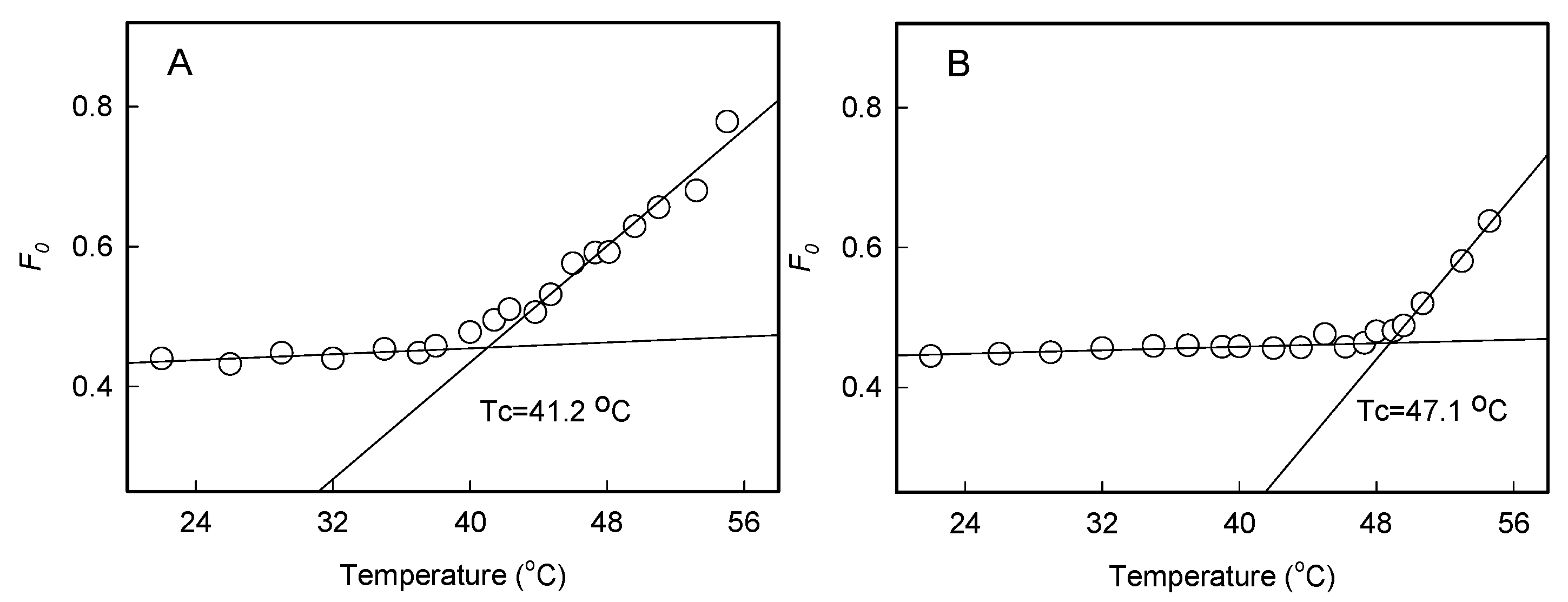
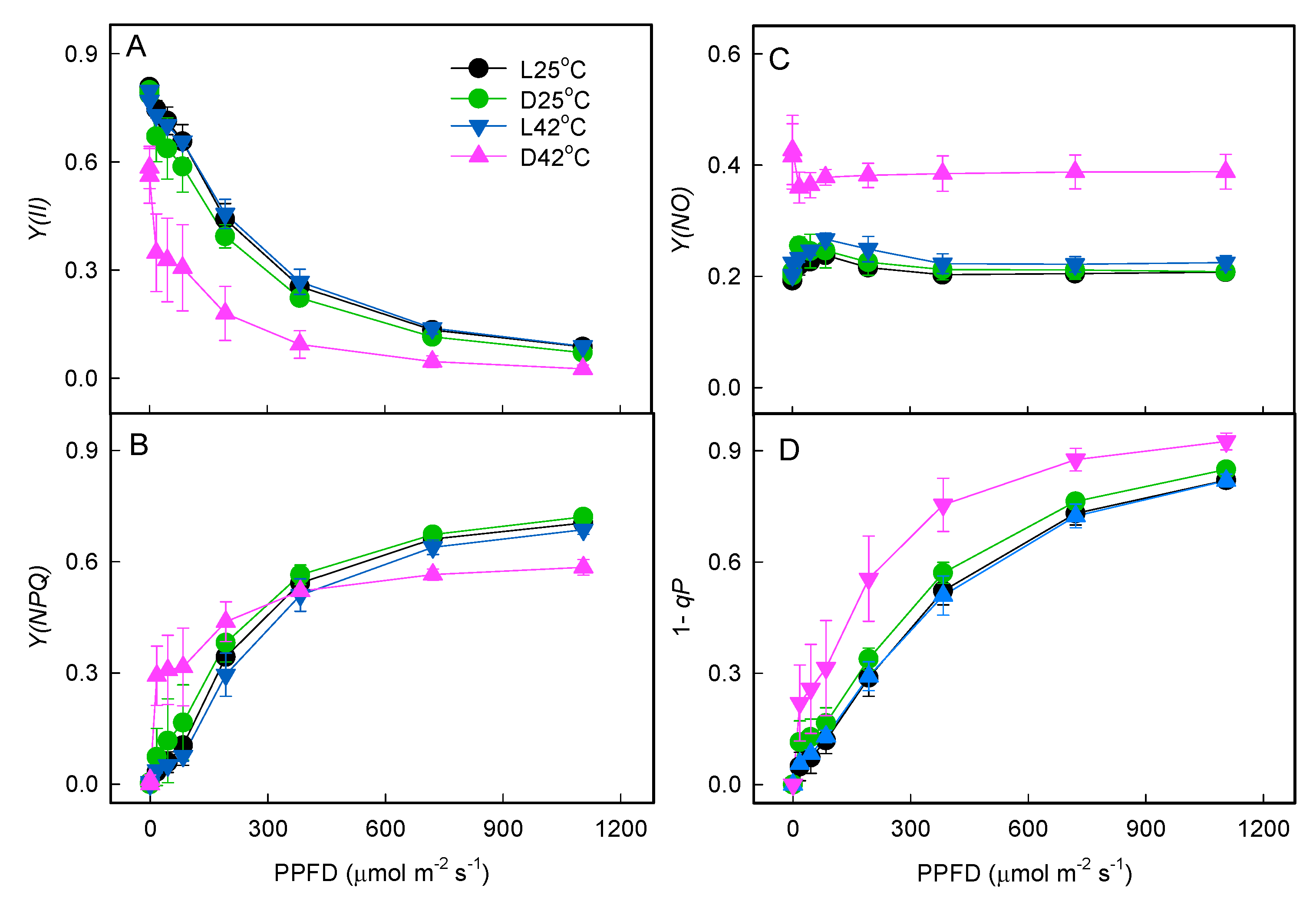
2.1. Effect of Heat Stress on Photosystem Activities
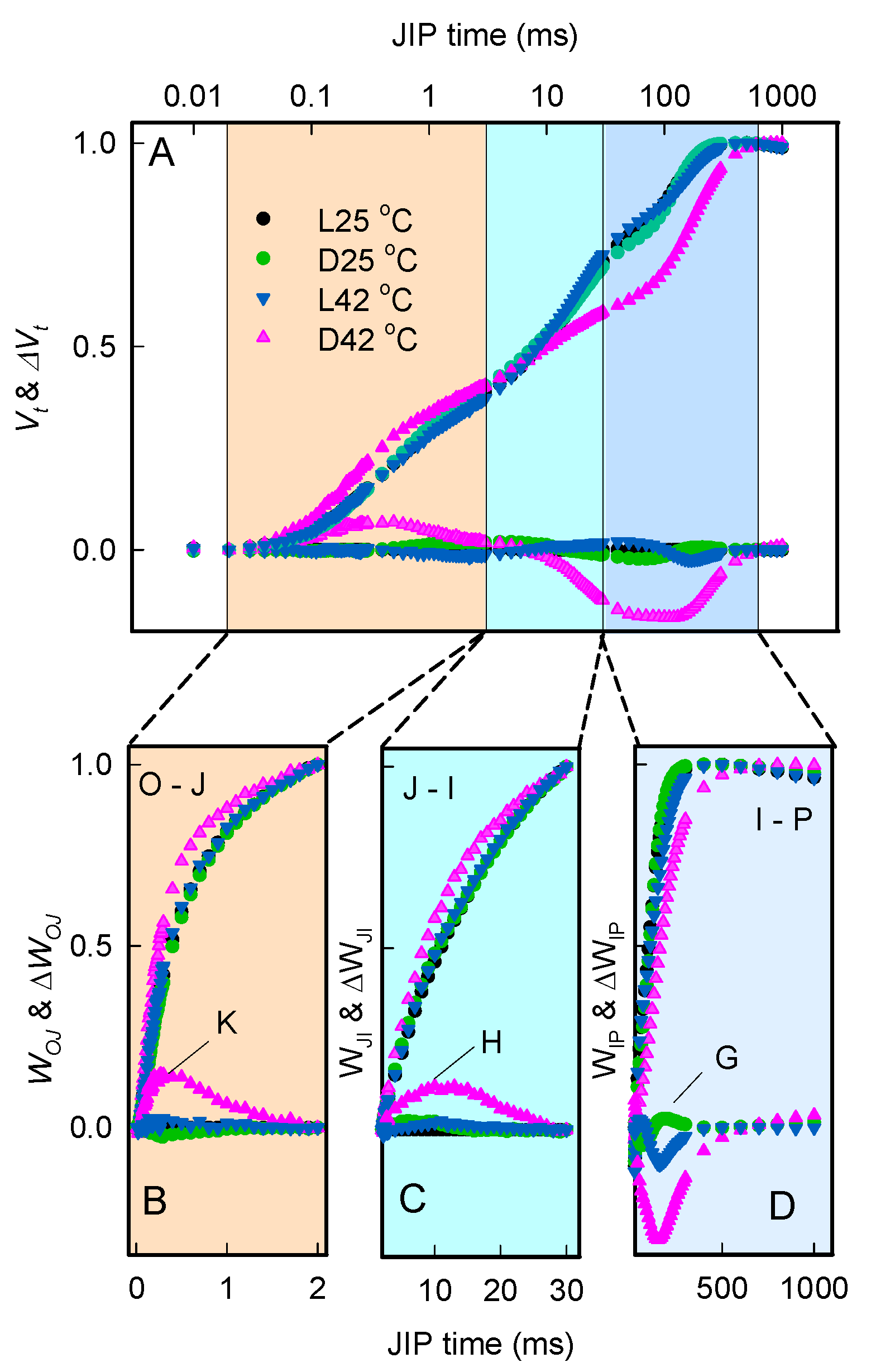
2.2. Effect of Heat Stress on Chlorophyll a Fluorescence Rise OJIP Kinetics
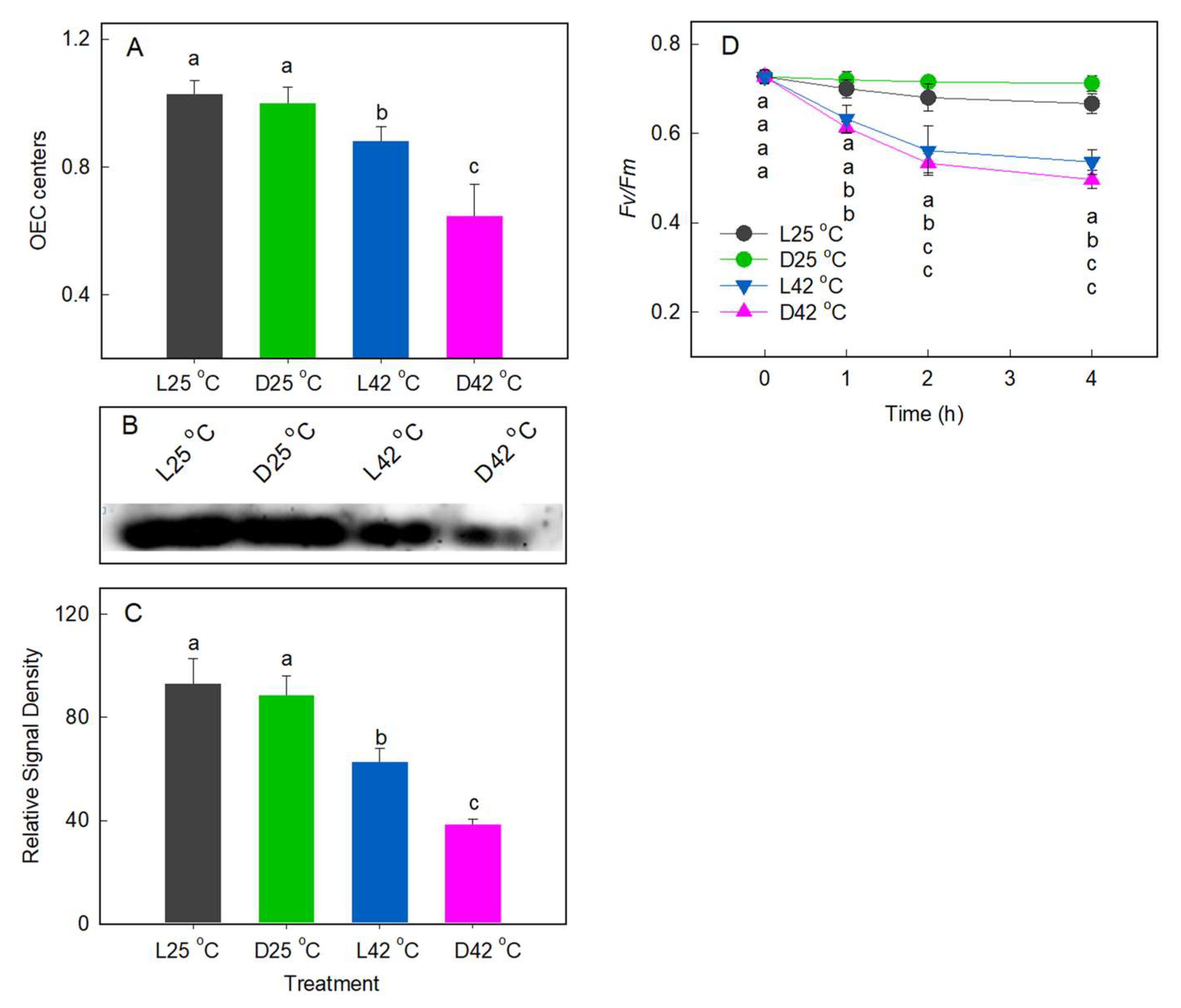
2.3. Effect of Heat Stress on D1 Protein Level
2.4. Effect of Heat Stress on the Re-Reduction Rate of P700+ after Far-Red Illumination and the Post-Illumination Chlorophyll Fluorescence
2.5. Energy Distribution in the Steady-State PSII following Heat Treatment
3. Discussion
3.1. Low Light Enhances PSII Thermostability via Repair Not Resistance Process
3.2. Low Light Enhances Cyclic Electron Flows around PSI under Heat Stress
4. Materials and Methods
4.1. Plant Material and Treatments
4.2. Measurement of Chlorophyll a Fluorescence
4.3. P700 and Chlorophyll Fluorescence Analysis
4.4. Detection of D1 Protein
4.5. Infiltration with Chloramphenicol
4.6. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sharkey, T.D. Effects of moderate heat stress on photosynthesis: Importance of thylakoid reactions, rubisco deactivation, reactive oxygen species, and thermotolerance provided by isoprene. Plant Cell Environ. 2005, 28, 269–277. [Google Scholar] [CrossRef]
- Salvucci, M.E. Association of Rubisco activase with chaperonin-60β: A possible mechanism for protecting photosynthesis during heat stress. J. Exp. Bot. 2008, 59, 1923–1933. [Google Scholar] [CrossRef] [PubMed]
- Berry, J.; Bjorkman, O. Photosynthetic response and adaptation to temperature in higher plants. Ann. Rev. Plant Physiol. 1980, 31, 491–543. [Google Scholar] [CrossRef]
- Tyystjärvi, E. Photoinhibition of photosystem II. Int. Rev. Cell Mol. Biol. 2012, 300, 243–303. [Google Scholar]
- Aro, E.M.; Virgin, I.; Andersson, B. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta 1993, 1143, 113–134. [Google Scholar] [CrossRef]
