Medicinal Plants in Semi-Natural Grasslands: Impact of Management
Abstract
:1. Introduction
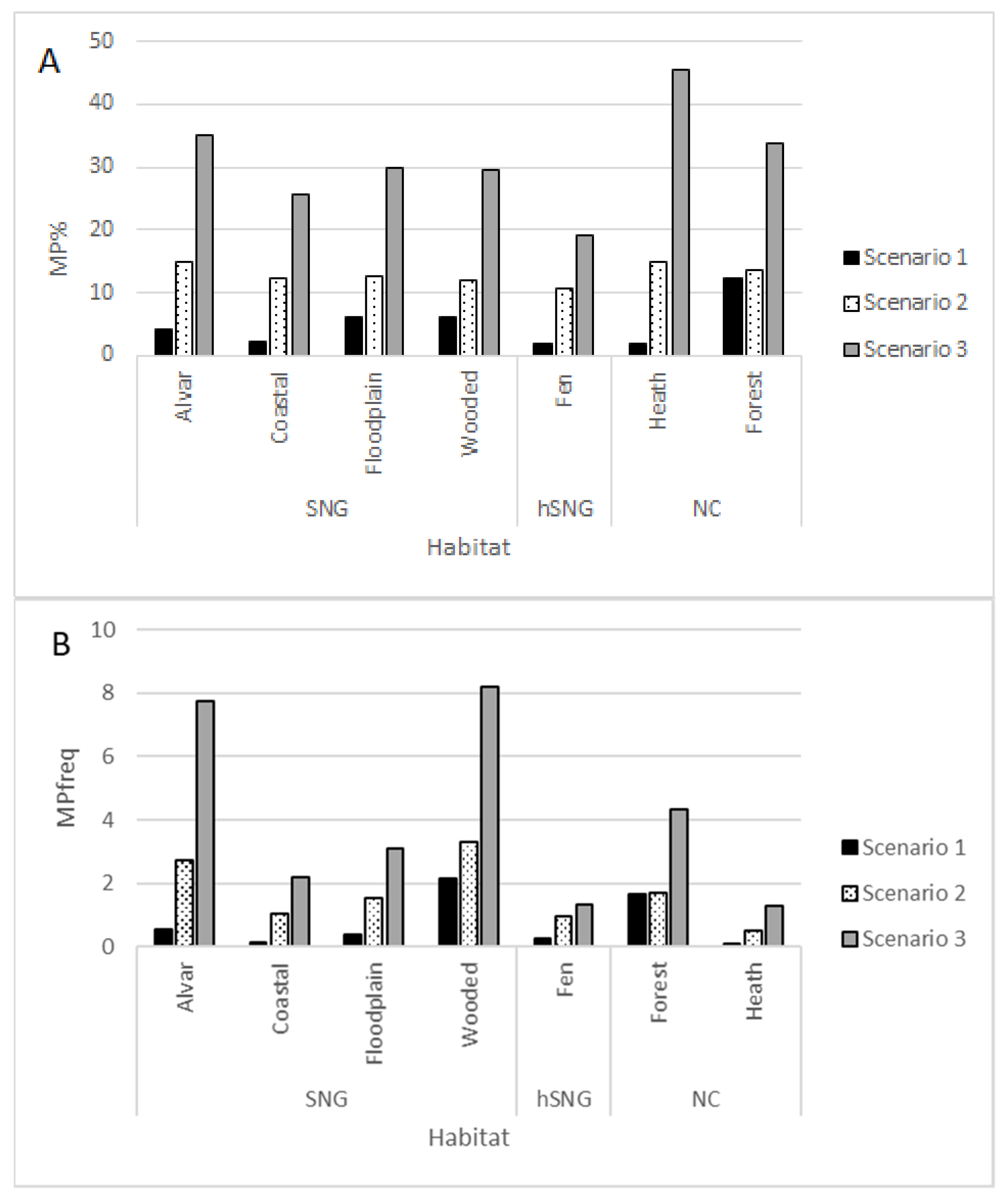
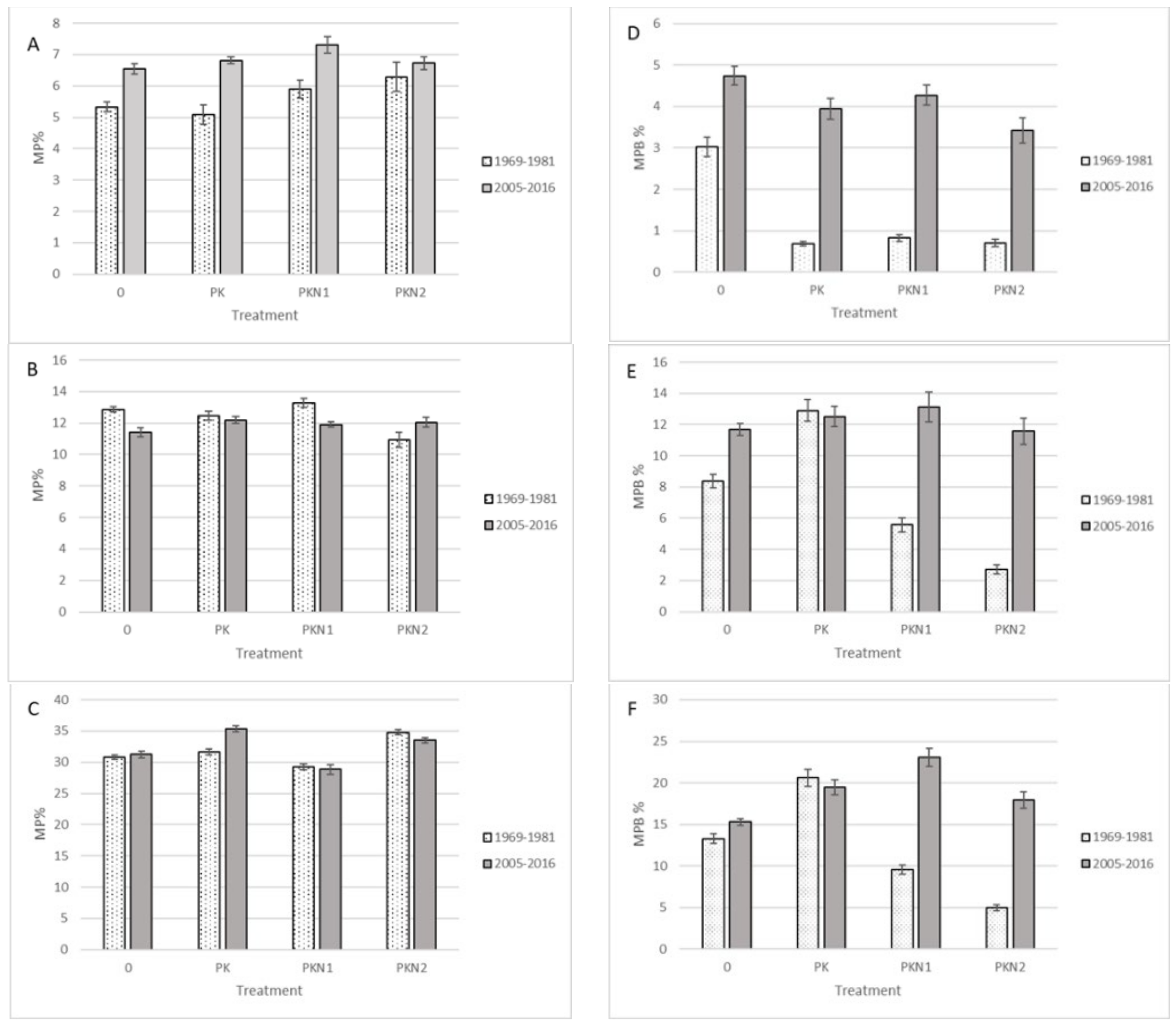
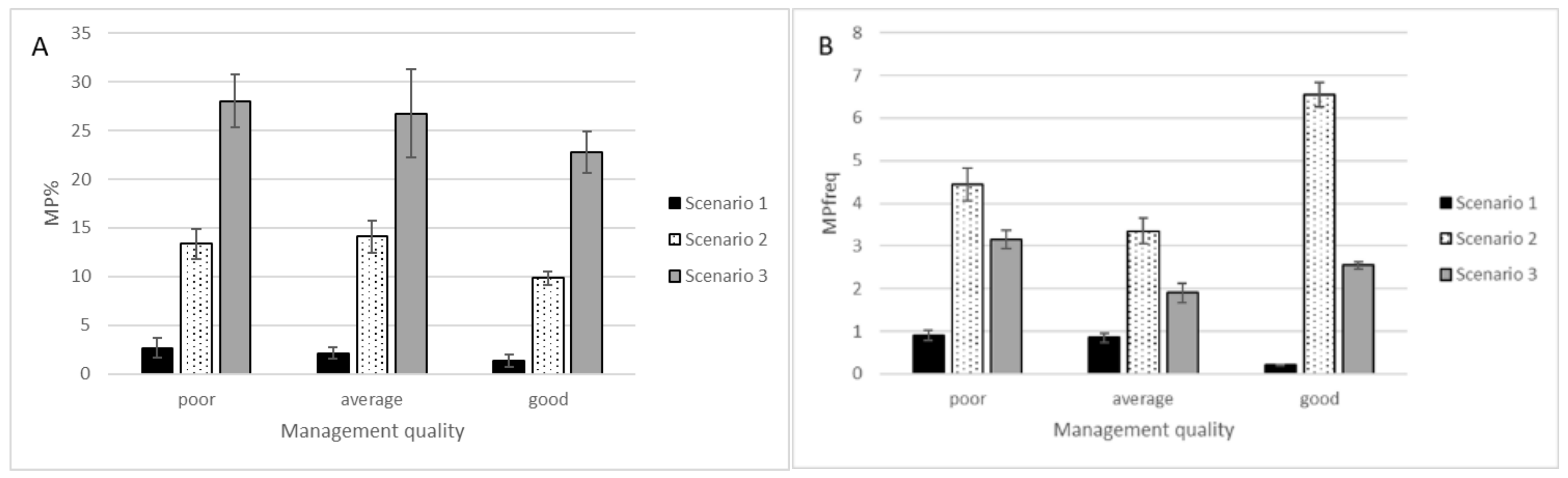
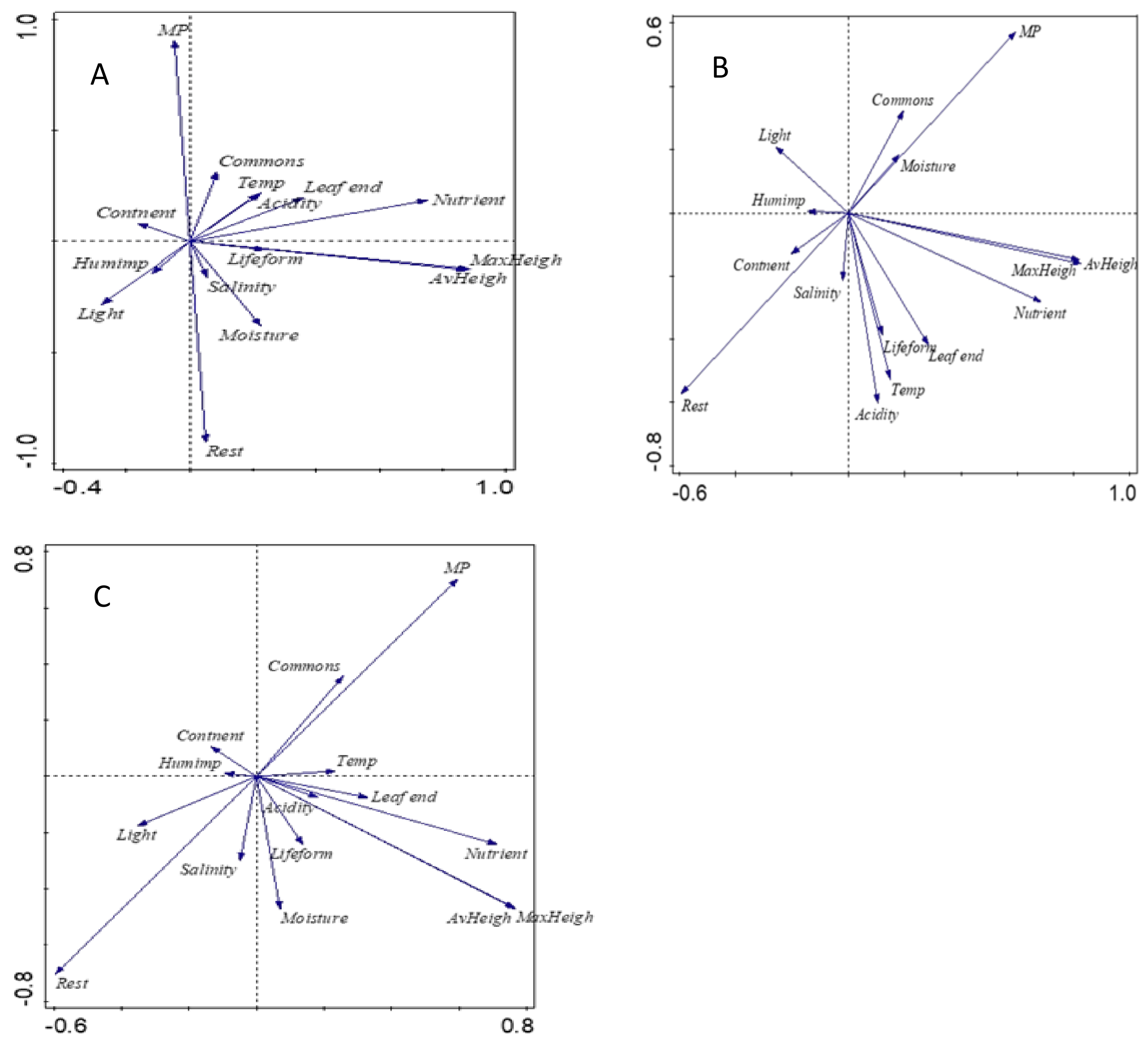
2. Results
3. Discussion
4. Materials and Methods
4.1. Medicinal Plant Species List
4.2. Floristic Databases and Created Parameters
4.3. Statistical Analyses
5. Conclusions
- The ratio of MP species in the local total plant species list was the largest in alvars, followed by floodplain and wooded meadows.
- The average number of MP species per study site in wooded meadows and alvars was about twice that found in naturally growing broad-leaved forest (according to the most detailed MP species list, 7.2, 7.8 and 4.3, respectively).
- Fertilization did not decrease MP species ratio in Estonian wooded meadow, but decreased the percentage of MP biomass in total yield.
- The frequency of MP species in Estonian coastal meadows depended on applied MP definition scenario.
- Principal component analysis revealed that MP species are more drought-tolerant, with higher commonness and more anthropophyte than the rest of studied grassland species.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valkó, O.; Deák, B.; Török, P.; Kelemen, A.; Miglécz, T.; Tóth, K.; Tóthmérész, B. Abandonment of croplands: Problem or chance for grassland restoration? case studies from hungary. Ecosyst. Health Sustain. 2016, 2, e01208. [Google Scholar] [CrossRef] [Green Version]
- Bartual, A.M.; Sutter, L.; Bocci, G.; Moonen, A.-C.; Cresswell, J.; Entling, M.; Giffard, B.; Jacot, K.; Jeanneret, P.; Holland, J.; et al. The potential of different semi-natural habitats to sustain pollinators and natural enemies in European agricultural landscapes. Agric. Ecosyst. Environ. 2019, 279, 43–52. [Google Scholar] [CrossRef]
