Adaptability and Stability of Safflower Genotypes for Oil Production
Abstract
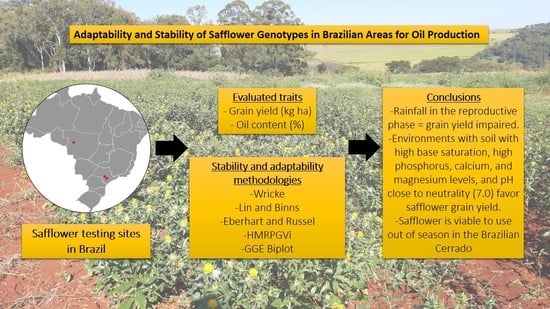
1. Introduction
2. Materials and Methods
2.1. Genetic Material, Experimental Design, and Traits Assessed
2.2. Statistical Analyses
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumari, S.; Choudhary, R.C.; Kumara Swamy, R.V.; Saharan, V.; Joshi, A.; Munot, J. Assessment of Genetic Diversity in Safflower (Carthamus tinctorius L.) Genotypes through Morphological and SSR Marker. J. Pharmacogn. Phytochem. 2017, 6, 2723–2731. [Google Scholar]
- Sharifi, R.S.; Namvar, A.; Sharifi, R.S. Grain Filling and Fatty Acid Composition of Safflower Fertilized with Integrated Nitrogen Fertilizer and Biofertilizers. Pesq. Agropec. Bras. 2017, 52, 236–243. [Google Scholar] [CrossRef]
- Tong, X.; Yang, J.; Zhao, Y.; Wan, H.; He, Y.; Zhang, L.; Wan, H.; Li, C. Greener Extraction Process and Enhanced In Vivo Bioavailability of Bioactive Components from Carthamus tinctorius L. by Natural Deep Eutectic Solvents. Food Chem. 2021, 348, 129090. [Google Scholar] [CrossRef] [PubMed]
- Paludo, J.T.S.; Bonfim-Silva, E.M.; Silva, T.J.A.; Souza, H.H.F.; Zanotto, M.D.; Fenner, W. Agronomic Performance of Safflower Genotypes (Carthamus tinctorius L.) under Different Soil Bulk Density Levels in the Oxisol of the Cerrado. Aust. J. Crop Sci. 2018, 12, 407–412. [Google Scholar] [CrossRef]
- de Oliveira Neto, S.S.; Zeffa, D.M.; Ebertz, O.F.; Zoz, T.; Zanotto, M.D. Genetic Variability, Adaptability and, Stability of Safflower Genotypes Developed for the Brazilian Conditions by REML/BLUP. Agron. J. 2021, 113, 3100–3109. [Google Scholar] [CrossRef]
- Oelke, E.A.; Oplinger, E.S.; Teynor, T.M.; Putnam, D.H.; Doll, J.D.; Kelling, K.A.; Durgan, B.R.; Noetzel, D.M. Safflower: Alternative Field Crops Manual; Purdue University: St. Paul, MN, USA, 1992. [Google Scholar]
- Singh, V.; Nimbkar, N. Safflower. In Breeding Oilseed Crops for Sustainable Production, 1st ed.; Gupta, S., Ed.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 147–165. [Google Scholar]
- Golkar, P.; Shahbazi, E.; Nouraein, M. Combining Ability X Environment Interaction and Genetic Analysis for Agronomic Traits in Safflower (Carthamus tinctorius L.): Biplot as a Tool for Diallel Data. Acta Agric. Slov. 2017, 109, 229–240. [Google Scholar] [CrossRef]
- Olivo, M.; Bassegio, D.; Zanotto, M.D. Combining Ability and Heterosis in a Diallel Cross of Safflower under Brazilian Tropical Conditions. Agron. J. 2020, 112, 1580–1588. [Google Scholar] [CrossRef]
- Espanani, S.; Majidi, M.M.; Saeidi, G.; Alaei, H.; Rezaei, V. Wide Hybridization and Introgression Breeding in Safflower: Effectiveness of Different Selection Methods. Plant Breed. 2019, 138, 846–861. [Google Scholar] [CrossRef]
- Polizel, A.C.; Juliatti, F.C.; Hamawaki, O.T.; Hamawaki, R.L.; Guimarães, S.L. Phenotypical Adaptability and Stability of Soybean Genotypes in the State of Mato Grosso. Biosci. J. 2013, 29, 910–920. [Google Scholar]