- Komayama, K.; Khatoon, M.; Takenaka, D.; Horie, J.; Yamashita, A.; Yoshioka, M.; Nakayama, Y.; Yoshida, M.; Ohira, S.; Morita, N. Quality control of Photosystem II: Cleavage and aggregation of heat-damaged D1 protein in spinach thylakoids. Biochim. Biophys. Acta 2007, 1767, 838–846. [Google Scholar] [CrossRef]
- Yoshioka, M.; Uchida, S.; Mori, H.; Komayama, K.; Ohira, S.; Morita, N.; Nakanishi, T.; Yamamoto, Y. Quality control of photosystem II. Cleavage of reaction center D1 protein in spinach thylakoids by FtsH protease under moderate heat stress. J. Biol. Chem. 2006, 281, 21660–21669. [Google Scholar] [CrossRef]
- Yamashita, A.; Nijo, N.; Pospisil, P.; Morita, N.; Takenaka, D.; Aminaka, R.; Yamamoto, Y.; Yamamoto, Y. Quality control of photosystem II: Reactive oxygen species are responsible for the damage to photosystem II under moderate heat stress. J. Biol. Chem. 2008, 283, 28380–28391. [Google Scholar] [CrossRef]
- Luo, H.B.; Ma, L.; Xi, H.F.; Duan, W.; Li, S.H.; Loescher, W. Photosynthetic responses to heat treatments at different temperatures and following recovery in grapevine (Vitis amurensis L.) leaves. PLoS ONE 2011, 6, e23033. [Google Scholar] [CrossRef]
- Sun, Y.; Geng, Q.; Du, Y.; Yang, X.; Zhai, H. Induction of cyclic electron flow around photosystem I during heat stress in grape leaves. Plant Sci. 2016, 256, 65–71. [Google Scholar] [CrossRef]
- Hu, M.J.; Guo, Y.P.; Shen, Y.G.; Guo, D.P.; Li, D.Y. Midday depression of photosynthesis and effects of mist spray in citrus. Ann. Appl. Biol. 2009, 154, 143–155. [Google Scholar] [CrossRef]
- Gao, Y.-B.; Zheng, W.-W.; Zhang, C.; Zhang, L.-L.; Xu, K. High temperature and high light intensity induced photoinhibition of bayberry (Myrica rubra Sieb. et Zucc.) by disruption of D1 turnover in photosystem II. Sci. Hortic. 2019, 248, 132–137. [Google Scholar] [CrossRef]
- Weis, E. Influence of light on the heat sensitivity of the photosynthetic apparatus in isolated spinach chloroplasts. Plant Physiol. 1982, 70, 1530–1534. [Google Scholar] [CrossRef]