- Parr, C.L.; Lehmann, C.; Bond, W.; Hoffmann, W.A.; Andersen, A. Tropical grassy biomes: Misunderstood, neglected, and under threat. Trends Ecol. Evol. 2014, 29, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Dzerefos, C.M.; Witkowski, E.; Kremer-Köhne, S. Aiming for the biodiversity target with the social welfare arrow: Medicinal and other useful plants from a Critically Endangered grassland ecosystem in Limpopo Province, South Africa. Int. J. Sustain. Dev. World Ecol. 2017, 24, 52–64. [Google Scholar] [CrossRef]
- Maseyk, F.J.F.; Demeter, L.; Csergő, A.M.; Buckley, Y.M. Effect of management on natural capital stocks underlying ecosystem service provision: A ‘provider group’ approach. Biodivers. Conserv. 2017, 26, 3289–3305. [Google Scholar] [CrossRef] [Green Version]
- Žuna Pfeiffer, T.; Špoljarić Maronić, D.; Petrošanec, S.; Štolfa Čamagajevac, I.; Stević, F. Steppe-like grassland as a refuge of the wild edible and medicinal plant species in anthropogenic landscape in northeastern Croatia. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2018, 152, 1059–1066. [Google Scholar] [CrossRef]
- Söderström, B.; Svensson, B.; Vessby, K.; Glimskär, A. Plants, insects and birds in semi-natural pastures in relation to local habitat and landscape factors. Biodivers. Conserv. 2001, 10, 1839–1863. [Google Scholar] [CrossRef]
- Vandebroek, I.; Pieroni, A.; Stepp, J.R.; Hanazaki, N.; Ladio, A.; Alves, R.R.N.; Picking, D.; Delgoda, R.; Maroyi, A.; Van Andel, T.; et al. Reshaping the future of ethnobiology research after the COVID-19 pandemic. Nat. Plants 2020, 6, 723–730. [Google Scholar] [CrossRef] [PubMed]
- Kalle, R.; Sõukand, R. Wild plants eaten in childhood: A retrospective of Estonia in the 1970s–1990s. Bot. J. Linn. Soc. 2013, 172, 239–253. [Google Scholar] [CrossRef]
- Aziz, M.A.; Khan, A.H.; Adnan, M.; Ullah, H. Traditional uses of medicinal plants used by Indigenous communities for veterinary practices at Bajaur Agency, Pakistan. J. Ethnobiol. Ethnomed. 2018, 14, 11. [Google Scholar] [CrossRef] [Green Version]