- Soares, I.O.; Bruzi, A.T.; Zambiazzi, E.V.; Guilherme, S.R.; Bianchi, M.C.; Silva, K.B.; Fronza, V.; Teixeira, C.M. Stability and Adaptability of Soybean Cultivars in Minas Gerais. Genet. Mol. Res. 2017, 16, gmr16039730. [Google Scholar] [CrossRef]
- Cruz, C.D.; Regazzi, A.J.; Carneiro, P.C.S. Modelos Biométricos Aplicados ao Melhoramento Genético, 3rd ed.; Editora UFV: Viçosa, Brazil, 2014; p. 668. [Google Scholar]
- Wricke, G. Zur Berechnung der Ökovalenz Bei Sommerweizen und Hafer. Z. Pflanzenzücht. 1965, 52, 127–138. [Google Scholar]
- Lin, C.S.; Binns, M.R. A Superiority Measure of Cultivar Performance for Cultivar X Location Data. Can. J. Plant Sci. 1988, 68, 193–198. [Google Scholar] [CrossRef]
- Eberhart, S.A.; Russel, W.A. Stability Parameters for Comparing Varieties. Crop Sci. 1966, 6, 36–40. [Google Scholar] [CrossRef]
- Yan, W.; Hunt, L.A.; Sheng, Q.L.; Szlavnics, Z. Cultivar Evaluation and Mega-Environment Investigation Based on the GGE Biplot. Crop Sci. 2000, 40, 597–605. [Google Scholar] [CrossRef]
- Resende, M.D.V. Software Selegen-REML/BLUP: A Useful Tool for Plant Breeding. Crop Breed. Appl. Biotechnol. 2016, 16, 330–339. [Google Scholar] [CrossRef]
- Sousa, A.M.C.B.; Silva, V.B.; Lopes, A.C.A.; Ferreira-Gomes, R.L.; Carvalho, L.C.B. Prediction of Grain Yield, Adaptability, and Stability in Landrace Varieties of Lima Bean (Phaseolus lunatus L.). Crop Breed. Appl. Biotechnol. 2020, 20, e295120115. [Google Scholar] [CrossRef]
- Golkar, P.; Rahmatabadi, N.; Mirmohammady Maibody, S.A.M. Improvement of Yield and Yield Stability in Safflower Using Multivariate, Parametric and Non-Parametric Methods under Different Irrigation Treatments and Planting Date. Acta Agric. Slov. 2020, 115, 315–327. [Google Scholar] [CrossRef]
- Ebrahimi, F.; Majidi, M.M.; Arzani, A.; Mohammadi-Nejad, G. Oil and Seed Yield Stability in a Worldwide Collection of Safflower under Arid Environments of Iran. Euphytica 2016, 212, 131–144. [Google Scholar] [CrossRef]
- Alizadeh, K.; Mohammadi, R.; Shariati, A.; Eskandari, M. Comparative Analysis of Statistical Models for Evaluating Genotype X Environment Interaction in Rainfed Safflower. Agric. Res. 2017, 6, 455–465. [Google Scholar] [CrossRef]
- Koç, H. Genotype-By-Environment Interaction for Seed Yield and Oil Content of Safflower (Carthamus tinctorius L.) Genotypes. Genetika 2021, 53, 11–22. [Google Scholar] [CrossRef]
- Shahrokhnia, M.H.; Sepaskhah, A.R. Physiologic and Agronomic Traits in Safflower under Various Irrigation Strategies, Planting Methods and Nitrogen Fertilization. Ind. Crops Prod. 2017, 95, 126–139. [Google Scholar] [CrossRef]
- OECD. ENV/JM/MONO(2020)14. Consensus Document on the Biology of Safflower (Carthamus tinctorius L.). 2020. Available online: https://www.oecd.org/officialdocuments/publicdisplaydocumentpdf/?cote=env/jm/mono(2020)14&doclanguage=en (accessed on 18 February 2022).