- Havaux, M.; Greppin, H.; Strasser, R.J. Functioning of photosystems I and II in pea leaves exposed to heat stress in the presence or absence of light: Analysis using in-vivo fluorescence, absorbance, oxygen and photoacoustic measurements. Planta 1991, 186, 88–98. [Google Scholar] [CrossRef]
- Buchner, O.; Stoll, M.; Karadar, M.; Kranner, I.; Neuner, G. Application of heat stress in situ demonstrates a protective role of irradiation on photosynthetic performance in alpine plants. Plant Cell Environ. 2015, 38, 812–826. [Google Scholar] [CrossRef]
- Krause, G.H.; Winter, K.; Krause, B.; Virgo, A. Light-stimulated heat tolerance in leaves of two neotropical tree species, Ficus insipida and Calophyllum longifolium. Funct. Plant Biol. 2014, 42, 42–51. [Google Scholar] [CrossRef]
- Krause, G.H.; Winter, K.; Krause, B.; Virgo, A. Protection by light against heat stress in leaves of tropical crassulacean acid metabolism plants containing high acid levels. Funct. Plant Biol. 2016, 43, 1061–1069. [Google Scholar] [CrossRef]
- Janda, T.; Prerostova, S.; Vankova, R.; Darko, E. Crosstalk between light- and temperature-mediated processes under cold and heat stress conditions in plants. Int. J. Mol. Sci. 2021, 22, 8602. [Google Scholar] [CrossRef]
- Roeber, V.M.; Bajaj, I.; Rohde, M.; Schmülling, T.; Cortleven, A. Light acts as a stressor and influences abiotic and biotic stress responses in plants. Plant Cell Environ. 2021, 44, 645–664. [Google Scholar] [CrossRef]
- Klughammer, C.; Schreiber, U. Complementary PS II quantum yields calculated from simple fluorescence parameters measured by PAM fluorometry and the Saturation Pulse method. PAM Appl. Notes 2008, 1, 27–35. [Google Scholar]
- Kramer, D.M.; Avenson, T.J.; Edwards, G.E. Dynamic flexibility in the light reactions of photosynthesis governed by both electron and proton transfer reactions. Trends Plant Sci. 2004, 9, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Murata, N.; Allakhverdiev, S.I.; Nishiyama, Y. The mechanism of photoinhibition in vivo: Re-evaluation of the roles of catalase, α-tocopherol, non-photochemical quenching, and electron transport. Biochim. Biophys. Acta Bioenerg. 