- Pavlova, D.; Karadjova, I.; Krasteva, I. Essential and toxic element concentrations in Hypericum perforatum. Aust. J. Bot. 2015, 63, 152–158. [Google Scholar] [CrossRef]
- Das, S.; Teja, K.C.; Mukherjee, S.; Seal, S.; Sah, R.K.; Duary, B.; Kim, K.-H.; Bhattacharya, S.S. Impact of edaphic factors and nutrient management on the hepatoprotective efficiency of Carlinoside purified from pigeon pea leaves: An evaluation of UGT1A1 activity in hepatitis induced organelles. Environ. Res. 2018, 161, 512–523. [Google Scholar] [CrossRef] [PubMed]
- Akula, R.; Ravishankar, G.A. Influence of abiotic stress signals on secondary metabolites in plants. Plant Signal. Behav. 2011, 6, 1720–1731. [Google Scholar] [CrossRef] [PubMed]
- Selmar, D.; Kleinwächter, M. Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind. Crop. Prod. 2013, 42, 558–566. [Google Scholar] [CrossRef]
- Hou, J.-L.; Li, W.-D.; Zheng, Q.-Y.; Wang, W.-Q.; Xiao, B.; Xing, D. Effect of low light intensity on growth and accumulation of secondary metabolites in roots of Glycyrrhiza uralensis Fisch. Biochem. Syst. Ecol. 2010, 38, 160–168. [Google Scholar] [CrossRef]
- Albergaria, E.T.; de Oliveira, A.F.M.; Albuquerque, U.P. The effect of water deficit stress on the composition of phenolic compounds in medicinal plants. S. Afr. J. Bot. 2020, 131, 12–17. [Google Scholar] [CrossRef]
- Yan, X.; Wu, S.; Wang, Y.; Shang, X.; Dai, S. Soil nutrient factors related to salidroside production of Rhodiola sachalinensis distributed in Chang Bai Mountain. Environ. Exp. Bot. 2004, 52, 267–276. [Google Scholar] [CrossRef]
- Jang, Y.K.; Jung, E.S.; Lee, H.-A.; Choi, D.; Lee, C.H. Metabolomic Characterization of Hot Pepper (Capsicum annuum “CM334”) during Fruit Development. J. Agric. Food Chem. 2015, 63, 9452–9460. [Google Scholar] [CrossRef]