- Mündel, H.-H.; Blackshaw, R.E.; Byers, J.R.; Huang, H.C.; Johnson, D.L.; Keon, R.; Kubik, J.; McKenzie, R.; Otto, B.; Rooth, B.; et al. Safflower Production on the Canadian Prairies: Revisited in 2004; Agriculture and Agri-Food Canada, Research Station: Lethbridge, AB, Canada, 2004; 43p. [Google Scholar]
- Prado, R.M. Nutrição de Plantas, 1st ed.; Editora UNESP: São Paulo, Brazil, 2008; 407p. [Google Scholar]
- Guedes, E.M.S.; Fernandes, A.R.; Lima, E.V.; Gama, M.A.P.; Silva, A.L.P. Fosfato Natural de Arad e Calagem e o Crescimento de Brachiaria Brizanta em Latossolo Amarelo sob Pastagem Degradada na Amazônia. Rev. Ciênc. Agrár. 2009, 1, 117–129. [Google Scholar]
- EMBRAPA. Sistema Brasileiro de Classificação de Solos, 5th ed.; Embrapa: Brasília, Brazil, 2014; 355p. [Google Scholar]
- Pavlov, D.C.; Tadorov, N.A. Safflower (Carthamus tinctorius L.). In Food and Feed from Legumes and Oilseeds, 1st ed.; Nwokolo, E., Smartt, J., Eds.; Springer: Boston, MA, USA, 1996; pp. 245–246. [Google Scholar]
- Abbadi, J.; Gerendas, J. Effects of Phosphorus Supply on Growth, Yield and Yield Components of Safflower and Sunflower. J. Plant Nutr. 2011, 34, 1769–1787. [Google Scholar] [CrossRef]
- Golzarfar, M.; Shirani Rad, A.H.; Delkhosh, B.; Bitarafan, Z. Safflower (Carthamus tinctorius L.) Response to Different Nitrogen and Phosphorus Fertilizer Rates in Two Planting Seasons. Žemdirb.-Agric. 2012, 99, 159–166. [Google Scholar]
- Rivas, J.; Matarazzo, R. Produccione de Cartamo: Consideraciones Generales; Instituto Nacional de Tecnología Agropecuaria: Buenos Aires, Argentina, 2009; p. 23. [Google Scholar]
- Koutroubas, S.D.; Papakosta, D.K.; Doitsinis, A. Phenotypic Variation in Physiological Determinants of Yield in Spring Sown Safflower under Mediterranean Conditions. Field Crop. Res. 2009, 112, 199–204. [Google Scholar] [CrossRef]
- Omidi, A.H.; Khazaei, H.; Monneveux, P.; Stoddard, F. Effect of Cultivar and Water Regime on Yield and Yield Components in Safflower (Carthamus tinctorius L.). Turk. J. Field Crops 2012, 17, 10–15. [Google Scholar]
- Singh, S.; Angadi, S.V.; Grover, K.; Begna, S.; Auld, D. Drought Response and Yield Formation of Spring Safflower under Different Water Regimes in the Semiarid Southern High Plains. Agric. Water Manag. 2016, 163, 354–362. [Google Scholar] [CrossRef]
- Bortolheiro, F.P.; Silva, M.A. Physiological Response and Productivity of Safflower Lines under Water Deficit and Rehydration. An. Acad. Bras. Ciênc. 2017, 89, 3051–3066. [Google Scholar] [CrossRef]
- Çamas, N.; Çirak, C.; Esendal, E. Seed yield, Oil Content and Fatty Acids Composition of Safflower (Carthamus tinctorius L.) Grown in Northern Turkey Conditions. J. Fac. Agric. Ondokuz Mayıs. Univ. 2007, 22, 98–104. [Google Scholar]
- Hussain, M.I.; Lyra, D.A.; Farooq, M.; Nikoloudakis, N.; Khalid, N. Salt and Drought Stresses in Safflower: A Review. Agron. Sustain. Dev. 2016, 36, 4. [Google Scholar] [CrossRef]
- Mattos, P.H.C. Evaluation of Sugarcane Genotypes and Production Environments in Paraná by GGE Biplot and AMMI Analysis. Crop Breed. Appl. Biotechnol. 2013, 13, 83–90. [Google Scholar] [CrossRef]