2012, 1817, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Feng, X.; Wang, H.; Chen, Y.; Sun, Y. Heat-induced down-regulation of photosystem II protects photosystem I in honeysuckle (Lonicera japonica). J. Plant Res. 2021, 134, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.Z.; Yin, Z.P.; Lu, T.; Yang, X.L.; Wang, F.; Qi, M.F.; Li, T.L.; Liu, Y.F. Cyclic electron flow modulate the linear electron flow and reactive oxygen species in tomato leaves under high temperature. Plant Sci. 2020, 292, 110387. [Google Scholar] [CrossRef]
- Munekage, Y.; Hojo, M.; Meurer, J.; Endo, T.; Tasaka, M.; Shikanai, T. PGR5 is involved in cyclic electron flow around photosystem I and is essential for photoprotection in Arabidopsis. Cell 2002, 110, 361–371. [Google Scholar] [CrossRef]
- DalCorso, G.; Pesaresi, P.; Masiero, S.; Aseeva, E.; Schünemann, D.; Finazzi, G.; Joliot, D.; Barbato, R.; Leister, D. A complex containing PGRL1 and PGR5 is involved in the switch between linear and cyclic electron flow in Arabidopsis. Cell 2008, 132, 273–285. [Google Scholar] [CrossRef]
- Yamori, W.; Sakata, N.; Suzuki, Y.; Shikanai, T.; Makino, A. Cyclic electron flow around photosystem I via chloroplast NAD(P)H dehydrogenase (NDH) complex performs a significant physiological role during photosynthesis and plant growth at low temperature in rice. Plant J. 2011, 68, 966–976. [Google Scholar] [CrossRef]
- Yamori, W.; Shikanai, T.; Makino, A. Photosystem I cyclic electron flow via chloroplast NADH dehydrogenase-like complex performs a physiological role for photosynthesis at low light. Sci. Rep. 2015, 5, 13908. [Google Scholar] [CrossRef]
- Yamori, W.; Shikanai, T. Physiological functions of cyclic electron transport around photosystem I in sustaining photosynthesis and plant growth. Ann. Rev. Plant Biol. 2016, 67, 81–106. [Google Scholar] [CrossRef]