- Jia, M.; Chen, L.; Xin, H.-L.; Zheng, C.-J.; Rahman, K.; Han, T.; Qin, L.-P. A Friendly Relationship between Endophytic Fungi and Medicinal Plants: A Systematic Review. Front. Microbiol. 2016, 7, 906. [Google Scholar] [CrossRef] [Green Version]
- Jurkiewicz, A.; Ryszka, P.; Anielska, T.; Waligórski, P.; Białońska, D.; Góralska, K.; Tsimilli-Michael, M.; Turnau, K. Optimization of culture conditions of Arnica montana L.: Effects of mycorrhizal fungi and competing plants. Mycorrhiza 2009, 20, 293–306. [Google Scholar] [CrossRef]
- Caruso, G.; Abdelhamid, M.; Kalisz, A.; Sekara, A. Linking Endophytic Fungi to Medicinal Plants Therapeutic Activity. A Case Study on Asteraceae. Agriculture 2020, 10, 286. [Google Scholar] [CrossRef]
- French, K. Species composition determines forage quality and medicinal value of high diversity grasslands in lowland England. Agric. Ecosyst. Environ. 2017, 241, 193–204. [Google Scholar] [CrossRef]
- Heinsoo, K.; Sammul, M.; Kukk, T.; Kull, T.; Melts, I. The long-term recovery of a moderately fertilised semi-natural grassland. Agric. Ecosyst. Environ. 2019, 289, 106744. [Google Scholar] [CrossRef]
- Kose, M.; Heinsoo, K.; Kaljund, K.; Tali, K. Twenty years of Baltic Boreal coastal meadow restoration: Has it been long enough? Restor. Ecol. 2020, 29, e13266. [Google Scholar] [CrossRef]
- Gotelli, N.J.; Colwell, R.K. Quantifying biodiversity: Procedures and pitfalls in the measurement and comparison of species richness. Ecol. Lett. 2001, 4, 379–391. [Google Scholar] [CrossRef] [Green Version]
- Sammul, M.; Kull, T.; Lanno, K.; Otsus, M.; Mägi, M.; Kana, S. Habitat preferences and distribution characteristics are indicative of species long-term persistence in the Estonian flora. Biodivers. Conserv. 2008, 17, 3531–3550. [Google Scholar] [CrossRef]
- Päretel, M.; Helm, A.; Roosaluste, E.; Zobel, M. Biological diversity of Estonian semi-natural grassland ecosystems. Probl. Contemp. Environ. Stud. 2007, 10, 223–302. [Google Scholar]