- Oda, M.C.; Sediyama, T.; Matsuo, E.; Nascimento, M.; Cruz, C.D. Estabilidade e Adaptabilidade de Produção de Grãos de Soja por Meio de Metodologias Tradicionais e Redes Neurais Artificiais. Sci. Agrar. Parana. 2019, 18, 117–124. [Google Scholar]
- Pacheco, R.M.; Duarte, J.B.; Vencovsky, R.; Pinheiro, J.B.; Oliveira, A.B. Use of Supplementary Genotypes in AMMI Analysis. Theor. Appl. Genet. 2005, 110, 812–818. [Google Scholar] [CrossRef]
- Shahzad, K.; Qi, T.; Guo, L.; Tang, H.; Zhang, X.; Wang, H.; Qiao, X.; Zhang, M.; Zhang, B.; Feng, J.; et al. Adaptability and Stability Comparisons of Inbred and Hybrid Cotton in Yield and Fiber Quality Traits. Agronomy 2019, 9, 516. [Google Scholar] [CrossRef]
- Resende, M.D.V. Métodos Estatísticos Ótimos na Análise de Experimentos de Campo, 1st ed.; Embrapa Florestas: Colombo, Brazil, 2004; p. 57. [Google Scholar]
- Yan, W.; Tinker, N.A. Biplot Analysis of Multi-Environment Trial Data: Principles and Applications. Can. J. Plant Sci. 2006, 86, 623–645. [Google Scholar] [CrossRef]
- Pourdad, S.S.; Moghaddam, M.J. Study on Seed Yield Stability of Sunflower Inbred Lines through GGE Biplot. Helia 2013, 36, 19–28. [Google Scholar] [CrossRef]
- Mahasi, M.J.; Pathak, R.S.; Wachira, F.N.; Riungu, T.C.; Kinyua, M.G.; Waweru, J.K. Genotype by Environment (GxE) Interaction and Stability in Safflower (Carthamus tinctorious L.). Asian J. Plant Sci. 2006, 5, 1017–1021. [Google Scholar] [CrossRef][Green Version]


| Characteristics | Environments | |||
|---|---|---|---|---|
| Bauru-SP | Botucatu-SP (1) | Botucatu-SP (2) | Campo Novo Do Parecis-MT | |
| Growing season | 2018 | 2018 | 2019 | 2019 |
| Geographic region | Southeast | Southeast | Southeast | Central–west |
| State | São Paulo | São Paulo | São Paulo | Mato Grosso |
| Soil taxonomy (USDA) | Oxisol | Oxisol | Oxisol | Oxisol |
| Base saturation (%) | 74.0 | 94.0 | 32.0 | 55.0 |
| pH (H2O) | 6.0 | 6.9 | 4.4 | 6.2 |
| H + Al (mmolc dm3) | 10.0 | 9.0 | 56.0 | 43.8 |
| K (mmolc dm3) | 1.0 | 2.0 | 2.0 | 1.6 |
| Ca (mmolc dm3) | 18.0 | 98.0 | 15.0 | 37.0 |
| Mg (mmolc dm3) | 9.0 | 41.0 | 9.0 | 13.0 |
| Al (mmolc dm3) | 0.0 | 0.0 | 1.0 | 0.0 |
| P (mg dm3) | 10.0 | 38.0 | 12.0 | 26.0 |
| Organic matter (%) | 1.1 | 0.0 | 3.0 | 3.0 |
| Climate classification 1 | Cwa | Cwa | Cwa | Aw |
| Annual average temperature (°C) | 21.6 | 20.2 | 20.2 | 22.7 |
| Accumulated annual rainfall (mm) | 1170.0 | 1300.0 | 1300.0 | 1940.0 |
| Altitude (m) | 526.0 | 760.0 | 770.0 | 572.0 |
| Environment | Grain Yield (kg ha−1) | Oil Content (%) | ||
|---|---|---|---|---|
| Average | CV (%) | Average | CV (%) | |
| Bauru-SP | 831.92 | 14.20 | 21.25 | 9.33 |
| Botucatu-SP(1) | 1226.16 | 17.71 | 19.91 | 10.46 |
| Botucatu-SP(2) | 621.81 | 18.19 | 18.85 | 8.60 |
| C. N Parecis-MT | 278.71 | 29.17 | 22.33 | 10.04 |
| Average | 739.65 | 19.82 | 20.58 | 9.61 |
| Mean square | ||||
| Source of variation | Df | Grain yield | Oil content | |
| Blocks | 2 | 1332.04 | 8.80 | |
| Genotype (G) | 10 | 309,434.76 ** | 70.10 ** | |
| Environment (E) | 3 | 5,186,528.38 ** | 76.55 ** | |
| G × E | 30 | 528,892.39 ** | 25.57 ** | |
| Residue | 80 | 20,126.31 | 3.98 | |