- Hertwig, B.; Streb, P.; Feierabend, J. Light dependence of catalase synthesis and degradation in leaves and the influence of interfering stress conditions. Plant Physiol. 1992, 100, 1547–1553. [Google Scholar] [CrossRef]
- Chen, J.H.; Chen, S.T.; He, N.Y.; Wang, Q.L.; Zhao, Y.; Gao, W.; Guo, F.Q. Nuclear-encoded synthesis of the D1 subunit of photosystem II increases photosynthetic efficiency and crop yield. Nat. Plants 2020, 6, 570–580. [Google Scholar] [CrossRef]
- Havaux, M. Short-term responses of Photosystem I to heat stress: Induction of a PS II-independent electron transport through PS I fed by stromal components. Photosynth. Res. 1996, 47, 85–97. [Google Scholar] [CrossRef]
- Wang, P.; Duan, W.; Takabayashi, A.; Endo, T.; Shikanai, T.; Ye, J.Y.; Mi, H. Chloroplastic NAD(P)H dehydrogenase in tobacco leaves functions in alleviation of oxidative damage caused by temperature stress. Plant Physiol. 2006, 141, 465–474. [Google Scholar] [CrossRef]
- He, J.; Chow, W.S. The rate coefficient of repair of photosystem II after photoinactivation. Physiol Plant. 2003, 118, 297–304. [Google Scholar] [CrossRef]
- Takahashi, S.; Murata, N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008, 13, 178–182. [Google Scholar] [CrossRef]
- Sonoike, K. Photoinhibition of photosystem I. Physiol. Plantarum. 2011, 142, 56–64. [Google Scholar] [CrossRef]
- Bukhov, N.G.; Dzhibladze, T.G.; Egorova, E.A. Elevated temperatures inhibit ferredoxin-dependent cyclic electron flow around photosystem I. Russ. J. Plant Physiol. 2005, 52, 578–583. [Google Scholar] [CrossRef]
- Marutani, Y.; Yamauchi, Y.; Kimura, Y.; Mizutani, M.; Sugimoto, Y. Damage to photosystem II due to heat stress without light-driven electron flow: Involvement of enhanced introduction of reducing power into thylakoid membranes. Planta 2012, 236, 753–761. [Google Scholar] [CrossRef]
- Bennoun, P. Evidence for a respiratory chain in the chloroplast. Proc. Natl. Acad. Sci. USA 1982, 79, 4352–4356. [Google Scholar] [CrossRef]