- Kull, K.; Zobel, M. High species richness in an Estonian wooded meadow. J. Veg. Sci. 1991, 2, 715–718. [Google Scholar] [CrossRef]
- Raal, A. Maailma Ravimtaimede Entsüklopeedia; Estonian Encyclopaedia Publishers: Tallinn, Estonia, 2010; ISBN 978-9985-70-313-7. [Google Scholar]
- Laasimer, L. Eesti NSV Taimkate; Valgus: Tallinn, Estonia, 1965. [Google Scholar]
- Terletskaya, N.V.; Korbozova, N.K.; Kudrina, N.O.; Kobylina, T.N.; Kurmanbayeva, M.S.; Meduntseva, N.D.; Tolstikova, T.G. The Influence of Abiotic Stress Factors on the Morphophysiological and Phytochemical Aspects of the Acclimation of the Plant Rhodiola semenowii Boriss. Plants 2021, 10, 1196. [Google Scholar] [CrossRef]
- Mahajan, M.; Kuiry, R.; Pal, P.K. Understanding the consequence of environmental stress for accumulation of secondary metabolites in medicinal and aromatic plants. J. Appl. Res. Med. Aromat. Plants 2020, 18, 100255. [Google Scholar] [CrossRef]
- Hassan, H.M.; Jiang, Z.-H.; Syed, T.A.; Qin, W. Review: Northern Ontario medicinal plants. Can. J. Plant Sci. 2012, 92, 815–828. [Google Scholar] [CrossRef]
- Kumar, K.H.; Razack, S.; Nallamuthu, I.; Khanum, F. Phytochemical analysis and biological properties of Cyperus rotundus L. Ind. Crop. Prod. 2014, 52, 815–826. [Google Scholar] [CrossRef]
- Cui, H.; Pan, H.-W.; Wang, P.-H.; Yang, X.-D.; Zhai, W.-C.; Dong, Y.; Zhou, H.-L. Essential oils from Carex meyeriana Kunth: Optimization of hydrodistillation extraction by response surface methodology and evaluation of its antioxidant and antimicrobial activities. Ind. Crop. Prod. 2018, 124, 669–676. [Google Scholar] [CrossRef]
- Kelly, T.R.; Bunn, D.A.; Joshi, N.P.; Grooms, D.; Devkota, D.; Devkota, N.R.; Paudel, L.N.; Roug, A.; Wolking, D.J.; Mazet, J.A.K. Awareness and Practices Relating to Zoonotic Diseases Among Smallholder Farmers in Nepal. EcoHealth 2018, 15, 656–669. [Google Scholar] [CrossRef] [PubMed]