| MSr+/MSr− | 7.13 | 1.91 | ||
| CVg average (%) | 20.99 | 11.40 | ||
| CVe average (%) | 19.18 | 9.70 | ||
| CVg/CVe ratio | 1.09 | 1.17 | ||
| Average | Wricke | Lin and Binns | Eberhart and Russel | HMRPGVi | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GY | %O | GY | %O | GY | %O | GY | %O | GY | %O | |||||
| Genotype | kg ha−1 | % | Wi% | Wi% | Pi | Pi | β | S2d 1/ | R2 | β | S2d 1/ | R2 | kg ha−1 | % |
| IMA7326 | 638.14 † a | 26.42 † a | 5.70 | 9.63 | 514,476.24 | 44.16 | 0.73 * | 12.69 ** | 48.48 | 0.02 * | 34.77 ** | 0.00 | 763.44 | 27.48 |
| P43 | 639.91 a | 23.40 a b | 6.88 | 7.29 | 529,319.47 | 33.60 | 0.82 | 16.74 ** | 47.47 | −1.34 ** | 14.60 ** | 28.11 | 693.56 | 24.25 |
| P30 | 870.98 a | 19.12 c | 4.00 | 10.28 | 338,413.37 | 15.11 | 0.65 ** | 7.00 ** | 56.46 | 2.01* | 5.10* | 68.59 | 977.54 | 16.82 |
| P28 | 764.14 a | 18.43 c | 2.82 | 7.32 | 207,086.42 | 15.65 | 1.00 | 6.78 ** | 75.83 | 1.38 | 3.08* | 59.94 | 741.44 | 16.65 |
| P7 | 754.77 a | 19.92 b c | 4.13 | 0.43 | 367,791.77 | 11.15 | 1.28 * | 8.43 ** | 80.85 | 0.71 | −1.23 | 94.82 | 761.28 | 19.30 |
| P35 | 543.13 a | 17.77 c | 1.29 | 48.87 | 470,723.28 | 45.23 | 0.93 | 2.64 ** | 86.10 | 2.01 * | 10.32 ** | 54.68 | 493.35 | 15.70 |
| P9 | 726.91 a | 20.78 b c | 0.41 | 5.11 | 290,372.29 | 23.02 | 1.11 | 0.10 | 97.42 | 1.15 | 7.65 ** | 33.75 | 695.76 | 19.96 |
| P11 | 1045.63 a | 19.71 b c | 26.33 | 1.23 | 55,876.87 | 5.69 | 1.54 ** | 62.12 ** | 47.06 | 0.89 | 4.53 * | 31.94 | 695.78 | 18.51 |
| P21 | 952.72 a | 20.64 b c | 37.26 | 4.42 | 117,960.49 | 9.89 | 0.87 | 97.49 ** | 15.53 | 1.60 | 1.77 | 74.35 | 566.32 | 19.09 |
| P31 | 563.06 a | 19.94 b c | 1.98 | 3.69 | 485,696.85 | 11.09 | 1.12 | 4.20 ** | 85.90 | 0.78 | −0.05 | 62.19 | 825.59 | 19.27 |
| P14 | 636.95 a | 20.27 b c | 9.19 | 1.72 | 565,202.05 | 13.48 | 0.95 | 23.58 ** | 46.52 | 1.80 | −1.32 | 99.89 | 559.88 | 18.79 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira Neto, S.S.; Zeffa, D.M.; Freiria, G.H.; Zoz, T.; da Silva, C.J.; Zanotto, M.D.; Sobrinho, R.L.; Alamri, S.A.; Okla, M.K.; AbdElgawad, H. Adaptability and Stability of Safflower Genotypes for Oil Production. Plants 2022, 11, 708. https://doi.org/10.3390/plants11050708
de Oliveira Neto SS, Zeffa DM, Freiria GH, Zoz T, da Silva CJ, Zanotto MD, Sobrinho RL, Alamri SA, Okla MK, AbdElgawad H. Adaptability and Stability of Safflower Genotypes for Oil Production. Plants. 2022; 11(5):708. https://doi.org/10.3390/plants11050708
Chicago/Turabian Stylede Oliveira Neto, Sebastião Soares, Douglas Mariani Zeffa, Gustavo Henrique Freiria, Tiago Zoz, Carlos Jorge da Silva, Maurício Dutra Zanotto, Renato Lustosa Sobrinho, Saud A. Alamri, Mohammad K. Okla, and Hamada AbdElgawad. 2022. "Adaptability and Stability of Safflower Genotypes for Oil Production" Plants 11, no. 5: 708. https://doi.org/10.3390/plants11050708
APA Stylede Oliveira Neto, S. S., Zeffa, D. M., Freiria, G. H., Zoz, T., da Silva, C. J., Zanotto, M. D., Sobrinho, R. L., Alamri, S. A., Okla, M. K., & AbdElgawad, H. (2022). Adaptability and Stability of Safflower Genotypes for Oil Production. Plants, 11(5), 708. https://doi.org/10.3390/plants11050708