- Yi, X.P.; Yao, H.S.; Fan, D.Y.; Zhu, X.G.; Losciale, P.; Zhang, Y.L.; Zhang, W.F.; Chow, W.S. The energy cost of repairing photoinactivated photosystem II: An experimental determination in cotton leaf discs. New Phytol. 2022, 235, 446–456. [Google Scholar] [CrossRef]
- Aihara, Y.; Takahashi, S.; Minagawa, J. Heat induction of cyclic electron flow around photosystem I in the symbiotic dinoflagellate Symbiodinium. Plant Physiol. 2016, 171, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Mano, J.; Miyake, C.; Schreiber, U.; Asada, K. Photoactivation of the Electron Flow from NADPH to Plastoquinone in Spinach Chloroplasts. Plant Cell Physiol. 1995, 36, 1589–1598. [Google Scholar]
- Bernhard Teicher, H.; Vibe Scheller, H. The NAD(P)H dehydrogenase in barley thylakoids is photoactivatable and uses NADPH as well as NADH. Plant Physiol. 1998, 117, 525–532. [Google Scholar] [CrossRef] [PubMed]
- Mi, H.; Deng, Y.; Tanaka, Y.; Hibino, T.; Takabe, T. Photo-induction of an NADPH dehydrogenase which functions as a mediator of electron transport to the intersystem chain in the cyanobacterium Synechocystis PCC6803. Photosynth. Res. 2001, 70, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Gao, Y.; Wang, H.; Yang, X.; Zhai, H.; Du, Y. Stimulation of cyclic electron flow around PSI as a response to the combined stress of high light and high temperature in grape leaves. Funct. Plant Biol. 2018, 45, 1038–1045. [Google Scholar] [CrossRef]
- Weng, J.H.; Lai, M.F. Estimating heat tolerance among plant species by two chlorophyll fluorescence parameters. Photosynthetica 2005, 43, 439–444. [Google Scholar] [CrossRef]
- Xu, H.; Liu, G.; Liu, G.; Yan, B.; Duan, W.; Wang, L.; Li, S. Comparison of investigation methods of heat injury in grapevine (Vitis) and assessment to heat tolerance in different cultivars and species. BMC Plant Biol. 2014, 14, 156. [Google Scholar] [CrossRef]
- Stirbet, A. Govindjee Chlorophyll a fluorescence induction: A personal perspective of the thermal phase, the J–I–P rise. Photosynth. Res. 2012, 113, 15–61. [Google Scholar] [CrossRef]