- Dold, A.P.; Cocks, M.L. The trade in medicinal plants in the Eastern Cape Province, South Africa. S. Afr. J. Sci. 2002, 98, 589–597. [Google Scholar]
- Tammeorg, J.; Kook, O.; Vilbaste, G. Eesti NSV Ravimtaimed, 5th ed.; Valgus: Tallinn, Estonia, 1984. [Google Scholar]
- Estonian Agency of Medicine. Ravimina Määratletud Raviomadustega Ainete ja Taimede Nimekiri. Available online: https://ravimiamet.ee/ravimina-m%C3%A4%C3%A4ratletud-raviomadustega-ainete-ja-taimede-nimekiri (accessed on 25 November 2020).
- European Commission DG Environment. Interpretation Manual of European Union Habitats version EUR 28; 2013. Available online: https://ec.europa.eu/environment/nature/legislation/habitatsdirective/docs/Int_Manual_EU28.pdf (accessed on 25 November 2020).
- Krall, H.; Kukk, T.; Kuusk, V.; Leht, M.; Oja, T.; Pihu, S.; Reier, Ü.; Zingel, H.; Tuulik, T.; Muuga, G. Eesti Taimede Välimääraja, 1st ed.; Leht, M., Ed.; Eesti Maaülikool: Tartu, Estonia, 2010. [Google Scholar]
| Habitat Type | Total Species Number | MP Species | ||
|---|---|---|---|---|
| Scenario 1 | Scenario 2 | Scenario 3 | ||
| Alvar | 187 | 9 | 20 | 51 |
| Coastal | 150 | 8 | 17 | 40 |
| Fen | 89 | 2 | 6 | 16 |
| Floodplain | 194 | 13 | 22 | 57 |
| Wooded | 330 | 19 | 29 | 78 |
| Broad-leaved forest | 181 | 13 | 20 | 50 |
| Heath | 51 | 2 | 7 | 20 |
| Habitat Type | Community n | % of Total Species List | % of MP Species List | ||
|---|---|---|---|---|---|
| Scenario 1 | Scenario 2 | Scenario 3 | |||
| Alvar | 36 | 67.6 | 40.0 | 66.7 | 55.0 |
| Coastal | 14 | 59.0 | 50.0 | 36.4 | 54.5 |
| Fen | 12 | 46.7 | 20.0 | 37.5 | 35.7 |
| Floodplain | 8 | 69.0 | 22.2 | 80.0 | 85.7 |
| Wooded | 71 | 72.3 | 50.0 | 68.8 | 73.1 |
| Broad-leaved forest | 10 | 58.0 | 83.3 | 80.0 | 60.0 |
| Heath | 10 | 43.5 | 0.0 | 0.0 | 25.0 |
| Class | Scenario 1 | Scenario 2 | Scenario 3 |
|---|---|---|---|
| Total number of MP species in list | 260 | 152 | >1700 |
| from these domestic | 62 | 113 | 207 |
| trees and bushes | 3 | 25 | 27 |