- Yusuf, M.A.; Kumar, D.; Rajwanshi, R.; Strasser, R.J.; Tsimilli-Michael, M.; Govindjee; Sarin, N.B. Overexpression of gamma-tocopherol methyl transferase gene in transgenic Brassica juncea plants alleviates abiotic stress: Physiological and chlorophyll a fluorescence measurements. Biochim. Biophys. Acta 2010, 1797, 1428–1438. [Google Scholar] [CrossRef]
- Zagorchev, L.; Traianova, A.; Teofanova, D.; Li, J.; Kouzmanova, M.; Goltsev, V. Influence of Cuscuta campestris Yunck. on the photosynthetic activity of Ipomoea tricolor Cav.–in vivo chlorophyll a fluorescence assessment. Photosynthetica 2020, 58, 237–247. [Google Scholar] [CrossRef]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageorgiou, G.C., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Appenroth, K.J.; Stockel, J.; Srivastava, A.; Strasser, R.J. Multiple effects of chromate on the photosynthetic apparatus of Spirodela polyrhiza as probed by OJIP chlorophyll a fluorescence measurements. Environ. Pollut. 2001, 115, 49–64. [Google Scholar] [CrossRef]
- Järvi, S.; Suorsa, M.; Tadini, L.; Ivanauskaite, A.; Rantala, S.; Allahverdiyeva, Y.; Leister, D.; Aro, E.M. Thylakoid-bound FtsH proteins facilitate proper biosynthesis of photosystem I. Plant Physiol. 2016, 171, 1333–1343. [Google Scholar]
- Li, Q.; Yao, Z.J.; Mi, H. Alleviation of photoinhibition by co-ordination of chlororespiration and cyclic electron flow mediated by NDH under heat stressed condition in tobacco. Front. Plant Sci. 2016, 7, 285. [Google Scholar] [CrossRef]
- Shikanai, T.; Endo, T.; Hashimoto, T.; Yamada, Y.; Asada, K.; Yokota, A. Directed disruption of the tobacco ndhB gene impairs cyclic electron flow around photosystem I. Proc. Natl. Acad. Sci. USA 1998, 95, 9705–9709. [Google Scholar] [CrossRef]
- Huang, W.; Fu, P.L.; Jiang, Y.J.; Zhang, J.L.; Zhang, S.B.; Hu, H.; Cao, K.F. Differences in the responses of photosystem I and photosystem II of three tree species Cleistanthus sumatranus, Celtis philippensis and Pistacia weinmannifolia exposed to a prolonged drought in a tropical limestone forest. Tree Physiol. 2013, 33, 211–220. [Google Scholar] [CrossRef]
- Schansker, G.; Ohnishi, M.; Furutani, R.; Miyake, C. Identification of twelve different mineral deficiencies in Hydroponically grown sunflower plants on the basis of short measurements of the fluorescence and P700 oxidation/reduction kinetics. Front. Plant Sci. 2022, 13, 894607. [Google Scholar] [CrossRef]
- Mi, H.; Endo, T.; Ogawa, T.; Asada, K. Thylakoid membrane-bound, NADPH-specific pyridine nucleotide dehydrogenase complex mediates cyclic electron transport in the cyanobacterium Synechocystis sp. PCC 6803. Plant Cell Physiol. 1995, 36, 661–668. [Google Scholar]
- Ueno, M.; Sae-Tang, P.; Kusama, Y.; Hihara, Y.; Matsuda, M.; Hasunuma, T.; Nishiyama, Y. Moderate heat stress stimulates repair of photosystem II during photoinhibition in Synechocystis sp. PCC 6803. Plant Cell Physiol. 2016, 57, 2417–2426. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Allakhverdiev, S.I.; Murata, N. Protein synthesis is the primary target of reactive oxygen species in the photoinhibition of photosystem II. Physiol. Plant. 2011, 142, 35–46. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Wang, Q.; Xiao, H.; Cheng, J. Low Light Facilitates Cyclic Electron Flows around PSI to Assist PSII against High Temperature Stress. Plants 2022, 11, 3537. https://doi.org/10.3390/plants11243537
Sun Y, Wang Q, Xiao H, Cheng J. Low Light Facilitates Cyclic Electron Flows around PSI to Assist PSII against High Temperature Stress. Plants. 2022; 11(24):3537. https://doi.org/10.3390/plants11243537
Chicago/Turabian StyleSun, Yongjiang, Qi Wang, Huijie Xiao, and Jin Cheng. 2022. "Low Light Facilitates Cyclic Electron Flows around PSI to Assist PSII against High Temperature Stress" Plants 11, no. 24: 3537. https://doi.org/10.3390/plants11243537
APA StyleSun, Y., Wang, Q., Xiao, H., & Cheng, J. (2022). Low Light Facilitates Cyclic Electron Flows around PSI to Assist PSII against High Temperature Stress. Plants, 11(24), 3537. https://doi.org/10.3390/plants11243537