| fungi | 3 | 1 | 3 |
| vine | 1 | 1 | 2 |
| lichen | 0 | 1 | 2 |
| low-height shrubs and herbaceous plants | 55 | 85 | 174 |
| Main purpose of the list | Import and market control | Growing and gathering | Encyclopedic knowledge |
| Habitat Type | Reason | NATURA 2000 Habitat | Number of Sites | Number of Plots | Average Shannon Index |
|---|---|---|---|---|---|
| Alvars | SNG | 6280* Nordic alvar and precambrian calcareous flatrocks | 18 | 920 | 0.93 |
| Coastal meadows | SNG | 1630* Boreal Baltic coastal meadows | 14 | 280 | 0.90 |
| Floodplain meadows | SNG | 6450 Northern boreal alluvial meadows | 8 | 640 | 0.85 |
| Fen meadows | Historic SNG | 72 Calcareous fens | 10 | 900 | 0.83 |
| 90 | |||||
| 7160 Fennoscandian mineral-rich springs and springfens | |||||
| Wooded meadows | SNG | 6530* Fennoscandian wooded meadows | 17 | 2800 | 0.91 |
| Heath | Natural habitat | 21 Sea dunes of the Atlantic, North Sea and Baltic coasts | 8 | 800 | 0.76 |
| 4030 European dry heaths | |||||
| Broad-leaved forest | Natural habitat | 9010 Western taiga | 8 | 420 | 0.88 |
| 9020* Fennoscandian hemiboreal natural old broad-leaved deciduous forests (Quercus, Tilia, Acer, Fraxinus or Ulmus) rich in epiphytes | |||||
| 9050 Fennoscandian herb-rich forests with Picea abies | |||||
| 9060 Coniferous forests on, or connected to, glaciofluvial eskers |
| Characteristic/Class | Abbreviation | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|---|
| Sensitivity to human impact | Humimp | Anthropophyte | Apophyte | Hemiradiaphore | Hemerophobe | |||||
| Commonness | Commons | Not known | Very rare | Rare | Uncommon | Scattered | Occasional | Common | Frequent | |
| Life form | Lifeform | Woody chamaephyte | Chamaephyte | Hemicrytophyte | Geophyte | Therophyte | Hydrophyte | |||
| Leaf endurance | Leaf end | Evergreen | Summergreen | Springgreen | ||||||
| Light | Light | Deep shade plant | Av. | Shade plant | Av. | Semi-shade plant | Av. | Plant in well-lit places | Light-loving plant | Plant in full light |
| Temperature | Temp | Cold, alpine | Av. | Cool, subalpine | Av. | Moderate heat indicators | Av. | Warm | ||
| Continentality | Contnent | Euroceanic | Oceanic | Between 2 and 4 | Near oceanic | Intermediate | Subcontinental | Av. | Continental | |
| Soil moisture | Moisture | Extreme dryness | Av. | Dry-site indicator | Av. | Moist-site indicator | Av. | Dampness indicator | Wet-site indicator | |
| Soil acidity | Acidity | Indicator of extreme acidity | Av. | Acidity indicator | Av. | Indicator of moderately acid soils | Av. | Weakly acid to weakly basic soils | Av. | Indicator of basic reaction |
| Nutrients demand | Nutrient | Indicator of extremely infertile sites | Av. | Indicator of more or less fertile soils | Av. | Intermediate fertility | Av. | Richly fertile soils | Av. | Extremely rich soils |
| Salinity | Salinity | Slightly salt-tolerant species | Species both in saline and non-saline | Common in coastal sites | Consistent but low salinity | Obligate halophytes | Species of mid-level saltmarsh | Species of lower saltmarsh | Permanently inundated by seawater |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kose, M.; Melts, I.; Heinsoo, K. Medicinal Plants in Semi-Natural Grasslands: Impact of Management. Plants 2022, 11, 353. https://doi.org/10.3390/plants11030353
Kose M, Melts I, Heinsoo K. Medicinal Plants in Semi-Natural Grasslands: Impact of Management. Plants. 2022; 11(3):353. https://doi.org/10.3390/plants11030353
Chicago/Turabian StyleKose, Marika, Indrek Melts, and Katrin Heinsoo. 2022. "Medicinal Plants in Semi-Natural Grasslands: Impact of Management" Plants 11, no. 3: 353. https://doi.org/10.3390/plants11030353
APA StyleKose, M., Melts, I., & Heinsoo, K. (2022). Medicinal Plants in Semi-Natural Grasslands: Impact of Management. Plants, 11(3), 353. https://doi.org/10.3390/plants11030353






